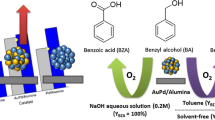
Abstract
Controlled synthesis of bimetallic catalysts has attracted much attention in heterogeneous catalysis because their catalytic activity depends on the size of nanoparticles and hence the methods of synthesis. In this work, one convenient method was proposed, with an aim to control the size and dispersion of bimetallic nanoparticles. In this method, Cu2+ (or Ni2+) configurational ion of hydrotalcites was used as directing reagent, which directed the position and dispersion of the final bimetallic nanoparticles by employing the metal interaction between Cu and Au as the driving force. The size, structure and composition of bimetallic nanoparticles were characterized using techniques of X-ray diffraction (XRD), nitrogen physisorption, X-ray photoelectron spectra (XPS) and scanning transmission electron microscopy (STEM). The mean size of bimetallic AuCu nanoparticles was 2.5 nm, which was 1/4 (Step-impregnation) or 1/10 (Co-impregnation) of that prepared by traditional methods. Even if the loading of Au was increased to 10 wt%, the obtained AuCu nanoparticles were still well dispersed. The catalytic activity of AuCu and AuNi nanoparticles in aerobic oxidation of benzyl alcohol was far higher than those prepared by traditional methods. The mechanism of forming bimetallic nanoparticles was investigated. It was found that the dispersion of Cu2+ (or Ni2+) and the interaction between Cu0 (or Ni0) and Au0 are two key factors affecting the dispersion of AuCu (or AuNi) nanoparticles.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Supported bimetallic catalysts have drawn increasing attention because of their promising in activity enhancement and improved stability in many reactions through forming new active sites and inducing synergistic effects [1, 2]. The catalytic activity of supported bimetallic catalysts is highly dependent on the size of nanoparticles [3, 4]. Generally a particle size less than 3–5 nm with a narrow size distribution and compositional homogeneity are considered to be most beneficial for the catalytic performance [5–8], and the size of nanoparticles is highly dependent on the method of synthesis [4].
The most traditional synthetic techniques for preparing supported bimetallic catalysts are co-impregnation and deposition–precipitation methods [2, 9, 10]. The two methods are simple, but they can not very well control over the size of metal particle [4]. Various new attempts in the literature were conducted to control the size of bimetallic nanoparticles by improving the conventional methods [4] or developing new methods [11]. Sol immobilization method have been used to control the particle growth by employing stabilizer-ligands [2, 4]. However, stabilizing polymer usually affects catalytic property of their stabilized nanoparticles and also increases the cost of catalysts [12]. In case industrial applications are targeted, the sol immobilization method is not very attractive due to the use of stabilizer-ligands [13].
In one array of the attempted methods, stepwise deposition of metals was often employed, in which one metal was first added to supports, followed by deposition of second metal (Scheme 1). The deposition of the second metal was controlled by employing a driving force, i.e., the interaction between first metal and second metal. Barbier research group utilized a driving force, i.e., surface redox reactions between second metal and H2 adsorbed on the pre-existing metal to control the deposition of second metal [14–17]. Besides, other driving forces were also employed, including electroless deposition [13, 18–22], galvanic replacement [23, 24], and complexation [25]. These works demonstrated that the procedure of controlling the deposition of a second metal on the surface of a pre-existing metallic surface are useful in controlling the degree of dispersion of the final bimetallic nanoparticles. They also implied that dispersion of first metal worked as the base of the final bimetallic nanoparticles (Scheme 1). Although the deposition of second metal was controlled in the modified methods shown above, the size of the pre-existing metal was not very well controlled because of its deposition by employing the conventional impregnation [13, 19, 25] or deposition–precipitation methods [22]. These modified methods have no control over the deposition of first metal, but concentrate on using the dispersion of first metal to control the deposition of second metal. The absence in controlling the dispersion of first metal leads to the deficiency in controlling the dispersion of final bimetal catalysts [26]. It will be deduced that if the size of the pre-existing metal is also well controlled besides the controlled deposition of second metal, bimetallic nanoparticles can be better dispersed.
