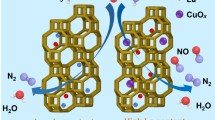
Abstract
Series of Ti/Cu-SSZ-13 zeolite catalysts with variable Ti content were prepared via convenient in-situ one-pot synthesizing strategy. Systematic evaluations of the NH3-SCR catalytic performance over the obtained catalysts were conducted. Results show that Ti/Cu-SSZ-13 with appropriate Ti content (in the current work Ti0.81/Cu2.15-SSZ-13) could serve as capable candidate for NH3-SCR application, as it exhibits highly efficient catalytic activity with expanded operation temperature window width from 140 to 540 °C, nearly 100% N2 selectivity, as well as superior tolerability against water vapor and SO2. Further structural/physicochemical characterizations demonstrate that the obtained Ti/Cu-SSZ-13 catalysts possess well-crystallized characteristic chabazite (CHA) structure. Isolated Cu2+ and monomeric Ti4+ are recognized as the primary active species, as the former mainly contributes to SCR reaction at low temperatures, while the latter are conducive for improving the high temperature SCR activity. Ti over doping would result in partial destruction of the zeolite structure, occupation of Cu2+ cation sites and formation of surface aggregated TiOx, thus leading to unsatisfactory NH3-SCR performances. Moreover, formation of agglomerated CuOx species during hydrothermal ageing and blockage of surface active sites by sulfate species formed during SO2 pretreatment are considered responsible for activity deterioration in the tolerability tests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The rapid development of modern transportation has brought great convivence to modern society, but the vehicle exhaust pollution has been come one of the most pressing threats to both environmental sustainability and human health simultaneously. Diesel engine vehicles with lean combustions technology have attracted more and more attentions in automobile industry, owning to their higher thermal efficiency as well as low hazardous exhaust emission [1, 2]. Yet, nitrogen oxides (NOx) emitted from diesel vehicles are recognized as main culprit for acid rain, photochemical smog, ozone layer depletion, as well as human respiratory and cardiovascular diseases [3, 4]. Among various techniques developed for eliminating NOx emission in diesel engine exhaust, selective catalytic reduction of NOx using NH3 as reductant (NH3-SCR) has been widely employed as the most practical and effective approach [5, 6]. Highly efficient catalysts are the critical materials for NH3-SCR technology. V2O5-WO3-TiO2 complex oxides (VWTi) have become the most widely employed commercial catalysts in SCR systems since early 1970s [7, 8]. Nevertheless, with more progressively strict emission regulations and fuel efficiency standards, serious challenges are raised against VWTi systems such as insufficient performance under lean combustion condition, limited operation temperature window width, unsatisfactory thermal stability, weak tolerability against SO2 let along the toxic effect of V5+ containing substance [9,10,11], therefore pushing forward the growing needs for investigating novel catalysts for SCR application.
Over the past few decades, Cu-based zeolites have aroused considerable research attention. For instance, Cu-ZSM-5, Cu-beta, Cu-USY, and Cu-SAPO-34 etc. [12,13,14] have all been proposed advantageous for NH3-SCR. Among various zeolites explored, Cu-SSZ-13 zeolites with characteristic chabazite (CHA) structure are currently the most state-of-the-art materials [15,16,17]. Gao et al. [18] reported that Cu-SSZ-13 presented superior NH3-SCR activity, low selectivity towards N2O and relative higher hydrothermal stability comparing to either Cu-beta or Cu-ZSM-5. Kwak et al. [19] reported Cu-SSZ-13 could maintain remarkable catalytic activity between 300 and 400 °C even after rigorous hydrothermal ageing. On the other hand, maintaining SCR reactivity at higher temperature range is of equal importance for developing novel SCR catalysts, since the exhaust gas could be extremely hot especially during vehicle rapid acceleration. Unfortunately, Cu/SSZ-13 catalysts display insufficient SCR reactivity at temperatures above 450 °C, which severely hinders its practical applications [20]. Various researchers [21,22,23] have proposed that one possible solution is doping transitional metals into Cu-SSZ-13. For example, Cu/Fe-SSZ-13 catalyst [24,25,26] is reported to exhibited enhanced activity with relatively broadened operation temperature window width, but its high-temperature selectivity and tolerability against H2O/SO2 still needs further alleviation [27,28,29]. Ti as transitional metal with weak acidity, presents similar chemical properties to Cu and Fe, and has good resistance to sulfur poisoning. Ti4+ ions can also better coordinate into Si-Al zeolites framework and sever as active centers without changing the original zeolite topology. Therefore, constructing Ti/Cu-SSZ-13 system with proper composition control might be potentially promising for optimizing NH3-SCR reactivity at more broadened temperature range. Also, the possible connections between the catalytic performance and active species requires further exploration to provide theoretical support. Moreover, the traditional method for synthesizing of SSZ-13 zeolite requires the addition of high-price organic template agent (N,N,N-trimethyl-1-adamantammonium hydroxide, TMAdaOH) and multi-step ion-exchange procedures [30], making the preparation of bimetallic SSZ-13 catalysts very complicated, costly and unpractical for industrial application. Thus, developing simple one-pot strategy for synthesizing Ti/Cu-SSZ-13 catalyst is also very necessary.
In the present work, Ti/Cu-SSZ-13 zeolite catalysts with variable Ti loading content were prepared via in-situ one-pot synthesizing strategy. The prepared Ti/Cu-SSZ-13 catalysts were subjected to NH3-SCR catalytic performances evaluation including activity tests as well as durability tests against H2O/SO2. Detailed characterizations such as X-ray diffraction (XRD), Hydrogen temperature-programmed reductions (H2-TPR), X-ray photoelectron spectroscopies (XPS) and ultraviolet visible spectroscopies (UV–Vis) were also performed. The scope of this work is (i) to propose Ti/Cu-SSZ-13 zeolite catalysts with excellent NH3-SCR activity and durability using effective one-pot synthesizing strategy along with proper composition tuning; and (ii) to gain possible new insights into the effect of Ti doping on the structure–activity correlations of NH3-SCR reaction over Ti/Cu-SSZ-13 zeolites.
