Abstract
Polygalacturonase (PG) catalyses the hydrolysis of pectin substances and is commonly used in the textile and food industries. Herein, PG was purified from Arabian balsam using three techniques (ammonium sulfate precipitation, ion exchange chromatography, and gel filtration) with a recovery of 11.2% and tenfold purification. The molecular weight of the purified PG was estimated to be 75.5 kDa using a Sephadex G-150 column. To improve the stability and reusability of the purified enzyme, a novel method to immobilize PG through calcium alginate-coated polypyrrole/silver nanocomposite was described. The immobilized PG was characterized by FTIR, TGA, SEM, EDX, and Raman spectroscopy. The immobilization efficiency was 84.4%. Excellent long-term storage stability of the immobilized PG was demonstrated with 83% of the initial activity preserved after 60 days. The immobilized PG was highly reusable, showing high activity (91% and 68%) after five and ten cycles. The immobilized PG showed improved stability to temperature and pH relative to that of the free enzyme. The Km and Vmax were determined to be 0.368 mg/mL and 5.33 µmol/mL for the immobilized PG and 0.667 mg/mL and 7.38 µmol/mL for free PG, respectively. Improved storage stability, catalytic efficiency (Vmax/Km), and reusability of the immobilized PG make it ideal for biotechnological and industrial applications.
Graphic Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Enzyme technology is increasingly used to meet various human needs. Pectinolytic enzymes are widely used in industry to improve fruit juice production, fruit texture, and enzymatic fruit peeling [1,2,3]. Enzymes have been used in the scouring of cotton and textiles as well as in the fermentation of coffee and tea [4, 5]. Pectinases are classified as de-esterifying and depolymerizing enzymes, depending on the degradation mechanism. De-esterifying enzymes are used to de-esterify the methoxy group of pectic acid, while depolymerizing enzymes cleave α-1,4 glycosidic bonds in pectin either by trans-elimination (pectin lyases) or by hydrolysis (polygalacturonases) [6, 7]. The biological decomposition of pectin is one of the most essential biomass degradation mechanisms [8]. Pectinolytic enzymes are generally known to hydrolyse pectinolytic substances [9]. The long and recalcitrant molecule pectin, which often serves as a structural polysaccharide in the middle lamella and cell walls of plants, is hydrolysed by these enzymes [4]. Polygalacturonase (pectinase, EC. 3.2.1.15 is one of the most common enzymes in fungi [10, 11], bacteria [12] and plants [13], and it hydrolyses the α-1-4-glycoside bond between residues of galacturonic acid to release D-galacturonic acid oligomers. This enzyme makes up 25% of enzyme sales in the food industry worldwide [4]. Notwithstanding their excellent catalytic characteristics, native enzymes as biocatalysts always have some disadvantages, such as poor stability under intensive operating conditions, difficulty in the recovery of products, and non-reusability in industrial processes [14]. While important advances have already been made in biotechnology, native biocatalysts face several challenges, such as a high cost, low stability, and poor reusability [15, 16]. To counteract these difficulties, enzyme immobilization has been used to improve the catalytic properties of the enzymes to prevent denaturation and to make them economically viable for several applications [17,18,19,20]. The use of immobilization to increase the stability and recyclability of enzymes has been shown to be beneficial [21]. Alginate, one type of polysaccharide, is generally used as a biopolymer in a variety of biomedical applications, including cell culture, drug release, tissue engineering, and enzyme or metal stabilization for catalysts [22, 23]. Due to their mild reducibility, excellent biocompatibility and low cost, alginate hydrogels have proven to be good stabilizers and synthesis templates for metal nanoparticles such as Ag, Ni, Co, and Fe [24, 25]. In this study, polygalacturonase was purified from Arabian balsam and immobilized on PPyAgNp/Ca-alginate. Physico-chemical characterization of purified and immobilized enzymes was performed. To the best of our knowledge, this is the first time that PG has been purified from Arabian balsam, immobilized, and assessed in terms of reusability and stability characteristics.
2 Materials and Methods
2.1 Chemicals and Techniques
2.1.1 Chemicals
Sephadex G-150, polygalacturonic acid (PGA), pyrrole, silver nitrate, sodium alginate, and DEAE-Sepharose were purchased from Sigma-Aldrich (USA). Other solvents used in our study were of analytical grade.
2.1.2 Plant Collection
Commiphora gileadensis also known as “Arabian balsam”, was used in our study and grows widely in Saudi Arabia especially in Makkah. Plant specimen collection was performed in April, 2020.
2.2 Polygalacturonase Purification
2.2.1 C. gileadensis Stem Peel Extract
Ten grams of plant stem peel specimens were collected and crushed into fine pieces using a mortar. The stem peel pieces were soaked in Na-acetate buffer (20 mM, pH 5.5), and crushed in a mortar again until totally smashed. The whole extract was filtered, the filtrate was centrifuged for 10 min at 1118 × g and the supernatant was pipetted into a clean and dry container to be used in further steps.