Inspired by the above deduction, we proposed a new strategy to control the degree of dispersion of the bimetallic nanoparticles by controlling both the degree of dispersion of a pre-existing metal as well as the second metal. As shown in Scheme 2, the first metal as configurational ion was well dispersed in the lattice of supports. The dispersion of second metal was controlled by employing a driving force, i.e., alloy formation between first metal and second metal. Hydrotalcite-like compounds (HTs) are common supports [27–29], here HTs were also employed as supports. HTs are crystalline anionic clays, which are-like the naturally occurring brucite (Mg(OH)2)-composed of metal hydroxide sheets, where the metal cations are octahedrally coordinated by hydroxide ions [30]. The general formula to describe the chemical composition is [MII 1−xMIII x(OH)2]An˗ x/n·mH2O, where MII and MIII represent metallic cations, An− indicates the interlayer anion, x is the molar ratio of MIII/(MII + MIII) and m represents the amount of water. The cations in the layers can be isomorphously substituted by many others, such as Zn, Co, Ni, Mn, Fe (divalents), and Al, Cr, Co, Fe, V, Y, Mn, Ga, lanthanides (trivalents). Herein, we prepared Mg-Al HTs with 2:1 of Mg:Al atomic ratio, wherein Mg2+ was partially isomorphously substituted by Cu2+ or Ni2+. And then, HAuCl4 was deposited on HTs by impregnation. The HAuCl4/HTs was reduced by H2 at the elevated temperature, obtaining well dispersed AuCu or AuNi nanoparticles. The Cu2+ or Ni2+, as configurational ion of HTs, was well dispersed in the lattice of HTs, which decided the highly dispersion of Cu0 or Ni0. Cu0 or Ni0 could interact with Au0 to form the corresponding alloy. Therefore, the well dispersed Cu0 or Ni0, and the metal interaction between Cu0 (or Ni0) and Au0, could well control the degree of dispersion of the final AuCu or AuNi nanoparticles. The oxidation of alcohols to their corresponding carbonyl compounds is one of the most important transformations in organic synthesis, and likely play a significant role in the development of value-added chemicals from biomass [31–35]. Recently, Liu et al. prepared Au/Cr-HTs catalysts. The strong synergy between chromium (III)-containing HTs and gold nanoparticles showed higher activity in the aerobic oxidation of alcohols [32]. Very recently, Du et al. [33] designed a flower-like hierarchical Au/NiAl-HTs catalyst, which promoted 60% higher activity than the common Au/NiAl-HTs nanoparticle catalyst in the oxidation of benzyl alcohol. The higher activity of the catalysts could be attributed to the shape selectivity of straight macropores and the synergistic between Ni and Au. Therefore, the development of efficient catalysts, which can aerobically oxidize a variety of alcohols to their corresponding aldehydes and ketones, is of both economic and environmental significance. In this work, the bimetallic catalyst by the proposed method exhibited good catalytic performance in the aerobic oxidation of benzyl alcohol (Scheme 3).
2 Experimental
2.1 Preparation of Supports
HTs with desired atomic ratios of Cu2+/Mg2+/Al3+ or Ni2+/Mg2+/Al3+ were prepared by a co-precipitation method as described in our previous report [36]. Typically, a 80 mL aqueous solution (Cu2+, Mg2+, and Al3+; or Ni2+, Mg2+, and Al3+) with a total concentration of 1.5 M and another 160 mL NaOH aqueous solution (1.5 M) was slowly dropwise added into 50 mL Na2CO3 aqueous solution under vigorous stirring at room temperature. The molar quantity of Na2CO3 employed was twice that of Al3+. The pH value of the mixture was kept constant, typically at values between 9.5 and 10.5 by adjusting the rate of NaOH aqueous solution. Then, the suspension was aged at 60 °C for 18 h with constant stirring to generate the heavy slurry. The slurry was then cooled down to room temperature, filtered, and washed with water until the pH value of the filtrate was near 8–9. The precipitate was dried at 110 °C overnight. The obtained HTs were denoted as HTs for Mg2+–Al3+HTs, Cu–HTs for Cu2+–Mg2+–Al3+HTs, and Ni–HTs for Ni2+–Mg2+–Al3+ HTs, respectively. The HTs was further calcined at 600 °C for 3 h in air. The calcined HTs were named Cu–HTO and Ni–HTO, respectively. Unless other special statement, atomic ratio of Cu2+/Mg2+/Al3+(or Ni2+/Mg2+/Al3+) was referred to 0.1:2:1.
2.2 Preparation of Catalysts
2.2.1 Catalysts Prepared by Proposed Methods
10 mg/mL AuCl4 − solution (AR, 99%, Sinopharm Chemical Reagent Co., Ltd) of desired volume was added to a beaker containing deionized water (15 mL) and supports (1 g) to form slurry. After 2 h of stirring at room temperature, the slurry was quickly dried on hot plate for 0.5 h. The sample after preliminary drying was further dried in oven and air atmosphere at 100 °C for 3 h. The dried samples were reduced by H2 (30 mL/min) at 300 or 600 °C for 1 h to obtain the final catalysts. The catalysts before reduction was denoted AuM/HTs-U, while the catalysts after reduction were named AuM/HTs-T or AuM/HTO-T, respectively. M was referred to Cu or Ni, U was referred to before reduction, and T was referred to reduction temperature. The samples with HTO meant that the HT supports have been pre-calcined as mentioned in the previous section. Unless other special statement was shown, loading amount of Au was referred to 1 wt% of supports.
2.2.2 Catalysts Prepared by Traditional Methods
For comparison, the catalysts were also prepared by traditional methods, such as co-impregnation method and stepwise impregnation method.
