Abstract
Herein, polyphenol oxidase (PPO) was purified from Coleus forskohlii via a three-step process involving precipitation by (NH4)2SO4, ion exchange chromatography and gel filtration. PPO was purified 15-fold with a total yield of 31% and a specific activity of 3168 U/mg, and the observed molecular weight was 42 kDa. To improve the reusability and stability of the purified enzyme, calcium alginate incorporated with zinc oxide nanoparticles (Ca-ALG-ZnO NPs) was successfully prepared and characterized through scanning electron microscopy and Fourier transform infrared spectroscopy and utilized as a support for PPO immobilization. The immobilization yield and enzyme activity reached 83% and 3950 U/g support, respectively. The results showed that immobilized PPO could be reused ten times while retaining 69% of its original activity. The purified and immobilized PPO exhibited an optimal pH of 7.0 and 7.5 and an optimal temperature of 40 °C and 50 °C, respectively. The purified and immobilized PPO showed Vmax values of 255.75 and 251.89 Units/ml, and Km values of 4.99 and 3.12 mM, respectively. As a result, this work provides an opportunity to understand and manage the influence of the coleus forskohlii PPO enzyme in key immobilization, industrial. Additionally, the results indicated that the Ca-ALG-ZnO NPs used as supports had a significant effect on the enzyme activity and stability of immobilized PPO.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Enzymes play an important role in a wide range of industries, including food and beverages, pharmaceuticals, household products, biofuels, biosensors, and biotech research and development [1, 2]. Soluble enzymes, however, are defective in many respects, such as stability, reusability, recovery, complexity, expensive manufacturing methods, narrow pH levels, and temperature sensitivity. Thus, the attempt to combine enzyme immobilization with insoluble matrices has become a proven approach to overcoming their application drawbacks [3]. According to green chemistry principles, the use of different biocatalysts not only produces target products and reduces side effects, but also improves selectivity and activity. Due to their high catalytic performance, environmentally friendly catalysts, and selectivity, enzyme-based biocatalysts are quite intriguing. Enzyme immobilization is shown to be beneficial to stability and recyclability [4]. Several materials for the immobilization of enzymes such as metal oxides, acrylic polymers, silica, and carbons have been examined as supports for enzymes [5,6,7,8,9]. Alginate is an anionic charged polysaccharide that is hydrophilic, biodegradable, and biocompatible. The primary drawback of alginate is its limitation of mechanical stability at high acidic and alkaline pH levels [10]. In this work, alginate and zinc oxide nanoparticles were mixed to improve the stability of alginate beads. Unique properties of zinc oxide nanoparticles include their great biocompatibility, non-toxicity, high specific surface area, chemical stability, and inexpensive cost [11]. Antibacterial nanomaterials such as nano zinc oxide was found in industry and food additives that are designed not to be released, acting as an antimicrobial agent [12]. These characteristics facilitate the use of nano zinc oxide as biomimetic support for immobilizing and modifying biomolecules.
Polyphenol oxidase (PPO: EC 1.14.18.1) belongs to the oxidoreductase family, a family of enzymes that contains copper and is widespread in plants, fruits, and seafood [13]. Such enzymes catalyze diphenol oxidation to quinones which are responsible for creating brown pigments when the fruit is exposed to air or shrimp are drained out of the water [14, 15]. Enzymatic brewing represents one of the biggest obstacles in the fruit and vegetable industry and is commonly seen as harmful in regard to sensory and nutritional terms of food quality [14]. Because of the significance of this reaction, PPO has been evaluated and characterized in many plants to develop methods to control browning reactions, which is necessary to increase product value and reduce losses following harvest [16]. However, no studies have reported the purification and characterization of coleus forskohlii PPO. Polyphenol oxidase was used in phenol-containing, artificial solutions. These enzymes are very effective biocatalysts and therefore have been proposed to remove phenolic substances from wastewater [17,18,19]. The methods of biological treatment are considerably cheaper than the methods of chemical or physical processing to reduce phenol concentrations [20].