2.2.2 C. gileadensis Polygalacturonase Precipitation
The protein fraction containing polygalacturonase was separated from the C. gileadensis peel extract by the ammonium sulfate precipitation method. Different weights of solid ammonium sulfate, to obtain 20 to 80% saturation, were added to the peel extract in a stepwise manner to dissolve and separate the polygalacturonase at 0.0 °C. The activity of the polygalacturonase was examined gradually to achieve the highest enzyme activity upon ammonium sulfate saturation. Highly active polygalacturonase was refined at 40% saturated ammonium sulfate and then centrifuged at 1118 × g for 20 min. The precipitated polygalacturonase was separated and resuspended in a few millilitres of Na-acetate buffer (20 mM, pH 5.5) for further enzyme purification. The suspended sample was dialyzed in the same buffer for 12 h to remove the excess ammonium sulfate and then centrifuged at 1118 × g for approximately 20 min. The partially purified polygalacturonase was refrigerated at −20 °C to be used in ion-exchange chromatography (IEC).
2.3 Purification of Polygalacturonase by Ion Exchange and Gel Filtration
The partially purified polygalacturonase fraction of the C. gileadensis peel extract obtained from the previous step was poured into a column containing DEAE-Sepharose as the stationary phase equilibrated with Na-acetate buffer (20 mM, pH 5.5) at room temperature. Then, the column was washed with a gradient of sodium chloride (0.0–0.3 M) dissolved in Na-acetate buffer to elute the polygalacturonase. Every eluted fraction of the NaCl gradient was analysed for enzyme activity according to the protein peak at 280 nm. Eluted fractions with high polygalacturonase activities were collected and then lyophilized. The lyophilized polygalacturonase was dissolved, poured into an equilibrated Sephadex-150 column with Na-acetate buffer (20 mM, pH 5.5), and eluted with the same buffer at a flow rate of 30 ml/h.
2.4 Protein Concentration Measurements
The concentration of protein was evaluated and standardized to bovine serum albumin according to the method of Bradford [26].
2.5 Polygalacturonase Activity Determination
PGA was used as a substrate in the colorimetric determination of polygalacturonase activity [27]. The enzymatic reaction was performed in a 0.5 ml final volume containing an appropriate quantity of purified or immobilized enzyme, 2% PGA, and Na-acetate buffer (50 mM, pH 5.5), and then incubated at 37 °C for 1 h, followed by the addition of DNS (0.5 ml). The reaction mixture was boiled for 10 min in a water bath then cooled immediately under tap water reach to room temperature. The color intensity was measured at 560 nm. The purpose from adding the DNS was to evaluate the D-galacturonic acid oligomers produced from PGA hydrolysis that yielded a stable colour with DNS [28]. As the enzyme activity increased, the PGA was consumed, and the color intensity increased.
2.6 Assessment of Enzyme Molecular Weight
The molecular weight of the purified polygalacturonase was determined by gel filtration using Sephadex G-150. The column was equilibrated using a gel filtration Markers Kit for Protein molecular weights (12,000–200,000 Da).
2.7 Immobilization of Polygalacturonase on PPyAgNp/Calcium Alginate Beads
PPyAgNp was synthesized by oxidative polymerization of pyrrole in the presence of silver nitrate as an oxidant according to a previously reported method [29]. Then, 100 mg of PPyAgNp nanocomposite was added to 100 units of purified enzyme dissolved in 10 mM Tris–HCl buffer (pH 7). Immobilization via ionic bonds was achieved by shaking at 90 rpm overnight at room temperature. Then, 10 mL of 2% (w/v) sodium alginate solution was added to the mixture and sonicated for 10 min. The mixture was carefully aspirated with a sterile syringe and dropped into a container containing 2% (w/v) CaCl2 solution. After 1 h, the beads were removed from the CaCl2 solution and washed with deionized water. The PG activity (units/g support) and immobilization efficiency (%) were determined using the following equations:
2.8 Morphological Characterization
An FT/IR-4600 spectrometer was used to obtain the attenuated total reflectance Fourier infrared (ATR–FTIR) spectrum of the immobilized enzyme. Raman spectroscopy of the PPyAgNp/Ca-alginate beads before and after immobilization was performed on a Raman: DXR (Thermo Scientific, USA) with a 532 nm laser as an excitation source. Thermogravimetric analysis (TGA) of the samples was accomplished using an SDT Q600 V20 9 Build 20 analyser. PPyAgNp/Ca-alginate beads with/without enzyme were gradually heated (20 °C min) until reaching a maximum temperature of 800 °C. The morphological features of the PPyAgNp/Ca-alginate beads before and after immobilization were measured by field emission scanning electron microscopy (FESEM, JEOL JSM 7600F FEG-SEM) coupled with energy dispersed X-ray spectroscopy (EDX). All samples were mounted on carbon tape over copper stubs and sputtered for 10 s with platinum prior to observation.