The details for co-impregnation method were shown below. A desired volume of HAuCl4·4H2O solution (10 mg/mL) and Cu(NO3)2·3H2O solution (10 mg/mL) was added simultaneously to a beaker containing deionized water (15 mL) and 1 g of HTs supports to form a slurry. After 2 h of stirring at room temperature, the slurry was quickly dried on hot plate for 0.5 h. The sample after preliminary drying was further dried in oven and air atmosphere at 100 °C for 3 h. The dried sample was reduced at 300 °C in H2 for 1 h to obtain final catalyst, denoted as AuCu/HTs-C.
The difference between stepwise impregnation method and co-impregnation method was the adding sequence of metal ions. For sequential impregnation, one ion (Au3+ or Cu2+/Ni2+) was first added to HTs supports. After drying, the other ion was added using similar procedure.
2.3 Characterization of Catalysts
X-ray diffraction (XRD) patterns were recorded using an X-ray powder diffractometer (Rigaku Co., Japan), and a Cu-Ka Source fitted with an Inel CPS 120 hemispherical detector. The patterns were taken over the 2θ range from 5° to 80°.
The surface area, pore characteristics and average pore diameter of the materials were analyzed with a Micromeritics ASAP 2020 HD 88 instrument. Pore volume and pore size distribution curves were obtained from analysis of desorption branches of the nitrogen isotherms using the BJH method. The samples were degassed at 140 °C for 6 h in N2 prior to surface area measurement. Nitrogen adsorption and desorption isotherms were measured at 77 K. Nitrogen adsorption data were obtained in the relative pressure range from 0.06 to 0.30. Total pore volumes were estimated from the amount of nitrogen adsorbed at a relative pressure of 0.995.
H2-temperature-programmed reduction (H2-TPR) was conducted over a H2-TPR instrument (Micromeritics Co. America) with thermal conductivity detector. After degassed in He atmosphere at 200 °C for 1 h, samples of 50 mg were heated at 10 °C/min from 40 to 800 °C in a flow of dry H2/Ar mixture (H2/Ar, 10/90 vol) at 50 mL/min.
X-ray photoelectron spectra (XPS) were measured to characterize the Cu, Ni, and Au elements valence of catalysts. XPS experiment was carried out using an Axis Ultra spectrometer (Kratos AnalyticalLtd., Japan) with Al Ka X-ray source (1486.6 eV, 15 kV, 5 mA) at energy of 150 W and ultrahigh vacuum conditions of 5 × 10−9 Torr. Data analysis was performed using Casa XPS software. The electron binding energy (BE) scale of all spectra was calibrated using C 1 s core excitation at 284.8 eV.
The scanning transmission electron microscopy (STEM), higher solution TEM (HRTEM), EELS elemental mapping observations and energy dispersive X-ray (EDX) were obtained using a FEI Tecnai G2 F20 instrument (USA).
2.4 Catalytic Reactions
The aerobic oxidations of benzyl alcohol were carried out using a 50 mL three-necked round-bottle flask equipped with a water-cooled condenser. Typically, the reactor was charged with 0.1 g of catalyst, 1 mmol of benzyl alcohol and 8 mL of toluene at 100 °C for 3 h under an oxygen flow (25–30 mL min−1). After cooling to room temperature, N-dodecane (1 mmol), as internal standard, was added. The resulting mixture was diluted to constant volume by toluene in 25 mL volumetric flask. The reaction products were analyzed by using a Shimadzu GC-2014 (Rtx-5 column, 30 m × 0.320 mm, df = 0.25 μm) gas chromatograph equipped with a flame ionization detector.
3 Results and Discussion
3.1 Catalyst Characterization
3.1.1 XRD Analysis
Figure 1 displays XRD patterns of Cu-HTs, Cu-HTO, and AuCu/HTs before and after reduction. Cu-HTs showed XRD patterns characteristic of HTs [37], with diffraction peak at 11.78°, 23.54°, 34.78°, 39.38°, 46.54°, 60.76° and 62.18° (Fig. 1a). Cu-HTO showed diffraction peaks characteristic of MgO [38], originated from calcination at high temperature (Fig. 1b). The diffraction patterns of AuCu/HTs-U before reduction (Fig. 1c) were similar with that of Cu-HTs (Fig. 1a). This indicated that the impregnation of Au3+ did not change the structure of HTs supports. The diffraction patterns of AuCu catalysts after reduction (Fig. 1d–g) were similar with that of Cu-HTO (Fig. 1b), irrespective of the reduction temperature and property of supports. No reflections corresponding to Cu or Au were observed for catalysts because of the low loadings and low size.
