Abstract
A new titanium(IV) complex [TiLCl2] {LH2 = 2-(3,5-di-tert-butyl-2-hydroxy-benzylamino)-4-methysulfanyl-butyric acid} having S-, N-, and O-bearing phenoxy-amine backbones has been synthesized and investigated for its efficient role in aqueous emulsion polymerization of polar olefins, methylacrylate and methylmethacrylate at ambient temperature. The catalyst microstructure has been supported by elemental analysis, 1H and 13C NMR, IR, and UV–visible spectroscopy. The catalyst is easy to synthesize and found to be moderately stable in water. Upon activation with BPh4 −, the activity of the complex as single-site catalytic species has been found to be high (in the order of 104 g mol−1 h−1) producing syndiotactic rich high molecular weight polymers with low molecular weight distribution (PDI values in the range of 1.2–1.4). Structure and molecular properties of the synthesized polymers were determined by 1H NMR, 13C NMR, gel permeation chromatography, and DSC analysis. DSC analysis reveals that the polymer product obtained is semi-crystalline with sharp melting temperature at 286.9 °C (T m). Kinetic parameters such as rate constant and activation energy of polymethylmethacrylate have also been studied, and the value of E app has been found to be 58.842 kJ mol−1 only. The lower value can be interpreted as a consequence of a high catalytic activity of the complex.

A new [ONSO]-type mono(phenolato) titanium(IV) complex [TiLCl2] {LH2 = 2-(3,5-di-tert-butyl-2-hydroxy-benzylamino)-4-methysulfanyl-butyric acid} with soft pendant donor has been synthesized for aqueous emulsion polymerization of polar olefins. Interestingly, it was reported in the literature that the side arm approach is the efficient strategy to design highly active metal catalysts. Therefore, in this paper, we have studied the influence of ligand with hard (N, O) and soft (S) pendant donors on the efficacy of present catalyst in achieving syndioselective homo and copolymerization of polar olefins (methyl acrylate and methyl methacrylate) under normal reaction conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Following the discovery and industrial applications of heterogeneous Ziegler–Natta catalysts in 1953 and with the birth of metallocene-based catalytic systems in 1980s by Kaminsky [1] and Sinn [2], development of effective catalysts for olefin polymerization with controlled stereochemistry and narrow molecular weight distribution has been of great interest. Over the last four decades, extensive work has been done on Ziegler–Natta type catalyst system in terms of catalyst design and the process technology involved in the polymerization process. Driven by the need of single-site high-performance catalysts for the polyolefin industries, the number of group 4 metallocene-based catalyst system was discovered [3]. These single-site catalytic systems contain active sites which behave in the same way for polymerization reactions and display higher polymerization activity with controlled molecular weight distribution [4]. For the first time, Ishihara et al. discovered syndiotactic polystyrene using homogeneous titanium(IV) half-sandwiched catalyst system and, thereafter, a number of group 4 metallocene complexes have been introduced. However, for a long time, the question regarding catalyst design for tailored polyolefins, mechanism of polymerization, origin of stereospecificity, and nature of active metal center remained elusive [5]. The search for stable ancillary ligands has provided an idea for the synthesis of “nonmetallocene” single-site catalysts [6]. After the success story of metallocene complexes, there was a huge outbreak and intensive investigation was made on early transition metal complexes by researchers in concern to nonmetallocene candidates. Fujita and Coates group studied a number of well-defined titanium complexes featuring fluorinated bis(phenoxy-imine) ligand system, one of the important families of nonmetallocene catalysts [7]. This new coordination catalyst (FI catalyst) shows very high catalytic activity than typical metallocene catalysts [8]. Thereafter, a number of group 4 catalyst precursor bearing noncyclopentadienyl ligands such as amidinate, amido, oxazolin, porphyrin, alkoxy, aryloxy, and ketonate have been synthesized. Admittedly, in comparison to metallocene catalysts, these complexes are reported to have a low catalytic activity for polymerization [9]. To improve the catalytic activity, side-arm effect is an efficient strategy for developing Ti/Zr complexes for olefin polymerization. An important family of group 4 metal complexes bearing bis(aryloxide) ligand with additional coordinating heteroatoms [10, 11] is one of the successful examples of side-arm effect to design highly active polymerization catalysts. Kol et al., Sudhakar and Sundararanjan, Li, and many more described the strong influence of pendant groups. For example, incorporation of pendant amino or pendant methoxy groups in ligand framework results in living polymerization of 1-hexene [12]. Heteroatom such as nitrogen and oxygen are reported to be common donor and reported to show lower activity due to strong coordination between hard donors and metal center. Thereafter, Okuda et al. in 2003 and Gibson in 2004 reveal that the metal complexes bearing soft pendant donor like sulfur increases their catalytic activity by stabilizing the reactive cationic metal center. Recently, it was also reported that the group 4 metal complexes bearing [OSSO]-linked ligand system were able to polymerize styrene efficiently to isotactic polystyrene and also, these sulfur-bearing complexes are capable of producing optically active oligostyrenes. Recently, Jun Okuda studied the synthesis and coordination chemistry of titanium complexes bearing tetradentate, [OSNO]-type hybrid ligand systems and explores their polymerization efficiency towards nonpolar olefins in organic solvent [13]. With the tremendous progress in olefin’s polymerization by coordination–insertion mechanism, homopolymerization of polar monomers like methylmethacrylate (MMA) and methylacrylate (MA) and incorporation of these polar functions into copolymers by single-site controlled insertive mechanism have generally proven incompatible and always remained a significant challenge in polymer synthesis [14]. Poly(methyl acrylate), poly(methyl methacrylate), and different poly(acrylates) are commercially very important polymers carrying a number of interesting features such as high surface sensitivity, biocompatibility, miscibility with other polymers, toughness, solvent resistance, interesting rheological properties, and many more. All these superior features make poly(acrylates) widely used as additive, coating and polishing agents, binder, sealer, optical fiber, etc. For almost half a century, free radical aqueous polymerization technology has been well known to produce poly(acrylates) and stable polymer latexes at commercial level because of their higher tolerance towards water than ionic counterparts [15].