2.9 Immobilized Polygalacturonase Reusability and Storage Stability Assessment
The reusability of the immobilized PG was assessed under the optimum reaction conditions. The immobilized enzyme was filtered out of the reaction mixture and then suspended in Na-acetate buffer (50 mM, pH 5.5). After that, the immobilized PG that remained active after filtration was suspended again in the reaction mixture and filtered. The activity measured after the first suspension was considered 100% and used as a control. The activities of all other subsequent reused enzymes were calculated compared to the control. To calculate the storage stability of the enzyme, the purified and immobilized PG was kept for approximately 60 days at 4 °C. Then, the enzyme activity was assessed every ten days under the optimum reaction conditions.
2.10 Physicochemical Characterization of the Enzyme
2.10.1 Determination of the Optimum pH
Different pH ranges were used to determine the enzyme’s optimal pH. Acetate buffer (50 mM) with pH values ranging from 4.0 to 6.0 and Tris–HCl buffer with pH values ranging from 6.5 to 9.0 were used in our experiments.
2.10.2 Determination of the Optimum Temperature and Thermal Stability
The optimum temperature at which the enzyme achieved the highest activity was determined by exposing the purified and immobilized PG to a series of temperatures (30–90 °C) and then measuring the enzyme activity. All other conditions were held constant throughout the experiments. To evaluate the thermal stability of the purified and immobilized PG, the samples were incubated at a series of temperatures (50–80 °C) for 10, 20, 30, 40, 50, and 60 min. After 24 h, an enzyme assay was carried out under standard assay conditions.
2.10.3 Kinetic Behavior Evaluation
Purified and immobilized enzyme were incubated with different concentrations of PGA substrate, and their kinetic behaviour was measured by plotting Line-weaver–Burk plots and then calculating the Km and Vmax values.
2.11 Statistical Analysis
To examine the analysis of statistical significance of differences in the results, we have used SPSS 18 to analyze the data. Analyzed using Wilcoxon Signed Ranks Test, we estimated that there is a statistically significant difference between relative activity (%) free enzyme and relative activity (%) immobilized enzyme across different conditions.
3 Results and Discussion
3.1 Polygalacturonase Purification
In this study, PG from the Arabian balsam plant was purified through ammonium sulfate (NH4)2SO4 precipitation, ion-exchange chromatography (DEAE-Sepharose column), and gel filtration (Sephadex G-150 column). The purification results are summarized in Table 1. In the initial purification step, the crude extract of PG was precipitated by (NH4)2SO4, and different fractions were collected from 20 to 80%. The PG activity was measured in all collected fractions, and significantly higher PG activity was detected in the fraction obtained by 40% (NH4)2SO4 precipitation. Compared to the crude extract, the PG obtained from 40% (NH4)2SO4 precipitation showed an increase in specific activity from 169.8 U/mg to 266.3 U/mg, with a recovery of 65% and 1.57-fold purification. Therefore, the fraction obtained by 40% (NH4)2SO4 precipitation was subjected to a DEAE-Sepharose column. Ammonium sulfate precipitation is a technique that is still commonly used to partially purify target proteins. For example, ammonium sulfate precipitation was used for purification of peroxidase and α-amylase from Commiphora gileadensis [30, 31] and peroxidase from haricot beans [32] and horseradish cv. Balady [33]. Since certain proteins have few hydrophilic areas, they can accumulate and precipitate at low ammonium sulfate concentrations (20–40% saturation). The protein, on the other hand, has significantly more hydrophilic areas, so it can stay in solution until the ammonium sulfate concentration is significantly higher (50–80% saturation) [34]. In the second step of purification, as shown in Fig. 1, five peaks with PG activity were eluted with 0.0, 0.05, 0.1, 0.2, and 0.3 sodium chloride and designated PG 1–5. PG3 showed an increase in specific activity to 671.43 U/mg, with a recovery of 21.8% and 3.95-fold purification. Therefore, fraction PG3 was subjected to Sephadex G-150 in the third step of purification. Figure 2a shows the elution profile. In this step, only one peak (PG3A) was observed, indicating that the purified enzyme was obtained successfully. The PG3A fraction exhibited specific activity of up to 1711.8 U/mg, with a recovery of 11.2% and tenfold purification. The molecular weight of the purified PG was estimated to be 75.5 kDa using the Sephadex G-150 column, as shown in Fig. 2b. Some polygalacturonase was stated to have a molecular weight between 69.7 and 110 kDa, which may be due to variations in amino acid sequence or glycosylation [35]. For example, the molecular weight of PG was 69.7 kDa for Aspergillus giganteus [36], 70 kDa for Botrytis cinerea [37], 92 kDa for Penicillium viridicatum [38], and 110 kDa for Bacillus paralicheniformis [39].