Figure 2 displays XRD patterns about Ni-HTs. The effect of calcinations and reduction on structure of Ni-HTs was similar with that of Cu-HTs. The XRD patterns of Ni-HTs and AuNi/HTs-U before reduction (Fig. 2a, c) were similar with that of HTs, while Ni–HTO, AuNi/HTs-600 and AuNi/HTO-600 (Fig. 2b, d, e) showed diffraction peaks similar with that of calcined HTs. XRD patterns of AuCu/HTs-300-C catalysts prepared by traditional co-impregnation and step-impregnation were also determined. As shown in Fig. 3, AuCu/HTs-300-C, irrespective of impregnation method, displayed diffraction patterns characteristic of MgO, which were similar with that of AuCu/HTs-300. To be worth mentioned, diffraction peaks ascribed to crystalline phase of Au was observed for catalysts prepared by traditional methods (Fig. 3 and Fig. S1). In contrast, no diffraction peaks ascribed to Au was observed for catalysts prepared by this method. This indicated that Au particles size by this work was smaller than that by traditional method.
3.1.2 BET Surface Area and Pore Size
HTs and isomorphously substituted HTs had similar surface area, which are in the range of 118–141 m2g−1 (Table 1, entries 1, 14, and 19). The calcined HTs displayed higher surface area than that of HTs, which were ascribed to the release of water and CO2 during calcinations. The surface of calcined HTs was 224 m2g−1 for Cu-HTO (Entry 2) and 270 m2g−1 for Ni-HTO (Entry 15), respectively. After impregnation of Au, the surface area of sample decreased. The surface area of AuCu/HTs-U and AuNi/HTs-U were 119 and 109 m2g−1 (Entries 3 and 16), respectively, which was less than their corresponding supports (141 and 130 m2g−1; entries 1 and 14). The surface area of bimetal catalysts after reduction was higher than that of both catalysts before reduction and intact supports. For example, the surface area of AuCu/HTs-300 (Entry 4) was 163 m2g−1, which was higher than that of AuCu/HTs-U (119 m2g−1, entry 3) and Cu-HTs (141 m2g−1, entry 1). With the increase of Au loading amount, the surface area of AuCu/HTs-300 decreased. It went down to 102 m2g−1 when the loading amount was 10 wt% (Entry 10). Furthermore, reduction temperature also affected surface area of catalysts. The surface area of AuCu/HTs-600 was higher than that of AuCu/HTs-300. Its surface area (Entry 11) was 195 m2g−1, which was less than that of AuCu/HTs-300 (163 m2g−1, entry 4). Besides, mean pore diameter of all samples were in the range of 7.3–26 nm. This indicated that all the catalysts were mesoporous. The pore size distributions are shown in the supporting information (Fig. S2). The results showed that the catalysts contained a large amount of mesopores and a small amount of micropores after thermal treatment.
3.1.3 H2-TPR Analysis
Figure 4 shows TPR profiles for Cu-HTs and Cu-HTO. Both of them displayed one major reduction peak and one minor reduction peak. Cu-HTs showed one major peak centered at around 348 °C and one minor peak around 433 °C. The reduction peak of Cu-HTO shifted to lower temperature, which centered at 318 °C (major peak) and 398 °C (minor peak), respectively. As shown in Fig. 5, TPR curves of Ni-HTs and Ni-HTO exhibited only one reduction peak centered at 690 °C. TPR profiles for supported Au also showed one reduction peak (Fig. 6), which located around 437 °C for Au/HTs-U and 416 °C for Au/HTO-U, respectively. TPR patterns for supported AuCu displayed one broad peak from 214 to 420 °C for AuCu/HTs-U, and from 168 to 367 °C for AuCu/HTO-U (Fig. 7). The broad peak may correspond to the reduction of Cu2+ to Cu0, and Au3+ to Au0 [39]. It is clear that Cu on the AuCu/HTs catalyst lowered the reduction temperature of Au3+. TPR patterns for supported AuNi contain more reduction peaks than that of AuCu catalysts (Fig. 8). Curves of AuNi/HTs-U displayed two sharp peaks located at 266 °C and 414 °C, and one broad peak around 654 °C. Profiles of AuNi/HTO-U displayed three sharp peaks located at 247, 315, and 443 °C, respectively, as well as one broad peak around 717 °C. Compared with HTs supported catalysts, the reduction peak of HTO samples started at lower temperature. For example, AuCu/HTO started at 168 °C, which was 36 °C lower than that of AuCu/HTs (Fig. 7). The difference in the reduction peak between HTs and HTO may be ascribed to their difference in metal dispersion, since dispersion affects reduction temperature of elements [40]. The difference in the dispersion between HTs and HTO may be due to calcination because calcination may result in sinter of nanoparticles [41].
3.1.4 XPS Analysis
Figure 9 shows Au 4f and Cu 2p XPS spectra of AuCu/HTs-300, AuCu/HTO-300, AuCu/HTs-600, and AuCu/HTO-600. XPS spectra of Au 4f region for all the samples could be fitted with maximum peaks at 87.4 and 83.8 eV binding energies, which are characteristic of Au0. No Au 4f spectra ascribed to Auδ+ was observed, indicating that Au3+ was completely reduced to Au0. XPS spectra of Cu 2p for all the samples exhibited characteristic peaks of metallic Cu. No Cuδ+ was detected, suggesting that Cu also had been reduced completely.