However, with growing environmental concern, increasing need of polymer materials at industrial scale, and limiting scope for tacticity control by radical polymerization, researcher’s interest has been developed in metal-catalyzed aqueous polymerization reactions. Claverie et al. have recently reported aqueous polymerization of ethylene catalyzed by nickel(II) phosphinoenolate complexes [16]. A major challenge is the use of extremely moisture sensitive early transition metal catalyst in which water blocks the coordination sites by binding as a ligand with metal center. Also, the use of extreme oxophilic, halophilic, and Lewis acidic type early transition metal-based catalyst system has proven to be incompatible with functional vinyl monomers like poly (methyl methacrylate) and poly (methyl acrylate) [17]. Recently, new titanium(IV) complexes, bearing tetradentate [OOOO] [18] and [ONNO]-type [19] ligands, have been synthesized and characterized which are also found to be active for polymerization in aqueous medium. Inspired by the success of this type of tetradentate ligand-based catalytic system and by interest to study the incompatibility of vinyl monomers with early transition metal complexes, in the present work, we report the synthesis of moderately water-stable titanium(IV) complex-bearing amino acid-based tetradentate [ONSO]-type ligand system to investigate the mixed effect of hard(nitrogen) and soft(sulfur) donors in presence of NaBPh4 towards aqueous homo and copolymerization of functional vinyl monomers such as methyl methacrylate and methyl acrylate.
Experimental
General remarks
Different chemicals and instrumental techniques were used for the synthesis and characterization of ligand, catalyst, and polymers. The ligand was synthesized by modified Mannich reaction between l-methionine, formaldehyde, and 2,4-di-tert-butyl phenol. Titanium tetrachloride (anhydrous fuming liquid, extra pure), sodium n-dodecyl sulfate (SDS), and sodium tetraphenylborate were obtained from Loba Chemie and were used without purification. l-methionine was purchased from Molychem. Tetrahydrofuran, also purchased from Molychem, was dried by refluxing with sodium wire and benzophenone and freshly distilled prior to use. Methyl acrylate and methyl methacrylate were purchased from Molychem and Loba Chemie respectively and were purified prior to use by the usual method.
Characterizations
1H and 13C NMR spectra of the complex were recorded on Bruker (1H frequency = 400 MHz) spectrophotometer. Chemical shift values (δ) are reported in parts per million and calibrated to the residual solvent peak CDCl3. All NMR spectra were recorded at ambient temperature (298 K). Polymer products were also characterized by high-resolution 1H and 13C NMR spectra, recorded on Bruker AVANCE III (1H frequency = 500 MHz) spectrophotometer in CDCl3 solutions at ambient temperature. Differential scanning calorimetry (DSC) measurements were conducted on a DSC Q20 V24.11 Build 124 and on NETZSCH STA 409 PC/PG. In Q20 V24.11 Build 124, accurately weighed samples (4 mg) were placed in hermetically sealed aluminum pan (40 μL) and scanned from 5 to 500 °C at a heating rate of 5 °C/min under a dry nitrogen atmosphere (flow rate 50 mL/min). The data were managed by TA Universal analysis. In NETZSCH STA 409 PC/PG, air was used as a sweeping gas. Sample mass of 5 mg was heated at a flow rate (1) of 1 mL /40 min and with a flow rate (2) of 2 mL/60 min. For elemental analysis, PerkinElmer 2400 Series II CHNS/O Analyzer was used. UV–visible spectra of the complex were recorded in methanol on a Shimadzu UV-1700 spectrophotometer. Fourier transform infrared (FT-IR) spectra were recorded using KBr pellets on a Thermo Nicolet FTIR Spectrometer (NEXUS-870). High-resolution mass spectrum (HRMS) has been obtained on a Micromass Q-Tof micromass spectrometer. Waters’ gel permeation chromatography (GPC) system equipped with a M515 HPLC pump, three styragel 4.6 × 300 mm columns connected in series, and a M2414 RI detector was used to determine molecular weights and polydispersities of the polymers synthesized. Commercially available HPLC grade tetrahydrofuran (THF) served as the mobile phase and was delivered at a flow rate of 0.3 mL/min. Sample concentrations were 1 mg/mL in THF, and the injection volume was 20 μL. The detector signals were simultaneously recorded, and absolute molecular weights were calculated using the Breeze software. Calibrations were done with narrow polystyrene standards.
Synthesis of the ligand L (L = 2-(3,5-di-tert-butyl-2-hydroxy-benzylamino)-4-methysulfanyl-butyric acid)
The tetradentate phenoxyamine-based ligand bearing sulfur as a soft donor was designed following side-arm approach. The synthesis of the ligand is outlined in Scheme 1. l-Methionine (2 g; 0.013 mol) was dissolved in basic solution of NaOH (0.536 g; 0.013 mol in 50 mL distilled water) in a round bottom flask. To this, 37% aqueous formaldehyde (1.99 mL; 0.026 mol) was added and stirred for 20 min. Following Mannich-based condensation mechanism, iminium ion (Schiff base) formation takes place by the reaction between primary amine (l-methionine) and formaldehyde. Simultaneously in another separate round bottom flask, ethanolic solution (50 mL) of 2,4-di-tert-butylphenol (2.76 g; 0.013 mol) was prepared. Then, this solution was transferred dropwise to the Schiff base solution. Schiff base undergoes electrophilic addition reaction with 2,4-di-tert-butylphenol. The reaction mixture was then refluxed for 24 h. Then, it was cooled below room temperature and an equimolar (0.013 mol) amount of HCl was added with continuous stirring resulting in precipitation of a brown semi-solid product. The product was then filtered out, washed with distilled water, dried, and re-crystallized from ethanol to give the ligand (LH2) in 87.82% yield. The ligand was characterized by 1H NMR and 13C NMR spectroscopic techniques.