a Chromatography of the polygalacturonase PG3 DEAE-Sepharose fraction using a Sephadex G-150 column (a), The molecular weight value for polygalacturonase PG3 was calculated from the calibration curve of the Sephadex G-150 column. Standard proteins: cytochrome C (12.4 kDa), carbonic anhydrase (29 kDa), bovine albumin (66 kDa), 4) alcohol dehydrogenase (150 kDa), and β-amylase (200 kDa). The void volume was determined with dextran blue (2000 kDa) (b)
3.2 Polygalacturonase Immobilization
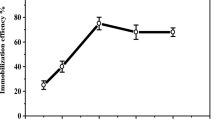
Purified PG has wide potential for use in the food industry. Therefore, purified PG was immobilized on polypyrrole/silver (PPyAgNp) via ionic bonds at pH 7 and entrapped into calcium alginate beads. Because polygalacturonase contains disulfide and/or thiol and amino acid groups within its chemical structure, it was therefore assumed that a PPy and AgNp composition would provide a good matrix for enzyme immobilization due to the positive charges on PPy that help to bind the enzyme via ionic bonds and the propensity of AgNp to bind to disulfide and/or thiol groups [29, 40]. The PG activity was 35.17 units/g support, and the immobilization efficiency was 84.4%. In a previous study, α-amylase was immobilized onto PPyAgNp/Fe3O4 and calcium alginate/Fe2O3 with immobilization efficiencies of 75% and 54.5% [30, 41]. In this study, the use of the PPyAgNp/Ca-alginate matrix increased the immobilization efficiency due to two types of interactions between the enzyme and the supporting matrix: ionic bonding and entrapment.
3.3 FT-IR Analysis
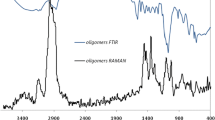
The FT-IR spectra of calcium alginate, PPyAgNp/Ca-alginate, and PPyAgNp/Ca-alginate with the enzyme are shown in Fig. 3. The peak at 3251 cm−1 corresponds to the O–H bond stretching vibrations of the alginate. The peak at 1595 cm−1 corresponds to the symmetric stretching vibrations of the COO − group. The peaks at 1460 and 1591 cm−1 correspond to the stretching vibration of the C–C and C–N bonds of the pyrrole ring, respectively. The peak at 1214 cm−1 corresponds to the breathing vibration of the pyrrole ring. The peak at 1319 cm−1 may result from the absorption assigned to doped NO3− in PPy or the interactions between AgNp and PPy. The peak at 795 cm−1 corresponds to C–H wagging. The broad peaks observed after immobilization at 3300 cm−1 and 1538 cm−1 were attributed to stretching of the OH and NH groups present in the PG enzyme. Amide bands I and II appear at 1600 cm−1 (CO stretch, amide I) and 1415 cm−1 (NH bend, amide II), respectively. The peak at 1027 cm−1 corresponds to the stretching vibrations of the C–O bond of the glycosidic linkage [30, 41, 42].
3.4 Raman Spectroscopy Analysis
Raman spectroscopy was further used to confirm the immobilization of the enzyme on PPyAgNp/Ca-alginate because the binding of the enzyme inside PPyAgNp/Ca-alginate would have an effect on the induced polarizability and the light dispersion characteristics of this matrix. The combination of IR and Raman spectroscopies is a more potent approach for sample analysis [43]. In Fig. 4a, the black spectrum corresponds to the enzyme with PPyAgNp/Ca-alginate. The intensities of the bands based on the concentration of the enzyme are compared to those of the supporting material. The significant vibrational modes of the enzyme are at 750, 1193, 1375, 1593, and 1882 cm−1. The bands of PPyAgNp/Ca-alginate are consistently lower in intensity than the vibrational bands of the enzyme. On the other hand, in Fig. 4b, it seems that the significant vibrational modes of calcium alginate are attributed to carboxyl (COO-) groups, whose vibrational bands are observed at 805 and 888 cm−1, and hydroxyl (OH-) groups, whose vibrational bands are observed at 1625 cm−1. Additionally, significant vibrational modes of polypyrrole are observed at 735, 810, 957, 1047, 1356, 1442, and 1550 cm−1. These results are agreement with the result of Liu and Hwang [44].
