Abstract
A facile method was proposed for one-step preparation of Au/MgO. In this method, the Au/MgO kept both basic sites of MgO crystal and low size of gold nanoparticles. Compared with those of Au/MgO prepared by conventional methods, Au/MgO by this method had the highest activity.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The selective oxidation of alcohols with molecular oxygen over catalysts offers a sustainable, environmentally benign alternative to traditional processes that use expensive inorganic oxidants [1]. Recently, considerable attention has been devoted to the application of nano gold catalysts in the selective aerobic oxidation of alcohols [2]. A key advantage in using gold, as compared to Pt and Pd, is the improved resistance of Au to over oxidation under liquid-phase oxidation conditions with O2 as the oxidant [3]. However, in alcohol oxidation with gold catalysts, a severe limitation arises because of the necessary addition of a homogeneous base to improve the oxidation kinetics and reduce deactivation [3]. The addition of homogeneous base presents negative environmental and economic impacts since the high pH of the medium in corrosive and the salts of product need to be neutralized to release free acid [4]. In recent years, there has been increasing emphasis on the design and use of environmentally-friendly heterogeneous base to reduce environmental and economic impacts arising from chemical processes, such as CeO2 [5], TiO2 [6], MgO [7], NiO [8], Al2O3 [9], Fe2O3 [6], and hydrotalcites [10].
MgO was a typical support of solid base for alcohol oxidation [7]. The methods of depositing Au on MgO support were various [11]. The typical methods include deposition–precipitation [12], sol-immobilisation [13], impregnation [7], and deposition–reduction [14]. Choudhary et al. [12] prepared Au/MgO by homogeneous deposition–precipitation and reported its catalytic activity for oxidation of benzyl alcohol in 2009. Brett et al. [13] reported glycerol oxidation on Au–Pt/MgO catalysts prepared using a sol-immobilisation method in 2011. In the same year, Boronat et al. [7] measured the kinetics of benzyl alcohol oxidation on a series of Au/MgO catalysts prepared by impregnation. Costa et al. [15] prepared Au/MgO by deposition–precipitation and investigated the activity of Au/MgO for the liquid-phase oxidation of a wide range of alcohols in 2012. In these typical methods of preparing gold supported catalysts, aqueous system was used. It was noted that MgO was used as the starting support, while the resulting catalyst is Au/Mg(OH)2, which is attributed to the hydrolysis of MgO in aqueous solution during the gold particle deposition process [16]. Therefore, most catalysts of Au/MgO investigated by the references above were actually Au/Mg(OH)2 although the starting support was MgO. However, in our recent work, we found that MgO support was better than its corresponding Mg(OH)2 support for Au catalyst in oxidation of benzyl alcohol [17]. This was because MgO was higher than Mg(OH)2 in terms of strength and number of basic sites. Thermal decomposition was a normal method of transforming Mg(OH)2 back to MgO [18, 19]. During the process of calcination, although the number and strength of basic sites of MgO was restored, the size of gold was also raised up because of the sintering of gold particles in the high temperature [17, 19]. The number of basic sites was proportional to the activity of gold, while the size of gold was reversely proportional to it [4, 20, 21]. If one method was designed to make the number of basic sites of MgO unchanged and the size of gold particles low during the process of preparing the Au/MgO catalyst, the obtained Au/MgO should show high activity [17]. Recently, Li et al. [22] investigated the effect of (111) facet of MgO on activity of Au particles for oxidation of benzyl alcohol. In order to avoid the hydrolysis of MgO in aqueous solution and keep the structure of MgO unchanged, the process of depositing Au particles was run in organic solvents. Although this method effectively kept the structure of MgO, the size of gold particles was high. This resulted in lower activity of Au/MgO for oxidation of benzyl alcohol. Enlightened by this, we designed a new method of preparing Au/MgO. In this new method, the Au/MgO kept both basic sites of MgO and low size of gold nanoparticles. The benzyl alcohol oxidation is an important model reaction to test for oxidative activity and selectivity over supported metals [4, 23]. The activity of Au/MgO was tested for oxidation of benzyl alcohol (Scheme 1).
2 Experimental
2.1 Catalyst Preparation
2.1.1 Preparation of Supports
MgO support was prepared by calcining commercially available Mg(OH)2 at 500 °C for 3 h in static air. The obtained MgO was denoted MgO-1.
