Abstract
The need for homogenous titanium compounds as catalysts has been increasing gradually due to their non-toxic properties and catalytic activities. In this article, titanium salicylate complexes 1–7 were produced by reaction of titanium(IV) isopropoxide with salicylic, 5-chlorosalicylic, 5-nitrosalicylic, 4-hydroxysalicylic, 3-methylsalicylic, 4-methylsalicylic, and 3,5-ditertbutylsalicylic acids with 1:2 mol ratio in isopropanol. These complexes 1–7 were structurally characterized using a variety of instrumental techniques such as high resolution mass spectrometry (HRMS), FTIR and NMR spectroscopies, elemental and thermogravimetric (TG) analysis. Also, complexes 1–7 were applied as catalysts in the ring-opening polymerization (ROP) of ε-caprolactone and were highly efficient catalysts. Polycaprolactone (PCL) was analyzed using NMR spectroscopy, differential scanning calorimetry (DSC), and gel permeation chromatography (GPC) techniques. In the ROP reactions of ε-CL monomer, the catalysts containing electron donating groups on salicylate ligands were more effective than the catalysts containing electron withdrawing groups.
Graphic Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Besides being a biodegradable and biocompatible polymer, poly(ε-caprolactone) (PCL) also possess features like hardness, extensibility, malleability and solubility which makes it popular in biomedical applications in tissue engineering and regenerative medical fields. Chain length, crystallinity, and degree of branching play an essential role in the applications of PCL. These parameters can be reached by using different polymerization conditions and catalysts. Different metal alkoxide catalysts were used for the polymerization of ε-caprolactone [1, 2]. Several homogenous titanium catalysts that were prepared with discrete ligands such as phenolate, perfluoroheptanoate, phthalate, and pyridine-2-carboxylate have been extensively studied over the past few years [3,4,5,6,7]. Among these investigations, titanium catalysts coordinated on carboxylate or phenolate groups have received considerable interest because these kinds of ligands can provide efficient bonding in order to modify the electronic and steric effect of titanium center of such complexes [8,9,10,11]. Salicylate derivatives which are one of the carboxylate ligands are also well-known for antipyretic, analgesic, anti-inflammatory characteristics but at the same time they are environmentally friendly [12]. In parallel with sustainable catalyst growth, titanium ion is not toxic and does not have any known biological side effects which makes it one of the most impressive metal ions to be used in sustainable polymerization catalysis and causes no problems even when the catalyst stays in residues [13]. Therefore, after taking the above properties into account, combining both non-toxic titanium ion and salicylate ligands and forming new catalysts was one of the purposes of this study. In the study published in 2014, the synthesis and characterization of Cl−, NO2−, and OH-substitute salicylate zirconium catalysts were carried out and their application in the ROP reactions of ε-CL were reported by our group [14]. They have demonstrated that they were able to polymerize ε-caprolactone with high catalytic activity and high level of stereocontrol. It has been reported that electron withdrawing groups (NO2 and Cl) on salicylate ligands increase the catalytic activity of zirconium catalysts in ε-CL polymerization due to the increase in the electrophilicity of the zirconium center. It is also important to note that there was a weak π-donor interaction between large zirconium ion and small oxide ion of alkoxide groups. Thus, we are interested in extending our previous study by using titanium alkoxide complexes which include both electron withdrawing and donating groups instead of zirconium complexes including only electron withdrawing groups.
To prevent multiple chain growing per metal center which is often observed in two or more alkoxide groups, catalysts containing a single site alkoxide group have been the choice for this investigation.
In this study, series of ligands with electron donating (CH3, tert-C(CH3)3, and OH) and electron withdrawing (NO2 and Cl) groups were aimed to be investigated for their steric and electronic influences over titanium complexes. Therefore, different substituted salicylate ligands were selected in order to investigate the correlation between the electronic effects of the salicylate ligands and the catalytic properties on the ROP reactions. Comprehensive information on the interaction of electronic influences and the catalytic activity of titanium ion can be useful to develop a higher level of control over the ε-caprolactone polymerization process. ε-Caprolactone polymers (PCL) are non-hazardous synthetic thermoplastic semi-crystalline aliphatic polyesters. They have low Tg and Tm values of ~ − 60 and 60 °C, respectively [15].
Therefore, it is necessary to design and synthesize novel class of titanium complexes as active catalysts in the ROP reactions of cyclic esters. The first aim of this work was to produce and elucidate the substituted salicylate titanium isopropoxide complexes. To the extent of our knowledge, these are the first examples of titanium catalysts containing substituted salicylate ligands with electron donating and withdrawing groups. The second aim was to investigate their applications as catalysts in the ROP reactions of ε-CL monomer. And, last but not least, studying the substituent effects (H, Me, t-Bu, Cl, NO2, and OH) on the ROP reaction of ε-CL to improve catalytic and controllability of ROP of ε-CL was our aim as well.
2 Experimental Section
2.1 Materials
Titanium(IV) isopropoxide (97%), salicylic acid (SAH), 3-methyl salicylic acid (3-Me-SAH, 97%), 4-methyl salicylic acid (4-Me-SAH, 99%), 3,5-di-tertbutyl salicylic acid (3,5-di-tBu-SAH, 98%), 5-chlorosalicylic acid (5-Cl-SAH, 98%), 4-hydroxysalicylic acid (4-HO-SAH, 97%), 5-nitrosalicylic acid (5-NO2-SAH, 99%), ε-caprolactone (ε-CL, 97%) and tetrahydrofuran (THF, 99.9%) were purchased from Aldrich or Merck and used as received. Drying of isopropanol (99.5%) was carried out over the activated 4 Å molecular sieves before using. All Ti-complex preparations were performed in closed flasks under ambient temperature. 1H and 13C{1H}NMR spectra were recorded on a Bruker 400 MHz instrument in CDCl3. Infrared spectra were recorded on Alpha-P Bruker spectrophotometer. The elemental analyses were accomplished by a LECO CHNS-932 analyzer. Mass spectra were taken by spectrometry (Waters SYNAPT G1 MS, HRMS) with electrospray ionization (ESI ± , in the range of 50–1100 Da) method. Thermogravimetric analysis was carried out on a Perkin Elmer Pyris 1 TGA. Samples were heated starting from 25 °C up to 950 °C at a rate of 10 °C min−1 in nitrogen atmosphere. Differential colorimetric measurements were performed by a Perkin Elmer DSC 8000 and employed to measure glass transition temperature (Tg) of PCL. Samples were heated from − 110 to 100 °C at heating scan of 10 °C min−1 in nitrogen medium.