Synthesis of catalyst precursor TiLCl2
Synthesis of the group 4 metal complex-bearing [ONSO]-type ligand was accomplished by treating ligand (LH2) with metal precursor in dry THF. In two separate round bottom flasks, l-methionine-based ligand (3.92 g; 0.010 mol) and TiCl4 (1.17 mL; 0.010 mol) were dissolved in 25 mL dry THF under nitrogen atmosphere by continuous stirring. Then, the ligand solution was added slowly to the clear yellow solution of the TiCl4 with the help of a cannula. The reaction mixture immediately turned dark red. Stirring was continued for one more hour, and then, the solvent (THF) was removed under reduced pressure. The residue was extracted with toluene and dried in vacuum to obtain complex 1 (Scheme 1) (Yield: 4.46 g, 86.3%). Complex 1 was characterized by 1H NMR, 13C NMR, IR, UV, and elemental analysis. Analysis calculated for C20H31O3NSTiCl2 (molecular weight = 484): H, 6.43; C, 49.79; N, 2.90; O, 9.95; S, 11.34 Found: H, 6.86; C, 49.79; N, 2.81; O, 9.61; S, 11.36. 1H NMR (400 MHz, CDCl3, ppm) (Fig. S1, Supporting Information): δ 7.29 (d, 1H, Ph J = 2.4 Hz), 7.08 (d, 1H, Ph, J = 2.4 Hz), 3.80 (s, 2H, −CH 2 NH), 3.43 (t,1H, -NHCHCOO, J = 5.6 Hz), 2.79 (m, 2H, -CH2(CH 2SCH3, J = 5.2 Hz), 2.06 (s,3H, –(CH2S)CH 3 ), 1.72 (m, 2H, -CH 2 (CH2SCH3), J = 5.2 Hz), 1.41 (s, 9H, Ph-C(CH 3 )3), 1.27 (s, 9H, Ph-C(CH 3 )3). 13C NMR (Fig. S2, Supporting Information): δ 16.60 –(CH2S)CH3, 25.60, 37.53 (4° carbons Ph-C (CH3)3), 29.65 -(CH2S)CH3 30.04, 31.61 (-C(CH3)3) for two t-but group, 34.04 -CH2(CH2SCH3), 45.60 (CH2(NH), 67.99 (NHCH (COO), 115.86–150.01(Ph Carbons),177.30 (-CHCOO).
Polymerization reactions
All the polymerization reactions were carried out at ambient temperature (25 °C) in the presence of air. Commercial methyl acrylate and methyl methacrylate were purified by washing three to four times with 5% NaOH and then several times with distilled water till its pH become neutral. After that, purified monomers were dried by adding anhydrous sodium sulfate and stored in refrigerator. Commercially available SDS was used as emulsifying agent without purification.
Homopolymerization of MMA and MA
The aqueous emulsion polymerization reactions were carried out at ambient temperature (25 °C) under normal atmospheric conditions. Polymerization reactions were carried out in round bottom flasks equipped with magnetic stirrer. Complex 1 (0.02 g; 0.05 mmol) was suspended in 15 mL water, and this suspension was allowed to stir for 1 h; an orange yellow solution was obtained. In another round bottom flask, SDS (sodium n-dodecyl sulfate) (0.23 g; 0.8 mmol) was dissolved in water (35 mL) for 15 min. To this, MA/MMA (0.02 mol) was added and the reaction mixture was stirred for one more hour. After complete 1 h of stirring, aqueous solution of complex 1 was added to the emulsified monomer (MA/MMA) solution followed by addition of large, soft, and noncoordinating anion, BPh4 − (0.017 g; 0.05 mmol). The reaction mixture was stirred for required time period at ambient temperature. The reaction was then quenched or terminated by pouring emulsion to methanol with continuous stirring. Precipitated polymer was then filtered and washed repeatedly with water and methanol and dried in vacuum at ambient temperature. Polymethylmethacrylate (PMMA) and polymethylacrylate (PMA) were further purified by dissolving in acetone, filtering, and reprecipitating by the addition of excess methanol. Finally, the polymers were dried under vacuum.
Copolymerization of MMA and MA
The copolymerization reactions were carried out under similar reaction conditions as described above for homopolymerization reactions. Premixed methyl acrylate and methyl methacrylate were added simultaneously in different proportions keeping the total amount of monomer fixed at 0.02 mol. The polymerization reactions were quenched in methanol resulting in white precipitates of copolymer. The copolymer was further purified by dissolving it in chloroform and then re-precipitated by the addition of methanol and finally dried in a vacuum.
Results and discussion
Synthesis and characterization of TiLCl2
The complex TiLCl2 synthesized by the reaction of the ligand with TiCl4 in dry THF afforded 86.27% yield. The complex synthesized was characterized by different instrumental techniques: elemental analysis, 1H NMR, 13C NMR, IR, and UV–visible spectroscopy. The proposed possible structure of the Ti(IV) complex with formula C20H31O3NSTiCl2 was well supported by the elemental analysis.
UV–visible spectrum
UV–visible spectrum of the nonmetallocene titanium complex 1 (Fig. S3, Supporting Information) with 10−3 M concentration was recorded in methanol. Solvent effect was not observed on λ max or on ε max values. The complex exhibits electronic transition in the visible range of the spectrum at λ 350 nm with ε ~ 250 due to phenoxyl to titanium charge transfer. The intense peak at λ 240 nm with ε value in the order of 103 mol−1 L cm−1 can be assigned to intra-ligand π → π* transition.
IR Spectrum
The IR spectrum of the complex 1 (Fig. S4, Supporting Information) exhibits broadband at 3234.61 cm−1 corresponding to υN–H(stretching). C−H stretching frequency of the tertiary-butyl group appears at 2958.34 cm−1. The band for C=O stretching vibration which appears at 1580 cm−1 is reported to shift to higher frequency for complexes of l-methionine as reported in literature. The sharp band at 1607.07 cm−1 is attributed to C=O stretching vibration which indicates covalent bonding between carboxyl group and metal ion [20]. The most characteristic C=C stretching band for 2,4-di-tert-butyl phenol is at 1507.08 cm−1. Titanium bonded to carboxylate oxygen of the ligand shows band at 1410.38 cm−1. C−N and C−O stretching bands are observed at 1086.56 and 1238.90 cm−1. C–S stretching vibration appears as a weak band at 682.53 cm−1. Involvement of the N in coordination to metal center (Ti) is supported by far IR band at 570.94 cm−1 and band at 484.32 cm−1 can be assigned to Ti−Cl stretching vibrations [21].