In order to study the effect of MgO structure, MgO with different structure were prepared by thermal decomposition of different precursors including freshly prepared Mg(OH)2, commercially available basic magnesium carbonate ((MgCO3)3Mg(OH)23H2O), and commercially available MgO samples. These precursors were calcined as commercially available Mg(OH)2. The obtained MgO materials were designated as MgO-2, MgO-3, and MgO-4, respectively. The freshly prepared Mg(OH)2 precursor was obtained by precipitating Mg(NO3)2 solution (150 mL; 0.4 mol L−1) with NaOH solution (120 mL; 1.0 mol L−1). The resulting mixture was aged at room temperature for 18 h, filtered, washed five times with distilled water, dried (100 °C; 12 h) and then calcined.
2.1.2 Preparation of Au/MgO
The Au/MgO catalysts were prepared by a new in situ deposition-reduction approach. In order to avoid the rehydration of MgO, the amount of water was controlled throughout the whole process. The typical procedure was given below. The obtained MgO (0.574 g) was added to 50 mL of methanol solution of HAuCl4 (Theoretical Au loading was 1 wt %). After stirring for 1 min, 1 mL of NaOH solution (1 mol L−1) was added and the resulting mixture was stirred at room temperature for 2 h. Then, the suspension was filtered and washed thoroughly with absolute ethanol. The solid was dried at 90 °C overnight. The effect of preparation conditions, including adding sequence of HAuCl4 and NaOH solution, concentration and volume of NaOH solution, water amount, organic solvents/reducing agents, and aging time of precipitate, were studied in detail.
2.2 Catalyst Characterization
X-ray powder diffraction patterns (XRD) were recorded using a D/Max-3C X-ray powder diffractometer (Rigaku Co., Japan), using a Cu–K source (40 kV, 30 mA) fitted with an Inel CPS 120 hemispherical detector with a scanning rate of 6° min−1 from 5° to 80° (2θ).
Elemental analysis was determined by an FEI Quanta 200 scanning electron microscope equipped with energy dispersive X-ray spectroscopy (EDS) capability.
The nitrogen adsorption and desorption isotherms were measured at −196 °C using a Micromeritics ASAP 2020 system. Specific surface areas of the samples were determined by nitrogen adsorption data in the relative pressure range from 0.06 to 0.30 using the BET (Brunauer–Emmett–Teller) equation. Total pore volumes were estimated from the amount of nitrogen adsorbed at a relative pressure of 0.995. Pore volume and pore size distribution curves were obtained from the analysis of the desorption branches of the nitrogen isotherms using the BJH (Barrett–Joyner–Halenda) method.
The gold loading of the catalysts was determined by flame atomic absorption spectrometry. A TAS986 atomic absorption spectrophotometer was used, the wavelength range detected was 190–900 nm. A hollow cathode lamp of gold and platinum (Ningqiang Source, Hengshui, China) was used as radiation source at 5.0 mA. The analytical line at 242.8 nm (Au) and 265.9 nm (Pt) was used in the measurements with 0.4 nm as the spectral bandwidth.
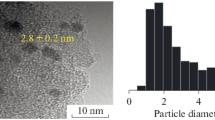
The scanning transmission electron microscopy (STEM) micrographs were obtained by using a FEI Tecnal G2 F20 instrument. Particle size distributions were obtained by the measurement of at least 100 particles, and the average diameter was calculated by dM = ∑dini/∑ni, where ni is the number of particles of diameter di.
X-ray photoelectron spectra (XPS) were obtained in an AXIS ULTRA spectrometer (Kratos Analytical Ltd.) using AlKa (hn = 1486.6 eV) X-ray radiation at an energy of 150 w. Spectra were collected at room temperature and at a pressure in the analyzer chamber of 5 × 10−9 torr. Kinetic energies of the photoelectron were measured with a hemispherical energy analyzer working at constant pass energy of 50 eV. The binding energy scale was calibrated by setting the C1s transition at 284.8 eV.
2.3 Catalytic Experiments
The aerobic oxidation of benzyl alcohols was carried out using a 50 mL three-necked round-bottle flask with a water-cooled condenser. Typically, the reactor was charged with 0.1 g of catalyst, 1 mmol of benzyl alcohol and 10 mL of toluene at 110 °C for 3 h under an oxygen flow (20 mL min−1). After cooling to room temperature, the resulting mixture was constant by toluene in 25 mL volumetric flask. The reaction products were quantitatively analyzed by a Shimadzu GC-2014 (RXI 5 ms, 30 m × 0.25 mm, df = 0.5 μm) gas chromatograph with a flame ionization Detector. N-dodecane (1 mmol) was used as an internal standard for analysis.
