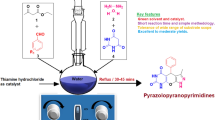
Abstract
Biginelli reaction is the most well-known and widely studied multicomponent reaction used for the direct synthesis of many biologically active 3,4-dihydropyrimidinones or thiones and their derivatives by reacting a β-keto ester/1,3-dicarbonyl compound, an aldehyde, and urea/thiourea. A new easily recoverable solid catalyst, Nafion-Ga (Gallium Nafionate, Ga(III) salt of Nafion-H, a solid polymeric perfluoroalkanesulfonic acid) was prepared from Nafion-K by metal exchange. Nafion-Ga is found to be an efficient and environmentally benign catalyst for the Biginelli reaction. A series of 3,4-dihydropyrimidinones and thiones were conveniently prepared by this green protocol using the catalyst under solvent free conditions. The wide scope of the catalyst for many other acid catalyzed organic transformations can be ascertained by further screening studies.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, contributions by Trost (atom economy principle) [1], Sheldon (E Factor) [2], Warner and Anastas (principles in green chemistry) [3], Olah and coworkers (safer acid catalyst systems) [4–44], and many others have raised awareness for the safe, environmentally benign and sustainable practice of chemistry. Synthetic procedures following the principles of green chemistry can deliver higher efficacy and reduce the environmental pollution during chemical synthesis. For example, by conducting reactions in water under ambient reaction conditions, both the use of organic solvents and energy input are minimized. As a result of many significant developments in genomics and proteomics, biocatalysis is emerging as one of the greenest technologies. Use of more sustainable chemistry encourages scientists to develop and adopt more benign synthetic methods in organic synthesis. Therefore, success of the green chemistry initiatives will reduce adverse environmental impacts, improve human safety and reduce the required time to reach the important goals to satisfy the needs of mankind. During our search for new “green” acid catalysts in organic synthesis to alleviate various problems associated with toxicity, hazardness and handling of acid catalysts such as HF and BF3, we used their aqueous as well as amine complexes for many synthetic organic transformations [4–18]. We found that amine supported HF complexes can act as efficient reservoir for HF in large amounts with significant reduction in its volatility making it a very convenient modification for various applications. HF immobilized on polymeric amines such as poly(4-vinylpyridine) was used as safer solid HF equivalents for various reactions such as isobutylene alkylation, hydrofluorination of alkenes, fluorination of alcohols [4–8] etc. Similarly, we used BF3–H2O as solvent as well as strong acid catalyst in many reactions including Friedel–Crafts hydroxyalkylation, Fries rearrangement and halogenation of deactivated aromatics so that their adverse effect on environment can be minimized [9–18].
Over the years, we have carried out extensive studies using perfluorinated resinsulfonic acid Nafion-H (1), a protic recyclable solid Brønsted acid prepared from Nafion-K developed by Du Pont [19–25]. Recent studies on gallium triflate proved it to be an aqueous-stable strong Lewis acid catalyst with unique stability and characteristic catalytic activity [26–44]. After studying the catalytic properties of both of these systems, anticipating that a suitable chemical modification of these systems can provide very efficient Lewis acid system, we prepared a series of Nafion-metal systems by metal exchange from Nafion-K (2). Recently, we have shown that Nafion-Ru is an effective catalyst for selective hydration of aryl nitriles to the corresponding amides [45] and Nafion-Fe has noticeably high catalytic efficacy in some ketonic Strecker reactions [46]. Many metal catalyst systems doped with Nafion have also been studied recently [47]. This encouraged us to prepare a series of Nafion-metal salts by similar cation exchange and their catalytic properties as heterogeneous catalysts are under investigation. We have found that the gallium salt, Nafion-gallium (Nafion-Ga, 3), is a highly efficient, aqeous stable and recyclable solid catalyst thermally stable up to 200 °C (Fig. 1).
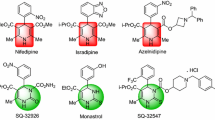
The development of synthetic methodologies and characterization of many molecular scaffolds that can function as potential targets for suitable drug design have been the major focus of synthetic chemists. Multi-component reactions are considered as important classes of reactions in organic synthesis contributing to this effort. Many 1,4-dihydropyridines (Hantzsch esters) [48, 49] are well known first or second generation cardiovasular agents that include nifedipine 4, nicardipine 5, felodipine 6 (Fig. 2) etc. They are shown to be very effective CCBs (calcium channel blockers) for lowering blood pressure, treatment of arrhythmias or angina [50, 51]. Their effective mode of action has been explained by proposing a suitable receptor bound conformation [52]. Molecular scaffolds with closely related structures such as 1,4-dihydropyrimidines were also later studied for similar potential therapeutic applications but the results were not very promising [53]. However, 3,4-dihydropyrimidinoid scaffolds were found to show not only calcium channel antagonistic properties, but many additional biological activities as well. Antihypertensive agents such as SQ 32926 (7), SQ 32547 (8) and Monastrol (9) are few examples (Fig. 2) showing vivid structure-actvity relationships (SARs) [54].