Mass Spectrum
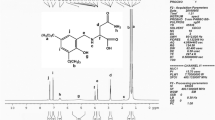
The HRMS (Fig. 1) of the complex shows a peak at m/z 506.0743 due to C20H31O3NSTiCl2Na+ [(M + Na)+]. The isotope pattern also matches with the composition. The peak at m/z 390.2098 may be assigned to the ligand C20H33O3NSNa+.
Homopolymerization of MA and MMA
Since 1980s, titanocene dichloride (Cp2TiCl2) has been attracting continuous attention in the biological world due to its anticancer activity or specialty. In this connection, the hydrolysis chemistry and stability of the titanocene dichloride were studied well. Experimental report suggested that it results in the formation of an activating agent called as aqua or hydroxy-titanocene [Cp2Ti(OH2)(OH)]+ [22]. By UV–visible spectrum, stability of another similar type of new class of antitumor agent [Ti (Ln)n-(OiPr)2] Ln~bis(phenolate) was also reported. Another interesting result revealing stability of Ti complex was that the band for (bis-phenolate) ligand to metal charge transfer shows only slight shift towards longer wavelength even after several hours [23]. E. Tshuva et al. synthesized [ONO]2 Ti homoleptic complex and reported that the complex is quite resistant to hydrolysis and do not show any sign of decomposition for several days [24]. Well-documented literature on [Cp2Ti(OH2)(OH)]+ as the major hydrolysis product and environmentally benign approach inspired us to check the newly synthesized complex 1, TiLCl2 for aqueous polymerization reactions. Therefore, catalytic polymerization reactions were carried out in dispersed aqueous system containing surface active agent, SDS. Like Cp2TiCl2, precatalyst TiLCl2 was also expected to produce same [LTi(OH2)(OH)]+ type of species upon addition to water and form a stable ion pair with soft donor BPh4 −. In fact, chloride estimation and pH measurement of an aqueous solution of the catalyst 1 indicated [LTi(OH2)(OH)]+ to be the most abundant species present in solution (details in page 11, Supporting Information). The nonmetallocene complex 1, TiLCl2 was used for homo and copolymerization of methyl methacrylate and methyl acrylate with NaBPh4 as a cocatalyst. Poly(acrylates) have been characterized by number of interesting features like outstanding water-clear color, stability of the properties upon severe conditions, high surface resistivity, resistance to weathering, and moisture [25]. Due to all these superior characteristics, PMMA and PMA have been used as additives, coatings and polishing, binder, optical fiber, etc. Influenced by all these interesting features, we carried out homo and copolymerization reactions of methyl methacrylate and methyl acrylate. To optimize the reaction parameters for polymerization of MMA and MA, a number of control experiments were performed using different concentrations of NaBPh4 and complex 1 and the results are summarized in Table 1.
It has been found that for optimum yield, Ti/B ratio of 1:1 is required (entry 1; Table 1) below which the yield was found to be average (entry 2; Table 1) and when Ti/B ratio of 1:2 was used, small change was observed in yield (entry 3; Table 1). The effect of change in concentration of precatalyst was also studied, and it was found that for higher yield 0.25 mol% catalyst loading (with respect to monomer) is required (entry 1; Table 1) below which the yield was found to be average (entry 4; Table 1) and when 0.5 mol% Ti was used, not much increase in yield was noticed (entry 5; Table 1). Individual role of the catalyst for polymerization reactions in the absence of counter anion NaBPh4 and vice versa was also studied but failed to afford any polymer (entries 6 and 7; Table 1). Interestingly, when similar polymerization reactions were carried out for methyl acrylate, optimum yield of PMA was found to be slightly higher than that of PMMA. In view of the higher yield of the polymer obtained, we decided to employ 0.25 mol% of the complex 1 and NaBPh4 to carry out the polymerization reactions of acrylates. The effect of temperature on polymerization was also studied. We could not detect any appreciable changes in yield when the temperature was raised to 30 °C. However, when the polymerization was carried out in ice bath(~ 5 °C), no polymer could be isolated and when the temperature was raised to 75 °C, and then very poor yield of polymer(14%) was obtained (entries 8 and 9; Table 1). So, the optimum yield is obtained around 25–30 °C.
To design a phenolate-based catalyst framework for olefin polymerization reactions, a number of sulfur linked precursors were reported. It has been investigated that soft sulfur atom interacts more weakly with hard titanium center, in contrast to hard donors like nitrogen and oxygen thus stabilizing metal center without deactivating it. Titanium complexes with tridentate dianionic [O−NX−] [X = S, P] ligand system was also reported to show higher activity [26]. Recently, researchers interest switch to tetradentate ligand system as they might mimic the steric environment of the surface Ti atoms in heterogeneous Ziegler–Natta system. Furthermore, the steric and electronic properties of the tetradentate ligand-based complexes can be modulated [27]. Therefore, [OSSO]-type complexes have been reported efficient in olefin polymerization [28]. Similar [ONNO]-type of complex has been studied by Kol et al. and considered to be good for the synthesis of another type of polyolefin, poly(1-hexene) [29]. In year 2011, G. Meppelder et al. had reported cyclohexanediyl-bridged [OSNO] bis(phenolate)-type hybrid ligand system to study their activity for styrene and 1-hexene polymerization. So, we were also curious to learn the combined effect of ligand-bearing sulfur and nitrogen donors for polymerization reactions of polar olefins, MMA, and MA. Having established optimum reaction conditions, the complex 1 was stabilized by counter-anion NaBPh4 and upon activation, the time dependence homopolymerization of MMA and MA was studied. The results are summarized in Tables 2 and 3.
In the polymerization reactions, the yield increases with time only within 60 min. But after 60 min, little change in yield was noticed reflecting moderate life time of the active species. From the yield versus time plot (Fig. S5, Supporting Information), it appears that the polymerization reactions are faster for MA producing 90% polymer product within half an hour whereas in case of MMA, yield increases to 90% after 60 min. Like yield, the turnover number [TON] also increases with time (Fig. S6, Supporting Information). Effect of time on catalyst activity was also studied, and it was observed that with increase in time, the catalyst activity declined slowly. This is because of the limited stability of the Ti(IV) complexes in aqueous solution. The complex 1 slowly hydrolyzed to catalytically inactive titanium oxides which was further confirmed by the fact that the orange yellow color of the polymerization mixture started to fade slowly with time (Fig. S7, Supporting Information). In comparison to the activity of the previously reported post-metallocene titanium complexes bearing hard donor groups (N, O), complex 1, bearing soft donor (sulfur) is found to be more active with activity in the range of 104. Gel permeation chromatography shows higher value of number average molecular weight (M n) in the range of 105 with narrow molecular weight distribution (M w/M n < 1.5) which suggests the presence of single-site catalytically active species for polymerization reactions (Figs. S8-S11, Supporting Information).