3 Results and Discussion
3.1 Characterization of Supports and Catalysts
3.1.1 BET Surface Area and Pore Size
Table 1 shows the nitrogen physisorption data of MgO supports from different precursors. The highest surface area was MgO-1 (174 m2 g−1), next was MgO-2 (75 m2 g−1). MgO-3 and MgO-4 showed the lowest surface area, which were in the range of 29–39 m2 g−1. The pore size distribution of MgO-1 was far narrower than that of the other three MgO supports (Fig. 1). The isotherms of the four samples had the characteristic Type IV shape. The Type IV isotherm and pore size distribution suggested that the four samples were mesoporous materials.
3.1.2 XRD Analysis
Figure 2 displays the XRD patterns of Mg(OH)2, MgO, and Au/MgO. The X-ray diffractograms of Mg(OH)2 (Fig. 2a) showed the typical X-ray diffractograms of Mg(OH)2 [18]. The thermal pre-treatment of Mg(OH)2 results in changed XRD patterns, caused by the structural changes associated with the loss of H2O from the starting material. The XRD patterns of MgO are presented in Fig. 2b. It can be seen that the major phases present in MgO were MgO. The X-ray diffractograms of Au/MgO (Fig. 2c) were similar to that of MgO, showing the typical X-ray diffractograms of MgO. This demonstrated that MgO kept its structure and did not rehydratate to form Mg(OH)2 in the process of doping. No diffractograms of gold were observed for the Au/MgO catalyst, due to its low loading amount.
Although the process of doping gold particles on the surface of MgO was run in non aqueous system, water was not avoidable. MgO or NaOH could react with HAuCl4 to form water and the corresponding salt. In order to study effect of water on the structure of MgO, different amount of water was added to the system of preparing Au/MgO. The XRD patterns of Au/MgO with different amount of water were shown in Fig. 3. Au/MgO prepared with no water and 4 % water (Volume ratio of water to methanol) showed the characteristic peaks of MgO. Au/MgO prepared with water above 4 % showed, beside MgO, diffraction peaks of Mg(OH)2. This indicated that MgO partly rehydred to form Mg(OH)2 when water amount was above 4 %.
3.1.3 Elemental Analyses
NaOH could promote the performance of Au catalyst in the alcohol oxidation [4]. In this work, NaOH was used in the process of preparing Au/MgO. In order to avoid the effect of residue NaOH, NaOH have to be completely removed from the surface of Au/MgO. The EDS method was used to analyze the elemental composition on the surface of Au/MgO (Fig. 4). Au/MgO catalyst mainly consisted of magnesium (58.16 %) and oxygen (39.10 %). None Na was found, indicating that NaOH was completely removed from Au/MgO. Accordingly, the effect of residue NaOH could be avoided in the alcohol oxidation.
3.1.4 STEM Analyses
Aging time may affect the size of gold [14]. The size of gold affected its activity [20]. Figure 5 shows the representative STEM micrographs of the gold catalysts prepared under different aging time. The mean size of gold on Au/MgO-1 was 3.5 nm for aging time of 0.5 h, and 5.3 nm for 2 h. Under aging time of 8 h, part of gold nanoparticles coagulated (Fig. 5c2). This indicated that the size of gold nanoparticles increased with aging time.
3.1.5 XPS Analyses
XPS spectra of Au 4f region for Au/MgO-1 are given in Fig. 6a. All spectra of Au 4f region could be fitted satisfactorily with peak maxima at 87.3 and 83.7 eV binding energies, which are characteristic of metallic Au. No Auδ+ was detected, suggested that Au was completely reduced.
The state of O ions could be used to investigate if MgO was rehydrated to form Mg(OH)2. In order to understand the state of O ions, the XPS spectra of O 1s for catalysts were determined (Fig. 6b). On the surface of Au/MgO, the main state of O ions was “O2−”. No “OH−” was detected, suggesting that the MgO support of Au/MgO did not rehydrated to form Mg(OH)2. The results were in agreement with those by XRD analysis.