Gel permeation chromatographic (GPC) analysis was carried out by a Shimadzu prominence GPC system assembled with a RID-10A refractive index detector (to analyze data based on narrow dispersity polystyrene standards covering the molecular mass 162- 34,300 Da, a LC-20AD solvent delivery unit, a CTO-10AS column oven and a set of two columns (PSS SDV 5µL 1000 Å and PSS SDV 5 µL 50 Å). The columns were balanced and operated at 30 °C when THF was used as the elution solvent with the flow rate of 1.0 mL/min. The sample concentrations were approximately 2 mg/mL and the injection volume was 50µL.
2.2 Synthesis of (SA)2Ti2O(OiPr)2 Compound (1)
Salicylic acid (1.00 g, 7.24 mmol, 2 equiv) was added into the solution of titanium(IV) isopropoxide (1.06 g, 3.62 mmol, 1 equiv) in isopropanol (20 mL). After stirring this obtained solution for 3 h at ambient temperature, the solvent isopropanol was removed from the solution by vacuo approximately around 35 °C. The resulting residue was washed with hexane (3 × 10 mL) and dried under reduced pressure and gave an orange solid product. Elemental analysis (C20H22O9Ti2, (SA)2Ti2O(OiPr)2 (1), Mw = 502.12 g/mol): Calcd.: C 47.84, H 4.42%. Found: C 48.28, H 4.24%. MS (m/z): [(SA)2Ti2O(OiPr)2+K+] or C20H22O9Ti2K+, Calcd.: 540.99, Found: 540.99(100.0%), 541.99(37.6%), 539.99(24.5%); [C20H22O9Ti2-TiO] or C20H22O8Ti: 438.08(100.0%), 439.08(29.3%), 436.08(11.20%). 1HNMR (CDCl3) δ/ppm: 7. 89 (d, 2H, J = 6.32 Hz, H-6, Ph), 7.50 (brd, 2H, H-4, Ph), 6.99 (t, 2H, J = 5.60 Hz, H-5, Ph), 6.91 (brd, 2H, H-3, Ph), 4.04 (brd, or m, 2H, OCH, OiPr), 1.21 (d, 12H, J = 5.78 Hz, CH3, OiPr). 13C-NMR (CDCl3) δ/ppm: 173.28 (COO), 162.09 (C-2, Ph), 136.42 (C-4, Ph), 130.78 (C-6, Ph), 119.33 (C-5, Ph), 117.61 (C-3, Ph), 111.68 (C-1, Ph), 64.83 (OCH, OiPr), 25.81 (CH3, OiPr). FTIR (cm−1): 3070 (C-H, asym, sp2, Ph), 2974 (C–H, asym, sp3, CH3), 2928 (C–H, sym, sp3, CH3), 1619 (COO, asym), 1598 (C=C, Ph), 1577, 1512, 1454 (C–H, bending, sp3, CH3), 1390 (COO, sym), 1316, 1234, 1146, 1105, 1030, 890, 837, 806, 754, 700, 668, 640, 594, 529, 450.
2.3 Synthesis of (Cl-SA)2Ti2O(OiPr)2 Compound (2)
The reaction between 5-chlorosalicylic acid (1.07 g, 6.08 mmol, 2 equiv) and titanium(IV) isopropoxide (0.88 g, 3.04 mmol, 1 equiv) in isopropanol (20 mL) was accomplished under exactly the same conditions to the previous reaction. It gave an orange solid product. Elemental analysis (C20H20Cl2O9Ti2, (5-Cl-SA)2Ti2O(OiPr)2 (2), Mw = 571.01 g/mol): Calcd.: C 42.07, H 3.53%. Found: C 43.32, H 3.39%. MS (m/z): [(5-Cl-SA)2Ti2O(OiPr)2+Na+] or C20H20O9Cl2Ti2Na+: Calcd.: 592.93, Found: 592.93(100.0%), 594.93(82.8%), 595.93(30.4%); [(C20H20O9Cl2Ti2-TiO)+Na+] or C20H20O8Cl2TiNa+: 528.99(100.0%), 530.99(68.8%), 529.99(33.1%). 1HNMR (CDCl3) δ/ppm: 7. 85 (brd, 2H, H-6, Ph), 7.44 (d, 2H, J = 8.89 Hz, H-4, Ph), 6.95 (d, 2H, J = 8.89 Hz, H-3, Ph), 4.08 (multiplet, 2H, J = 6.09 Hz, OCH, OiPr), 1.22 (d, 12H, J = 5.73 Hz, CH3, OiPr). 13C-NMR (CDCl3) δ/ppm: 171.91 (COO), 160.64 (C-2, Ph), 136.3 (C-4, Ph), 129.88 (C-6, Ph), 124.06 (C-5, Ph), 119.24 (C-3, Ph), 112.63 (C-1), 65.13 (OCH, OiPr), 24.99 (CH3, OiPr). FTIR (cm−1): 3062, 2971, 2919, 1603 (COO, asym), 1567 (C=C, Ph), 1506, 1461, 1420, 1369 (COO, sym), 1290, 1225, 1107, 1002, 898, 826, 793, 728, 669, 616, 472.
2.4 Synthesis of (NO2-SA)2Ti2O(OiPr)2 Compound (3)
The reaction of 5-nitrosalicylic acid (1.02 g, 5.45 mmol, 2 equiv) with titanium(IV) isopropoxide (0.78 g, 2.74 mmol, 1 equiv) in isopropanol (20 mL) was accomplished under exactly the same conditions to the previous reaction. It gave an orange solid the product. Elemental analysis (C20H20N2O13Ti2, (5-NO2-SA)2Ti2O(OiPr)2 (3), Mw = 592.11 g/mol): Calcd.: C 40.57, H 3.40, N 4.73%. Found: C 41.74, H 4.02, N 5.47%. MS (m/z): [C20H20O13N2Ti2K+-TiO2] or C20H20O11N2TiK+: Calcd.: 551.02, Found: 551.02(100.0%), 552.02(31.0%), 553.02(18.4%). 1HNMR (CDCl3) δ/ppm: 8. 85 (d, 2H, J = 2.66 Hz, H-6, Ph), 8.36–8.39 (dd, 2H, J = 9.18 Hz, J = 2.68 Hz, H-4, Ph), 7.11 (d, 2H, J = 9.2 Hz, H-3, Ph), 4.09 ( sept., 2H, J = 6.1 Hz, OCH, OiPr), 1.24 (d, 12H, J = 6.1 Hz, CH3, OiPr). 13C-NMR (CDCl3) δ/ppm: 170.89 (COO), 166.80 (C-2, Ph), 139.93(C-5, Ph), 130.68 (C-4, Ph), 127.28 (C-6, Ph), 116.7 (C-3, Ph), 116.1 (C-1, Ph), 64.91 (OCH, OiPr), 25.17 (CH3, OiPr). FTIR (cm−1): 3088, 2971, 2932, 1603 (COO, asym), 1578 (C=C, Ph), 1506, 1461, 1434, 1382 (COO, sym), 1310, 1251, 1146, 1107, 1075, 1002, 924, 839, 799, 715, 662, 623, 466, 415.