3.5 Thermogravimetric Analysis
The thermal behaviour of calcium alginate, PPyAgNp/Ca-alginate, and PPyAgNp/Ca-alginate with the enzyme was studied by TGA from room temperature to 900 °C at a rate of 20 °C min−1 under a nitrogen atmosphere. These thermal estimations helped to evaluate the fabrication of Ca-alginate surfaces with PPyAgNp and the immobilization of the enzyme to PPyAgNp/Ca-alginate. For this purpose, the prepared compounds were subjected to TGA, and the thermograms obtained are shown in Fig. 5. A significant mass loss at 300 °C was observed for Ca-alginate, with a mass loss of 50%, while the same mass loss was observed for PPyAgNp/Ca-alginate and PPyAgNp/Ca-alginate-enzyme at 390 °C and 340 °C, respectively. This significant difference in the thermal behaviour of the studied support provides evidence that the enzyme has been successfully immobilized onto PPyAgNp/Ca-alginate.
3.6 Field Emission Scanning Electron Microscopy Analysis
Figure 6 shows the surface morphologies of calcium alginate beads coated with polypyrrole/silver nanocomposites before and after immobilization with PG at various magnifications. The FESEM images demonstrate a disparity between the morphological characteristics of the beads with and without immobilized PG. Figure 6a shows that there were no observable pores on the surface of the control bead. Figure 6b indicates that PPyAg nanoparticles were very uniformly distributed on the bead surface. Following immobilization of the enzyme, the bead surface coated with PPyAg was covered with dense PG particles displaying an irregular morphology and a rugged surface compared to the control bead, as shown in Fig. 6c. The presence of silver nanoparticles was proven using EDX, as shown in Fig. 6d, e, f.
High and low magnification FESEM images of a calcium alginate beads, b calcium alginate beads coated with polypyrrole/silver nanocomposites c immobilization of polygalacturonase onto calcium alginate beads coated with polypyrrole/silver nanocomposites, d, e, f the SEM–Energy-dispersive X-ray (EDX) spectra of calcium alginate beads, calcium alginate beads coated with polypyrrole/silver nanocomposites, and polygalacturonase onto calcium alginate beads coated with polypyrrole/silver nanocomposites, respectively
3.7 Stability and Reusability of Immobilized Enzyme
The operational stability of immobilized PG has been investigated because of its significance in production cost reduction. The immobilized PG preserved 91% of its initial activity after 5 cycles and 68% after 10 cycles (Fig. 7a), indicating the high reusability of the PG immobilized on PPyAgNp/Ca-alginate. The operating stability of the immobilized enzyme was higher than that of immobilized α-amylase in calcium alginate/Fe2O3 (43% activity remained after 10 cycles) [30]. Generally, over time, free enzyme activity decreases. Enzyme immobilization is intended to improve enzyme stability during storage. A comparison of the activity of free and immobilized PG during storage at 4 °C for 60 days shows that the reduction in the activity of immobilized PG was smaller than that of the free enzyme (17% vs. 74%) (Fig. 7b).
3.8 Physico-Chemical Characterization of Purified and Immobilized Enzymes
To study the effect of pH on the activity of free and immobilized PG, different pH values from 4.0 to 9.0 were applied; the results can be seen in Fig. 8a. The optimum pH for the free enzyme was determined to be pH 5.0, while that of the immobilized form was pH 5.5. At both low and high pH values, the immobilized PG activity was higher than that of the free form. Increasing the enzyme activity of immobilized PG relative to that of the free form can be attributed to decreased autolysis of the enzyme and possible stabilization of PG immobilized on a high-surface-area support material and confinement, resulting in improved substrate binding at active PG enzyme sites. Another study showed that the optimum pH of PG immobilized on calcium alginate was 4.5 [45]. The optimum pH of PG purified from Aspergillus flavus was 5.0 [46].
The Lineweaver–Burk plot was applied to evaluate the kinetic parameters of free and immobilized PG. The Km and Vmax values for immobilized PG were lower than those for free PG (Fig. 8b). This may be due to improvement in the enzyme structure following ionic interaction with the matrix support that increased the thermal stability of the PPyAgNp/Ca-alginate-PG. The Km is relevant to the enzyme and substrate affinity. After immobilization, the affinity of the enzyme to the substrate increased by 1.38-fold. The catalytic efficiency (Vmax/Km) of immobilized PG was higher than that of the free form (11.06 and 14.48, respectively). This result suggests that the enzyme on the PPyAgNp/Ca-alginate surface has more accessible potential active sites, thus increasing PG’s affinity to the substrate. It demonstrates that immobilized enzymes can tolerate higher temperatures than free form [47, 48]. Changes in the physical and chemical properties of immobilized PG cause temperature shifts. In addition, the ionic interaction via disulfide and/thiol groups of immobilized PG might decrease conformational flexibility, leading to the higher activation energy for the formation of the desired configuration, making the substrate more easily bind to the molecule [48]. Rehman et al. reported that the Km and Vmax values for polygalacturonase were 1.055 mg ml−1 and 11.4 mM min−1 after entrapment within the calcium alginate beads [49].