2 Experimental Section
2.1 Catalysts Preparation
2.1.1 Syntheses of Ti/Cu-SSZ-13 Zeolites
Ti/Cu-SSZ-13 zeolites with variable Ti loading content were synthesized via in-situ one-pot strategy according to following steps: (i) certain quantity of CuSO4·was dissolved in deionized water and kept stirring for 0.5 h; (ii) then 1.2 g Tetraethylenepentamine (TEPA) was injected and stirred for another 1 h; (iii) C10H14O5Ti was chosen as Titanium source and was added into the solution along with certain amount of NaOH and NaAlO sequentially; (iv) 3.6 mL colloidal silica was dripped dropwise under 3 h continuous stirring; (v) the mixed solution was transferred to a 100 mL Teflon lined stainless steel autoclave and hold on at 140 °C for 96 h hydrothermal reaction; (vi) the hydrothermal product was dried at 100 °C overnight followed by 12 h acid-leaching with dilute nitric acid at 80 °C; (vii) the obtained precursor was finally calcined at 550 °C in a muffle furnace for 4 h to acquire the designed catalyst.
The different Ti loading content was controlled by varying C10H14O5Ti quantity (i.e., x = 0, 0.05, 0.1, 0.15, 0.2, 0.25) and the actual Ti/Cu content of the prepared Ti/Cu-SSZ-13 zeolites was validated using ICP-AES analyses. The obtained catalysts were denoted according to the corresponding ICP results as Ti0.40/Cu2.53-SSZ-13, Ti0.52/Cu2.38-SSZ-13, Ti0.81/Cu2.15-SSZ-13, Ti1.08/Cu1.68-SSZ-13 and Ti1.34/Cu1.47-SSZ-13, respectively (subscript numbers representing different weight percentage).
2.1.2 Hydrothermal Ageing and SO2 Pretreatment
The obtained Ti/Cu-SSZ-13 sample with the most outstanding SCR activity was subjected to hydrothermal ageing at 750 °C for 24 h using air flow with 10 vol. % steam. The hydrothermal aged catalysts were designated using suffix “-aged”.
Similarly, the optimized Ti/Cu-SSZ-13 sample were also put through SO2 pretreatment at 350 °C for 16 h using 100 ppm SO2 with Ar as balancing gas flow. Catalysts after SO2 pretreatment were designated using as suffix “-SO2”.
All the mentioned catalysts were tableted, grinded, and sieved to approximately 40 to 60 mesh before conducting further tests.
2.2 NH3-SCR Catalytic Performance Tests
NH3-SCR catalytic performance tests were performed using self-construct fixed bed reactor. The reaction atmosphere contains 5 vol. % O2, 500 ppm NOx (490 ppm NO + 10 ppm NO2), 500 ppm NH3 and Ar are used as balancing gas. The inlet reaction gas flow was set at an hourly space velocity (GHSV) of 50,000 h− 1. Actual composition of both inlet and outlet gas was analyzed online quantitatively using an FTIR spectrometer (Bruker EQUINOX 55, Bruker Corporation, United States) attached with a multi-pass gas cell (1.33 L cell volume and 10.0 m light path length). NOx conversions and N2 selectivity were determined based on the following Eqs. (1) and (2):
2.3 Catalysts Characterizations
X-ray diffraction analyses (XRD) was performed on an X-ray diffractometer (Shimazu XRD 7000, SHIMADZU Corporation, Japan) equipped with monochromated Cu Kα radiation. The diffractograms were recorded from 5° to 70° (2θ) at 0.02° step size. H2 temperature-programmed reductions (H2-TPR) were carried out on an automatic chemisorption apparatus (ChemBET3000, Quantachrome Instruments, USA). 100 mg sample was first pretreated at 400 °C using 40 mL min− 1 Ar flow for 30 min. Upon cooling down to 50 °C, 5 vol. % H2/Ar was introduced into the reactor with 40 mL min− 1 flow rate till baseline stabilization. The reactor temperature was elevated to 950 °C at 10 °C min− 1 ramping rate and H2-TPR profiles were collected synchronously by measuring thermal conductivity detector (TCD) signals. X-ray photoelectron spectroscopies (XPS) were performed on a Thermo ESCALAB 250Xi apparatus (Themo Fisher Scientific Corporation, USA) employed with monochromatic Al Kα source. C 1 s peak at 284.8 eV were used as internal label to calculate binding energies of each element tested. Ultraviolet visible spectroscopies (UV–Vis) were performed on a UV/Vis spectrometer (UV 2450, SHIMADZU Corporation, Japan). Sepctra were recorded at room temperature with wavelength ranging from 200 to 800 nm and BaSO4 was referenced as background.
3 Results and Discussion
3.1 NH3-SCR Catalytic Performances
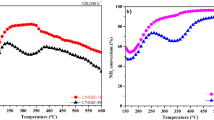
Figure 1a presents the NOx conversion plots of the synthesized Ti/Cu-SSZ-13 catalysts. The operation temperature window width (corresponding temperature ranges wherein NOx conversion stays above 90%) follows the sequence as: Ti0.81/Cu2.15-SSZ-13 (140–540 °C) > Ti1.08/Cu1.68-SSZ-13 (140–530 °C) > Ti0.52/Cu2.38-SSZ-13 (140–520 °C) > Ti1.34/Cu1.47-SSZ-13 (145–505 °C) > Ti0.40/Cu2.53-SSZ-13 (145–495 °C) > Cu3.25-SSZ-13 (145–490 °C). In comparison with Cu3.25-SSZ-13 catalysts, although the low temperature activity has no obvious change (T90 value are quite similar at temperature below 200 °C), the high temperature activity of Ti/Cu-SSZ-13 is clearly improved first and then starts to decrease with increasing Ti content, making the operation temperature window width broadened first and then narrowed. It is worth noticing that the Ti0.81/Cu2.15-SSZ-13 catalyst (i.e., with 0.81 wt.% Ti content) displays the most broadened operation temperature window width from 140 to 540 °C, which suggests that doping appropriate amount of Ti into Cu-SSZ-13 can significantly promote the high-temperature activity for NH3-SCR reaction. Figure 1b shows the N2 selectivity over the prepared Ti/Cu-SSZ-13 catalysts. As can be seen, all the Ti/Cu-SSZ-13 catalysts possess over 95% N2 selectivity, especially for Ti0.81/Cu2.15-SSZ-13 catalyst which shows nearly 100% N2 selectivity during the whole testing temperature range. These results suggest that Ti/Cu-SSZ-13 catalyst with proper Ti doping content (in this work 0.81 wt.%) presents catalytic advantages comparing to single Cu-SSZ-13 and could be capable for efficent NOx elimination.