2.5 Synthesis of (4-HO-SA)2Ti2O(OiPr)2 Compound (4)
The reaction of 4-hydroxysalicylic acid (1.04 g, 6.54 mmol, 2 equiv) with titanium(IV) isopropoxide (0.96 g, 3.27 mmol, 1 equiv) in isopropanol (20 mL) was accomplished under exactly the same conditions to the previous reaction. It gave the product as an orange solid. Elemental analysis (C20H22O11Ti2, (4-HO-SA)2Ti2O(OiPr)2 (4), Mw = 534.12 g/mol): Calcd.: C 44.97, H 4.15%. Found: C 45.50, H 4.39%. MS (m/z): [((4-HO-SA)2Ti2O(OiPr)2-TiO)+Na+] or C20H22O10TiNa+: Calcd.: 493.06, Found: 493.06(100.0%), 494.06(29.4%), 491.06(11.2%). FTIR (cm−1): 3177 (OH), 3070, 2967, 2930, 1606 (COO, asym), 1587 (C=C, Ph), 1542, 1476, 1437, 1378 (COO, sym), 1233, 1147, 1095, 977, 937, 845, 773, 629, 491, 425.
2.6 Synthesis of (3-Me-SA)2Ti2O(OiPr)2 Compound (5)
The reaction of 3-methylsalicylic acid (1.05 g, 6.68 mmol, 2 equiv)) with titanium(IV) isopropoxide (0.99 g, 3.34 mmol 1 equiv) in isopropanol (20 mL) was accomplished under exactly the same conditions to the previous reaction. The product was an orange solid. Elemental analysis (C22H26O9Ti2, (3-Me-SA)2Ti2O(OiPr)2 (5), Mw = 530.17 g/mol): Calcd.: C 49.84, H 4.94%. Found: C 50.54, H 4.80%. MS (m/z): [(3-Me-SA)2Ti2O(OiPr)2- (TiO)+Na+] or C22H26O8TiNa+: Calcd.: 489.10, Found: 489.10(100.0%), 490.10 (31.4%), 487.11(11.2%). 1HNMR (CDCl3) δ/ppm: 7.75 (d, 2H, J = 7.94 Hz, H-6, Ph), 7.36 (d, 2H, J = 7.22 Hz, H-4, Ph), 6.81 (t, 2H, J = 7.65 Hz, H-5, Ph), 4.07 ( m, J = 6.12 Hz, OCH, OiPr), 2.28 (s, CH3, 3-Me-SA), 1.20 (d, J = 6.12 Hz, CH3, OiPr). 13C-NMR (CDCl3) δ/ppm: 174.16 (COO), 160.57 (C-2, Ph), 137.23 (C-4, Ph), 128.32 (C-6, Ph), 126.68 (C-3, Ph), 118.67 (C-5, Ph), 110.94 (C-1, Ph), 64.86 (OCH, OiPr), 25.05 (CH3, OiPr), 15.64 (CH3, 3-Me-SA). FTIR (cm−1): 2967, 2921, 2862, 1614 (COO, asym), 1594 (C=C, Ph), 1535, 1502, 1450, 1391 (COO, sym), 1306, 1220, 1147, 1082, 1003, 879, 793, 753, 675, 622, 524, 451, 425.
2.7 Synthesis of (4-Me-SA)2Ti2O(OiPr)2 Compound (6)
The reaction of 4-methylsalicylic acid (1.03 g, 6.66 mmol, 2 equiv) with titanium(IV) isopropoxide (0.98 g, 3.33 mmol 1 equiv) in isopropanol (20 mL) was accomplished under exactly the same conditions to the previous reaction. The resulting product was a yellow solid. Elemental analysis (C22H26O9Ti2, (4-Me-SA)2Ti2O(OiPr)2 (6), Mw = 530.17 g/mol): Calcd.: C 49.84, H 4.94%. Found: C 50.56, H 4.80%. MS (m/z): [(4-Me-SA)2Ti2O(OiPr)2-(OTi)+Na+] or C22H26O8TiNa+: Calcd.: 489.10, Found: 489.10(100.0%), 490.10 (31.4%), 487.11(11.2%). 1HNMR (CDCl3) δ/ppm: 7. 75 (d, 2H, J = 8.09 Hz, H-6, Ph), 6.80 (s, 2H, H-3, Ph), 6.71 (d, 2H, J = 8.10 Hz, H-5, Ph), 4.05 ( sept, 2H, J = 6.13 Hz, OCH, OiPr), 2.35 (s, 6H, CH3, 4-Me-SA), 1.20 (d, 12H, J = 6.12 Hz, CH3, OiPr). 13C-NMR (CDCl3) δ/ppm: 173.48 (COO), 162.07 (C-2, Ph), 147.91 (C-4, Ph), 130.60 (C-6, Ph), 120.64 (C-5, Ph), 117.71 (C-1, Ph), 109.12 (C-3, Ph), 64.75 (OCH, OiPr), 25.10 (CH3, OiPr), 21.92 (CH3, 4-Me-SA). FTIR (cm−1): 2974, 2915, 2862, 1607 (COO, asym), 1575 (C = C, Ph), 1489, 1424, 1378 (COO, sym), 1318, 1246, 1167, 1108, 1010, 957, 859, 780, 753, 701, 629, 537, 438.