In this study, the catalytic activity of free and immobilized PG was measured at different temperatures (Fig. 9a). The optimum temperature for free PG shifted was 50 °C, whereas that for PG immobilized on PPyAgNp/Ca-alginate was 60 °C. Several studies revealed that the immobilized enzymes can tolerate higher temperatures than free form [47, 48]. At temperatures from 50 °C to 90 °C, the activity of the free PG decreased significantly, while the activity of the immobilized enzyme decreased less at temperatures from 60 °C to 90 °C. The increase in immobilized PG activity can be related to the reduction in the alteration of the 3D structure of PG upon heating due to binding of the enzyme on the PPyAgNp surface and entrapment within calcium alginate. Compared to that of immobilized PG, the enzyme activity of free PG decreased much further t temperatures greater than 50 °C because the enzyme was denatured, resulting in structural deterioration and decreased enzyme activity at increasing temperatures [50].
The thermal stability of free and immobilized PG was evaluated by preincubation of both enzymes at different temperatures ranging from 50 °C to 80 °C for various periods of time. After 24 h, aliquots were taken for determination of the residual enzyme activity. Compared to the free enzyme at varying temperatures, the immobilized enzyme was inactivated at a much lower rate (Fig. 9b, c). After 60 min at 50, 60, 70, and 80 °C, the immobilized PG maintained approximately 86%, 85%, 70%, and 41% activity, respectively, while the free enzyme displayed only 75%, 59%, 29%, and 6% residual activity under the same conditions. The increase in the thermal stability could be due to the stabilizing effect of the support system that limits thermal denaturation conformational changes. Rehman reported that the immobilization process increased the thermal stability of polygalacturonase [25].
3.9 The Wilcoxon Signed Ranks Statistic analysis
The Wilcoxon Signed Ranks Test results displays that there is statistically significant difference between relative activity (%) of free enzyme and relative activity (%) of immobilized enzyme across different conditions. The results have been demonstrated a significant difference between activity of free enzyme and immobilized enzyme during different pH values, temperatures and kinetics were (P < 0.05) (Tables 1S, 2S, 3S: Supplementary materials). In addition, there is difference of storage stability during days between free and immobilized PG at 4 °C (b) was (P < 0.05) (Table 4S: Supplementary materials).
4 Conclusion
Enzymes with distinct physicochemical characteristics and economical production for downstream applications have often been attractive for research projects. In the current study, three purification steps (ammonium sulfate precipitation and DEAE-Sepharose and Sephadex-150 columns) were successfully used to purify PG from Arabian balsam with a significant level of purification (tenfold). In various aspects of biological science, polymers play an important role, and in the current study, PPyAgNp/Ca-alginate was used to immobilize polygalacturonase by ionic bonding and trapping techniques with efficiency of 84.4%. The immobilized PG showed good stability in terms of reusability (68% of its initial activity after 10 cycles) and storage (remained 83% of its initial activity after storage for 60 days). The immobilization of PG on the carrier increased the thermal stability, pH value, and substrate affinity. In terms of reusability and immobilization efficiency, this approach might have beneficial applications in various textile and food industries. Furthermore, substantial research will be required to evaluate the viability of this method in different industrial processes.
Abbreviations
- FTIR:
-
Fourier transform infrared spectroscopy
- TGA:
-
Thermogravimetric analysis
- SEM:
-
Scanning electron microscopy
- EDX:
-
Energy-dispersive X-ray spectroscopy
References
Gupta R, Beg Q, Khan S, Chauhan B (2002) An overview on fermentation, downstream processing and properties of microbial alkaline proteases. Appl Microbiol Biotechnol 60:381–395
Mohamed SA, AL-Malki AL, Kumosani TA (2009) Characterization of a polygalacturonase from Trichoderma harzianum grown on citrus peel with application for apple juice. Aust J Basic Appl Sci 3:2770–2777
Tochi BN, Wang Z, Xu SY, Zhang W (2009) The influence of pectinase and pectinase/hemicellulases enzyme preparations on percentage pineapple juice recovery, particulates and sensory attributes. Pak J Nutr 8:1184–1189
Jayani RS, Saxena S, Gupta R (2005) Microbial pectinolytic enzymes: a review. Process Biochem 40:2931–2944
Kobayashi T, Higaki N, Yajima N, Suzumatsu A, Hagihara H, KAWAI S, ITo S (2001) Purification and properties of a galacturonic acid-releasing exopolygalacturonase from a strain of Bacillus. Biosci Biotechnol Biochem 65:842–847
Fontana RC, da Silveira MM (2012) Production of polygalacturonases by Aspergillus oryzae in stirred tank and internal- and external-loop airlift reactors. Bioresour Technol 123:157–163