3.2 Catalysts Tolerability Against H2O/SO2
Tolerability of the zeolite-based catalysts against H2O/SO2 is another crucial aspect for evaluating the practicality regarding the purification of diesel engine exhaust. On one hand, these catalysts generally operate long-time in high temperature exhaust gas containing large proportion of water vapor, which are prone to problems such as dealumination, structure collapse or active species sintering, causing activity deterioration. On the other hand, trace amount of sulfur-containing components in the exhaust gas can easily cause catalysts poisoning, which has long been critical issues for elongating catalysts lifetime. Based on the activity results above, to further evaluating the tolerability against H2O/SO2, we herein choose Ti0.81/Cu2.15-SSZ-13 catalyst as the most outnstanding candidate and subsquentially underwent hydrothermal ageing as well as SO2 pretreatment before conducting NH3-SCR tests.
3.2.1 Hydrothermal Ageing
Figure 2 shows the NH3-SCR catalytic performance of Ti0.81/Cu2.15-SSZ-13 and Cu3.25-SSZ-13 catalysts beofore and after hydrothermal ageing. For Cu3.25-SSZ-13, drastically diminution of the operation temperature window width occurs after hydrothermal ageing (fresh: 145–490 °C and aged: 160–450 °C). While for Ti0.81/Cu2.15-SSZ-13 catalyst, the operation temperature window can be preserved from 175 to 490 °C after 24 h hydrothermal ageing. Although the the operation temperature window of Ti0.81/Cu2.15-SSZ-13-aged was deteriorated about 70 °C compared to the fresh catalyst, it still shows clear advantages over single Cu3.25-SSZ-13. Meanwhile, Fig. 2b also shows that N2 selecvitiy of Ti0.81/Cu2.15-SSZ-13 at high temperature range (> 450 °C) is slightly dropped after hydrothermal ageing, but it still remains quite advantagous comparing to Cu3.25-SSZ-13-aged. These results validate that doping an appropriate amount of Ti into Cu-SSZ-13 catalyst can also benefiting its tolerability against water vapor as well as maintaining excellent N2 selecvitiy.
3.2.2 SO2 Pretreatment
The tolerability against sulfur poisoning of Ti0.81/Cu2.15-SSZ-13 catalyst was evaluated by SO2 pretreatment tests. As shown in Fig. 3a, the operation temperature window of Cu3.25-SSZ-13 ranges from 165 to 470 °C after 16 h SO2 pretreatment at 350 °C. While for Ti0.81/Cu2.15-SSZ-13 catalyst, the operation temperature window stays from 180 to 520 °C after SO2 pretreatment. The operation temperature window deterioration induced by SO2 pretreatment is relatively more moderate than that of hydrothermal ageing. Similarly, despite its slightly hindered low temperature activity after SO2 pretreatment, Ti0.81/Cu2.15-SSZ-13-SO2 still maintains much better NOx eliminating capability at high temperatures than Cu3.25-SSZ-13-SO2 along with more broadened operation temperature window, as notably its T90 value in the high temperature range was only deteriorated by 20 °C. Besides, high N2 selectivity (~ 100%) over Ti0.81/Cu2.15-SSZ-13-SO2 can still be observed after SO2 pretreatment (Fig. 3b). These resutls demonstrate that the superior toleratbility againsts sulfur-poisoning can be achieved by doping appropriate amount of Ti.
In order to better simulate actual operating condition for exhaust purification of diesel engines, SO2 poisoning tolerability of Ti0.81/Cu2.15-SSZ-13 catalyst was further evaluated by applying SO2 “on/off” testing method during SCR reaction. As shown in Fig. 4, for both Ti0.81/Cu2.15-SSZ-13 and Cu3.25-SSZ-13 samples, the NOx conversions remain stable around 100% before injecting SO2 into the SCR reaction inlet gas. Once SO2 is injected, immediate drop of NOx conversion over both catalysts can be observed. Higher concentration of injected SO2 could result in more drastically deteriorated SCR reactivity. For Cu3.25-SSZ-13, only about 35% NOx conversion gets to be retained after 30 h reaction with 10 ppm SO2 injection. Increasing SO2 concentration above 50 ppm causes more severe damage as it takes less than 5 h for the NOx conversion to drop below 50%. When SO2 concentration increased to 100 ppm, the SCR activity was almost completely deteriorated after 5 h reaction. These results indicate poor tolerability against sulfur poisoning of single Cu-SSZ-13 catalyst. For Ti0.81/Cu2.15-SSZ-13 catalyst, NOx conversion can hold on to nearly 100% for 12 h with 10 ppm SO2 injection and can remain above 75% after 30 h. Increasing SO2 concentration to 50 ppm, full conversion of NOx (~ 100%) can still be retained for more than 5 h. Continuing to increase SO2 concentration to 100 ppm, NOx conversion can maintain about 20% after the initial 5 h reaction. Such significant differences further demonstrate the superiority of Ti0.81/Cu2.15-SSZ-13 in toleratbility againsts sulfur-poisoning. Besides, once SO2 is remove away from the reaction inlet, NOx conversion over neither Ti0.81/Cu2.15-SSZ-13 nor Cu3.25-SSZ-13 was able to return to the original stage. These results imply that the SO2 poisoning cannot be simply explained by the competitive adsorption between SCR reactant and SO2, but rather attributed to active sites blockage by the formation sulfate species.
Based on all the above results, it is well demonstrated that doping Cu-SSZ-13 catalysts with appropriate amount of Ti can not only improve the NH3-SCR activity, but also increase its tolerability against hydrothermal ageing and SO2 poisoning. Ti0.81/Cu2.15-SSZ-13 catalyst in this work presents widely broadened operation temperature window, excellent N2 selectivity as long with superior H2O/SO2 tolerability, making it potentially capable candidates for NH3-SCR application in diesel engine vehicles.