Coleus forskohlii is a widespread family of Lamiaceae medicinal plants and has been distributed worldwide, including in Saudi Arabia, China, India, Pakistan, and Africa [21, 22]. This plant's extract has been utilized in Ayurvedic medicine for millennia to cure a variety of ailments [23]. Antioxidants, including antioxidant enzymes, may help the body defend itself against various types of reactive oxygen damage brought on by a variety of factors [22]. The aim of this study was to purify and immobilize PPO from Coleus forskohlii. The purification process was performed in three steps via (NH4)2SO4 precipitation, ion exchange and gel filtration chromatography. Characterization of the purified and immobilized enzyme was determined by investigating reusability, storage stability, temperature, optimum pH, and kinetic behavior.
2 Materials and Methods
Catechol, DEAE-sepharose, Sephacryl S-200, sodium alginate, zinc oxide nanoparticles (particle size < 100 nm, surface area 10–25 m2/g), ammonium sulfate (NH4)2SO4 and other chemicals used in this study were purchased from Sigma-Aldrich Limited.
2.1 Plant Collection
Coleus forskohlii is a wild plant available in Saudi Arabia. The stem of this plant was collected from Khulis city in June 2021.
2.2 C. forskohlii Polypenol Oxidase Purification
2.2.1 C. forskohlii Stem Peel Extract
Thirty grams of C. forskohlii stem peels were pulverized in pH 7.0, 20 mM Tris–HCl buffer. This crude extract was filtered and centrifuged for 15 min at 12,000 rpm, and the pellet was discarded. The supernatant was retained and used in subsequent stages.
2.2.2 C. forskohlii Polyphenol Oxidase Precipitation
The first step of partial purification was carried out by precipitation of the crude extract containing the C. forskohlii PPO enzyme via 80% saturation of solid (NH4)2SO4 at 4 °C. The precipitated PPO was collected and redissolved in a few milliliters of Tris–HCl buffer (pH 7.0, 20 mM). To eliminate excess ammonium sulfate, the suspended sample was dialyzed for 8 h in the same buffer and then centrifuged at 12,000 rpm for 15 min. The partially purified PPO was kept at − 20 °C to be utilized in the next step.
2.3 Purification of PPO by Ion Exchange and Gel Filtration
The partially purified PPO fraction produced in the preceding stage was homogenized with Tris–HCl buffer (20 mM, pH 7.2) applied onto DEAE-Sepharose column. Then, the column was washed with a gradient of sodium chloride (0.0–0.3 M) prepared in Tris–HCl buffer. The enzyme activity was determined by measuring the protein peak at 280 nm in each eluted portion of the NaCl gradient. Eluted fractions with significant PPO activity were collected and stored as lyophilized powder. The powdered PPO was dissolved in Tris–HCl buffer (20 mM, pH 7.2), applied onto an equilibrated Sephacryl S-200 column, and eluted with the same buffer at a flow rate of 30 ml/h.
2.4 Protein Concentration Measurements
The protein content was determined and standardized to bovine serum albumin using Bradford’s technique [24].
2.5 Polyphenol Oxidase Assay
The activity of polyphenol oxidase was investigated using catechol as a substrate according to the method of Dahham et al. [25]. A reaction mixture containing 2.8 mL of catechol (20 mM) prepared in sodium phosphate buffer (10 mM, pH 6.8) and 0.2 mL crude extract was added. A spectrophotometer was used to record the absorbance at 400 nm for 3 min. Under standard assay conditions, 1 unit of enzyme activity was defined as a change in absorbance of 0.001 per minute.
2.6 Molecular Weight Assessment
The gel filtration technique with Sephacryl S-200 was used to measure the molecular weight of purified PPO. A gel filtration Markers Kit for Protein molecular weights (12,000–200,000 Da) was used to equilibrate the column.
2.7 Immobilization Process
Different concentrations (0.2–1.2% w/w) of zinc oxide nanoparticles (ZnO NPs) were mixed and sonicated for 10 min with sodium alginate (2% w/v) and 100 units of purified enzyme. With a sterile syringe, the mixture was delicately evacuated and dumped into a glass container containing CaCl2 (2% w/v). After 1 h, the beads were washed in deionized water after being withdrawn from the CaCl2 solution. The PPO activity (units/g support) and immobilization efficiency (IE %) were calculated using the following formulas:
2.8 FT–IR and SEM Analysis
The FT–IR spectra of the Ca-ALG-ZnO NP beads before and after immobilization were obtained using an FT/IR-4600 spectrometer. Field emission scanning electron microscopy (FESEM, JEOL JSM 7600F FEG-SEM) and energy dispersive X-ray spectroscopy (EDX) were used to examine the morphological characteristics of the Ca-ALG-ZnO NP beads before and after immobilization. All samples were mounted on carbon tape over copper stubs and sputtered for 10 s with platinum prior to observation.
