Abstract
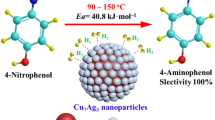
Bimetallic nanoparticles have shown a lot of promise as catalysts in the recent years. This piece of work highlights the use of nanocatalyst for the chemoselective reduction of nitro group in presence of other reducible functional groups. CuNi bimetallic nanoparticles of varying composition (1:1, 1:2) have been synthesized by polyol method using metal sulphates with hydrazine hydrate as reducing agent. The characterization of synthesised nanoparticles was done by XRD and FESEM with EDX, which confirm FCC structure and composition of synthesised bimetallic nanoparticles. Particle size varies with composition i.e. 23 nm for (1:1) and 20 nm for (1:2). The selective reduction of p-nitrophenol, p-nitrobenzaldehyde and 2,4 dinitrophenol was performed in water using both single Ni nanoparticles and bimetallic CuNi nanoparticles (1:1, 1:2) with hydrazine hydrate as reducing agent at room temperature. The various amino products obtained has various industrial applications. UV–visible spectroscopy was used to monitor the progress of the reaction. The bimetallic nanoparticles facilitated the rate of reaction much as compared to single Ni nanoparticles.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nitrosubstituted aromatic hydrocarbons are generally toxic and very difficult to be degraded in the environment. The conversion of aromatic nitro compounds to aromatic amino compounds obey with the demands of green chemistry [1]. Para nitrophenol and its derivatives are used to manufacture herbicides, insecticides and synthetic dyestuffs and they can substantially damage the ecosystem along with the common organic pollutants [2]. They are harmful for our health as they can damage the liver, kidney, central nervous system. Therefore removal of nitrophenol and its derivatives is very necessary. The reaction product 4-aminophenol and its derivatives are useful compounds which are used as intermediates for manufacturing analgesics, antipyretics, photographic developer, anti-corrosion lubricant etc. [3].
Nanomaterials are the cornerstones of nanoscience and technology. The potential applications of nanomaterials are in nanocatalysis [4], nanomedicine [5], chemical and biosensors [6], and in drug delivery [7]. In this context, metal nanoparticles based on noble metals (Pt, Pd, Au etc.) have shown high potential towards the reduction of nitro groups using H2, NaBH4, N2H4·H2O etc. as reducing agents [8,9,10,11,12,13,14,15]. But the high cost of noble metals makes them undesirable to use them as catalyst, despite showing high selectivity. This paved the way for the development of inexpensive non noble metal based catalysts, which will undoubtedly be highly economical [16,17,18,19,20,21,22,23].
In this piece of work, the selective reduction of the nitro group into amino group has been carried out using nickel nanoparticles and bimetallic CuNi nanoparticles of different compositions (1:1, 1:2) with hydrazine hydrate as reducing agent using water as green solvent at room temperature. Bimetallic nanoparticles displayed enhanced catalytic activity as compared to pure nanoparticles due to synergistic effect [24].

2 Experimental section
Copper sulphate and nickel sulphate were purchased from CDH, solvents were of analytical grade and used as received. p-nitrophenol, p-nitrobenzaldehyde and 2,4 dinitrophenol were purchased from Merck.
Powder X-ray diffraction (XRD) measurements were performed using Bruker D8 advance X-Ray diffractometer using CuKα radiation (λ = 0.154 nm) and the interpretation was done with by Origin software. FESEM images were taken using “FEI NoVa NanoSEM 450” FESEM instrument operated at 18 kV. Compositional analysis were made using “Bruker” made X-Flash 6130 EDS attachment and “Esprit” software. UV–visible spectral studies were performed using Perkin Elmer Lamda 25 UV–visible Spectrometer. IR spectral study was performed using Perkin Elmer Spectrum Version 10.5.1.
2.1 Synthesis of CuNi bimetallic nanoparticles (1:1, 1:2) by polyol method
0.1 M (0.2496 g) copper sulphate and 0.1 M (0.1547 g) nickel sulphate (1:1)/0.2 M (0.3095) nickel sulphate (1:2) were dissolved in 50 mL of ethylene glycol, followed by addition of 3 mL hydrazine hydrate. Few drops of 1 M NaOH was added with continuous stirring for maintaining pH = 11.5 and then the temperature of the reaction mixture was maintained at 180 °C for 2 h. The nanoparticle suspension was centrifuged at 10,000 rpm, washed with ethanol and dried at 60 °C overnight. Shiny green coloured crystals were obtained. Yield = 0.38 gm.