3.3 Crystalline Structure by XRD
The crystal phase structures of the Ti/Cu-SSZ-13 catalysts were characterized by XRD analyses (Fig. 5). Characteristics diffraction peaks locating at (2θ = 9.5°, 12.8°, 14.0°, 16.1°, 17.8°, 20.7°, 25.0° and 30.7° can be observed on all the samples, which can be assigned to typical chabazite (CHA) structure (JCPDS #34-0137) [20, 31]. The sharp and clear peak shapes along with relatively high intensity reflects the formation of good crystallinity and further demonstrates that the in-situ one-pot synthesis strategy in this work are quite successful. Neither of the diffraction peaks assigning to CuOx (2θ = 35.6° and 38.8°) nor TiOx (2θ = 36.8°, 37.6 o, and 39.1°) can be observed, which can be explained as the content are too low to be detected by XRD or the copper/titanium oxide species are well distributed in the zeolite matrix [32]. With increasing Ti content, the intensity of CHA characteristic peaks gradually decreased with broaden peak shape, indicating that higher Ti introduction can lead to relatively weakened zeolite crystallinity. Such crystallinity decrease could result from the incorporation of Ti ions into the zeolite framework. Due to the same coordinate numbers of Ti4+ and Si4+ ions, Ti4+ ions are most likely to substitute part of Si4+ ions in the zeolite framework to form a tetrahedral coordination structure [33]. The ionic radius of Ti4+ is larger than Si4+ ions, thus Ti4+ incorporation will expand the interplanar spacing and cause partial destruction of the zeolite structure. On the other hand, with increasing Ti content, some of the cation sites in the zeolite cages will also be occupied by Ti4+ ions, which could impair the capacity to accommodate Cu2+ species, therefore the number of Cu species entering the SSZ-13 cages will also decreased. Moreover, textural characterization results of Cu3.25-SSZ-13 and Ti0.81/Cu2.15-SSZ-13 (see the supplymentary information) show that Ti introduction would lead to slightly decreased specific surface area along with and pore volume, which might be related to the blockage of microporous channels by part of the Ti species. SEM photos of Cu3.25-SSZ-13 and Ti0.81/Cu2.15-SSZ-13 were also recorded to give direct observation of the catalysts’ morphologies (Fig. S1 in the supplymentary information), and the results shown that both samples present similar morphologies consisting of cube-shaped crystals, which suggests that doping small amount of Ti into Cu-SSZ-13 zeolites does not change the zeolite morphology. Correlating the activity results of Cu3.25-SSZ-13 and Ti0.81/Cu2.15-SSZ-13, it’s reasonable to say that such textural/morphological properties change here in our work may not be the key factor determining SCR activity.
XRD profiles of Ti0.81/Cu2.15-SSZ-13 catalysts after hydrothermal ageing and SO2 pretreatment are also presented in Fig. 5. For Ti0.81/Cu2.15-SSZ-13-aged, although obvious broadened peaks with decreased intensity can be seen, most of the featured CHA structure can be preserved in a certain degree, indicating that the zeolite structure is not completely destroyed during hydrothermal ageing but rather partial crystallinity damage and formation of amorphous phase. Such structure evolutions are likely to be one of the reasons explaining the inferior SCR activity upon hydrothermal ageing. For Ti0.81/Cu2.15-SSZ-13-SO2, there is no significant changes in the diffraction profiles, suggesting that SO2 pretreatment does not bring much impact to the crystalline structure of Ti0.81/Cu2.15-SSZ-13 catalyst, which is also an important reason explaining why its SCR performance does not deteriorate significantly after SO2 pretreatment. It’s worth noticing that the diffraction peak at 2θ = 26° becomes much higher after SO2 treatment. Although it is quite difficult to give precise attribution of this single peak, a reasonable assumption could be that this peak indicate the formation of Titanium sulfate (JCPDS # 18-1406) or Aluminum Titanium sulfate (JCPDS # 28-0035).
3.4 Redox Properties by H2-TPR
The redox properties of the prepared Ti/Cu-SSZ-13 catalysts were examined by H2-TPR analyses (Fig. 6). Four major reduction peaks can be observed over all the samples. Based on our previous reports [20, 24] and other reports [25, 34, 35], three peaks (α, β, γ) at temperatures below 500 °C reflect the reduction of different isolated Cu2+ species coordinated in various sites of the CHA structure. To be specific, peak α at 181 °C is attributed to the reduction of isolated Cu2+ near octagonal window (Cu1 species in site IV); peak β at 249 °C is ascribed to the reduction of isolated Cu2+ inside the super cage diverging from the dual hexatomic ring (Cu2 species in Site I); peak γ at 366 °C is assigned to the reduction of isolated Cu2+ located in the hexagonal prism center (Cu3 species in Site III). The high temperature peak η above 700 °C corresponds to the reduction of monovalent Cu+ to metallic Cu0. No reduction peak assigning to Ti species is observed since titanium oxides are generally reported to be reduced at temperatures higher than 1000 °C. With increasing Ti content, areas of both peak α and peak β diminishes moderately while peak γ is expanded with enlarged area. Various reports [34, 36, 37] have clarified that Cu3 species located at site III are more stable due to greater steric hindrance of the hexagonal prism. Therefore, the peak diminishment with increasing Ti content indicates that Cu1 and Cu2 species gradually disappear due to site occupation by Ti species or possible migration of Cu species, which then leads to unsatisfying low temperature SCR activity of Ti/Cu-SSZ-13 with high Ti contents.
H2-TPR profiles of Ti0.81/Cu2.15-SSZ-13 catalysts after hydrothermal ageing and SO2 pretreatment are also shown in Fig. 6. Comparing to the fresh catalyst, both peaks α and peak β disappear substantially in the H2-TPR profile of Ti0.81/Cu2.15-SSZ-13-aged sample, indicating significant loss of isolated Cu2+ species. Meanwhile, a new peak δ around 460 °C overlapping peak γ appears, which is related to the reduction of small CuOx agglomerates [38]. These agglomerated CuOx clusters are most likely to form in the course of hydrothermal ageing period and are considered responsible for facilitating unfavorable NH3 oxidation [39], thus causing narrower operation temperature windows. Similar TPR profile can be seen for Ti0.81/Cu2.15-SSZ-13-SO2 sample as all three peaks (α, β and γ) diminish and shift to higher temperatures, accompanied by the appearance of peak δ with much smaller area than that of Ti0.81/Cu2.15-SSZ-13-aged. These results demonstrate that the relative content of isolated Cu2+ species also declined after SO2 pretreatment, but the formation of CuOx species was not as severe as that after hydrothermal ageing. Therefore, the high temperature SCR activity of Ti0.81/Cu2.15-SSZ-13-SO2 is relatively better than that of Ti0.81/Cu2.15-SSZ-13-aged.