2.9 Reusability and Storage Stability
The activity of the immobilized PPO was assessed several times under optimal assay conditions to test its reusability. Immobilized PPO was withdrawn after each assay, washed with distilled water, and resuspended in fresh catechol solution. The same method was repeated ten times. To measure the percentage activity during each repeated use, the initial activity was used as the control (100%). Purified and immobilized PPO storage stability at 4 °C was investigated over an 8-week period by evaluating residual activity every 7 days. The initial activity was used as the control (100%).
2.10 Physico-Chemical Characterization of Purified and Immobilized PPO
To evaluate the optimal pH and temperature values for free and immobilized PPO, activity was determined at pH values ranging from 4.0 to 9.0 (0.05 M sodium acetate buffer pH 4.0–6.0; Tris–HCl buffer pH 6.5–9.0), and temperatures ranging from 30 to 80 °C. The pH and temperature values with the highest activity were assumed to be 100%, and the relative activity of the others was determined accordingly. To evaluate the kinetic parameters, purified and immobilized PPO extracts were incubated with different concentrations of catechol (4–20 mM), and their kinetic behavior was measured by plotting Line-weaver–Burk plots and then calculating the Km and Vmax values.
3 Results and Discussion
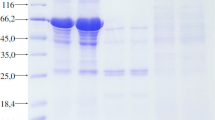
In the present study, PPO was purified and characterized from C. forskohlii. The crude supernatant precipitated with 80% ammonium sulfate showed 310 units/mg protein and 1.45-fold purification with a recovery of 64% (Table 1). DEAE-Sepharose was utilized as a column filling substance for the ion exchange chromatography method. From the elution profile of the enzyme on DEAE–Sepharose, three phases with PPO activity were identified (Fig. 1): the negative adsorbed phase and the phases eluted with 0.05 and 0.2 M sodium chloride and classified as C. forskohlii polyphenol oxidases PPO 1, PPO 2, and PPO 3 for the three phases, respectively. The C. forskohlii polyphenol oxidase PPO 3 with the maximum activity was isolated on a Sephacryl S-200 column to obtain polyphenol oxidase PPO 3A (Fig. 2) with a specific activity of 3168 units/mg protein and 15-fold purification (Table 1). In a work by Benaceur et al. [26], the ammonium sulfate (80%) precipitation technique was enforced in the initial stage of purification. The PPO truffles were purified 1.3-fold with 78% recovery yield and specific activity equal to 25 unit/mg protein [26]. Various studies have demonstrated that the characteristics and purity of enzymes may be improved by using ammonium sulfate precipitation, as well as ion exchange and gel filtration chromatography [27,28,29,30,31,32]. Compared to previous literature reports, the fold purification was 11.7 for potato PPO [33], 19.77 for C. sinensis PPO [34], and 12.38 for Indian pink guava PPO [35]. The recovery rate was close to the recovery rate seen for PPO from Terfezia arenaria of 34% [26]. The molecular weight of the purified PPO was determined to be 42 kDa using a Sephacryl S-200 column (Fig. 2). These results agree with the PPO produced from tea leaf (Camellia sinensis) (42 kDa) [36].