2.2 General procedure for the reduction of nitro group using hydrazine hydrate at room temperature and CuNi (1:1, 1:2) nanoparticles as catalysts
5 mmol of nitro substrate in 30 ml distilled water was taken in a round bottom flask. To this 0.5 mL hydrazine hydrate was added and the reaction mixture was stirred for sometime at room temperature. After this 0.001 g of catalyst (synthesised nanoparticles) was added and the contents were stirred. The progress of the reaction was monitored every 3 min by UV–visible spectroscopy. After completion, the reaction mixture was centrifuged at 10,000 rpm to separate out the catalyst [25].
3 Results and discussion
The XRD patterns of bimetallic CuNi nanoparticles with various ratios (1:1, 1:2) are shown in Fig. 1. Both the samples possess FCC structure with the diffraction lines at (111), (200), (220), (311) planes. The average particle size decreases from 23 nm to 20 nm on increasing the nickel content. The lattice parameter (a) is found to decrease with increase in Ni content from 3.602 to 3.580. The lattice parameters of bimetallic nanoparticles are located in between the lattice parameter of pure nickel i.e. 3.517 (JCPDS 65-0380) and pure copper i.e. 3.615 (JCPDS 04-0836) which confirms the formation of bimetallic nanoparticles. With the increase in nickel content, the diffraction lines are shifted to high angle and more close to the position of pure nickel. There is a distinct peak at 110 plane (2 theta = 37) in Fig. 1a which corresponds to Cu2O (Cuprite) confirmed using Match 3 software for XRD. No peaks corresponding to pure copper and pure nickel can be seen in the XRD pattern thereby excluding the possibility that the synthesised bimetallic nanoparticles are simply the mixtures of the two metal nanoparticles [26] (Fig. 2).
The synthesised CuNi bimetallic nanoparticles were further analysed by EDX (Energy Dispersive X-ray Analysis) and EDX spectrum are shown in Fig. 3a, b to confirm the formation and elemental composition of the nanoparticles. The elemental composition i.e. copper (49.30%) and nickel (51.90%) (Table in Fig. 3a) clearly indicates that 1:1 composition of CuNi bimetallic nanoparticles are present in sample 1. Similarly, the elemental composition i.e. copper (30.72) and nickel (69.28) (Table in Fig. 3a) clearly indicates that 1:2 composition of CuNi bimetallic nanoparticles are present in sample 2.
The catalytic activity of the synthesised nanoparticles was evaluated for the reduction of nitro groups in various substrates using hydrazine hydrate as reducing agent at room temperature under aerobic conditions (Table 1).
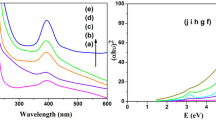
The efficacies for the conversion of nitro group to amino group was studied for the model nitro substrate p-nitrophenol using the synthesised Ni, CuNi (1:1, 1:2) nanoparticles. The catalytic reaction was monitored by UV–visible spectroscopy (as shown in Fig. 4) where a gradual decrease in the intensity of the characteristic absorbance peak of p-nitrophenol at 405 nm was observed along with the development of new absorbance peak at 310 nm which corresponds to the formation of p-amino phenol which has wide range of applications in photographic development of white and black films, drying agents, antipyretic and analgesic drugs and specially an important constituent of paracetamol. The reaction was completed in 45 min with Ni Nanoparticles, 30 min with CuNi Nanoparticles (1:1) and in 20 min with CuNi Nanoparticles (1:2). The reaction did not proceed either in the absence of catalyst or in the absence of hydrazine hydrate. We have performed experiment in water only without the addition of any organic solvent which is the most desirable condition. This finding can also be used to selectively reduce nitro group in those drugs which share the same basic structure, but differ only in their substituent groups as in case of benzodiazepines [27].
The synthesised nanocatalyst showed excellent chemoselectivity for the reduction of nitro group in p-nitrobenzaldehyde even in the presence of other reducible group. (i.e. CHO) to p-aminobenzaldehyde. The absorbance at 267 nm which was due to nitro group gradually decreased with time and a new peak appear at 313.95 nm which was due to amino group. The reaction was completed in 15 min with CuNi nanoparticles (1:1) and in 8 min with CuNi nanoparticles (1:2) using hydrazine hydrate as reducing agent.
In case of 2,4-dinitrophenol, the synthesised bimetallic nanoparticles showed selective reduction of only one nitro group which can be a desirable product in any reaction. The UV spectrum of 2, 4 dinitrophenol shows the absorbance at 245 nm and 358 nm which is due to OH and NO2 group respectively. But with due course of time with the use of reducing agent and nanocatalyst, a new absorbance peak at 383 nm was seen which was due to NH2 group along with the presence of absorbance peak at 353 nm. The results indicate that reduction of only one nitro group occurred. The reaction was completed in 75 min with Ni Nanoparticles, 45 min with CuNi Nanoparticles (1:1) and in 30 min with CuNi Nanoparticles (1:2). The results show the role of bimetallic nanoparticles to facilitate the reaction.