The structural analysis of the polymers have been unequivocally established by 1H and 13C NMR spectroscopy, gel permeation chromatography (GPC), and differential scanning calorimetry (DSC). Important information related to polymer structure in amorphous and semi-crystalline state can be determined by high-resolution 1H and 13C NMR spectra.
The 1H NMR spectra of the PMMA samples (Fig. S12, Supporting Information) show peaks at 0.84, 1.01, and 1.21 ppm due to α-CH 3 protons. The analysis of the rr, mr, and mm stereo-triad distributions indicated MMA polymerization to yield PMMA with moderate syndiotactic microstructure with an average composition being [rr] = ca. 63%, [mr] = ca. 33%, and [mm] = ca. 4%. The chemical shift signals appearing at 3.59 and 1.89 ppm can be assigned to the resonances of methoxy (–OCH 3) and methylene (–CH 2) protons in MMA units, respectively. Triad tests {4[rr][mm]/[mr]2 ≈ 0.92}provide appropriate information that the stereochemistry of the polymerization is chain-end controlled.
The 13C NMR spectrum also shows characteristic chemical shift signals of the carbon types of syndiotactic PMMA samples as represented by (Fig. S13, Supporting Information). The α-CH3 carbons and quaternary carbons of triad sequences [rr] and [mr] show peaks at 16.55 and 18.73 ppm and at 44.56 and 44.90 ppm, respectively. The chemical shift signals appearing at 51.79, 54.17–54.39, and 176.98–178.09 can be assigned to the resonances of methoxy (–OCH3), methene (–CH2) and carbonyl carbons in MMA units, respectively. The peaks at 176.98, 177.79, and 178.09 ppm can be assigned to [mmrr + rrmr], [rrrr], and [rrrm] pentad sequences [30]. The relative intensities of these peaks also suggest that the PMMA samples are syndiotactic rich.
High-resolution proton NMR spectra are widely used to study the tacticity of the PMA samples (Fig. S14, Supporting Information). Peaks in the region 1.41–1.94 ppm are assigned for –CH 2 protons. For methylene protons, the cross peaks around 1.93 and 1.47 ppm are assigned to the meso (m) dyad and the cross peak at 1.68 ppm is assigned to the racemic (r) dyad. This methylene dyad distribution also shows that the PMA samples are syndiotactic rich [31]. The –CH proton appears at 2.31 ppm, and the methoxy proton (–OCH 3) appears at 3.66 ppm as a sharp singlet.
The 13C NMR spectra of the PMA samples (Fig. S15, Supporting Information) obtained at 400 MHz report that the methylene and methine carbon resonances appear at 34.2–35.0 and 41.4 ppm. The sharp peak for –OCH3 carbon appears at 51.9 ppm, and carboxy carbon appears at 175.0 ppm. It was reported by Matsuzaki et al. that the carboxy and methylene carbons show splitting because of sensitivity to configurational sequences.
Thermal analysis applications (DSC)
Thermal analysis of polymer samples was carried out using differential scanning calorimetry under N2 atmosphere which supports the syndiotactic rich microstructure of polymeric materials. T g of PMMA and PMA are known to increase with increase in syndiotacticity and molecular weight [32, 33]. A clear glass transition temperature (T g) of ~ 24 °C (Fig. S16, Supporting Information) and ~ 136 °C (Fig. S17, Supporting Information) in comparison to 9 °C [34] and 110 °C [35] for random PMA and PMMA reveals higher syndiotactic contents and high molecular weight of PMA and PMMA samples, respectively.
Copolymerization of MA and MMA
Copolymerization of functional monomers, e.g., alkyl acrylate is of practical interest as incorporation of polar groups in copolymer materials exercise great control over number of important properties such as toughness, adhesion, surface, and barrier properties. Copolymers of acrylates carry excellent chemical and optical properties therefore used for the preparation of coatings, membranes, adhesives, etc. The efficiency and performance of newly synthesized titanium(IV) complex 1 for the copolymerization of methyl acrylate with methyl methacrylate are summarized in Table 4. The soft pendant donor (S) group also influences the copolymerization activity as well as %MMA incorporation in copolymers. All the copolymerization reactions were carried out under normal atmospheric condition by varying feed ratios of MA and MMA. Both the monomers were mixed prior to addition. After simultaneous addition of both the monomers, reaction mixture was stirred for one more hour. The crude copolymers were washed thoroughly with water and reprecipitated from chloroform and dried under vacuum.
Characterizations
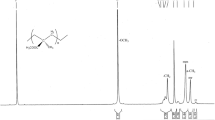
The copolymers were characterized and supported by different instrumental techniques, gel permeation chromatography (GPC), 1H and 13C NMR spectroscopy, and DSC. The average molecular weight and molecular weight distribution was determined by GPC at room temperature using pure THF as solvent and calibrated against standard polystyrene. GPC analysis reveals that the copolymer products posses narrow molecular weight distribution (PDI) value in the range of 1.2–1.4 with average molecular weight in the range 7.3 × 105 to 15.7 × 105 at different feed ratios. High-resolution 1H NMR spectrum analysis (Fig. 2) of methyl acrylate-co-methyl methacrylate was carried out with the purpose of estimation of chemical and stereochemical compositions as function of conversion. In the 1H NMR spectra of the copolymers, the three peaks for α-CH3 protons of the enchained MMA units, which appear in the region of 0.81 to 1.12 ppm for homo-PMMA, split into six peaks in the region 0.83 to 1.25 ppm which proves that the MMA units are enchained units and the polymers are not mixtures of homopolymers [36].