2.8 Synthesis of (3,5-di-tBu-SA)2Ti2(μ2-OiPr)2(OiPr)2 Compound (7)
The reaction of 3,5-di-tert-butylsalicylic acid (1.00 g, 3.90 mmol, 2 equiv) with titanium(IV) isopropoxide (0.57 g, 1.95 mmol, 1 equiv) in isopropanol (20 mL) was accomplished under exactly the same conditions to the previous reaction. The product was a solid with brown color. Elemental analysis (C42H68O10Ti2, (3,5-di-tBu-SA)2Ti2(μ2-OiPr)2(OiPr)2 (7), Mw = 828.72 g/mol): Calcd.: C 60.87, H 8.27%. Found: C 62.48, H 7.92%. MS (m/z): C21H34O5TiK+: Calcd.: 453.15, Found: 453.15(100.0%), 454.15(23.9%), 455.15(14.2%). 1HNMR (CDCl3) δ/ppm: 8.06–7.19 (doublets, 4H, J = 9.07 Hz, Ph), 4.72 (septet, H, J = 6.14 Hz, OCH, OiPr), 4.67 (septet, H, J = 6.19 Hz, OCH, OiPr), 4.03 (septet, 2H, J = 6.13 Hz, OCH, OiPr), 1.54–1.35 (a few singlets, 36H, CH3, tert-butylsalycilate), 1.29 (d, 6H, J = 6.19 Hz, CH3, OiPr), 1.26 (d, 6H, J = 6.88 Hz, CH3, OiPr), 1.20 (d, 12H, J = 6.14, CH3, OiPr). 13C-NMR (CDCl3) δ/ppm: 176.04 (COO), 162.72, 142.51, 136.61, 130.74, 126.06, 115.55, 86.09, 83.99, 64.53 (OCH, OiPr), 35.34–2947 (3,5-tBu), 25.23–24.02 (three peaks, CH3, OiPr). FTIR (cm−1): 3006, 2954, 2908, 2869, 1614 (COO, asym), 1578 (C=C, Ph), 1522, 1437, 1391 (COO, sym), 1358, 1299, 1233, 1194, 1122, 1016, 912, 852, 806, 760, 734, 681, 642, 563, 498, 445,400.
2.9 Ring—Opening Polymerization of ε-Caprolactone Using (3-Me-SA)2Ti2O(OiPr)2 Catalyst
The catalyst 3-Me-SA)2Ti2O(OiPr)2 (5) (20 mg) was mixed with ε-CL (1.0 g) in a vial under nitrogen. The solvent-free ring opening polymerization of ε-caprolactone was carried out at 100 °C for 5 and 16 h as indicated in Table 1. 1H NMR (CDCl3, ppm), δ: 4.08 (t, J = 7.0 Hz, εCH2-O), 2.32 (t, J = 7.0 Hz, αCH2-C=O), 1.66 (m, J = 7.0 Hz, β,δCH2), 1.40 (m, J = 7.0 Hz, γCH2). 13C NMR (CDCl3), δ/ppm: 173.8 (C=O), 64.4 (εCH2O), 34.3 (αCH2), 28.6 (δCH2), 25.8 (βCH2), 24.8 (γCH2).[O=C-αCH2βCH2γCH2δCH2εCH2O-].
The ROP reactions of ε-caprolactone with the remaining compounds of 1–4, 6, and 7 were performed under the identical conditions like in the previous reaction.
3 Results and Discussion
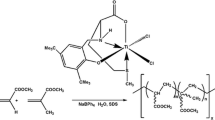
Reactions of Ti(OiPr)4 with salicylic or substituted salicylic acid in 1:2 mol ratio in isopropanol at ambient temperature produced the products of (SA)2Ti2O(OiPr)2 (1), (5-Cl-SA)2Ti2O(OiPr)2 (2), (5-NO2-SA)2Ti2O(OiPr)2 (3), (4-HO-SA)2Ti2O(OiPr)2 (4), (3-Me-SA)2Ti2O(OiPr)2 (5), (4-Me-SA)2Ti2O(OiPr)2 (6), and (3,5-di-tBu-SA)2Ti2(μ2-OiPr)2(OiPr)2 (7). Complexes 1–7 were formulated as dimeric based on their mass measurement results. The formulation of complexes was also supported by integration of 1H NMR spectra, the interpretation of 13C NMR spectra, FT-IR data, elemental analysis, and TGA studies. Several attempts were made to prepare single crystals of complexes but the results were unsuccessful.
In the existence of salicylic or substituted salicylic acids, a number of the isopropoxide groups from the initial titanium precursor underwent a substitution reaction by salicylate groups to give new complexes exhibiting dimeric structures (Scheme 1). Since the mass spectroscopy used in this study could measure between 50 and 1100 daltons, it indicated that the molecular formulas of these complexes might be dimeric as formulated. However, with the same composition, these compounds may also be oligomeric such as tetramer [(SA)4Ti4(OiPr)4(O)2] or hexamer [(SA)6Ti6(OiPr)6(O)3]. The new compounds 1–6 except 7 included oxo groups which are common in many metal alkoxide complexes. The presence of small amounts of moisture in the reaction medium causes hydrolysis and condensation reactions. The condensation reactions between metal alkoxides units, via ether or alcohol elimination, led to the formation of oxo-bridges [16]. The tendency to form oxo bridges disappeared with the bulky size of substituted groups (tBu) on salicylate ligands.
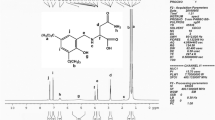
The 1H, 13C NMR spectra of salicylate or substituted salicylate titanium compounds showed the predicted chemical signals and peak multiplicities. For instance, 1H NMR spectrum of (3-Me-SA)2Ti2O(OiPr)2 compound (5) presented doublet and triplet at 7.75–6.81 ppm with J = 7.94–7.22 Hz for phenyl protons, multiplet at 4.07 ppm with J = 6.12 Hz for CH protons, singlet at 2.28 ppm for CH3 on salicylate, and doublet at 1.20 ppm with J = 6.12 Hz for CH3 protons of OCH(CH3)2 moieties in titanium compounds (Fig. 1).
Salicylic acid provides binding flexibility where it can be a monoanion with only the deprotonated carboxylate group or a dianion with deprotonation of the phenol OH as well [17]. The signals for the carboxyl group (COOH) and hydroxide group (OH) protons were absent from the 1H NMR spectra. Absence of carboxylic acid and hydroxide proton signals at around ~ 11 and ~ 8.6 ppm indicated that salicylate ligands were completely coordinated to titanium atom in bidentate modes.