3.5 Surface Chemistry by XPS
The surface chemistry of the prepared Ti/Cu-SSZ-13 catalysts was characterized by XPS analyses. Figure 7 shows the XPS spectra processed via multi-peak fitting method and Table 1 summaries the corresponding element distribution data. Figure 7a presents the Ti 2p spectra. Two sets of characteristic peaks around 458.0 eV (Ti 2p1/2) and 464.5 eV (Ti 2p3/2) can be observed, which can be further differentiated and attributed to Ti4+ sepcies (458.5 and 464.5 eV) and Ti3+ sepcies (460.2 and 466.2 eV) resepectively [40]. As shown in Table 1, with increasing Ti content, the amount of Ti4+ species on the catalyst surfaces increases first and then decreases, following the order of Ti0.81/Cu2.15-SSZ-13 > Ti1.08/Cu1.68-SSZ-13 > Ti0.52/Cu2.38-SSZ-13 > Ti1.34/Cu1.47-SSZ-13 > Ti0.40/Cu2.53-SSZ-13, which is in consistent with their order of SCR operation temperature window width. Considering that the low temperature activity of all the Ti/Cu-SSZ-13 catalysts are very close, these results suggest that the abundant surface Ti4+ species are conducive for elevating the high temperature SCR reactivity. Figure 7b presents the Cu 2p spectra. All the catalysts present typical Cu 2p3/2 characteristic peaks around 933.0 eV. The Cu 2p3/2 peaks can be further deconvoluted into two peaks, whereas peak around 933.2 eV represents the surface Cu+ species, while peak around 936.2 eV is attributed to isolated Cu2+ species coordinated in the SSZ-13 zeolite frame structure [24, 41, 42] as Ti doping content increases, the total amount of Cu species gradually decreases. Meanwhile, the relative content of isolated Cu2+ species coordinated in the zeolite framework also slightly decreases possbily due to the occupation of charge compensation sites by Ti species. Since isoltaed Cu2+ species are generally recognized as the predominant active species of Cu zeolite catalyts contributing to SCR reactiviy in low temperature ranges, therefore the over-doping of Ti slightly hinders the low temperature NH3-SCR activities of Ti/Cu-SSZ-13.
3.6 Identification of Ti Species by UV–Vis
To gain better insights regarding the identification of different Ti species in the synthesized Ti/Cu-SSZ-13 catalysts, UV–Vis analyses were conducted, and the obtained spectra are shown in Fig. 8 below together with the peak deconvolution results. The bands observed around 210 nm are assigned to the charge transfer of Ozeolite → Cu2+. Two bands assigning to Ti species can be seen around 220 nm and 280 nm, respectively. The former is attributed to the charge transfer from the excitation of O 2p valent electron to the empty d orbitals of monomeric Ti4+ ions, while the latter represents the charge transfer of Ti = O group in the aggregated TiOx particles over the SSZ-13 surface [43,44,45]. Table 2 present the relative content of these two Ti species estimated semi-quantitatively based on peak-deconvoluting results. It is quite clear that the monomeric Ti4+ species are predominant at lower Ti loading content; while the amount of monomeric Ti4+ species turns to decrease and more aggregated TiOx appears with increasing Ti content. Such evolution between different Ti species be elucidated as: at low Ti content, the majority of doped Ti prefers to occupy either protonic charge-compensating H+ sites or the Si4+ sites in the frame structure of SSZ-13 zeolite; Increasing Ti loading leads to saturation of these protonic H+ sites and the Si4+ sites, thus the over doped Ti is very likely to aggregate over catalyst’s surface and thereby decreasing the relative content of monomeric Ti4+ species. Combining activity evaluation results above, we can see that the distribution and chemical state of different Ti species mainly affect the high temperature NH3-SCR reactivity of the Ti/Cu-SSZ-13 catalyst. Higher content of mononuclear Ti4+ species could improve the high temperature reactivity, while the presence of more aggregated TiOx would lead to the narrowed operation temperature window instead. Ti0.81/Cu2.15-SSZ-13 sample synthesized via in situ one-pot strategies not only contains more mononuclear Ti4+ species, but also has relatively low content of surface aggregated TiOx specie, therefore presents the best NH3-SCR catalytic performances.
3.7 Discussions of the Correlations Between Active Species and SCR Reactivity
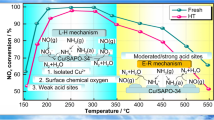
Various previous studies have focused on the identification of active Cu species for the NH3-SCR over Cu-SSZ-13 zeolites. Series of different forms of Cu species such as isolated Cu2+/Cu+ species, dimeric Cu species as well as CuOx have all been suggested to be active sites. Wang et al. [14, 46] demonstrated that for Cu-based small-pore zeolites, agglomerated CuOx clusters are very unlike to be the primary active sites as CuOx clusters would accelerate the unfavorable NH3 oxidation. Giordanino et al. [47] reported that dimeric Cu species contribute very little to SCR reactivity as they are quite unlikely to exist stably in Cu-SSZ-13. Bates et al. [48] suggested that the kinetically rate-determining steps for standard SCR mainly take place on the isolated Cu2+ species located in exchange sites via UV–Vis-NIR and XAS identifications. Zhang et al. [26] reported that the isolated Cu2+ ions play significant roles in maintaining higher NH3-SCR activity at low-temperature in Fe-Cu-SSZ-13 catalyst using UV–Vis and EPR analyses. Our previous research [20] demonstrated that for Cu-SSZ-13 isolated Cu2+ species exist in three different forms, which are contributive for low-temperature NH3-SCR activity improvement. In the current work, neither dimeric Cu species nor CuOx clusters are observed, but instead isolated Cu2+ species along with Cu+ are evidenced as the primary existing Cu species. Considering the reduction temperature of Cu+ goes high above 700 °C, Cu+ species can be reasonably ruled out to be possible active sites. Hence, we hereby propose that excellent low temperature reactivity of Ti/Cu-SSZ-13 relies on to the contribution of isolated Cu2+ species (Fig. 9). With increasing Ti doping content, these isolated Cu2+ species partially decreases possbily due to sites occupation by Ti, thus the low temperature SCR reactivity is slightly deterred.