This methyl triad distribution is designated as coisotactic [mm], coheterotactic [mr] and cosyndiotactic [rr] MMA units respectively [37]. As the molar fraction of the MMA in the copolymer increases, intensity of the signal due to α-CH3 proton increases along with % cosyndiotacticity (Fig. 2). The signal for –OCH3 groups appears in the range of 3.5–3.6 ppm. The percentage of methyl methacrylate incorporation was determined by using the formula (Aα-methyl/AO–CH3) × 100 where A α-methyl = area of MMA α-methyl proton resonances and AO–CH3 = area of the O–CH3 proton resonances in 1H NMR spectra.
13 C NMR
The 13CNMR spectral pattern of copolymers is studied and shown in Fig. S18 (Supporting Information). The resonance peaks for carbons were assigned by comparing with the spectra of analogous chemical groups reported in the literature and also by comparing with 13C NMR of homopolymers of MMA and MA. It was reported that carbonyl carbon of poly (methyl acrylates) does not provide any structural information, but the α-methylene carbon and C=O carbon of methyl methacrylate may be sensitive to stereochemical configuration and therefore may split into different signals assigned to tactic dyad, triad or tetrad distribution [38]. The α-CH3 carbons of [rr] and [mr] triad sequences reported in 13C NMR spectrum of MMA homopolymer were analyzed to split into five resonance bands [16.4 ppm–20.5 ppm], out of which the first, third, and fifth correspond to the chemical shift of [rr] [mr or rm] and [mm] triads of polymethyl methacrylate. The –CH2 carbon appears between 35.06 and 36.98 ppm of the spectrum. The quaternary carbon gives rise to three peaks with following stereochemical configuration: syndiotactic triads [rr], 44.89 ppm; heterotactic triads [mr], 45.26 ppm; and isotactic triads [mm], 45.59 ppm. The –CH carbon appears at 47.21 ppm and sharp peak for –OCH3 carbon appears at 51.9 ppm. The peak for C=O carbon of copolymer splits into seven peaks as reported in the literature [39].
DSC
Thermal transitions to obtain glass transition temperature and crystalline melting temperature of semi-crystalline PMA-co-PMMA were recorded on instrument NETZSCH STA 409 PC/PG. Air was used as sweeping gas. Sample mass of 5 mg was heated at flow rate (1) of 1 mL/40 min and with flow rate (2) of 2 mL/60 min. Glass transition temperature and crystalline melting temperature of semi-crystalline polymers are important temperatures of phase
transformation because it is associated with the change in mechanical behavior as well as with many key properties such as brittleness, toughness, long term stability, optical clarity, and many more [40]. Therefore, the influence of PMA incorporation on melting (T m), on glass transition (T g), and on enthalpy were investigated. PMA-co-PMMA were characterized by only one intermediate T g value at 73.8 °C when both the monomers were premixed and added simultaneously in polymerization which indicates that the copolymers are random in nature and not a block copolymers. DSC analysis shows the melt transition with a sharp peak at 286.9 °C (T m) corresponding to higher molecular weight semi-crystalline copolymer product. The evaluation of percent crystallinity of polymers is very important at commercial levels. Therefore, from representative DSC curves for random MA-co-MMA copolymer, percent crystallinity is calculated to be 40.06%. A large endothermic peak at 384.2 °C is due to depolymerization of PMA-co-PMMA (Fig. 3).
Kinetic studies
The conversion data obtained from the polymerization of MMA, using complex 1 and cocatalyst NaBPh4, was analyzed to study the kinetics parameters such as rate constant and activation energy. Following “classical free radical polymerization” model, the values of the rate constant and activation energy at different time intervals were calculated. The apparent rate constant polymerization, k app (s−1) can be expressed as
The value of apparent rate constant k app (s−1) can be determined via linear regression analysis of the experimental data. Figure 4 shows the plots of \( \ln \left(\frac{M_0}{M}\right) \) versus time at constant temperature, and the results of the kinetic studies are summarized in Table 5. As depicted from the plot of \( \ln \left(\frac{M_0}{M}\right) \) versus time, the polymerization of MMA is well described as being first-order kinetics irrespective of the polymerization time.
The catalytic activity of the complex for the polymerization reactions was also studied with the help of the Arrhenius equation as given as follows
where k x = k app and E x = E app .
The results of kinetic parameters for MMA polymerization catalyzed by complex 1 were obtained and were discussed by analogy to conventional radical initiator such as AIBN (Fig. 5). It has been concluded that the minimum energy E app = 58.842 KJ mol−1 required for the catalytic polymerization reactions is lower than conventional radical polymerization method. The lower value can be interpreted as a consequence of a high catalytic activity of the complex. Moreover, values of k app are found to be of the order of 10−4 (s−1) which is reported to be higher than several commonly used radical initiators such as AIBN [41].
Comparison between [Ti(ONSO)Cl2]-, [Ti(ONNO)Cl2]-, and [Ti(OOOO)Cl2]-type catalytic systems
The importance and efficiency of complexes bearing additional sulfur donor was reported by Okuda et al. [42] and many other researchers. Therefore the catalytic activity of newly synthesized titanium(IV) complex bearing tetradentate [ONSO]-type ligand has been compared with previously reported similar types of Ti(IV) complexes bearing [ONNO] and [OOOO]-type ligands (Table 6). For comparison, we have studied the effect of catalytic complexes on yield and activity under similar reaction conditions. It has been found that in the newly synthesized [ONSO]-type titanium(IV) complex, TiLCl2 having sulfur donor shows more activity in comparison to [ONNO] [19] and [OOOO] [18]-type titanium(IV) complexes. This may be explained by the fact that the soft-donor sulfur may stabilize the reactive metal center more efficiently as they interact more weakly with hard cationic metal center in contrast to hard donors like O and N which form a strong bond with metal center, hence lowering the activity of the metal complex for polymerization reactions. 1H NMR spectrum reveals that the stereoselectivity of the present catalytic system in polymerization reactions is almost comparable to Ti (IV) [ONNO and OOOO]-type complexes, producing ~ 63% syndiotactic PMMA and ~ 67% cosyndiospecific MMA units in copolymers. Interestingly, DSC analysis of PMA-co-PMMA synthesized by Ti(IV) [ONSO]-type complex shows sharp T m value at 286.9 °C which corresponds to semi-crystalline copolymer product which was not found when [ONNO]-type Ti(IV) complex was used for polymerization reactions.