Ni 2p and Au 4f XPS spectra of AuNi/HTs-300 and AuNi/HTs-600 are shown in Fig. 10. Ni 2p XPS spectra of AuNi catalysts varied with reduction temperature. AuNi/HTs-300 exhibited maximum peak at 872 and 855 eV binding energies, which are characteristic of Ni2+, while AuNi/HTs-600 presented maximum peak characteristic of Ni0. In the case of Au 4f XPS spectra, both AuNi/HTs-300 and AuNi/HTs-600 showed maximum peaks at 87.4 and 83.8 eV binding energies, which are characteristic of Au0. This indicated that metal Ni on AuNi/HTs could not be reduced at 300 °C, and the reduction could be completed at 600 °C. This result was in accordance with that of H2-TPR.
3.1.5 STEM Analysis of Supports and Catalysts
3.1.5.1 Mean Size of Bimetallic Nanoparticles
Figure 11 shows STEM images for Cu-HTs-300 and Cu-HTs-600. The size of Cu nanoparticles was 4.7 nm when reduced at 300 °C. When reduced at 600 °C, the size of Cu nanoparticles was increased to 6.7 nm. This indicated that reduction temperature affected the dispersion of Cu nanoparticles. After impregnation of Au, the formed AuCu nanoparticles were observed. As shown in Fig. 12, AuCu/HTs-300 and AuCu/HTO-300 nanoparticles were well distributed. Their mean size was 2.5 nm and 2.8 nm, respectively. In contrast, AuCu/HTs-600 and AuCu/HTO-600 displayed higher size, which were 7.3 nm and 8.1 nm, respectively. The high size of the latter two AuCu catalysts may be due to the effect of calcinations temperature since they were reduced at 600 °C. Effect of Au loading amount was also investigated. As shown in Fig. S3 (Supporting information), AuCu nanoparticles had narrow size distribution in the investigated scope of loading amount although their size increased slowly with loading amount. The mean size was 2.7 nm for 2 wt% loading (Fig. S3a) and 2.9 nm for 2.5 wt% loading (Fig. S3b). Those sizes were slightly higher than 2.5 nm of AuCu/HTs-300 with 1 wt% loading (Fig. 12a). It rose to 4.6 nm when loading amount was increased to 10 wt% (Fig. S3f). For comparison, AuCu was also prepared using traditional methods. As shown in Fig. 13, the mean size of AuCu by co-impregnation was higher than that by sequential impregnation. The former was around 23.1 nm, while the latter was 10 nm. Compared with those prepared by the proposed method, AuCu nanoparticles by traditional work showed far higher size whether the used method was co-impregnation or sequential impregnation. For the same amount of Au loading, the size of AuCu/HTs-300-C (10.4 ~ 10.7 nm, Fig. 13a and b) by sequential impregnation was four times higher than that of AuCu/HTs-300 by this work (2.5 nm, Fig. 12a). The size of AuCu/HTs-300-C (23.1 nm, Fig. 13c) by co-impregnation was almost 10 times higher than that of AuCu/HTs-300 by this work (2.5 nm, Fig. 12a).
Effect of Cu content was investigated. As shown in Fig. 14a, when Cu:Mg:Al molar ratio was 0.05:2:1, the obtained size of AuCu nanoparticles was 2.4 nm, which was similar with that for 0.1:2:1 (Fig. 12a). When the molar ratio was above 0.1:2:1, the mean size was increased. It went up to 10.2 nm for 0.5:2:1 and 25.7 nm for 1:2:1 molar ratio. This indicated that Cu amount affected the size of AuCu nanoparticles.
The mean size of AuNi catalysts was also determined. As shown in Fig. 15, the mean size of AuNi/HTs-300 was 7.8 nm, which slightly decreased to 6.3 nm (i.e. AuNi/HTs-600) when reduction temperature was increased to 600 °C. The mean size of AuNi/HTs-600-C, i.e., the AuNi catalysts by traditional method, was 8.7 nm, which was slightly higher than that of AuNi/HTs-600 by traditional method. It was obvious that the property in size of AuNi catalysts was different from that of AuCu catalysts. The size of AuNi reduced at 600 °C was smaller than that at 300 °C, while contrary phenomenon was observed for AuCu catalysts. Besides, the mean size of AuCu catalysts by this work was far smaller than that by traditional work, however, the discrepancy between this work and traditional work was small for AuNi catalysts. The mechanism for the specialty of AuNi catalysts was discussed in the section about mechanism.