On the other hand, effective improvement of NH3-SCR activity at high temperatures by doping transitional metal ions into Cu-SSZ-13 system have also been confirmed by many studies. Our previous studies [24] demonstrated that doping Fe into Cu-SSZ-13 could generate sufficient monomeric Fe3+ species, acting as critical sites for sustaining the high temperature SCR activity, which are in good consistent with other researchers’ reports [21,22,23]. However, the strong oxidizing ability of multi valent transitional metal ions (for example Fe3+) would facilitate undesired NH3 oxidation [49]. Hence, Ti with moderate oxidation ability and with weak acidity could be proper substitution. On one hand, Gu et al. [50] and Geng et al. [51] reported that the introduction of Ti species can improve the surface acidity, which is beneficial for the adsorption of NH3, and finally enhances the SCR performance. Song et al. [52] revealed that Ti doping can also lower the activation energy for NO oxidation step via DFT calculations. In the present work, the monomeric Ti4+ species are verified as predominant Ti species. According to the above literatures and combing the activity-structure results, it can be proposed that the dominant active sites for low temperature SCR reaction can be ascribed to isolated Cu2+ species, while high temperature SCR reactivity mainly relies on the contribution of monomeric Ti4+ species, therefore by introducing proper amount of Ti into Cu-SSZ-13 can significantly expand its operation temperature windows width. On the other hand, Kunitake et al. [53] proposed that incorporation of a small amount of Ti into the CHA framework may have decreased the stress of the zeolite framework, thus being very useful to improve the thermal/hydrothermal stability. Imasaka [54] revealed that crystal structure of the zeolites collapsed because of the desorption of Al from the framework after ageing while improvement of the thermal stability over Ti-CHA zeolite was expected owing to the he incorporation of Ti and substitution of aluminum into the zeolite framework. Therefore, Ti introduced Cu/Ti-SSZ-13 also exhibited high hydrothermal stability. However, Ti over doping would result in partial destruction of the zeolite structure, occupation of Cu2+ cation sites and formation of surface aggregated TiOx species, thus leading to unsatisfactory NH3-SCR performances.
Moreover, although the affection of hydrothermal ageing and SO2 poisoning on active species evolution requires further in-depth studies and was not deeply discussed in the current manuscript, a brief explanation is proposed as follows. For Cu species, the above H2-TPR results suggest agglomerated CuOx species formed during hydrothermal ageing possibly due to migration of isolated Cu2+ species. While after SO2 pretreatment, our previous EPR studies [20] on SO2 pretreated Cu-SSZ-13 samples shows that the coordination environment of Cu2+ species didn’t change a lot and the content of isolated Cu2+ species decreased due to blockage by sulfate species formed during SO2 pretreatment, which are considered responsible for activity deterioration in the tolerability tests. For Ti species, since most of the featured CHA structure can be preserved in a certain degree during hydrothermal ageing, thus it’s reasonable to deduce that most of Ti could still be incorporated into zeolite framework after hydrothermal ageing. While after SO2 pretreatment, both the XRD and ex-situ FITR (see the supplymentary information) results indicate the existence of surface sulfates, thereby deteriorating NH3-SCR activity.
4 Conclusion
Ti/Cu-SSZ-13 zeolite catalysts with variable Ti loading content were prepared via convenient in-situ one-pot synthesizing strategy and underwent systematic NH3-SCR performance evaluations. Among the Ti/Cu-SSZ-13 catalysts tested, the optimized Ti0.81/Cu2.15-SSZ-13 catalyst in this work presents wide-expanded operation temperature window width ranging from 140 to 540 °C, excellent N2 selectivity along with superior H2O/SO2 tolerability, making it potential candidate for diesel-engines exhaust purification. Detailed characterizations demonstrate that the synthesized Ti/Cu-SSZ-13 catalysts present well-crystallized characteristic CHA structure. The catalytic advantages of Ti0.81/Cu2.15-SSZ-13 catalyst can be ascribed to the contribution of both Cu and Ti active species, as the isolated Cu2+ species serve as major active sites for SCR reaction at low temperatures, while the abundant monomeric Ti4+ species are conducive for improving the high temperature SCR activity. Ti over doping contrarily leads to inferior NH3-SCR reactivity, resulting from partially- destructed zeolite structure, occupation of Cu2+ cation sites as well as formation of surface aggregated TiOx species. Besides, hydrothermal ageing causes formation of agglomerated CuOx while sulfate species generated during SO2 pretreatment block surface active sites, therefore explaining the corresponding activity deterioration.