The 13C NMR spectra of the titanium compounds presented shifts for the substituted salicylate carbon peaks when it was compared with the free salicylic acid derivatives. For instance, the 13C NMR spectrum of the (3-Me-SA)2Ti2O(OiPr)2 compound (5) showed characteristic carbon peaks at 174.16 ppm for COO, 160.57–110.94 ppm for phenyl carbons, and 15.64 ppm for CH3 on salicylate groups (Fig. 2). The other two peaks at 25.05 and 64.86 ppm in the Fig. 2 belonged to the CH3 and OCH of OiPr group, respectively. In contrast to compounds 1–6, the compound 7 was in dimeric structure with isopropoxide bridges instead of oxo bridges. The 1H NMR peaks at 4.72, 4.67 ppm (septet, H, J = 6.19 Hz, OCH, OiPr, bridges), and 13C NMR peaks at 86.09, 83.99 ppm (OCH, OiPr, bridges) confirmed the presence of bridge isopropoxide groups. Generally, the bridge protons and carbons appear at low field or high values in the NMR spectra. Due to the steric effect of bulky group (tert-butyl) on salicylate ligands and the higher organic content, compound 7 preferred to be dimeric structure with isopropoxide bridges.
The chemical shifts and spin–spin coupling constants provided in the experimental section were fully consistent with the literature values for similar salicylate zirconium propoxide and salicyl aldehyde titanium isopropoxide compounds [14, 18].
In the FTIR spectra of titanium complexes 1–7, the band in carboxyl region indicated the coordination of carboxylate group. The FTIR spectrum of free salicylic acids and its derivatives shows strong bands at around 1670 and 1440 cm–1 matching asymmetrical and symmetrical stretching resonance of the carboxylate groups. After the successful salicylate ligand complexation to titanium isopropoxide, the band around 1670 cm−1 shifted to lower wave number around 1600–1620 cm−1. For instance, coordinated carboxyl peaks for (3-Me-SA)2Ti2O(OiPr)2 were observed around 1614 cm−1 for νCOOasym and 1380 cm−1 for νCOOsym. The Δυasym-sym value of 234 cm−1 was higher compared to appropriate ones for sodium salts (ΔυCO2Na = ~ 211 cm−1) [19]. This indicates that in the (3-Me-SA)2Ti2O(OiPr)2 compound (5), the titanium ion was coordinated by monodentate carboxylate group. The IR spectrum of (3-Me-SA)2Ti2O(OiPr)2 compound (5) showed no band around ~ 3200–3300 cm−1 because of the OH stretching vibration and suggested the absence of hydroxide part. Those values given above were in agreement with the ones reported for a number of carboxylate transition metal(IV) derivatives [20, 21]. In contrast to phenolic OH at 2-position, spectroscopic measurements support the presence of free phenolic OH at 4-position in compound (4), which did not react with other Ti-OiPr moiety. Actually, in literature there are a few Ti and Zr salicylate compounds which have free phenolic OH group even at 2-position [22, 23]. This can happen for a number of reasons. One of the reasons as mentioned in literature could be the spatial effect of the molecular structure of Ti-complex preventing the acid–base reaction of OH and OiPr [24]. The other reason can be mesomeric effects of phenolic OH at 2- and 4-positions. The phenolic OH at 2-position decreases the acidity of OH group at 4-position so OH group at 4-position does not react with isopropoxide group.
The molecular weights of salicylate titanium complexes 1–7 were obtained from high resolution mass spectrometry (HRMS). These complexes were examined under positive and negative ionization settings. For the compounds 1–7, using mass measurement results, the dimeric structures were proposed. The potassium adduct molecular ion peak, [(SA)2Ti2O(OiPr)2 + K+], was present at 541.2657 (for mol weight) in the mass spectrum (calculated to be 541.2156 for C20H22O9Ti2K+). The molecular ion adducted with K+ were at m/z 540.99(100.0%), 541.99(37.6%), and 539.99(24.5%) because of different exact mass of each isotope. The second peak appeared at m/z 438.08(100.0%), 439.08(29.3%), 436.08(11.20%) for [C20H22O9Ti2-TiO] or C20H22O8Ti (calculated to be 438.08) (Fig. 3). In contrast to mass spectrum of [(SA)2Ti2O(OiPr)2] compound (1), the molecular ion of [(4-Me-SA)2Ti2O(OiPr)2] compound (6) was not present in the spectrum, but the sodium adduct ion [(4-Me-SA)2Ti(OiPr)2+Na]+ resulting from the loss of TiO units of molecular ion was present at m/z 489.10(100.0%), 490.10 (31.4%), and 487.11(11.2%). The formula for this ion was [C22H26O8Ti+Na]+. The salicylate zirconium complexes were also formulated as dimer in a similar reaction condition [14]. As mentioned before, these compounds were formulated as dimer however with the same percentage composition they can also be tetramer or hexamer. These kind of compounds have a generic formula like [(SA)xTiy(OiPr)zOt]n. For instance, reactions between titanium alkoxides and ligands such as 2,2′-biphenol, pyrocatechol, and cyclobutane carboxylic acid resulted in the formation of hexamer structures containing oxo group [25, 26]. Carboxylate complexes of Ti, Zr, Sn, and Al alkoxides possess different structures like monomeric, dimeric, trimeric etc. depending on various parameters such as carboxylate/metal alkoxide ratio, purity of starting materials, reaction time, exposing the reaction to air, solvent types, evaporation temperatures, and measurement conditions. The parameters mentioned above are highly effective in determining which kinds of complexes are formed in a particular system [27, 28].
The mass spectrum of (3,5-di-tBu-SA)2Ti2(μ2-OiPr)2(OiPr)2 compound (7) showed the mass–charge ratio for the molecular ion at 485.24. It was equal to half of the molecular weight of the predicted formula. It can be attributed to the weakness of the bridged isopropoxide bonds in this compound. The percentage composition was compatible with interpretation of 1H-NMR spectrum and elemental analysis data of (3,5-di-tBu-SA)2Ti2(μ2-OiPr)2(OiPr)2 compound (7).