3.1.5.2 Composition of AuCu Nanoparticles
The microstructure and compositions of AuCu nanoparticles were characterized by a STEM with EDS. As shown in Fig. 16, the EDS line scan analysis indicated the nanoparticles were homogeneously AuCu mixed structure irrespective of loading amount and reduction temperature. The EDX mapping images of a selected nanoparticle on the surface of AuCu/HTs-600 were also determined (Fig. 17). The mapping images further confirmed that Au and Cu mixed homogeneously. The homogeneous structure of AuCu bimetallic nanoparticles indicated that there was one kind of interaction between Cu and Au which resulted in their mixture, since Cu and Au was added to supports stepwise. In thermodynamics, the process of forming alloy between Cu and Au is spontaneous [41]. Therefore, it is possible that the interaction is the force of forming alloy. In order to verify the inference, HRTEM was carried out to further reveal the microstructure of the nanoparticles. As shown in Fig. 18, the interplanar spacing of nanoparticles was 2.12 Å for AuCu/HTs-300 and 2.19 Å for AuCu/HTs-600, which was between 2.09 Å (Cu111) and 2.35 Å (Au111). This indicates that Au and Cu mixed homogeneously and formed the structure of alloy.
3.2 Mechanism Forming Bimetallic Nanoparticles and Factors Affecting the Dispersion of AuCu Nanoparticles
Based on analysis of XRD, XPS, TPR, and STEM, the mechanism to prepare bimetallic nanoparticles was proposed, as shown in Scheme 2. The dispersion of Cu2+ as configurational ion of HTs, which isomorphously substituted for Mg2+ in the crystal lattice of HTs, could be well controlled by adjusting its contents in HTs. After impregnation, Au3+ was randomly deposited on the surface of HTs supports. Upon reduction, there is one kind of interaction, i.e., interaction between Cu0 and Au0. The interaction drove Au0 to the position of Cu0 and hereafter formed alloy. Thereafter, configurational ion Cu2+of HTs directed the position of the final AuCu nanoparticles on the surface of HTs supports. Accordingly, the dispersion of Cu2+decided the dispersion of the final AuCu nanoparticles. The direction was realized by the interaction between Cu0 and Au0. Hence, dispersion of Cu2+and the interaction between Cu0 and Au0 are two key factors affecting the dispersion of AuCu nanoparticles.
Dispersion of Cu2+ could be tuned by adjusting its contents in the HTs supports. The change in dispersion of Cu2+ could affect the dispersion of the final AuCu nanoparticles. That is, Cu2+ amount affect the dispersion of the final AuCu nanoparticles. As shown in Fig. 14, it was found that Cu amount affected the size of AuCu nanoparticles. These experimental results verified our deduction.
The interaction between Cu0 and Au0 could be tuned by controlling the reduction temperature of catalysts. The appropriate reduction temperature for each bimetallic ion should be near each other. TPR analysis showed that the appropriate reduction temperature for Cu2+ and Au3+ was near other, which corresponded to the low size of AuCu/HTs-300. If the discrepancy between reduction temperatures of two metals was too large, dispersion of configurational ion, i.e., the first metal, and the interaction between first metal and Au0 could not play their role in directing the position and dispersion of the targeted bimetallic nanoparticles (Scheme 4). As shown in Figs. 5 and 6, there is a big discrepancy in the optimum reduction temperature between single Ni and single Au. The XPS spectra for AuNi bimetal in Fig. 10 showed that the reduction of Au3+ was completed at 300 °C, while none Ni2+ was reduced at 300 °C, and Ni2+ was completely reduced at 600 °C. The discrepancy in the reduction temperature could explain the property of AuNi/HTs (Fig. 15) reduced at 300 °C and 600 °C, respectively. The nanoparticles of AuNi/HTs-300 had a broader distribution than that of AuNi/HTs-600. As shown in Scheme 4, at 300 °C, the interaction between Ni0 and Au0 was weak since the reductive degree of Ni2+ was low. Consequently, the dispersion of Ni2+and the interaction between Ni0 and Au0 did not play their role well in directing the position and dispersion of AuNi nanoparticles. At 600 °C, Ni2+ was completely reduced, so the metal interaction was increased. This explained the reason of the lower size and narrower distribution of AuNi/HTs-600. However, the size of AuNi/HTs-600 was bigger than that of AuCu/HTs-300 although alloying force did work. The higher in the size of AuNi/HTs-600 was due to the increased size of Au nanoparticles. Before Ni2+ was completely reduced and metal interaction between Au and Ni did not work, the single Au nanoparticles were already sintered because of the increased reduction temperature.
Besides guaranteeing the complete reduction of every bimetallic ion, appropriate reduction temperature could avoid the sinter of bimetallic nanoparticles. If reduction temperature was set above the appropriate reduction temperature, bimetallic nanoparticles would sinter although bimetallic ions could be completely reduced. As shown in Fig. 12, 300 °C was appropriate reduction temperature for AuCu bimetal, while 600 °C was highly above 300 °C and led to the sinter of the bimetallic nanoparticles.