References
Boretti A (2017) The future of the internal combustion engine after “diesel-gate.” SAE Technical Paper, p 1933
Joshi A (2019) Review of vehicle engine efficiency and emissions. SAE Int J Adv Curr Prac Mobility 1:734–761
Pourvakhshoori N, Khankeh HR, Stueck M, Farrokhi M (2020) The association between air pollution and cancers: controversial evidence of a systematic review. Environ Sci Pollut Res 27:38491–38500
Qu Y, An J, He Y, Zheng J (2016) An overview of emissions of SO2 and NOx and the long-range transport of oxidized sulfur and nitrogen pollutants in East Asia. J Environ Sci 44:13–25
Shelef M, McCabe RW (2000) Twenty-five years after introduction of automotive catalysts: what next? Catal Today 62:35–50
Zhang NQ, He H, Wang DS, Li YD (2020) Challenges and opportunities for manganese oxides in low-temperature selective catalytic reduction of NOx with NH3: H2O resistance ability. J Solid State Chem 289:121464
Lian ZH, Li YJ, Shan WP, He H (2020) Recent progress on improving low-temperature activity of vanadia-based catalysts for the selective catalytic reduction of NOx with ammonia. Catalysts 10:1421
Topsøe NY (1994) Mechanism of the selective catalytic reduction of nitric oxide by ammonia elucidated by in situ on-line Fourier transform infrared spectroscopy. Science 265:1217–1219
Liu JN, Huang Y, Li HY, Duan HR (2022) Recent advances in removal techniques of vanadium from water: a comprehensive review. Chemosphere 287:132021
Szymaszek A, Samojeden B, Motak M (2020) The deactivation of industrial SCR catalysts—a short review. Energies 13:3870
Zhang QJ, Wu YF, Yuan HR (2020) Recycling strategies of spent V2O5-WO3/TiO2 catalyst: a review. Resour Conserv Recy 161:104983
Ma YY, Liu Y, Li ZF, Geng C, Bai XF, Cao DX (2020) Synthesis of CuCe co-modified mesoporous ZSM-5 zeolite for the selective catalytic reduction of NO by NH3. Environ Sci Pollut Res 27:9935–9942
Mohan S, Dinesha P, Kumar S (2020) NOx reduction behaviour in copper zeolite catalysts for ammonia SCR systems: a review. Chem Eng J 384:123253
Wang L, Gaudet JR, Li W, Weng D (2013) Migration of Cu species in Cu/SAPO-34 during hydrothermal ageing. J Catal 306:68–77
Andana T, Rappe KG, Gao F, Szanyi J, Pereira-Hernandez X, Wang Y (2021) Recent advances in hybrid metal oxide–zeolite catalysts for low-temperature selective catalytic reduction of NOx by ammonia. Appl Catal B Environ 291:120054
Liu ZQ, Jiang H, Guan B, Wei YF, Wu XZ, Lin H, Huang Z (2022) Optimizing the distribution and proportion of various active sites for better NH3-SCR property over Cu/SSZ-13. Environ Sci Pollut Res 29:19447–19459
Shan YL, Du JP, Zhang Y, Shan WP, Shi XY, Yu YB, Zhang RD, Meng XJ, Xiao FS, He H (2021) Selective catalytic reduction of NOx with NH3: opportunities and challenges of Cu-based small-pore zeolites. Natl Sci Rev 8:nwabo10
Gao F, Mei DH, Wang YL, Szanyi J, Peden CHF (2017) Selective catalytic reduction over Cu/SSZ-13: linking homo- and heterogeneous catalysis. J Am Chem Soc 139:4935–4942
Kwak JH, Tonkyn RG, Kim DH, Szanyi J, Peden CHF (2010) Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J Catal 275:187–190
Chen JW, Zhao R, Zhou RX (2018) A new insight into active Cu2+ species properties in one-pot synthesized Cu-SSZ-13 catalysts for NOx reduction by NH3. ChemCatChem 10:5182–5189
Yin CY, Cheng PF, Li X, Yang RT (2016) Selective catalytic reduction of nitric oxide with ammonia over high-activity Fe/SSZ-13 and Fe/one-pot-synthesized Cu-SSZ-13. Catal Sci Technol 6:7561–7568
Wang JC, Peng ZL, Qiao H, Yu HF, Hu YF, Chang LP, Bao WR (2016) Cerium-stabilized Cu-SSZ-13 catalyst for the catalytic removal of NOx by NH3. In Eng Chem Res 55:1174–1182
Zhao ZC, Yu R, Shi C, Gies H, Xiao FS, De Vos D, Yokoi T, Bao XH, Kolb U, McGuire R, Parvulescu AN, Maurer S, Müller U, Zhang WP (2019) Rare-earth ion exchanged Cu-SSZ-13 zeolite from organotemplate-free synthesis with enhanced hydrothermal stability in NH3-SCR of NOx. Catal Sci Technol 9:241–251
Wan J, Chen JW, Zhao R, Zhou RX (2021) One-pot synthesis of Fe/Cu-SSZ-13 catalyst and its highly efficient performance for the selective catalytic reduction of nitrogen oxide with ammonia. J Environ Sci 100:306–316
Zhang RR, Li YH, Zhen TL (2014) Ammonia selective catalytic reduction of NO over Fe/Cu-SSZ-13. RSC Adv 4:52130–52139
Zhang T, Li JM, Liu J, Wang DX, Zhao Z, Cheng K, Li JH (2015) High activity and wide temperature window of Fe-Cu-SSZ-13 in the selective catalytic reduction of NO with ammonia. AIChE J 61:3825–3837
Hammershøi PS, Jangjou Y, Epling WS, Jensen AD, Janssens TVW (2018) Reversible and irreversible deactivation of Cu-CHA NH3-SCRcatalysts by SO2 and SO3. Appl Catal B Environ 226:38–45
Shan YL, Shi XX, Yan ZD, Liu JJ, Yu YB, He H (2019) Deactivation of Cu-SSZ-13 in the presence of SO2 during hydrothermal ageing. Catal Today 320:84–90
Wang AY, Wang YL, Walter ED, Washton NM, Guo YL, Lu GZ, Peden CHF, Gao F (2019) NH3-SCR on Cu, Fe and Cu+ Fe exchanged beta and SSZ-13 catalysts: Hydrothermal ageing and propylene poisoning effects. Catal Today 320:91–99
Zones SI, Van Nordstrand RA (1988) Novel zeolite transformations: the template mediated conversion of cubic-P zeolite to SSZ-13. Zeolites 8:166–174
Di Iorio JR, Gounder R (2016) Controlling the isolation and pairing of aluminum in chabazite zeolites using mixtures of organic and inorganic structure-directing agents. Chem Mater 28:2236–2247
Deka U, Juhin A, Eilertsen EA, Emerich H, Green MA, Korhonen ST, Weckhuysen BM, Beale AM (2012) Confirmation of isolated Cu2+ ions in SSZ-13 zeolite as active sites in NH3-selective catalytic reduction. J Phys Chem C 116:4809–4818
Mrak M, Tušar NN, Logar NZ, Mali G, Kljajić A, Arčon I, Launay F, Gedeon A, Kaučič V (2006) Titanium containing microporous/mesoporous composite (Ti, Al)-Beta/MCM-41: synthesis and characterization. Micropor Mesopor Mat 95:76–85