3.2 Effect of Preparation Conditions on Catalytic Activity of Au/MgO
3.2.1 Effect of NaOH Concentration and Volume
Figure 7 shows the effect of concentration and volume of NaOH solution on the activity of gold catalysts. When Au/MgO-1 was prepared without the addition of NaOH, the conversion of benzyl alcohol was 45 %. When NaOH was added, the activity of Au/MgO-1 was dramatically increased and the conversion of benzyl alcohol was above 88 % (Fig. 7a). In order to further study the effect of concentration and volume of NaOH solution, the catalyst amount and reaction time was reduced from 0.1 g and 3 h to 0.05 g and 1 h, respectively. It was found that the activity of Au/MgO-1 varied with the concentration and volume of NaOH solution (Fig. 7b). Its activity was proportional to the volume of NaOH solution when the concentration of NaOH was less than 1 mol L−1. When the concentration of NaOH was increased to 2.5 mol L−1, its activity increased with volume of NaOH up to 0.4 mL. The activity of Au/MgO-1 decreased a little above 0.4 mL of NaOH solution.
3.2.2 Effect of the Organic Solvents/Reducing Agents
The organic solvents in the system of preparing Au/MgO, beside as solvents, had another functionality of reducing agent. The effect of organic solvents/reducing agents was investigated and shown in Table 2. Using methanol as solvents/reducing agents (Entry 1), the obtained Au/MgO showed the highest activity (Conversion of benzyl alcohol, 99 %). When methanol was replaced by ethanol or 2-propanol, the activity of Au/MgO decreased. The conversion of benzyl alcohol was 89 % (Entry 2) and 80 % (Entry 3) for ethanol and 2-propanol, respectively. The conversion of benzyl alcohol by acetone (Entry 4) was similar to that of 2-propanol. The lowest conversion of benzyl alcohol (47 %) was from tetrahydrofuran (Entry 5). The organic solvents/reducing agents had different electric potential which affected the rate of oxidation–reduction reaction between Au(III) and reducing solvents. The difference in the activity may result from the difference in the reduction potential of organic solvents/reducing agents.
3.2.3 Effect of Water Amount
The effect of water amount in the process of preparing Au/MgO was studied and shown in Table 3. In the absence of water, the mole of benzyl alcohol converted per time per mole of gold was 608 for Au/MgO (Entry 1). When water amount was increased to 4 %, mole of benzyl alcohol converted per time per mole of gold was increased to the maximum of 752 (Entry 2). Above 4 %, mole of benzyl alcohol converted per time per mole of gold decreased with water amount. When water amount was increased to 50 %, the mole of benzyl alcohol converted per time per mole of gold was decreased to 197. The results indicated that water amount affected the catalytic activity of Au/MgO. The effect may be ascribed to the structure of Au/MgO. XRD analysis of structure of Au/MgO versus water amount showed that water amount affect the structure of Au/MgO (Fig. 3). When water amount was below 4 %, the support of Au/MgO kept the crystalline structure of MgO. However, when water amount was above 4 %, part of MgO support rehydrated to form Mg(OH)2. The transformation decreased the number of basic sites on the surface of Au/MgO. The decrease in the number of basic sites accordingly reduced the conversion of benzyl alcohol [17].
3.2.4 Effect of Supports
Supports may disperse and fix the gold nanoparticles [24]. Besides, the structure of supports also affects the catalytic activity of gold nanaparticles [4]. The effect of supports were investigated and shown in Table 4. MgO supports of MgO-1 to MgO-4 were prepared from different precursors. They showed different activity. Au/MgO-1 showed the maximum activity. The activity decreased from MgO-1 down to MgO-4. Mole of benzyl alcohol converted per time per mole of gold of Au/MgO-1 was 752, which was five times higher than that of Au/MgO-4 (148). The effect of MgO precursors was in accordance with the surface area of MgO. The surface area of MgO decreased from MgO-1 down to MgO-4.
3.2.5 Effect of Aging Time of Precipitate
The variation of conversions of benzyl alcohol versus aging time of precipitates was shown in Fig. 8. Aging time of freshly precipitated Au nanoparticles exhibited an influence on the catalytic properties of the resulting Au/MgO catalysts. The activity of Au/MgO catalysts increased from 0.5 h of aging time (Conversion of benzyl alcohol, 50 %) to 2 h of aging time (Conversion of benzyl alcohol, 63 %). Above 2 h of aging, an increase in aging time resulted in a slightly decrease in the conversion of benzyl alcohol. The conversion of benzyl alcohol went down to 42 % at 16 h of aging time. The trends in the evolution of benzyl conversion as a function of aging time of precipitate were in agreement with that of gold size versus aging time. The gold size affected its activity [20]. The prolonged aging time resulted in the increase of the size of gold particles, which accordingly decreased the activity of gold catalysts.