In order to verify the mechanism shown above, AuCuNi/HTs-600 was prepared (Fig. 19). The size of AuCuNi/HTs-600 was 2.1 nm and similar or even smaller than that of AuCu/HTs-300, which was far smaller than that of both AuCu/HTs-600 and AuNi/HTs-600. This indicated that AuCuNi/HTs-600 avoided both the sinter of AuCu/HTs-600 arising from the increased temperature, and the high size of AuNi/HTs-600 resulting from the sinter of single Au nanoparticles at increased temperature. At temperature below 600 °C, dispersion of Cu2+and the interaction between Cu0 and Au0 directed the dispersion of AuCu nanoparticles. At 600 °C, dispersion of Ni2+and the interaction between Ni0 and AuCu directed the dispersion and inhibited the sinter of AuCu nanoparticles. The low size and narrow distribution of nanopaticles on AuCuNi/HTs-600 proved our deduction.
3.3 Performances of the Bimetal Catalysts in Aerobic Oxidation of Benzyl Alcohol
The catalytic performance of bimetallic catalysts prepared by this work were tested and compared with those prepared by traditional methods. As shown in Table 2, benzaldehyde yield was below 46% and TOF was < 256 h− 1 for bimetallic catalysts prepared by traditional methods in the aerobic oxidation of benzyl alcohol (Entries 1–2). 92% of benzaldehyde yield and TOF (704 h− 1) was obtained by AuCu/HTs-300 (Entry 3), which was far higher than that by traditional method. The 704 h− 1 of TOF was also far higher than 400 h− 1 of Au/Cu-HTs in reference [32]. This indicated that the catalysts prepared by this work showed higher activity than those by traditional methods. The benzaldehyde yield by AuNi (i.e., AuNi/HTs-300 and AuNi/HTs-600) was between 67 ~ 85% (Entries 4–5), which was higher than that by AuCu/HTs-300-C or AuNi/HTs-600-C (Traditional method) but lower than that by AuCu/HTs-300 (This work). The medium activity of AuNi catalyst was ascribed to its high size. The reason for its high size has been discussed in the previous section. However, the activity of AuCuNi/HTs-600 (Entry 6) was similar with that of AuCu/HTs-300. The high activity of AuCuNi/HTs-600 was also in accordance with the low size of AuCuNi nanoparticles.
The reusability of AuCu/HTs-300 catalysts was also investigated. Table S1 displays the results of recycling experiments of catalysts. The conversions of benzyl alcohol by fresh catalysts were 92%. The conversion decreased from 92% in the first run to 40% in the second run. After second run, the yield did not change apparently. The lower reusability of the catalysts may be due to the leaching of Mg2+ and the stability of gold particles [42]. In the future work, we will continue to improving our methods to solve this problem.
4 Conclusions
In this work, one convenient method was proposed to prepare bimetallic catalysts. The mean size of AuCu/HTs-300 by this new method was 2.5 nm, which was 1/4 (Sequential impregnation) or 1/10 (Co-impregnation) of the size of catalysts by traditional method. Furthermore, the former yield of benzaldehyde was 92%, while the latter was below 46%. The prepared catalysts showed higher activity than that of the catalysts by traditional methods. This demonstrated that the proposed method was successful. In this proposed method, configurational ion Cu2+of HTs directed the position and dispersion of the final AuCu nanoparticles on the surface of HTs supports. The direction was realized by employing the interaction between Cu0 and Au0. It was found that dispersion of Cu2+ (or Ni2+) and the interaction between Cu0 (or Ni0) and Au0 are two key factors affecting the dispersion of AuCu nanoparticles. The dispersion of Cu2+ (or Ni2+) could be controlled by adjusting their contents in HTs supports. The interaction between Cu0 (or Ni0) and Au0 could be controlled by regulating the reduction temperature. This method could be applied to the controlled synthesis of other bimetallic or trimetallic catalysts.