The thermogravimetric analysis of (3-Me-SA)2Ti2O(OiPr)2 and (3,5-di-tBu-SA)2Ti2(μ2-OiPr)2(OiPr)2 complexes (5 and 7) were measured to provide an additional support to the elemental analysis and mass measurements. The thermal degradation profile of (3-Me-SA)2Ti2O(OiPr)2 compound (5) (under nitrogen flow) showed stability up to 270 °C. 2% of weight loss was noticed until the temperature reached 100 °C. This 2% loss might be attributed to the solvent impurities or moisture which might have remained in the titanium complex during the drying process. When the temperature kept being increased, it gave two peaks at around 390 and 495 °C with 35% and 55% weight loss. These peaks were assigned to degradation of (3-Me-SA)2Ti2O(OiPr)2 compound (5) to oxide including some organic ligands. 68.51% of total weight loss was observed until the temperature reached 950 °C and the resulting residue was titanium dioxide (31.49%) (Fig. 4). The weight loss (68.51%) confirmed the suggested formula and the molecular weight of 530.17 g/mol. Based on stoichiometry, a theoretical weight % loss and residue were calculated for the compound-5, and found to be 69.87% and 30.13% (2 × 79.865 g TiO2/530.17 gmol−1 compound × 100 = 30.13%) respectively. Theoretical values were in good agreement with that of the measured values. This weight loss supports not only dimeric formula of the compound but also oligomeric formula with the same composition of the [(3-Me-SA)2Ti2O(OiPr)2]n (n = 2,3) compound. In a similar study, the reaction between titanium isopropoxide and benzene dicarboxylate was resulted in the formation of titanium-oxo-cluster [29]. The weight loss for (3,5-di-tBu-SA)2Ti2(μ2-OiPr)2(OiPr)2 compound (7) was found 81.15% (the residue was 18.85%) until 950 °C. Based on stoichiometry, % residue was calculated for the compound-7 and found to be 19.28% (2 × 79.865 g TiO2/828.7173 gmol−1 compound × 100 = 19.28%). Theoretical value (19.28%) was in a very good agreement with that of the measured value (18.85%). These TGA results supported the proposed formula of (3-Me-SA)2Ti2O(OiPr)2 and (3,5-di-tBu-SA)2Ti2(μ2-OiPr)2(OiPr)2 compounds (5 and 7) based on the mass measurements and other techniques.
These complexes 1–7 were utilized as catalysts in the ROP reaction of ε-caprolactone. The titanium center of these catalysts 1–7 acted as a Lewis acid to activate the ε-CL whereas the OiPr group behaved as an initiating nucleophile in the ROP. First, ε-caprolactone coordinates to titanium center and then OiPr group attacks ε-CL’s carbonyl group. Consequently, PCL with an isopropoxide end group derived from the catalyst is obtained (Scheme 2). Salicylate titanium complexes performed the ROP of ε-caprolactone through the medium of the mechanism of coordination-insertion. This suggestion is consistent with the literature data reported for a coordination-insertion mechanism for ROP of cyclic organic compounds [30, 31].
The optimum polymerization rates of ε-CL and the optimum recovery rate for PCL took place at 100 °C for Ti-complexes 1–7. The conversion of the monomer ε-CL to poly-CL, the determination of average molecular mass (Mw), and the molecular mass distribution index (PDI) of PCL were detected by gel permeation chromatographic (GPC) measurements. By varying the reaction times from 5 to 16 h, the PCL with different weight average molecular weights or number average molecular (Mn) weights were obtained (Table 1).
These novel SA-Ti-OiPr catalysts showed excellent activity in the ROP of ε-CL monomers. When taking a closer look at the values in Table 1, it can be seen that average molecular weights of PCL prepared with (4-Me-SA)2Ti2O(OiPr)2 compound (6) were 13,264 Da and 16,612 Da for 5 and 16 h stirring and the molecular mass distribution indexes were 1.14 and 1.26, respectively. The molecular masses of the PCL polymers increased approximately linearly with monomer conversions. The conversion of ε-CL monomers into the PCL polymers rose from 90 to 100% as the time was increased from 5 to 16 h. The ε-CL polymerization with catalysts 1–7 exhibited controlled characters having narrow molecular mass distributions (1.0–1.26). The turnover numbers (TON = moles of monomer per mole of catalyst) and turnover frequencies (TOF = TON/time(h)) in polymerization of ε-CL by catalysts 1–7 were calculated to be 2.13 × 102–3.52 × 102 and 13–22 h−1, respectively. The Ti-OiPr catalysts 4–7 containing electron-donating groups proceeded efficiently showing monomer conversion > 90% within only 5 h. The ε-CL monomer conversion by using SA-Zr-OnPr catalysts was higher than 90% within 12 h [14]. The other important note to mention is the behavior of catalyst (7). Since catalyst (7) was highly active, the ε-CL polymerization reaction was almost completed in about 5 h. Therefore, 16 h of stirring caused trans-esterification reaction between PCL chains, which also led to bimodal MWD.
In such complexes, it is also necessary to remind that chelate bonded ligands remain inactive in polymerization reactions due to the strong binding. In some carboxylate complexes that do not contain active groups such as alkoxide or halide groups, the carboxy group can act as an initiator at high temperatures (80–140 °C) [32].
It is also important to mention that most catalysts reported in literature showed an activity with the presence of nucleophilic co-catalysts such as 4-dimethylaminopyridine (DMAP), bis(triphenylphosphine)-iminium salts ([PPN]X), and benzyl alcohol [33]. Co-catalysts mentioned above behave as external nucleophiles and they increase the labilization of the initiating or propagating groups [34, 35]. In this study, the ROP reactions of ε-caprolactone proceeded readily at 100 °C in controlled manner, delivering PCL with narrow PDIs (Mw/Mn = 1.04–1.26) and moderate molar mass values controlled by monomer to catalyst ratio. Consequently, substituted salicylate titanium complexes in this study were highly active without external co-catalysts or initiators in the ε-CL ROP as seen in Table 1.
In a similar manner, a titanium complex having tellurium-bridged chelating bis(aryloxo) ligand, [TiCl2{2,2´-Te(4-Me-6-tBu-C6H2O)2}]2, was prepared and used in the ROP of ε-CL. In this catalytic system, polymerization was observed to be solvent dependent. When the ε-CL polymerization was carried out in toluene at 100 °C, titanium catalyst was found to give polymers with rather broad PDI due to back-biting [36]. In another titanium study, bulky bis(phenolate) titanium complex was obtained from [Ti (OCHMe2)4] and 2,2′-methylenebis(6-tert-butyl-4-methylphenol) ligand in ether and used as catalyst in the ROP of ε-CL at the ratio of 100:1 ([M]/[I]) in CH2Cl2 at 25 °C. The ε-CL conversion proceeded to 23 and 67% in 6 and 24 h, respectively, and was completed in 75 h, affording a polymer with Mn and Mw/Mn of 5600 Da and 1.10, respectively [37]. The conversion was very low for 6 or 24 h stirring and molecular weight of polymer was also low when compared to salicylate titanium complexes (1–7).
GPC curve of PCL synthesized at 100 °C with (3-Me-SA)2Ti2O(OiPr)2 compound (5) was given in Fig. 5. As it can be seen from Fig. 5, the GPC chromatogram of the PCL exhibited mono-modal molecular mass distribution.