3.2.6 Effect of Adding Sequence of HAuCl4 and NaOH Solution
In the process of preparing Au/MgO, beside the solvent/reducing agents, HAuCl4 and NaOH were used. The adding sequence of HAuCl4 and NaOH was investigated and shown in Table 5. When HAuCl4 was added before NaOH, the conversion of benzyl alcohol decreased with the interval between addition of HAuCl4 and NaOH solution (Table 5, path 1). When the interval was 1 min, the conversion of benzyl alcohol was 69 % at reaction time of 0.5 h. When the interval was increased to 20 min, the conversion of benzyl alcohol decreased to 42 %. In contrast, when NaOH was added before HAuCl4, the interval between addition of HAuCl4 and NaOH solution did not affect the conversion of benzyl alcohol (Table 5, path 2). At reaction time 0.5 h, the conversion of benzyl alcohol was around 69 % for the different interval. The difference between the two paths was ascribed to the effect of basic reagents. In the section above, effect of NaOH in the process of preparing Au/MgO has been demonstrated. Supports of MgO are solid base. In the path 1 of HAuCl4 added before NaOH, solid base of MgO took part in the formation of Au particles before NaOH. The longer the interval between addition of HAuCl4 and NaOH solution, the more the extent to which MgO affected. However, for the path 2 of NaOH added before HAuCl4, there was no HAuCl4 in the system before the addition of HAuCl4. No Au particles were formed in the absence of HAuCl4. So the interval between addition of HAuCl4 and NaOH solution could not affect the formation of Au particles. Path two was more easily manipulated for the repetition of Au/MgO catalysts than path one, since the activity of Au/MgO catalysts was not affected by the interval between addition of HAuCl4 and NaOH solution. The results about adding sequence of HAuCl4 and NaOH solution further demonstrated that base played an important role in preparing Au/MgO. In the process of preparing Au/MgO, the equation of chemical reaction was shown below:
The Eqs. (1) and (2) indicated that NaOH took part in both the formation of precipitate of Au(OH)3 and the reduction of Au(III) by methanol. Accordingly, the forming rate and number of crystal nucleus of Au was affected by NaOH, which affected the size of gold particles. More importantly, the size of gold particle is also changed by NaOH due to the dependency of the adsorptivity of the support on pH [14, 24].
3.3 Catalytic Activity of Au/MgO for Alcohol Oxidation
Au/MgO by deposition–reduction method of reference showed very low activity [22], the conversion of benzyl alcohol was 3.05 % (Table 6, entry 1). The reaction condition and structure of MgO of this reference was different from that of this work, so the activity of Au/MgO by deposition–reduction method of reference could not be directly compared with that by this work. The difference between this deposition–reduction method and the method proposed by this work was the use of NaOH in the process of preparing Au/MgO. The reported deposition–reduction method did not use NaOH, while NaOH was used in this method. In the section above, the role of NaOH had been demonstrated by analysis of mechanism and results of experiments. When NaOH was not added in the process of preparing Au/MgO, low conversion of benzyl alcohol was obtained. This indicated that the activity Au/MgO by deposition–reduction method of reference was far lower than that of Au/MgO prepared by this work. So, compared with the deposition–reduction method of reference, the high activity of Au/MgO by this method was ascribed to the use of NaOH in the process of preparing catalysts.