Gel filtration of (PPO 3A) DEAE-Sepharose fractions using a Sephacryl S-200 column (a), The molecular weight value for PPO (PPO 3A) was calculated from the calibration curve of the Sephacryl S-200 column. Standard proteins: cytochrome C (12.4 kDa), carbonic anhydrase (29 kDa), bovine albumin (66 kDa), 4) alcohol dehydrogenase (150 kDa), and β-amylase (200 kDa). The void volume was determined with dextran blue (2000 kDa) (b)
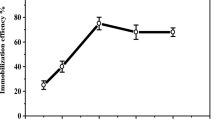
The characteristics of PPO of being unstable, nonreusable, and expensive are the main factors that hinder its use, which is widely adopted on an industrial basis. One of the most promising and intriguing methods for overcoming these constraints is the immobilization of enzymes in solid materials [4]. Immobilization is known to be a beneficial strategy for improving the recyclability and stability of enzymes. Calcium alginate incorporated with metal oxide is an intriguing class of support matrices for the immobilization of various enzymes. Purified PPO was encapsulated into calcium alginate beads incorporated with different concentrations of ZnO nanoparticles (Table 2). To obtain the optimal conditions for the immobilization process, different concentrations of zinc oxide nanoparticles 0.2–1.2% (w/w) were mixed with alginate 2% (w/v). Immobilization efficiency (IE%) and enzyme activity are affected by the nanoparticle concentrations and the PPO concentration in solution, and they were optimized in this study. IE% and PPO activity (U/g Support) increased with increasing concentration of ZnO up to 0.4% and then decreased (Table 2). The optimized values were detected to be 83% IE and 3950 U/g support. Additionally, IE% and PPO activity (U/g Support) increased with increasing initial concentrations of PPO up to 100 units and then decreased (Table 1S, supplementary materials). Enzyme activity and IE% are essential criteria for immobilization evaluation. One of the major features that may be influenced by the immobilization process is the enzyme activity of the immobilized enzyme [37]. IE% denotes the enzyme's ability to bind to the support and is often used to choose an immobilization parameter to be utilized [26]. The decrease in immobilization yield obtained by immobilization enzyme on alginate beads might be attributed to a lack of stability of the obtained beads as well as enzyme leakage caused by the loss of nanocomposite bead structure. ZnO Np was combined with alginate to increase immobilization yield. The strong interaction between the carboxyl group in alginate and zinc oxide can be attributed to the enhancement in the immobilization enzyme.
Because cost reduction is a primary objective of industrialization, the reusability of immobilized enzymes has attracted considerable attention. In this study, for the first five cycles, the decreasing rate remained essentially unchanged, ranging between 91–98%, after which the conversion rate began to decline. The residual activity was 69% after ten repeated cycles (Fig. 3a). The decrease in enzyme activity might be due to the enzyme's active site being blocked by accumulating reaction products, which might have hampered the enzyme’s ability to attach to its substrate [38, 39]. Among the several important studies on PPO immobilizations, few studies be compared to this study. Wang et al. studied the reusability of immobilized PPO on chitosan-gold nanoparticles/montmorillonite. They found that 59% of the initial activity of the immobilized enzyme was preserved after 10 cycles [40]. Lončar and Vujčić synthesized a carrier for PPO immobilization. They observed that the reusability was decreased to 60% after 6 cycles [41]. Compared to prior studies, our results show greater reusability. To determine the storage stability, the immobilized and purified enzymes were incubated in phosphate buffer (pH 6.8, 10 mM) for a period of 8 weeks at 4 °C. Every seven days, the residual activity was examined under normal circumstances. After 8 weeks of storage, the activity of the purified enzyme was 17% of its initial activity, and the immobilized enzyme on alginate/ZnO nanoparticle beads retained 68% of its initial activity (Fig. 3b). As a result, immobilization greatly reduced enzyme deactivation and improved enzyme storage stability. Shao et al. revealed that immobilized PPO on an alginate–SiO2 hybrid gel retained 93% of its initial activity after 4 weeks of storage at 4 °C [42]. In other studies, immobilized α-amylase on amidrazone acrylic fabric retained approximately 65% of its activity after 8 weeks [43].
Figure 4 depicts the infrared spectra of Ca-alginate, modified alginate, and immobilized PPO. All samples had comparable absorption peaks with discernible variations. Because these peaks can be found in all the investigated samples, it can be concluded that alginate does not lose its primary structure throughout the immobilization process or when it is presoaked with the various buffers utilized in the procedures. Peaks at 3339 cm−1 represent the stretching vibrations of the O–H bonds for Ca-alginate [44]. The symmetric stretching vibrations of the COO- group were recognized as the band peaks at 1587 cm−1 and 1412 cm−1, whereas the asymmetric stretching vibrations of the C–O band were detected as the band peaks at 1080 cm−1 and 1030 cm−1. After treatment of alginate with ZnO NPs, the stretching vibrations of ZnO NPs are indicated by the absorption peaks at 732 and 608 cm−1 [39]. After immobilization, the peak at 3307 cm−1 is represented as the N–H stretching vibration of PPO. The peak at 2962 cm−1 corresponds to the C–H stretching vibration. Other characteristic peaks at 1624 and 1290 cm−1 are represented as amide I and amide II, respectively [44, 45].