The identification of final product was done by melting point determination and IR spectrum. The melting point of the product is 127 °C which corresponds to 4-amino-2-nitrophenol (125–127 °C). In IR spectrum (Fig. 5) broad peak observed at 3193.15 cm−1 is assigned to hydrogen bonded phenolic group i.e. nitro group at ortho position to phenolic group forms strong hydrogen bond, which confirms the retention of nitro group at ortho position. Hence, regioselective reduction at para position is confirmed.
The results indicate that the efficiency of CuNi (1:2) bimetallic nanoparticles as catalyst is more in comparison to CuNi (1:1) nanaoparticles and pure Ni nanoparticles. The catalytic activity enhanced in case of CuNi bimetallic nanoparticles (1:1, 1:2) as compared to pure nickel nanoparticles due to synergy between the two metals. The bimetallic CuNi Np of 1:2 composition facilitated the rate of reaction in all the substrates. This is due to increase in surface area of catalyst with decrease in average particle size of 1:2 bimetallic CuNi Nanoparticles. Greater the surface area of the catalyst, faster is the reaction.
Langmuir- Hinshelwood mechanism for heterogeneous catalysis advocates the adsorption of substrate and reducing agent on the surface of catalyst. The presence of nanoparticle facilitates the transfer of electron from reducing agent to the substrate [28].
As indicated in table the fastest reaction is observed with substrate containing CHO functional group which is electron withdrawing and simultaneously provide facile orientation to the substrate molecule in comparison to OH containing substrate (Fig. 6).
The mechanism of conversion of nitrosubstrates to amino products has been illustrated in Fig. 7.
4 Conclusion
The CuNi bimetallic nanoparticles of different ratios (1:1, 1:2) have been synthesised successfully by polyol method with 93% yield. The XRD data confirms the face centred cubic arrangement of the synthesised nanoparticles and EDX analysis confirms the composition of the synthesised nanoparticles. The inexpensive non noble metal nanocatalyst displayed chemoselective reduction of range of nitro substrates into respective amines in the presence of other reducible functional groups. The efficiency of CuNi (1:2) bimetallic nanoparticles as catalyst is more in comparison to CuNi (1:1) nanaoparticles which is turn is more than pure Ni nanoparticles. The catalytic system is less expensive, easy to prepare, air stable, energy efficient and reproducible. Particle size and surface area of catalyst played a significant role in facilitating the rate of the reaction. Electrostatic interactions between catalyst surface and substrate can be monitored by selecting the substituent which provides more facile orientation thereby enhancing the rate of the reaction. All the catalytic reactions were performed at room temperature in aqueous medium—which is a greener route. Water and nitrogen are the only by-products from the catalytic reaction which is a great advantage.
References
Pradhan N, Pal A, Pal T (2002) Silver nanoparticle catalysed reduction of aromatic nitrocompounds. Colloids Surf A Physicochem Eng Asp 196:247–257
Gerelbaatar K, Tsogoo A, Tsedev N, Ganbold EO (2017) Reduction of 2,4 dinitrophenol to 2,4 diaminophenol using AuNanoparticles and AgNanoparticles as catalyst. Solid State Phenom 271:76–84
Ju KS, Parales RE (2010) Nitroaromatic compounds, from synthesis to biodegradation. Microbiol Mol Biol Rev 74(2):250–272
Braun T, Mark L, Ohmacht R, Sharma U (2007) Olive oil as a biocompatible solvent for pristine C60. Fuller Nanotub Carbon Nanostruct 15:311–314
Sharma S, Sharma U (2013) Synthesis, characterization and determination of encapsulation efficiency of chitosan nanoparticles for terbinafine. Indo Am J Pharm Res 3(12):1564
Zhang X, Guo Q, Cui D (2009) Recent advances in nanotechnology applied to biosensors. Sensors 9:1033–1053
Seleci M, Seleci DA, Joncyzk R, Stahl F, Blume C (2016) Smart multifunctional nanoparticles in nanomedicine. BioNanoMaterials 17(1–2):33–41
Corma A, Serna P (2006) Chemoselective hydrogenation of nitro compounds with supported gold catalysts. Science 313:332–334
Xiang Y, Meng Q, Li X, Wang J (2010) In situ hydrogen from aqueous-methanol for nitroarene reduction and imine formation over an Au–Pd/Al2O3 catalyst. J Chem Commun 46:5918–5920