Conclusion
In concern to increasing need for environmental benign polymerization pathways, a new titanium (IV) complex bearing amino acid-based ligand with extra soft sulfur donor has been synthesized for aqueous polymerization of acrylates at room temperature. In contrast to hard pendant donors, sulfur, a soft pendant donor, is expected to increase the efficacy of the metal complexes as a catalyst. Therefore, the polymerization of acrylates, catalyzed by transition metal [Ti (ONSO)] complex, yields high molecular weight polymers with narrow PDI value. The yield of the polymer product and hydrolytic stability of the [ONSO]-type catalytic system is found to be higher in comparison to the previously reported post metallocene complexes. The higher catalytic activity and stability of the complex are also verified by kinetic study. It was found that the minimum energy which is required for the catalytic polymerization reactions is lower than conventional radical polymerization method. Therefore, the lower E app value can be interpreted as a consequence of a higher catalytic activity of the complex. 1H NMR spectrum of copolymer shows that with increase in MMA mole fraction in copolymer, percentage of cosyndiotacticity increases. Many characteristic properties of polymers such as surface resistivity, brittleness, toughness, long-term stability, optical clarity, and many more are associated with polymer’s thermal behavior. DSC analysis has been done to study the thermal behavior of the polymers; a sharp peak at 286.9 °C (T m) appears which reveals high molecular weight copolymer product with semi-crystalline domain which was not previously reported in [ONNO]- and [OOOO]-type catalytic systems.
References
Sinn H, Kaminsky W (1980) Ziegler-Natta catalysis. Adv Organomet Chem 18:99–149. https://doi.org/10.1016/S0065-3055(08)60307-X
Scheirs J, Kaminsky W (2000) Metallocene-based polyolefins, preparation, properties and technology. Wiley & Sons, Chichester 1:526 2:571
Brintzinger HH, Fischer D, Mulhaupt RB, Waymouth RM (1995) Stereospecific olefin polymerization with chiral metallocene catalysts. Angew Chem 34(11):1143–1170. https://doi.org/10.1002/anie.199511431
Ziegler K, Gellert HG, Zosel K, Lehmkuhl W, Pfohl W (1955) Herstellung von aluminiumalkylen und dialkylaluminiumhydriden. Angew Chem 67:424
Bajgur Chandrasekhar S, Sivaram S (2000) The evolution of new generation ‘single-site’ Ziegler-Natta polymerization catalysts. Current Sci 78:11
H latky, Gregory G (2000) Heterogeneous single-site catalysts for olefin polymerization. Chem Rev 100:1347–1376
Cheng Z, Nie Y, Yan X, Lei R, Lin S (2011) Prepare of novel zirconium complex and catalytic performance for ethylene polymerization. Adv. Mat. Res 179-180:1091–1095
ChunHong WU, HuaYi L, YuQi F, YouLiang HU (2008) The influence of the metal net charge of non-metallocene early transition metal catalyst on the ethylene polymerization activity. Chin Sci Bull 53:3164–3168
Ma Lifu, Yaping S, Qigu H, Yangfeng Z, Deng K, Junlong L, Yang W (2008) A kind of novel nonmetallocene catalysts for ethylene polymerization. J Polymer Sci Part A Polym Chem 46:33–37
Cohen Ad, Kopilov J, Goldberg I, Kol M (2009) C1-symmetric zirconium complexes of [ONNO′]-type salan ligands: accurate control of catalyst activity, isospecificity and molecular weight in 1-hexene polymerization. Organometallics 28(5):1391–1405. https://doi.org/10.1021/om801058w
Agapie T, Henling LM, Dipasquale AG, Rheingold AL, Bercaw JE (2008) Zirconium and titanium complexes supported by tridentate LX2 ligands having two phenolates linked to furan, thiophene and pyridine donors: precatalysts for propylene polymerization and oligomerization. Organometallics 27(23):6245–6256. https://doi.org/10.1021/om800136y
Xu Tieqi, Jie L, Wu G-p, Lu X-b (2011) Highly active ethylene polymerization and regioselective 1-hexene oligomerization using zirconium and titanium catalysts with tridentate [ONO] ligands. Inorg Chem 50:10884–10892
Meppelder Geert-Jan M, Hong-Tao F, Spaniol Thomas P, Jun O (2009) Synthesis, structure and olefin polymerization activity of titanium complexes bearing asymmetric tetradentate [OSNO]-type bis(phenolato) ligands. Inorg Chem 48(15):7378–7388. https://doi.org/10.1021/ic900903b
Jensen Tryg R, Cheol YS, Dash Aswini K, Luo L, Marks Tobin J (2003) Organotitanium-mediated stereoselective coordinative/insertive homopolymerizations and copolymerizations of styrene and methyl methacrylate. J Am Chem Soc 125(47):14482–14494. https://doi.org/10.1021/ja0363664
Seok LW, Kyun NS, Hyun YJ, Chan KG, Do GH, Lee J, Chul JB (2004) Preparation of high molecular weight poly(methyl methacrylate) with high yield by room temperature suspension polymerization of methyl methacrylate. Fibers and Polymers 5:75–81
Wehrmann Peter, Martin Z, Ralf T, Stefan M (2006) Copolymerization of ethylene with 1-butene and norbornene to higher molecular weight copolymers in aqueous emulsion. Macromolecules 39(18):5995–6002. https://doi.org/10.1021/ma060813t
Foley SR, Stockland Jr RA, Shen H, Jordan RF (2003) Reaction of vinyl chloride with late transition metal olefin polymerization catalysts. J Am Chem Soc 125:4350–4361 and references therein
Sharma K, Lunawat G, De SK (2016) Environmentally benign stereoselective polymerizations of polar as well as nonpolar olefins by a new postmetallocene Ti(IV) salicylate complex at ambient temperature in aqueous emulsion. J Polym Res 23(3):41. https://doi.org/10.1007/s10965-016-0924-6