Figure 5a shows that there was a homogenous structure with an undulant and tortuous surface. The addition of ZnO NPs to the calcium alginate matrix resulted in some particle conurbations, as seen in Fig. 5b. In addition, the ZnO NPs were found to be uniformly dispersed on the surface of the bead. Following PPO immobilization, the bead surface coated with ZnO NPs displayed an irregular morphology and a rugged surface accompanied by an increased level of agglomeration, as shown in Fig. 5c. The presence of ZnO NPs was proven using EDX, as shown in Fig. 5d.
The influence of pH was studied using catechol substrates over wide pH intervals (4–9) (Fig. 6A). The optimal pH values for purified and immobilized PPO correlate to maximal activity at pH 7. After pH 7, the catalytic activity was decreased significantly in the case of purified PPO, while after immobilization, the enzyme had a broader pH activity profile than that of the free enzyme. The ability of an enzyme to oxidize a substrate is related to its catalytic efficiency. Both the binding force and affinity are affected by the ionization state of the amino acids in the active site. The pH impact is caused by a shift in this ionization state, which results in a reduction in enzyme activity. Generally, the optimal pH of vegetable and fruit PPO changes depending on the substrate utilized and the enzyme source [46, 47]. For truffle PPO, the optimal pH value was pH 7 using catechol as the substrate [26], while Borage PPO had an optimal pH value of 7.5 [46].
The catalytic activity of purified and Ca-ALG-ZnO-PPO was measured at temperatures of 30–80 °C (Fig. 6B). Purified PPO exhibits maximal relative activity at a temperature of approximately 40 °C, and at temperatures higher than that, there is a reduction in enzyme activity. On the other hand, PPO cross-linked to Ca-ALG-ZnO NPs demonstrated maximal activity at 50 °C and preserved approximately 87% of its relative activity at 60 °C. According to the current results, PPO exhibited greater heat resistance after cross-linking onto Ca-ALG-ZnO NPs. The enhancement in immobilized PPO activity can be ascribed to a reduction in PPO conformational change during heating because of encapsulation of PPO in Ca-ALG-ZnO NPs. Compared to immobilized PPO, the enzymatic activity of purified PPO decreases after 40 °C because enzyme denaturation causes structural deterioration. The stability of immobilized PPO at higher temperatures may be advantageous for reduced autolysis, as Ca-ALG-ZnO NPs functional enzyme molecules have substantially reduced contacts between themselves [48].
A Lineweaver–Burk plot was used to calculate the Michaelis–Menten constant (Km) and the maximal reaction rate (Vmax) for immobilized and purified PPO (Fig. 1S). The Vmax and Km values for purified and immobilized PPO with catechol substrate were 255.75 U/mL and 4.99 mM and 251.89 U/mL and 3.12 mM, respectively. These results demonstrated that immobilized PPO shows a lower Km and Vmax values than soluble enzymes, indicating enhanced activity upon immobilization [30, 43, 49]. With catechol substrate, PPO from guava afflicted with fruit rot disease has a Km of 4.4 mM [50]. Jia et al. [51] reported that the Km value for PPO from sour cherry pulp was 3.5 mM. In another study carried out by Öztürk et al. [34], the Km value for PPO from tea leaves was found to be 3.782 mM using catechol. The Vmax for immobilized PPO on CTS-AuNPs/MMT was found to be 215.5 U/mL [40]. The PPO from Indian pink guava showed a Vmax of 454.55 U/mL [35]. The Vmax/Km value of immobilized PPO was greater than that of purified PPO, indicating that immobilized PPO more easily interacted with the substrate and had a better catalytic efficiency (Table 3) [52].
4 Conclusion
Polyphenol oxidase is an important enzyme in the biosynthesis of melanin. As a result, understanding the kinetic characteristics of PPO, a food quality-related enzyme, is significant. In this study, purification of C. forskohlii PPO through ion exchange and gel filtration chromatography was reported for the first time. The enzyme exhibited a molecular weight of 42 kDa. PPO was purified 15-fold with a specific activity of 3168 U/mg and a total yield of 31%. After purification, the purified PPO was encapsulated onto Ca/ALG-ZnO NPs. The detailed characterization of purified and immobilized PPO was investigated. The immobilization process improved and enhanced the reusability ten times, retaining 69% of its original activity. Immobilization also improved the thermal stability, pH levels, and substrate affinity. The overall results of immobilized PPO suggest that Ca-ALG-ZnO NPs would be an ideal support for the effective immobilization of PPO in a variety of applications.