Tepe O, Dursun AY (2014) Exo-pectinase production by Bacillus pumilus using different agricultural wastes and optimizing of medium components using response surface methodology. Environ Sci Pollut Res 21:9911–9920
Khan M, Nakkeeran E, Umesh-Kumar S (2013) Potential application of pectinase in developing functional foods. Annu Rev Food Sci Technol 4:21–34
Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr Res 344:1879–1900
Mohamed SA, Al-Malki AL, Khan JA, Kabli SA, Al-Garni SM (2013) Solid state production of polygalacturonase and xylanase by Trichoderma species using cantaloupe and watermelon rinds. J Microbiol 51:605–611
Niture SK, Pant A (2004) Purification and biochemical characterization of polygalacturonase II produced in semi-solid medium by a strain of Fusarium moniliforme. Microbiol Res 159:305–314
Kashyap DR, Soni SK, Tewari R (2003) Enhanced production of polygalacturonase by Bacillus sp.DT7 using solid state fermentation. Bioresour Technol 88:251–254
Srivastava S, Nighojkar A, Kumar A (1994) Multiple forms of pectin methylesterase from Cuscuta reflexa filaments. Phytochemistry 37:1233–1236
Sheldon RA, Schoevaart R, Van Langen LM (2005) Cross-linked enzyme aggregates (CLEAs): a novel and versatilemethod for enzyme immobilization (a review). Biocatal Biotransform 23:141–147
Bilal M, Asgher M, Iqbal HMN, Hu H, Zhang X (2016) Gelatin-immobilized manganese peroxidase with novelcatalytic characteristics and its industrial exploitation for fruitjuice clarification purposes. Catal Lett 146:2221–2228
Bilal M, Iqbal HMN, Hu H, Wang W, Zhang X (2017) Enhanced bio-catalytic performance and dye degradation potential of chitosan-encapsulated horseradish peroxidase in a packed bed reactor system. Sci Total Environ 575:1352–1360
Abdulaal WH, Almulaiky YQ, El-Shishtawy RM (2020) Encapsulation of HRP enzyme onto a magnetic Fe3O4 Np–PMMA film via casting with sustainable biocatalytic activity. Catalysts 10:181
Almulaiky YQ, Aqlan FM, Aldhahri M, Baeshen M, Khan TJ, Khan KA, Alayafi AA (2018) α-Amylase immobilization on amidoximated acrylic microfibres activated by cyanuric chloride. R Soc Open Sci 5:172164
Al-Najada AR, Almulaiky YQ, Aldhahri M, El-Shishtawy RM, Mohamed SA, Baeshen M, Al-Harbi SA (2019) Immobilisation of α-amylase on activated amidrazone acrylic fabric: a new approach for the enhancement of enzyme stability and reusability. Sci Rep 9:1–9
Jia YJ, Feng BZ, Sun WX, Zhang XG (2009) Polygalacturonase, pectate lyase and pectin methylesteraseactivity in pathogenic strains of Phytophthora capsici incubatedunder different conditions. J Phytopathol 157:585–591
Gan J, Bagheri AR, Aramesh N, Gul I, Franco M, Almulaiky YQ, Bilal M (2020) Covalent organic frameworks as emerging host platforms for enzyme immobilization and robust biocatalysis–A review. Int J Biol Macromol 167:502–515
Brayner R, Vaulay M-J, Fiévet F, Coradin T (2007) Alginate-mediated growth of Co, Ni, and CoNi nanoparticles: influence of the biopolymer structure. Chem Mater 19:1190–1198
Krueger KM, Al-Somali AM, Mejia M, Colvin VL (2007) The hydrodynamic size of polymer stabilized nanocrystals. Nanotechnology 18:475709
Otari SV, Patil RM, Waghmare SR, Ghosh SJ, Pawar SH (2013) A novel microbial synthesis of catalytically active Ag-alginate biohydrogel and its antimicrobial activity. Dalton Trans 42:9966–9975
Sayo K, Deki S, Hayashi S (1999) A novel method of preparing nano-sized gold and palladium particles dispersed in composites that uses the thermal relaxation technique. Eur Phys J D 9:429–432
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Contreras-Esquivel JC, Hours RA, Voget CE, Mignone CF (1999) Aspergillus kawachii produces acidic pectin releasing enzyme activity. J Biosci Bioeng 88:48–52
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analyt chem 31:426–428
Omastová M, Mosnáčková K, Fedorko P, Trchová M, Stejskal J (2013) Polypyrrole/silver composites prepared by single-step synthesis. Synth Met 66:57–62
Al-Harbi SA, Almulaiky YQ (2020) Purification and biochemical characterization of Arabian balsam α-amylase and enhancing the retention and reusability via encapsulation onto calcium alginate/Fe2O3 nanocomposite beads. Int J Biol Macromol 160:944–952
Almulaiky YQ, Al-Harbi SA (2019) A novel peroxidase from Arabian balsam (Commiphora gileadensis) stems: its purification, characterization and immobilization on a carboxymethylcellulose/Fe3O4 magnetic hybrid material. Int J Biol Macromol 133:767–774
Köktepe T, Altın S, Tohma H, Gülçin I, Köksal E (2017) Purification, characterization and selected inhibition properties of peroxidase from haricot bean (Phaseolus vulgaris L.). Int J Food Prop 20:1944–1953
Mohamed SA, Abulnaja KO, Ads AS, Khan JA, Kumosani TA (2011) Characterisation of an anionic peroxidase from horseradish Cv. Balady Food Chem 128:725–730
Wingfield PT (Ed.) (2016) Protein precipitation using ammonium sulfate. Curr Protoc Protein Sci, 84(1), A-3F.