Sharma K, De Sudip K (2016) A post-metallocene titanium(IV) complex bearing asymmetric tetradentate [ONNO]-type amino acid-based ligand and its activity toward polymerization of polar monomers at room temperature in aqueous emulsion. Colloid Polym Sci 294(12):2051–2070. https://doi.org/10.1007/s00396-016-3970-z
Ali A-JFH, Mussa A-STA, Noori JOM (2013) Synthesis and characterization of some essential amino acid metal complexes having biological activity. J Chem Pharma Res 5(10):172–176
Clark RJH (1965) Metal-halogen stretching frequencies in inorganic complexes. Spectrochim Acta 21(5):955–963. https://doi.org/10.1016/0371-1951(65)80163-1
Chen Xiang, Lixin Z (2010) The hydrolysis chemistry of anticancer drug titanocene dichloride: an insight from theoretical study. J Mol Struct THEOCHEM 940(1-3):45–49. https://doi.org/10.1016/j.theochem.2009.10.007
Peri Dani, Sigalit M, Michal S, Tshuva Edit YC (2009) Synthesis characterization and cytotoxicity and hydrolytic behavior of C2- and C1-symmetrical Ti(IV) complexes of tetradentate diamine bis(phenolate) ligands: a new class of antitumor agents. Chemistry 15(10):2403–2415. https://doi.org/10.1002/chem.200801310
Tshuva Edit Y, Miriam Versano, Goldberg Israel, Kol Moshe, Weitman Hana, Goldschmidt Zeev (1999) Titanium complexes of chelating dianionic amine bis (phenolate) ligands: an extra donor makes a big difference, 2:371–373
Lyoo Won Seok, Noh Seok Kyun, Yeum Jeong Hyun, Kang, Gu Chan, Ghim Han Do, Lee Jinwon, Ji Byung Chul (2004) Preparation of high molecular weight poly (methyl methacrylate) with high yield by room temperature suspension polymerization of methyl methacrylate 5:75–81
Wang C, Sun XL, Guo YH, Gao Y, Liu B, Ma Z, Xia W, Shi LP, Tang Y (2005) Novel titanium catalysts bearing an [O, N, S] tridentate ligand for ethylene homo- and copolymerization. Macromol Rapid Commun 26(20):1609–1614. https://doi.org/10.1002/marc.200500403
Ciancaleoni Gianluca, Natascia F, Budzelaar Peter HM, Vincenzo B, Alceo M (2011) Structure and dynamics in solution of bis (phenoxy-amine) zirconium catalysts for olefin polymerization. Organometallics 30(11):3096–3105. https://doi.org/10.1021/om2001926
Capacchione C, Proto A, Ebeling H, Mulhaupt R, Molle K, Spaniol TP, Okuda J (2003) Ancillary ligand effect on single-site styrene polymerization: isospecificity of group 4 metal bis(phenolate) catalysts. J Am Chem Soc 125:4964–4965
Tshuva EY, Goldberg I, Kol M (2000) Isospecific living polymerization of 1-hexene by a readily available non-metallocene C2-symmetrical zirconium catalyst. J Am Chem Soc 122(43):10706–10707. https://doi.org/10.1021/ja001219g
De SK, Bhattacharjee M (2011) Synthesis of high molecular weight polymer nanoparticles by [Cp2ZrCl2] catalyzed emulsion polymerization. J Poly Sci Part A Poly Chem 49:3920–3927
Matsuzaki K, Kanai T, Kawamura T, Matsumoto S, Uryu T (1973) 13C nuclear magnetic resonance spectra of polyacrylates and their model compounds. J Polym Sci Part A Chem Ed 11:961–969
Kitayama T, Masuda E, Yamaguchi M, Nishiura T, Hatada K (1992) Syndiotactic-specific polymerization of methacrylates by tertiary phosphine triethylaluminium. Polym J 24:817–827
Marshall EL, Gibson VC (2008) Stereoselective acrylate polymerization Taylor and Francis 593–626
Wood LA (1958) Glass transition temperatures of copolymers. J Polym Sci 28(117):319–330. https://doi.org/10.1002/pol.1958.1202811707
Hatada K (1999) Stereoregular uniform polymers. J Polym Sci Part A Polym Chem 37:245–260
Lopez-Gonzalez MMC, Fernandaz-Garcia M, Barrales-Rienda JM, Madruga EL (1993) Sequence distribution and stereoregularity in methyl methacrylate-methyl acrylate copolymers at high conversions. Polymer 34:14
Jensen TR, Yoon SC, Dash AK, Luo L, Marks TJ (2003) Organotitanium mediated stereoselective coordinative/insertive homopolymerizations and copolymerizations of styrene and methyl methacrylate. J Am Chem Soc 125:14482–14494
Kim Y, Harwood JH (2002) Analysis of sequence distribution in methyl methacrylate-methyl acrylate copolymers by 13C NMR spectroscopy. Polymer 43(11):3229–3237. https://doi.org/10.1016/S0032-3861(02)00153-2
San RJ, Miguel V (1990) Quantitative evaluation of sequence distribution and stereoregularity in ethylacrylate-methyl methacrylate copolymers by 13C n.m.r spectroscopy. Polymer 31:1216–1221
Sichina WJ (2000) DSC as problem solving tool: measurement of percent crystallinity of thermoplastics. PerkinElmer Inc.
Harrar-Ferfera Hafida, Farouk A (2008) Polymerization of methyl methacrylate with nickel (II) a-benzoinoxime complex. J Appl Polym Sci 108(3):1514–1522. https://doi.org/10.1002/app.24711
Bajgur Chandrasekhar S, Sivaram S (2000) The evolution of new generation ‘single-site’ Ziegler-Natta polymerization catalysts. Current. Sci. 78:11
Acknowledgments
We thank Mr. Chitresh Kumar Bhargava, Research Fellow, Department of Chemical Engineering, IIT Bombay for providing some of the characterizations presented in this paper.
Funding
This study was funded by Jaypee University of Engineering and Technology, Guna, 473226, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No human and animals were involved in the present study.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 2437 kb)
Rights and permissions
About this article
Cite this article
De, S.K., Sharma, K. & Sharma, C. Synthesis and catalytic performance of a new post-metallocene titanium complex having asymmetric tetradentate [ONSO]-type amino acid-based chelating ligand for acrylate polymerization at room temperature in aqueous emulsion. Colloid Polym Sci 296, 107–119 (2018). https://doi.org/10.1007/s00396-017-4234-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-017-4234-2