References
Das R, Talat M, Srivastava ON, Kayastha AM (2018) Covalent immobilization of peanut β-amylase for producing industrial nano-biocatalysts: A comparative study of kinetics, stability and reusability of the immobilized enzyme. Food chem 245:488–499
Singh K, Srivastava G, Talat M, Srivastava ON, Kayastha AM (2015) α-Amylase immobilization onto functionalized graphene nanosheets as scaffolds: Its characterization, kinetics and potential applications in starch based industries. Biochem Biophys Rep 3:18–25
Das R, Mishra H, Srivastava A, Kayastha AM (2017) Covalent immobilization of β-amylase onto functionalized molybdenum sulfide nanosheets, its kinetics and stability studies: A gateway to boost enzyme application. Chem Eng J 328:215–227
Gan J, Bagheri AR, Aramesh N, Gul I, Franco M, Almulaiky YQ, Bilal M (2021) Covalent organic frameworks as emerging host platforms for enzyme immobilization and robust biocatalysis–A review. Int J Biol Macromol 167:502–515
Mohamed SA, Al-Harbi MH, Almulaiky YQ, Ibrahim IH, El-Shishtawy RM (2017) Immobilization of horseradish peroxidase on Fe3O4 magnetic nanoparticles. Electron J Biotechnol 27:84–90
Almulaiky YQ, Aqlan FM, Aldhahri M, Baeshen M, Khan TJ, Khan KA, Alayafi AA (2018) α-Amylase immobilization on amidoximated acrylic microfibres activated by cyanuric chloride. R. Soc. Open sci. 5:172164
Attard GS, Bartlett PN, Coleman NR, Elliott JM, Owen JR, Wang JH (1997) Mesoporous platinum films from lyotropic liquid crystalline phases. Science 278:838–840
Sun J, Zhang H, Tian R, Ma D, Bao X, Su DS, Zou H (2006) Ultrafast enzyme immobilization over large-pore nanoscale mesoporous silica particles. Chem Commun 12:1322–1324
Abdel-Mageed HM, El-Laithy HM, Mahran LG, Fahmy AS, Mäder K, Mohamed SA (2012) Development of novel flexible sugar ester vesicles as carrier systems for the antioxidant enzyme catalase for wound healing applications. Process Beachem 47:1155–1162
Strand BL, Morch YA, Skjak-Braek G (2000) Alginate as immobilization matrix for cells. Minerva Biotecnologica 12:223
Kamaldeep DK, Kashyap KD (2012) Optimization of zinc oxide nanoparticles synthesis to fabricate glucose oxidase sensor. Adv Appl Sci Res 3:3081–3088
Alfadul SM, Elneshwy AA (2010) Use of nanotechnology in food processing, packaging and safety review. Afr J Food Agric Nutr Dev 10:2719–2739
Gong Z, Li D, Liu C, Cheng A, Wang W (2015) Partial purification and characterization of polyphenol oxidase and peroxidase from chestnut kernel. LWT-Food Sci Technol 60:1095–1099
Falguera V, Sánchez-Riaño A, Quintero-Cerón J, Rivera-Barrero C, Méndez- Arteaga J, Ibarz A (2012) Characterization of polyphenol oxidase activity in juices from 12 underutilized tropical fruits with high agroindustrial potential. Food Bioproc. Technol. 5:2921–2927
Al-Najadaa AR, Mohamed SA (2014) Changes of antioxidant capacity and oxidoreductases of Saudi date cultivars (Phoenix dactylifera L.) during storage. Sci Hortic 170:275–280
Queiroz C, Mendes Lopes ML, Fialho E, Valente-Mesquita VL (2008) Polyphenol oxidase: characteristics and mechanisms of browning control. Food Rev Int 24:361–375
Aitken MD, Massey IJ, Chen T, Heck PE (1994) Characterization of reaction products from the enzyme catalyzed oxidation of phenolic pollutants. Water Res 28:1879–1889
Edalli VA, Kamanavalli CM (2010) Removal of phenolic compounds by mushroom polyphenol oxidase from Pleurotus species. Bioscan 4:89–92
Mukherjee S, Basak B, Bhunia B, Dey A, Mondal B (2013) Potential use of polyphenol oxidases (PPO) in the bioremediation of phenolic contaminants containing industrial wastewater. Rev Environ Sci Biotechnol 12:61–73