References
Divins NJ, Angurell I, Escudero C, Pérez-Dieste V, Llorca J (2014) Science 346:620–623
Bracey CL, Ellis PR, Hutchings GJ (2009) Chem Soc Rev 38:2231–2243
Baldizzone C, Mezzavilla S, Carvalho HWP, Meier JC, Schuppert AK, Heggen M, Galeano C, Grunwaldt J, Schüth F, Mayrhofer KJJ (2014) Angew Chem Int Ed 53:14250–14254
Paalanen P, Weckhuysen BM, Sankar M (2013) Catal Sci Technol 3:2869–2880
Fang W, Chen J, Zhang Q, Deng W, Wang Y (2011) Chem Eur J 17:1247–1256
Liu X, Wang A, Yang X, Zhang T, Mou C, Su D, Li J (2008) Chem Mater 21:410–418
Falsig H, Hvolbæk B, Kristensen IS, Jiang T, Bligaard T, Christensen CH, Nørskov JK (2008) Angew Chem 120:4913–4917
Li L, Gao Y, Li H, Zhao Y, Pei Y, Chen Z, Zeng X (2013) J Am Chem Soc 135:19336–19346
Liao F, Lo TWB, Tsang SCE (2015) ChemCatChem 7:1998–2014
Bauer JC, Mullins D, Li M, Wu Z, Payzant EA, Overbury SH, Dai S (2011) Phys Chem Chem Phys 13:2571–2581
Lu J, Low KB, Lei Y, Libera JA, Nicholls A, Stair PC, Elam JW (2014) Nat Commun 5:3264–3272
Zhong R, Sun K, Hong Y, Xu B (2014) ACS Catal 4:3982–3993
Rebelli J, Detwiler M, Ma S, Williams CT, Monnier JR (2010) J Catal 270:224–233
Barbier J, In: Ertl G, Knözinger H, Weitkamp J (Eds) (1999) Preparation of solid catalysts, Wiley-VCH Verlag GmbH
Lamy-Pitara E, Ouazzani-Benhima LEl, Barbier J (1992) Appl Catal A 81:47–65
Pieck CL, Marecot P, Barbier J (1996) Appl Catal A 143:283–298
Barbier J, Marécot P, Del Angel G, Bosch P, Boitiaux JP, Didillon B, Dominguez JM, Schifter I, Espmosa G (1994) Appl Catal A 116:179–186
Rebelli J, Rodriguez AA, Ma S, Williams CT, Monnier JR (2011) Catal Today 160:170–178
Beard KD, Borrelli D, Cramer AM, Blom D, Van Zee JW, Monnier JR (2009) ACS Nano 3:2841–2853
Ohashi M, Beard KD, Ma S, Blom DA, St-Pierre J, Van Zee JW, Monnier JR (2010) Electrochim Acta 55:7376–7384
Beard KD, Van Zee JW, Monnier JR (2009) Appl Catal B 88:185–193
Rodriguez AA, Williams CT, Monnier JR (2014) Appl Catal A 475:161–168
Jia Q, Zhao D, Tang B, Zhao N, Li H, Sang Y, Bao N, Zhang X, Xu X, Liu H (2014) J Mater Chem A 2:16292–16298
Guan Y, Zhao N, Tang B, Jia Q, Xu X, Liu H, Boughton RI (2013) Chem Commun 49:11524–11526
Hakim SH, Sener C, Alba-Rubio AC, Gostanian TM, O’Neill BJ, Ribeiro FH, Miller JT, Dumesic JA (2015) J Catal 328: 75–90
Wang H, Liu D, Xu C (2016) Catal Sci Technol 6:7137–7150
Liu P, Degirmenci V, Hensen EJM (2014) J Catal 313: 80–91
Debecker DP, Gaigneaux EM, Busca G (2009) Chem Eur J 15:3920–3935
Xu Z, Zhang J, Adebajo MO, Zhang H, Zhou C (2011) Appl Clay Sci 53:139–150
Fan G, Li F, Evans DG, Duan X (2014) Chem Soc Rev 43:7040–7066
Zope BN, Hibbitts DD, Neurock M, Davis RJ (2010) Science 330:74–78
Liu P, Guan Y, Santen RA, Li C, Hensen EJM (2011) Chem Commun 47:11540–11542
Wang J, Lang X, Zhaorigetu B, Jia M, Wang J, Guo X, Zhao J (2014) ChemCatChem 6:1737–1747
Zhao J, Yu G, Xin K, Li L, Fu T, Cui Y, Liu H, Xue N, Peng L, Ding W (2014) Appl Catal A 482:294–299
Du Y, Jin Q, Feng J, Zhang N, He Y, Li D (2015) Catal Sci Technol 5:3216–3225
Xu C, Sun J, Zhao B, Liu Q (2010) Appl Catal B 99:111–117
Liu P, Derchi M, Hensen EJM (2014) Appl Catal B 144:135–143
Pojanavaraphan C, Luengnaruemitchai A, Gulari E (2013) Appl Catal A 456:135–143
Luo M, Bian P, Zheng X (1998) Chin J Appl Chem 15:113–114
Liu J, Qiao B, Song Y, Huang Y, Liu J (2015) Chem Commun 51:15332–15335
Kirkeminde A, Spurlin S, Draxler-Sixta L, Cooper J, Ren S (2015) Angew Chem Int Ed 54:4203–4207
Wang Z, Xu C, Wang H (2014) Catal Lett 144:1919–1929
Acknowledgements
This work was supported by projects funded by the Major Research Plan of National Natural Science Foundation of China (Program No. 91545130).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, H., Liu, N., Xu, C. et al. Directed Dispersion of Au Based Catalysts at H2 Reduction Process for Aerobic Oxidation of Benzyl Alcohol. Catal Lett 147, 547–565 (2017). https://doi.org/10.1007/s10562-016-1938-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-016-1938-8