The methods of sol-immobilisation, deposition–precipitation, and impregnation are widely used for preparing gold supported catalysts [11, 23]. The size of gold by these three methods was in the range of 8.2–45.8 nm (Table 6, entries 2–4), which was higher than that of gold by this work (Entry 5). The mole of benzyl alcohol converted per time per mole of gold of Au/MgO by these three methods was less than 433, while mole of benzyl alcohol converted per time per mole of gold of Au/MgO by this work was 752. This indicated that Au/MgO by this work had the highest activity. These three conventional methods were conducted in aqueous solution. In the aqueous solution, MgO was rehydrated to form Mg(OH)2 [16, 19]. Au/Mg(OH)2 was the product of first step in the process of preparing Au/MgO by these three methods [16, 17]. The transformation of Au/Mg(OH)2 to Au/MgO was done through the calcination of second step. During the process of calcination, although the number of basic sites was increased, the size of gold was also raised up because of the sintering of gold particles in the high temperature [17]. The number of basic sites of Au/MgO by the three conventional methods was similar to that of Au/MgO. The lower activity of Au/MgO by the three conventional methods was ascribed to its lower size of gold nanoparticles. This demonstrated that Au/MgO by this method kept the crystal phase of MgO, displayed low size of Au particles, and had high catalytic activity. Besides, Au/MgO by this method was prepared in one step. The procedure of this work was simpler and more easily manipulated than that of the three conventional methods.
3.4 Study of the Formation of Byproduct Toluene in the Presence of Au/MgO-1
Benzyl alcohol can be transformed to benzaldehyde by oxidative dehydrogenation and to toluene byproduct by disproportionation, respectively. The formation of toluene byproduct has been widely investigated [25–29]. It was reported that the formation of toluene was affected by both the supports and catalysts [27]. Two methods were used to study the effect of MgO as a support on toluene formation. One method used solvent free condition as the literatures used [26]. The other method used a different solvent of benzene. The results by both of the two methods showed that toluene was not detected in the presence of Au/MgO catalysts (Table 7). This indicated that MgO as a support suppressed the toluene formation in the presence of gold nanoparticles catalysts. These results were in consistence with the results in the literature. Nowicka et al. [27] utilized in situ spectroscopic method to study the toluene formation in the presence of gold–palladium nanoparticles catalysts. They found that acid–basic property of supports affected toluene formation. The acidic supports, like TiO2, promote the disproportionation of benzyl alcohol to toluene, while basic supports, like ZnO and MgO, switch off disproportionation completely. In this work, MgO support was used. It was also found that MgO suppressed the toluene formation in the presence of gold catalysts.
3.5 Reusability of Au/MgO-1
The stability of catalysts was investigated. Table 8 displays the results of recycling experiments of catalysts. The conversions of benzyl alcohol by fresh catalysts were 98 %. The conversion decreased from 98 % in the first run to 50 % in the second run. In the third run, the conversion went down to 40 %. The reusability of Au/MgO by this work was similar with that by sol-immobilisation method [30]. The activity of Au/MgO by the last method also decreased apparently after first run. The conversion of benzyl alcohol by sol-immobilisation method was 95 % for first run. In the second run, the conversion decreased to 31 %. The leaching of Mg2+ was evaluated by atomic absorption spectroscopy analysis of the reaction solution after 3 h reaction. Small quantities of Mg2+ were found in the ending up reaction mixture. The concentration of Mg2+ in the reaction mixture was 86 ppm. This indicated that the leaching of Mg2+ in the oxidation of benzyl alcohol in toluene solvents was similar with it in methanol solvents [19].
4 Conclusions
A simple and quick method was proposed for the one-step preparation of Au/MgO. The Au/MgO kept the crystal phase of MgO and displayed low size of Au particles. The activity of Au/MgO was far higher than that prepared by conventional methods. The Mole of benzyl alcohol converted per time per mole of gold of Au/MgO by this work was 752, while Mole of benzyl alcohol converted per time per mole of gold of Au/MgO by conventional methods was less than 433. It was found that NaOH played a key role in the control of gold size and high activity of gold catalysts. In addition, MgO support suppressed the formation of byproduct toluene in the presence of Au nanoparticles.