Guimarães CO, França AB, Samanamud GRL, Baston EP, Lofrano RCZ, Loures CCA, Naves FL (2019) Optimization of treating phenol from wastewater through the TiO 2-catalyzed advanced oxidation process and response surface methodology. Environ Monit Assess 191:349
Shukla PK, Misra A, Kumar M, Jaichand SK, Akhtar J, Srivastava S, Agrawal PK, Singh AK (2018) Rawat, Simultaneous quantification of forskolin and iso-forskolin in Coleus forskohlii (Wild.) Briq. and identification of elite chemotype, collected from eastern ghats (India). Pharmacogn Mag 13:881–885
Almulaiky YQ, Kuerban A, Aqlan F, Alzahrani SA, Baeshen MN, Afifi M, Alkhaled M (2017) In vitro antiglycation, antioxidant properties of coleus forskohlii “Balady” leaves and stem and their antioxidant enzyme activities. Annu Res Rev 19:1–11
Ding X, Staudinger JL (2005) Induction of drug metabolism by forskolin: the role of the pregnane X receptor and the protein kinase a signal transduction pathway. J Pharmacol Exp Ther 312:849–856
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Dahham SS, Al-Rawi SS, Ibrahim AH, Majid ASA, Majid SAM (2018) Antioxidant, anticancer, apoptosis propertiesand chemical composition of black truffle Terfezia claveryi. Saudi J. Biol. Sci. 25:1524–1534
Benaceur F, Chaibi R, Berrabah F, Neifar A, Leboukh M, Benaceur K, Gargouri A (2020) Purification and characterization of latent polyphenol oxidase from truffles (Terfezia arenaria). Int J Biol Macromol 145:885–893
Almulaiky YQ, Al-Harbi SA (2019) A novel peroxidase from Arabian balsam (Commiphora gileadensis) stems: Its purification, characterization and immobilization on a carboxymethylcellulose/Fe3O4 magnetic hybrid material. Int J Biol Macromol 133:767–774
Eddehech A, Zied Z, Aloui F, Smichi N, Noiriel A, Abousalham A, Gargouri Y (2019) Production, purification, and biochemical characterization of a thermoactive, alkaline lipase from a newly isolated Serratia sp. W3 Tunisian strain. Int J Biol Macromol 123:792–800
Govindarajan RK, Krishnamurthy M, Neelamegam R, Shyu DJ, Muthukalingan K, Nagarajan K (2018) Purification, structural characterization and biotechnological potential of tannase enzyme produced by Enterobacter cloacae strain 41. Process Biochem 77:37–47
Al-Harbi SA, Almulaiky YQ (2020) Purification and biochemical characterization of Arabian balsam α-amylase and enhancing the retention and reusability via encapsulation onto calcium alginate/Fe2O3 nanocomposite beads Int. J Biol Macromol 160:944–952
Almulaiky YQ (2020) Peroxidase from coleus forskohlii: purification and biochemical characterization. Int J Nutr 5:9–20
Almulaiky YQ, Al-Harbi SA (2021) Preparation of a calcium alginate-coated polypyrrole/silver nanocomposite for site-specific immobilization of polygalacturonase with high reusability and enhanced stability. Catal Letters. https://doi.org/10.1007/s10562-021-03631-7
Aksoy MA (2020) new insight into purification of polyphenol oxidase and inhibition effect of curcumin and quercetin on potato polyphenol oxidase. Protein Expr Purif 171:1–6
Öztürk C, Aksoy M, Küfrevioğlu OI (2020) Purification of tea leaf (Camellia sinensis) polyphenol oxidase by using affinity chromatography and investigation of its kinetic properties. J Food Meas Charact 14:31–38
Vishwasrao C, Ananthanarayan L (2018) Partial purification and characterization of the quality deteriorating enzymes from Indian pink guava (Psidium guajava L.), var. Lalit. J. Food Sci. Technol. 55:3281–3291
Teng J, Gong Z, Deng Y, Chen L, Li Q, Shao Y, Xiao W (2017) Purification, characterization, and enzymatic synthesis of theaflavins of polyphenol oxidase isozymes from tea leaf (Camellia sinensis). LWT 84:263–270