Huang J, Yu L, He L, Liu Y-M, Cao Y, Fan K-N (2011) Direct imine formation by oxidative coupling of alcohols and amines using supported manganese oxides under an air atmosphere. Green Chem 13:2672–2677
Layek K, Kantam ML, Shirai M, Nishio-Hamane D, Sasaki T, Maheswaran H (2012) Gold nanoparticles stabilized on nanocrystalline magnesium oxide as an active catalyst for reduction of nitroarenes in aqueous medium at room temperature. Green Chem 14:3164–3174
Lin X, Wu M, Wu D, Kuga S, Endoe T, Huang Y (2011) Platinum nanoparticles using wood nanomaterials: eco-friendly synthesis, shape control and catalytic activity for p-nitrophenol reduction. Green Chem 13:283–287
Luo P, Xu K, Zhang R, Huang L, Wang J, Xing W, Huang J (2012) Highly efficient and selective reduction of nitroarenes with hydrazine over supported rhodium nanoparticles. J Catal Sci Technol 2:301–304
Fan G, Huang W, Wang C (2013) In situ synthesis of Ru/RGO nanocomposites as a highly efficient catalyst for selective hydrogenation of halonitroaromatics. Nanoscale 5:6819–6825
Cai S, Duan H, Rong H, Wang D, Li L, He W, Li Y (2013) Highly active and selective catalysis of bimetallic Rh3Ni1 nanoparticles in the hydrogenation of nitroarenes. ACS Catal 3:608–612
Zhang N, Xu Y (2013) Aggregation- and leaching-resistant, reusable, and multifunctional Pd@CeO2 as a robust nanocatalyst achieved by a hollow core-shell strategy. J Chem Mater 25:1979–1988
Chen Z, Liu S, Yang M-Q, Xu Y (2013) Aggregation- and leaching-resistant, reusable, and multifunctional Pd@CeO2 as a robust nanocatalyst achieved by a hollow core–shell strategy. J ACS Appl Mater Interfaces 1:1258–1266
Westerhaus FA, Jagadeesh RV, Wienhofer G, Pohl M-M, Radnik J, Surkus A-E, Rabeah J, Junge K, Junge H, Nielsen M, Bruckner A, Beller M (2013) Synthesis of uniform cds nanospheres/graphene hybrid nanocomposites and their application as visible light photocatalyst for selective reduction of nitro organics in water. Nat Chem 5:537–543
Vernekar AA, Patil S, Bhat C, Tilve SG (2013) Heterogenized cobalt oxide catalysts for nitroarene reduction by pyrolysis of molecularly defined complexes. RSC Adv 3:13243–13250
Saha A, Ranu B (2008) Magnetically recoverable catalytic Co–Co2B nanocomposites for the chemoselective reduction of aromatic nitro compounds. J Org Chem 73:6867–6870
Kadam HK, Tilve SG (2012) Convenient reduction of nitrobenzenes to anilines using electrochemically generated nickel. RSC Adv 2:6057–6060
Cantillo D, Baghbanzadeh M, Kappe CO (2012) Copper (II) bromide as a procatalyst for in situ preparation of active Cu nanoparticles for reductionof nitroarenes. Angew Chem Int Ed 51:10190–10193
Rezaei SJ, Malekzadeh AM, Ramazani A, Khorramabadi H (2018) Chemo-selective reduction of nitro and nitrile compounds using Ni nanoparticles immobilized on hyperbranched polymer-functionalized magnetic nanoparticles. Appl Organomet Chem 32:3975–3987
Moganavally P, Suresh R, Deepa M (2015) Synthesis and characterization of bimetallic CuNi, CuNi2, CuNi3 nanoparticles. Int J ChemTech Res 8(5):109–112
Rai RK, Mahata A, Gupta S, Nguyen KT, Zhao Y, Pathak B, Singh SK (2014) Room temperature chemoselective reduction of nitro groups using non-noble metal nanocatalysts in water. Inorg Chem 53:2904–2909
Saravanan P, Jose TA, Thomas PJ, Kulkarni GU (2001) Submicron particles of Co, Ni and Co–Ni alloys. Bull Mater Sci 24(5):515–521
Rang HP, Dale MM (1999) Pharmacology, 2nd edn. Churchill Livingstone, Edinburg
Begum R, Rehan R, Farooqi ZH, Butt Z, Ashraf S (2016) Physical chemistry of catalytic reduction of nitroarenes using various nanocatalytic systems: past, present and future. J Nanopart Res 18:231–255
Acknowledgements
We would like to thank the Centre-director, Dr. V. Ganeshan, UGC-DAE, Indore for allowing us to utilise available national facilities, Dr. M. Gupta, Dr. D. M. Phase and Dr. R. Venkatesh for providing XRD facilities, FESEM with EDX analysis respectively. Thanks to Prof. and Head, School of Studies in Chemistry and Biochemistry, Vikram University, Ujjain for providing research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sanyal, M., Sharma, U. Selective reduction of nitro group using CuNi bimetallic nanoparticles. SN Appl. Sci. 1, 353 (2019). https://doi.org/10.1007/s42452-019-0318-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0318-6