Welinder KG (1992) Superfamily of plant, fungal and bacterial peroxidases. Curr Opin Struc Biol 2:388–393
Pedrolli DB, Carmona EC (2010) Purification and characterization of the exopolygalacturonase produced by Aspergillus giganteus in submerged cultures. J Ind Microbiol Biot 37:567–573
Rha E, Park HJ, Kim MO, Chung YR, Lee CW, Kim JW (2001) Expression of exo-polygalacturonases in Botrytis cinerea. FEMS Microbiol Lett 201:105–109
Gomes E, Leite RSR, Da Silva R (2009) Purification of an exopolygalacturonase from Penicillium viridicatum RFC3 produced in submerged fermentation. Int J Microbiol. https://doi.org/10.1155/2009/631942
Rahman M, Choi YS, Kim YK, Park C, Yoo JC (2019) Production of novel polygalacturonase from Bacillus paralicheniformis CBS32 and application to depolymerization of ramie fiber. Polymers 11:1525
Ernest V, Gajalakshmi S, Mukherjee A (2014) Enhanced activity of lysozyme-AgNP conjugate with synergic antibacterial effect without damaging the catalytic site of lysozyme. Artif Cells Nanomed Biotechnol 42:336–343
Mohamed SA, Al-Harbi MH, Almulaiky YQ, Ibrahim IH, Salah HA, El-Badry MO, El-Shishtawy RM (2018) Immobilization of Trichoderma harzianum α-amylase on PPyAgNp/Fe3O4-nanocomposite: chemical and physical properties. Artif Cells Nanomed Biotechnol 46:201–206
Almulaiky YQ, El-Shishtawy RM, Aldhahri M, Mohamed SA, Afifi M, Abdulaal WH, Mahyoub JA (2019) Amidrazone modified acrylic fabric activated with cyanuric chloride: a novel and efficient support for horseradish peroxidase immobilization and 401 phenol removal. Int J Biol Macromol 140:949–958
Lin SY, Li MJ, Cheng WT (2007) FT-IR and Raman vibrational microspectroscopies used for spectral biodiagnosis of human tissues. Spectroscopy 21:1–30
Liu YC, Hwang BJ (2000) Identification of oxidized polypyrrole on Raman spectrum. Synth Met 113:203–207
Buga ML, Ibrahim S, Nok AJ (2010) Physico-chemical characteristics of immobilized polygalacturonase from Aspergillus niger (SA6). Artif Cells Nanomed Biotechnol 9:8934–8943
Anand G, Yadav S, Yadav D (2017) Purification and biochemical characterization of an exo-polygalacturonase from Aspergillus flavus MTCC 7589. Biocatal Agric Biotechnol 10:264–269
Almulaiky YQ, Khalil NM, El-Shishtawy RM, Altalhi T, Algamal Y, Aldhahri M, Mohammed MM (2021) Hydroxyapatite-decorated ZrO2 for α-amylase immobilization: toward the enhancement of enzyme stability and reusability. Int J Biol Macromol 167:299–308
Mazlan SZ, Hanifah SA (2017) Effects of temperature and pH on immobilized laccase activity in conjugated methacrylate-acrylate microspheres. Int J Polym Sci. https://doi.org/10.1155/2017/5657271
Rehman HU, Aman A, Nawaz MA, Karim A, Ghani M, Baloch AH, Qader SA (2016) Immobilization of pectin depolymerising polygalacturonase using different polymers. Int J Boil Macromol 82:127–133
Aldhahri M, Almulaiky YQ, El-Shishtawy RM, Al-Shawafi WM, Salah N, Alshahrie A, Alzahrani HA (2020) Ultra-thin 2D CuO nanosheet for HRP immobilization supported by encapsulation in a polymer matrix: characterization and dye degradation. Lett Catal. https://doi.org/10.1007/s10562-020-03289-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Almulaiky, Y.Q., Al-Harbi, S.A. Preparation of a Calcium Alginate-Coated Polypyrrole/Silver Nanocomposite for Site-Specific Immobilization of Polygalacturonase with High Reusability and Enhanced Stability. Catal Lett 152, 28–42 (2022). https://doi.org/10.1007/s10562-021-03631-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03631-7