Biginelli reaction [55–58] is the most well-known and widely studied multicomponent reaction used for the direct synthesis of biologically active 3,4-dihydropyrimidinones (DHPMs) and their derivatives. Originally, Biginelli reaction was carried out using HCl as an acid catalyst, which resulted in low yields and long reaction times. Other catalytic methods include use of ionic liquids and complexes [59–61], trifluoroethanol [62], microwave irradiation [63], and Lewis acid catalysts such as BiCl3, BF3·OEt2–CuCl/H+ and InCl3 [64]. Recently, highly efficient enantioselective organocatalytic Biginelli reaction has also been reported [65–68]. When we explored the efficacy of Nafion-Ga in Biginelli reaction, the results obtained clearly manifested the characteristic efficacy and the green nature of this catalyst. Since classical methods require conventional acid catalysts, which are less efficient and commonly environmentally detrimental, Nafion-gallium as a solid, recoverable, and potentially reusable catalyst, plays elegant role in increasing efficacy in transformations requiring acid catalysis, while minimizing chemical wastes.
2 Results and Discussion
Utility of Nafion-H and Nafion SAC 13 (Nafion H-silica nanocomposite with enhanced surface area) [69] as green catalysts due to their profound catalytic efficacy, recyclability, safe and mild reaction conditions has been well studied. Nafion-gallium was prepared using metal exchange between powdered Nafion-K (potassium salt of Nafion-H, particle size <500 μm) and gallium chloride in CH2Cl2 under reflux (Scheme 1). After cooling, the solid residue was filtered, washed with acetone and distilled water several times till the filtrate became Cl free and dried under vacuum using a P2O5 trap for several hours. Elemental analysis showed effective incorporation of gallium though trace amounts of chloride and potassium were found. Since it is known that similar Nafion systems have trace amounts of free acidic sites [19], presence of a few -OH sites cannot be completely ruled out. Due to the presence of high numbers of F atom, the analysis of oxygen atom could not be carried out accurately. SEM studies show that the surface morphologies of Nafion-K and Nafion-Ga are quite comparable, Fig. 3.
As our study was focused towards more useful sustainable and safer Biginelli process, we decided to avoid the use of solvents and noxious liquid acid systems. Though we require solvents for the complete extraction and purifcation process of the product from the reaction mixture, considerable amount of solvent can be avoided in the reaction step by conducting the reactions with the neat mixture of all three reacting components. Nafion-Ga, the solid perfluororesin sulfonate of Ga can be considered as a higher polymeric analog of Ga(III) triflate [26–46].
Using Nafion-Ga, we carried out the Biginelli reaction with a series of benzaldehyde derivatives, urea/thiourea, and 1,3-dicarbonyl compounds such as ethyl/methyl acetoacetate and pentan-2,4-dione. Aromatic aldehydes containing both electron-donating as well as electron-withdrawing substituents displayed good reactivity resulting in the formation of the corresponding 2,4-dihydropyrimidines in good yields and sterically hindered β-keto esters also reacted without noticeably affecting the yields (Scheme 2).
A mixture of aldehyde 10, 1,3-dicarbonyl compound 11 and urea/thiourea 12 was taken in a pressure tube fitted with a magnetic stir bar and the catalyst Nafion-Ga was added. Depending on the substrates, the mixture was stirred at 120 °C for 1–3 h. With solid substrates with high melting points, the reaction was conducted in a small amount of CHCl3 (see the Section 3, Experimental). In all the cases, the products were obtained in good to excellent yields (63–98 %). Tables 1 and 2 show the results from the Biginelli reaction using Nafion-gallium as the catalyst.


In order to study the recyclability of catalyst, the reaction using p-chlorobenzaldehyde, urea and ethyl acetoacetate (Table 1, entry b) was repeated using the same catalyst. The recovered catalyst was used for further four susequent runs (Scheme 3). Even after the 5th run, the yield was found to be 82 %. After each reaction, the catalyst was washed with dichloromethane and dried for use for the next run.
3 Experimental
3.1 General
Unless otherwise mentioned, all chemicals were purchased from commercial sources. 1H, 13C and 19F NMR spectra were recorded on Varian 500 MHz or 400 MHz NMR spectrometers. 1H NMR chemical shifts were determined relative to the signal of the residual protonated solvent, CDCl3 (δ 7.26) and/or DMSO-d 6 (δ 2.50). 13C NMR chemical shifts were determined relative to the 13C signal of solvent, CDCl3 at δ 77.0 or DMSO-d 6 (δ 39.52). 19F NMR chemical shifts were determined relative to CFCl3 as an internal standard at δ 0.0. Mass spectral data were recorded on Trace GC-DSQ or Brucker GC–MS spectrometers with a mass selective detector at 70 eV.