Li S, Zhong L, Wang H, Li J, Cheng H, Ma Q (2021) Process optimization of polyphenol oxidase immobilization: Isotherm, kinetic, thermodynamic and removal of phenolic compounds. Int J Biol Macromol 185:792–803
Zhang F, Zheng B, Zhang J, Huang X, Liu H, Guo S, Zhang J (2010) Horseradish peroxidase immobilized on graphene oxide: physical properties and applications in phenolic compound removal. J Phys Chem 114:8469–8473
El-Shishtawy RM, Ahmed NS, Almulaiky YQ (2021) Immobilization of catalase on chitosan/zno and chitosan/ZnO/Fe2O3 nanocomposites: A comparative study. Catalyst 11:820
Wang H, Li S, Li J, Zhong L, Cheng H, Ma Q (2020) Immobilized polyphenol oxidase: Preparation, optimization and oxidation of phenolic compounds. Int J Biol Macromol 160:233–244
Lončar N, Vujčić Z (2011) Tentacle carrier for immobilization of potato phenoloxidase and its application for halogenophenols removal from aqueous solutions. J Hazar Mater 196:73–78
Shao J, Huang LL, Yang YM (2009) Immobilization of polyphenol oxidase on alginate–SiO2 hybrid gel: stability and preliminary applications in the removal of aqueous phenol. J Chem Technol Biotechnol 84:633–635
Al-Najada AR, Almulaiky YQ, Aldhahri M, El-Shishtawy RM, Mohamed SA, Baeshen M, Al-Harbi SA (2019) Immobilisation of α-amylase on activated amidrazone acrylic fabric: a new approach for the enhancement of enzyme stability and reusability. Sci Rep 9:1–9
Almulaiky YQ, Khalil NM, El-Shishtawy RM, Altalhi T, Algamal Y, Aldhahri M, Mohammed MM (2021) Hydroxyapatite-decorated ZrO2 for α-amylase immobilization: Toward the enhancement of enzyme stability and reusability. Int J Biol Macromol 167:299–308
Shi C, Dai Y, Liu Q, Xie Y, Xu X (2003) The FT-IR spectrometric analysis of the changes of polyphenol oxidase II secondary structure. J Mol Struct 644:139–144
Alici EH, Arabaci G (2016) Purification of polyphenol oxidase from borage (Trachystemo norientalis L.) by using three-phase partitioning and investigation of kinetic properties. Int J Biol Macromol 93:1051–1056
Derardja AE, Pretzler M, Kampatsikas I, Barkat M, Rompel A (2017) Purification and characterization of latent polyphenol oxidase from apricot (Prunus armeniaca L.). J Agric Food Chem 65:8203–8212
Atiroğlu V, Atiroğlu A, Özacar M (2021) Immobilization of α-amylase enzyme on a protein@ metal–organic framework nanocomposite: A new strategy to develop the reusability and stability of the enzyme. Food Chem 349:129127
Akgol S, Kacar Y, Denizli A, Arica MY (2001) Hydrolysis of sucrose by invertase immobilized onto novel magnetic polyvinyl alcohol microspheres. Food Chem 74:281–288
Razzaque MA, Saud ZA, Absar N (2000) Purification and characterization of polyphenoloxidase from guava infected with fruit-rot disease. Pak J Biol Sci 3:407–410
Jia G, Baogang W, Xiaoyuan F, Haoru T, Wensheng L, Kaichun Z (2011) Partial properties of polyphenol oxidase in sour cherry (Prunus cerasus L.) Pulp. World J. Agric. Sci. 7:444–449
Cao SL, Li XH, Lou WY, Zong MH (2014) Preparation of a novel magnetic cellulose nanocrystal and its efficient use for enzyme immobilization. J Mater Chem B 2:5522–5530
Acknowledgements
The work was funded by the University of Jeddah, Saudi Arabia, under Grant No. (UJ-20- 076-DR). The authors, therefore, acknowledge with thanks the university technical and support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Almulaiky, Y.Q., Almaghrabi, O. Polyphenol Oxidase from Coleus forskohlii: Purification, Characterization, and Immobilization Onto Alginate/ZnO Nanocomposite Materials. Catal Lett 152, 3089–3099 (2022). https://doi.org/10.1007/s10562-022-03916-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-03916-5