In the titanium complexes 2 and 3 containing electron-withdrawing substituents NO2 and Cl at 5-position on the phenyl ring, there was a moderate π-donor interaction between titanium and isopropoxide group. This interaction restricted the coordination-insertion of ε-CL monomers and also decreased the activity of the isopropoxide group as the initiator. These two factors can be the main cause of the low polymerization rate. Since it is known that NO2 and Cl groups at 4-position on the salicylate ligand can cause stronger π-bonds between titanium and isopropoxide ion and cause lower polymerization, they were not chosen as ligands in this study.
In order to solve the low polymerization rates encountered with titanium complexes 2 and 3, titanium complexes 4–7 containing electron-donating substituents methyl, tert-butyl and hydroxyl on the phenyl ring were synthesized which reduced the formation of π-backbonding between titanium metallic center and isopropoxide group. For the salicylate titanium complexes 4–7, there were significant activity improvements in comparison with catalysts 2 and 3 under the similar conditions. These observations are consistent with metal alkoxide complexes containing electron-withdrawing and electron-donating groups present in literature [38, 39]. In the future, the usage of these catalysts can be expanded in the polymerization of lactides and epoxides or copolymerization of CO2 with epoxides and lactones as in literatures [40, 41].
DSC analysis was carried out to find out the melting point and the degree of crystallinity of PCL (Fig. 6). The melting point and the melting enthalpy of PCL were 62.60 °C and 104.8578 Jg−1, respectively. The degree of crystallinity was calculated as 75.27% by assuming the enthalpy of 139.3 Jg−1 of 100% crystalline PCL [42]. DSC thermogram of PCL showed a peak without shoulder peak suggesting homophase system. As it can be seen from the Fig. 6, PCL has a glass transition temperature of about − 62 °C.
4 Conclusions
A novel class of substituted salicylate titanium (IV) complexes 1–7 was prepared with the aim of searching their capabilities of catalyzing ε-caprolactone monomers to form PCL. The structures of titanium complexes were elucidated by spectroscopic and thermal analysis. These novel salicylate titanium complexes with isopropoxy groups were efficient catalysts for the ROP reactions of ε-caprolactone at 100 °C with different time intervals. These catalysts displayed considerably efficient activities in the ε-CL ROP by having a good molecular weight control and narrow molecular weight distributions (PDI < 1.26). PCLs were characterized by GPC, DSC measurements, and spectroscopic methods. The salicylate titanium catalysts 4–7 with electron donating group were more effective in activity when compared to catalysts 2 and 3 with electron withdrawing groups under similar condition.
In summary, the titanium complexes 1–7 acting as catalysts display some advantages such as displaying high catalytic activities, having a good temperature stability and being able to synthesize easily. In addition, these titanium complexes are environmentally friendly and non-toxic which make them very attractive. The PCL obtained by these titanium catalysts have moderate molecular mass and semi crystallinity features which can make it a very good candidate for drug release and other medical applications.
References
Malikmammadov E, Tanir TE, Kiziltay A, Hasirci V, Hasirci N (2018) PCL and PCL-based materials in biomedical applications. J Biomater Sci Polym E 29:863–893
Nofar M, Sacligil D, Carreau PJ, Kamal MR, Heuzey MC (2019) Poly (lactic acid) blends: Processing, properties and applications. Int J Biol Macromol 125:307–360
Yalcin G, Yildiz U, Kayan A (2012) Preparation of Al, Ti, Zr-perfluoroheptanoate compounds and their use in ring opening polymerization. App Catal A 423:205–210
Yalcin G, Kayan A (2012) Synthesis and characterization of Zr, Ti, Al-phthalate and pyridine-2-carboxylate compounds and their use in ring opening polymerization. App Catal A 433:223–228
Chmura AJ, Davidson MG, Jones MD, Lunn MD, Mahon MF (2006) Group 4 complexes of amine bis (phenolate)s and their application for the ring opening polymerization of cyclic esters. Dalton Trans 7:887–889
Su CK, Chuang HJ, Li CY, Yu CY, Ko BT, Chen JD, Chen MJ (2014) Oxo-bridged bimetallic group 4 complexes bearing amine-bis (benzotriazole phenolate) derivatives as bifunctional catalysts for ring-opening polymerization of lactide and copolymerization of carbon dioxide with cyclohexene oxide. Organometallics 33:7091–7100
Ligny R, Guillaume SM, Carpentier JF (2019) Yttrium-mediated ring-opening copolymerization of oppositely configurated 4-alkoxymethylene-β-propiolactones: effective access to highly alternated isotactic functional PHAs. Chem Eur 25:6412–6424
Yu CJ, Li CY, Tsai CY, Ko BT (2019) Titanium, zirconium and hafnium complexes bearing amino-benzotriazole phenolate ligands as efficient catalysts for ring-opening polymerization of lactides. Inorg Chem Commun 109:107561
Tshuva EY, Peri D (2009) Modern cytotoxic titanium (IV) complexes; Insights on the enigmatic involvement of hydrolysis. Coord Chem Rev 253:2098–2115
Kim S, Sarkar D, Kim Y, Park MH, Yoon M, Kim Y, Kim M (2017) Synthesis of functionalized titanium-carboxylate molecular clusters and their catalytic activity. J Ind Eng Chem 53:171–176
Li CY, Yu CJ, Ko BT (2013) Facile synthesis of well-defined titanium alkoxides based on benzotriazole phenoxide ligands: efficient catalysts for ring-opening polymerization of cyclic esters. Organometallics 32:172–180
Kovala-Demertzi D, Hadjikakou SK, Demertzis MA, Deligiannakis Y (1998) Metal ion–drug interactions. Preparation and properties of manganese (II), cobalt (II) and nickel(II) complexes of diclofenac with potentially interesting anti-inflammatory activity: behavior in the oxidation of 3, 5-di-tert-butyl-o-catechol. J Inorg Biochem 69:223–229
Le Roux E (2016) Recent advances on tailor-made titanium catalysts for biopolymer synthesis. Coord Chem Rev 306:65–85