References
Zope BN, Hibbitts DD, Neurock M, Davis RJ (2010) Science 330:74–78
Pina CD, Falletta E, Rossi M (2012) Chem Soc Rev 41:350–369
Villa A, Veith GM, Prati L (2010) Angew Chem Int Ed 49:4499–4502
Davis SE, Ide MS, Davis RJ (2013) Green Chem 15:17–45
Tanaka A, Hashimoto K, Kominami H (2012) J Am Chem Soc 134:14526–14533
Enache DI, Knight DW, Hutchings GJ (2005) Catal Lett 103:43–52
Boronat M, Corma A, Illas F, Radilla J, Ródenas T, Sabater MJ (2011) J Catal 278:50–58
Villa A, Chan-Thaw CE, Veith GM, More KL, Ferri D, Prati L (2011) ChemCatChem 3:1612–1618
Hallett-Tapley GL, Silvero MJ, Bueno-Alejo CJ, González-Béjar M, McTiernan CD, Grenier M, Netto-Ferreira JC, Scaiano JC (2013) J Phys Chem C 117:12279–12288
Fang W, Chen J, Zhang Q, Deng W, Wang Y (2011) Chem Eur J 17:1247–1256
Takei T, Akita T, Nakamura I, Fujitani T, Okumura M, Okazaki K, Huang J, Ishida T, Haruta M (2012) In: Gates BC, Jentoft FC (eds) Advances in catalysis, vol 55. Elsevier Inc., San Diego, pp 1–126
Choudhary VR, Dumbre DK (2009) Catal Commun 10:1738–1742
Brett GL, He Q, Hammond C, Miedziak PJ, Dimitratos N, Sankar M, Herzing AA, Conte M, Lopez-Sanchez JA, Kiely CJ, Knight DW, Taylor SH, Hutchings GJ (2011) Angew Chem Int Ed 123:10318–10321
Sunagawa Y, Yamamoto K, Takahashi H, Muramatsu A (2008) Catal Today 132:81–87
Costa VV, Estrada M, Demidova Y, Prosvirin I, Kriventsov V, Cotta RF, Fuentes S, Simakov A, Gusevskaya EV (2012) J Catal 292:148–156
Jia C, Liu Y, Bongard H, Schüth F (2010) J Am Chem Soc 132:1520–1522
Huangfu X (2013) The effect of preparation methods and basicity of supports on catalytic performance of Au in oxidation of benzyl alcohol. Thesis of Shaanxi Normal University, PR China
Bartley JK, Xu C, Lloyd R, Enache DI, Knight DW, Hutchings GJ (2012) Appl Catal B 128:31–38
Estrada M, Costa VV, Beloshapkin S, Fuentes S, Stoyanov E, Gusevskaya EV, Simakov A (2014) Appl Catal A 473:96–103
Wang M, Ma J, Chen C, Lu F, Du Z, Cai J, Xu J (2012) Chem Commun 48:10404–10406
Min BK, Friend CM (2007) Chem Rev 107:2709–2724
Li Z, Ciobanu CV, Hu J, Palomares-Báez J, Rodríguez-López J, Richards R (2011) Phys Chem Chem Phys 13:2582–2589
Alhumaimess M, Lin Z, He Q, Lu L, Dimitratos N, Dummer NF, Conte M, Taylor SH, Bartley JK, Kiely CJ, Hutchings GJ (2014) Chem Eur J 20:1701–1710
Corma A, Garcia H (2008) Chem Soc Rev 37:2096–2126
Cao E, Sankar M, Nowicka E, He Q, Morad M, Miedziak PJ, Taylor SH, Knight DW, Bethell D, Kiely CJ, Gavriilidis A, Hutchings GJ (2013) Catal Today 203:146–152
Meenakshisundaram S, Nowicka E, Miedziak PJ, Brett GL, Jenkins RL, Dimitratos N, Taylor SH, Knight DW, Bethell D, Hutchings GJ (2010) Faraday Discuss 145:341–356
Nowicka E, Hofmann JP, Parker SF, Sankar M, Lari GM, Kondrat SA, Knight DW, Bethell D, Weckhuysen BM, Hutchings GJ (2013) Phys Chem Chem Phys 15:12147–12155
He Q, Miedziak PJ, Kesavan L, Dimitratos N, Sankar M, Lopez-Sanchez JA, Forde MM, Edwards JK, Knight DW, Taylor SH, Kiely CJ, Hutchings GJ (2013) Faraday Discuss 162:365–378
Yan Y, Chen Y, Jia X, Yang Y (2014) Appl Catal B 156–157:385–397
Xu C, Wang Z, Huangfu X, Wang H (2014) RSC Adv 4:27337–27345
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Program No. 21343015), Natural Science Basic Research Plan in Shaanxi Province of China (Program No. 2013JM2003), and the Fundamental Research Funds for the Central Universities (Program No. GK201302016).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Z., Xu, C. & Wang, H. A Facile Preparation of Highly Active Au/MgO Catalysts for Aerobic Oxidation of Benzyl Alcohol. Catal Lett 144, 1919–1929 (2014). https://doi.org/10.1007/s10562-014-1344-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1344-z