Gao F, Walter ED, Karp EM, Luo JY, Tonkyn RG, Kwak JH, Szanyi J, Peden CHF (2013) Structure–activity relationships in NH3-SCR over Cu-SSZ-13 as probed by reaction kinetics and EPR studies. J Catal 300:20–29
Kwak JH, Zhu HY, Lee JH, Peden CHF, Szanyi J (2012) Two different cationic positions in Cu-SSZ-13? Chem Commun 48:4758–4760
Chen ZQ, Guo L, Qu HX, Liu L, Xie HF, Zhong Q (2020) Controllable positions of Cu2+ to enhance low-temperature SCR activity on novel Cu-Ce-La-SSZ-13 by a simple one-pot method. Chem Commun 56:2360–2363
Xie LJ, Liu FD, Ren LM, Shi XY, Xiao FS, He H (2014) Excellent performance of one-pot synthesized Cu-SSZ-13 catalyst for the selective catalytic reduction of NOx with NH3. Environ Sci Technol 48:566–572
Kim YJ, Lee JK, Min KM, Hong SB, Nam IS, Cho BK (2014) Hydrothermal stability of CuSSZ13 for reducing NOx by NH3. J Catal 311:447–457
Han J, Wang AY, Isapour G, Härelind H, Skoglundh M, Creaser D, Olsson L (2021) N2O formation during NH3-SCR over different zeolite frameworks: effect of framework structure, copper species, and water. Ind Eng Chem Res 60:17826–17839
Shi YJ, Guo XL, Wang YY, Kong FZ, Zhou RX (2022) New insight into the design of highly dispersed Pt supported CeO2-TiO2 catalysts with superior activity for VOCs low-temperature removal. Green Energy Environ online. https://doi.org/10.1016/j.gee.2022.03.009
Han S, Cheng J, Zheng CK, Ye Q, Cheng SY, Kang TF, Dai HX (2017) Effect of Si/Al ratio on catalytic performance of hydrothermally aged Cu-SSZ-13 for the NH3-SCR of NO in simulated diesel exhaust. Appl Surf Sci 419:382–392
Yu R, Zhao ZC, Huang SJ, Zhang WP (2020) Cu-SSZ-13 zeolite–metal oxide hybrid catalysts with enhanced SO2-tolerance in the NH3-SCR of NOx. Appl Catal B Environ 269:118825
Bourezgui A, Kacem I, Daoudi M, Al-Hossainy AF (2020) Influence of gamma-irradiation on structural, optical and photocatalytic performance of TiO2 nanoparticles under controlled atmospheres. J Electron Mater 49:1904–1921
Thangaraj A, Kumer R, Mirajkar SP, Ratnasamy P (1991) Catalytic properties of crystalline titanium silicalites I. Synthesis and characterization of titanium-rich zeolites with MFI structure. J Catal 130:1–8
Zhou DH, Zhang HJ, Zhang JJ, Sun XM, Li HC, He N, Zhang WP (2014) Density functional theory investigations into the structure and spectroscopic properties of the Ti4+ species in Ti-MWW zeolite. Micropor Mesopor Mat 195:216–226
Wang L, Li W, Qi GS, Weng D (2012) Location and nature of Cu species in Cu/SAPO-34 for selective catalytic reduction of NO with NH3. J Catal 289:21–29
Giordanino F, Vennestrøm PNR, Lundegaard LF, Stappen FN, Mossin S, Beato P, Bordigaa S, Lamberti C (2013) Characterization of Cu-exchanged SSZ-13: a comparative FT-IR, UV-Vis, and EPR study with Cu-ZSM-5 and Cu-β with similar Si/Al and Cu/Al ratios. Dalton Trans 42:12741–12761
Bates SA, Verma AA, Paolucci C, Parekh AA, Anggara T, Yezerets A, Schneider WF, Miller JT, Delgass WN, Ribeiro FH (2014) Identification of the active Cu site in standard selective catalytic reduction with ammonia on Cu-SSZ-13. J Catal 312:87–97
Ellmers I, Vélez RP, Bentrup U, Brückner A, Grünert W (2014) Oxidation and selective reduction of NO over Fe-ZSM-5–How related are these reactions? J Catal 311:199–211
Gu JL, Duan RD, Chen WB, Chen Y, Liu LL, Wang XD (2020) Promoting effect of Ti species in MnOx-FeOx/silicalite-1 for the low-temperature NH3-SCR reaction. Catalysts 10:566
Geng Y, Chen XL, Yang SJ, Liu FD, Shan WP (2017) Promotional effects of Ti on a CeO2–MoO3 catalyst for the selective catalytic reduction of NOx with NH3. ACS Appl Mate Inter 9:16951–16958
Song ZJ, Wang B, Yu J, Ma C, Chen T, Yang W, Liu S, Sun LS (2018) Effect of Ti doping on heterogeneous oxidation of NO over Fe3O4 (1 1 1) surface by H2O2: a density functional study. Chem Eng J 354:517–524
Kunitake Y, Takata T, Yamasaki Y, Yamanaka N, Tsunoji N, Takamitsu Y, Sadakane M, Sano T (2015) Synthesis of titanated chabazite with enhanced thermal stability by hydrothermal conversion of titanated faujasite. Micropor and Mesopor Mat 215:58–66
Imasaka S, Ishii H, Hayashi JI, Araki S, Yamamoto H (2019) Synthesis of CHA-type titanosilicate zeolites using titanium oxide as Ti source and evaluation of their physicochemical properties. Micropor and Mesopor Mat 273:243–248
Acknowledgements
This work was financially supported by the Key Program of Science Technology Department of Zhejiang Province (No.2018C03037), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (20KJB610005), the Natural Science Foundation of Jiangsu Province (BK20201037, BK20190705), Key Research and Development Program of Anhui Province (202104g01020006) and the Scientific Research Fund of Nanjing Institute of Technology (No. YKJ2019111 and No. YKJ2019110).
Author information
Authors and Affiliations
Contributions
All authors have contributed the creation of this manuscript. JW: Conceptualization, Methodology, Validation, Investigation, Data curation, Visualization, Writing—original draft, Funding acquisition. JC: Methodology, Validation, Investigation, Data curation, Visualization. YS: Investigation, Validation, Data curation. YW: Methodology, Validation, Investigation, Data curation. YL: Investigation, Funding acquisition, Writing—review & editing. JZ: Investigation, Funding acquisition, Writing—review & editing. GW: Resources, Investigation, Funding acquisition. RZ: Conceptualization, Resources, Funding acquisition, Project administration, Supervision, Writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wan, J., Chen, J., Shi, Y. et al. In-situ One-Pot Synthesis of Ti/Cu-SSZ-13 Catalysts with Highly Efficient NH3-SCR Catalytic Performance as Well as Superior H2O/SO2 Tolerability. Catal Surv Asia 26, 346–357 (2022). https://doi.org/10.1007/s10563-022-09374-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10563-022-09374-8