Mert O, Kayan A (2014) Synthesis and characterization of substituted salicylate zirconium compounds and their catalytic activity over ε-caprolactone. J Incl Phen Macrocycl Chem 80:409–416
Dash TK, Konkimalla VB (2012) Poly-є-caprolactone based formulations for drug delivery and tissue engineering: a review. J Control Release 158:15–33
Kayan A (2019) Inorganic-organic hybrid materials and their adsorbent properties. Adv Compos Hybrid Mater 2:34–45
Stavila V, Thurston JH, Whitmire KH (2009) Selective arylation reactions of bismuth-transition metal salicylate complexes. Inorg Chem 48:6945–6951
Sanwaria AR, Gopal R, Jain J, Nagar M, Chaudhary A (2020) Highly pure brookite phase of TiO2 from salicylaldehyde modified titanium (IV) isopropoxide: synthesis, characterization and photocatalytic applications. J Inorg Organomet Polym Mater 30:1393–1403
Swislocka R, Kalinowska M, Ferenc W, Sarzynski J, Lewandowski W (2012) Spectroscopic and magnetic properties of Cu (II) complexes with selected biologically important ligands. Cent Eur J Chem 10:1095–1105
Edwards DA, Mahon MF, Paget TJ, Summerhill NW (2001) The bis (η-cyclopentadienyl) titanium (IV)-salicylate system revisited and the characterization of two 3,5-di-t-butylsalicylate analogues. The molecular structure of [Cp2Ti (sal)],(sal=O2CC6H4O2-). Trans Metal Chem 26:116–119
Zhu BC, Fang WH, Emayavaramban P, Zhang L, Zhang J (2018) Structures and photophysical performances of (fluoro) salicylate stabilized polyoxo-titanium clusters. CrystEngComm 20:5964–5968
Kickelbick G, Schubert U (1999) Hydroxy carboxylate substituted oxozirconium clusters. J Chem Soc, Dalton Trans 8:1301–1306
Baul TSB, Manne R, Tiekink ER (2019) Mono-and di-anionic coordination modes of arylazosalicylates in their bis (η5-cyclopentadienyl) titanium (IV) complexes: syntheses and crystal structures. Inorg Chim Acta 484:469–480
Jiang MT, Kosuru SR, Lee YH, Lu WY, Vandavasi JK, Lai YC, Chen HY (2018) Factors influencing catalytic behavior of titanium complexes bearing bisphenolate ligands toward ring-opening polymerization of L-lactide and å-caprolactone. Express Polym Lett 12:126–135
Gigant K, Rammal A, Henry M (2001) Synthesis and molecular structures of some new titanium (IV) aryloxides. J Am Chem Soc 123:11632–11637
Czakler M, Artner C, Schubert U (2018) Two new hexanuclear titanium oxo cluster types and their structural connection to known clusters. New J Chem 42:12098–12103
Janek M, Radtke A, Muzioł T, Jerzykiewicz M, Piszczek P (2018) Tetranuclear oxo-titanium clusters with different carboxylate aromatic ligands: optical properties, DFT calculations, and photoactivity. Materials 11:1661
Mert O, Kayan A (2013) Synthesis of silyliminophenolate zirconium compounds and their catalytic activity over lactide/epoxide. App Catal A Gen 464:322–331
Wu YY, Luo W, Wang YH, Pu YY, Zhang X, You LS, Dai J (2012) Titanium–oxo–clusters with dicarboxylates: single-crystal structure and photochromic effect. Inorg Chem 51:8982–8988
Fuoco T, Pappalardo D (2017) Aluminum alkyl complexes bearing salicylaldiminato ligands: versatile initiators in the ring-opening polymerization of cyclic esters. Catalysts 7:64
Medina DA, Contreras JM, López-Carrasquero FJ, Cardozo EJ, Contreras RR (2018) Use of samarium (III)–amino acid complexes as initiators of ring-opening polymerization of cyclic esters. Polym Bull 75:1253–1263
Mandal M, Monkowius U, Chakraborty D (2016) Cadmium acetate as a ring opening polymerization catalyst for the polymerization of rac-lactide, ε-caprolactone and as a precatalyst for the polymerization of ethylene. J Polym Res 23:220
Proverbio M, Galotto NG, Losio S, Laura IT (2019) Influence of co-catalysts and polymerization conditions on properties of poly (anhydride-alt-epoxide)s from ROCOP using salen complexes with different metals. Polymers 11:1222
Trott G, Saini PK, Williams CK (2016) Catalysts for CO2/epoxide ring-opening copolymerization. Philos Trans R Soc A Math Phys Eng Sci 374:20150085
Carpentier JF, Guillaume SM, Li H, Shakaroun R (2020) Recent advances in metal-mediated stereoselective ring-opening polymerization of functional cyclic esters towards well-defined poly (hydroxy acid)s. Chem A Eur J 26:128–138
Takashima Y, Nakayama Y, Watanabe K, Itono T, Ueyama N, Nakamura A, Okuda J (2002) Polymerizations of cyclic esters catalyzed by titanium complexes having chalcogen-bridged chelating diaryloxo ligands. Macromolecules 35:7538–7544
Takeuchi D, Nakamura T, Aida T (2000) Bulky titanium bis (phenolate) complexes as novel initiators for living anionic polymerization of ε-caprolactone. Macromolecules 33:725–729
Chen HY, Liu MY, Sutar AK, Lin CC (2010) Synthesis and structural studies of heterobimetallic alkoxide complexes supported by bis (phenolate) ligands: efficient catalysts for ring-opening polymerization of L-lactide. Inorg Chem 49:665–674
Kayan A (2020) Recent studies on single site metal alkoxide complexes as catalysts for ring opening polymerization of cyclic compounds. Catal Surv Asia 24:87–103
Della Monica F, Paradiso V, Grassi A, Milione S, Cavallo L, Capacchione C (2020) A novel [OSSO]-type chromium (III) complex as versatile catalyst for copolymerization of carbon dioxide with epoxides. Chem A Eur J 26:5347–5353
Solé-Daura A, Zhang T, Fouilloux H, Robert C, Thomas CM, Chamoreau LM, Poblet JM (2020) Catalyst design for alkene epoxidation by molecular analogues of heterogeneous titanium-silicalite catalysts. ACS Catal 10:4737–4750
Yildiz BC, Kayan A (2017) Preparation of single-site tin (IV) compounds and their use in the polymerization of ε-caprolactone. Des Monomers Polym 20:89–96
Acknowledgements
This work was supported by the research foundation of Kocaeli University, (project No: 2017/107). The MS, TGA, and DSC analyses were performed by the METU central laboratory R&D training and measurement center. We thank the stuff working on this center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yildiz, B.C., Kayan, A. Non-Toxic and Environmentally Friendly Titanium Complexes and Their Effects on Ɛ-Caprolactone Polymerization. Catal Surv Asia 24, 313–324 (2020). https://doi.org/10.1007/s10563-020-09307-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10563-020-09307-3