3.2 General Procedure for the Preparation of Dihydropyrimidinones and Thiopyrimidinones (13a–p) by Biginelli Reaction
Aldehyde 10 (1 mmol), 1,3-dicarbonyl compound 11(1 mmol), urea/thiourea 12 (1.5 mmol) were added to Nafion-Ga (0.2 g) into a pressure tube fitted with a magnetic stir bar. The mixture was stirred at 120 °C for 1 h. With liquid components and components with lower melting points, the reactions were conducted in the absence of a solvent. Tables 1 and 2 show the results for the Biginelli reaction using Nafion-Ga as the catalyst. For entries a–h in Table 1 and k–m in Table 2, the reaction mixture was stirred under neat conditions for 1 h at 120 °C. For entries i, j in Table 1 and n–p in Table 2, 2 mL CHCl3 was added into the pressure tube in order to facilitate the stirring. An increased reaction time of 3 h was also required in these cases because of the subsequent dilution of the system. In the case of reactions with urea, the reaction mixture was cooled (after completion of the reaction), 5 mL of hot ethanol was added into the resulting mixture and the solution was filtered to remove the catalyst. The residue was washed with 1:1 mixture of ethyl acetate and CH2Cl2 till the product was completely transferred into the filtrate (~20 mL). The filtrate was washed with water, dried (anhydrous Na2SO4) and the solvent was removed under reduced pressure.
It has been found that products from thiourea had increased solubility as compared to products from urea in organic solvents. After the reaction, the residue was dissolved in CH2Cl2 with minimal ethyl acetate and filtered to remove the catalyst. The filtrate was washed with water to remove excess thiourea, dried (anhydrous Na2SO4), and the solvent was removed under reduced pressure. The residue was triturated with hexane and recrystallized from ethylacetate-CH2Cl2 (1:1) to get the pure product. All products were fully characterized by spectral analysis (NMR and GC–MS) and the spectral data were compared with those of the authentic samples [59, 65, 70–75].
3.3 Spectroscopic Data
3.3.1 Ethyl 6-methyl-2-oxo-4-phenyl-1,2,3,4-tetrahydropyrimidine-5-carboxylate (13a)
White solid. 1H NMR (500 MHz, DMSO-d6) δ 9.19 (brs, 1H, NH), 7.73 (brs, 1H, NH), 7.32 (m, 2H), 7.24 (m, 3H), 5.14 (d, J = 3.5 Hz, 1H), 3.98 (q, J = 7.1 Hz, 2H), 2.25 (s, 3H), 1.08 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.35, 152.17, 148.39, 144.88, 128.41, 127.28, 126.27, 99.26, 59.21, 53.98, 17.81, 14.10. MS (EI, m/z): 260.76 (M+); 231.60; 183.44; 155.50.
3.3.2 Ethyl 4-(4-chlorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (13b)
Yellow solid. 1H NMR (400 MHz, CDCl3) δ 8.68 (brs, 1H), 7.18 (m, 4H), 6.74 (brs, 1H), 5.24 (brs, 1H), 3.97 (q, J = 7.0 Hz, 2H), 2.25 (s, 3H), 1.08 (t, J = 7.1 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 165.16, 152.50, 147.22, 142.54, 132.50, 127.94, 127.59, 99.61, 59.21, 54.06, 17.89, 13.63. MS (EI, m/z): 294.83 (M+); 265.65; 221.55; 183.55.
3.3.3 Ethyl 4-(4-bromophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (13c)
Yellow solid. 1H NMR (500 MHz, CDCl3) δ 8.49 (brs, 1H), 7.37 (m, 2H), 7.15 (m, 2H), 6.39 (brs, 1H), 5.28 (d, J = 2.9 Hz, 1H), 4.02 (q, J = 7.1 Hz, 2H), 2.28 (s, 3H), 1.12 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 165.69, 153.21, 147.38, 143.31, 131.69, 128.52, 121.59, 100.56, 60.03, 54.98, 18.65, 14.26. MS (EI, m/z): 338.82 (M+); 309.78; 265.69; 183.59.
3.3.4 Ethyl 6-methyl-4-(4-nitrophenyl)-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (13d)
Pale yellow solid. 1H NMR (500 MHz, CDCl3) δ 8.95 (s, 1H, NH), 8.06 (d, J = 8.8 Hz, 2H), 7.45 (d, J = 8.8 Hz, 2H), 7.38 (s, 1H, NH), 5.33 (d, J = 3.4 Hz, 1H), 3.97 (q, J = 7.1 Hz, 2H), 2.26 (s, 3H), 1.09 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 164.98, 152.06, 151.40, 148.62, 146.43, 127.14, 123.03, 98.40, 59.18, 53.82, 17.85, 13.66. MS (EI, m/z): 305.29 (M+), 276.22; 260.2; 183.19.
3.3.5 Ethyl 4-(4-cyanophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (13e)
Yellow solid. 1H NMR (400 MHz, CDCl3) δ 8.35 (s, 1H, NH), 7.59 (d, J = 8.1 Hz, 2H), 7.41 (d, J = 8.1 Hz, 2H), 6.09 (s, 1H, NH), 5.43 (brs, 1H), 4.06 (q, J = 7.4 Hz, 2H), 2.31 (s, 3H), 1.14 (t, J = 7.4 Hz, 3H). 13C NMR (100 MHz, CDCl3) δ 165.43, 153.49, 148.73, 147.33, 132.84, 127.61, 118.73, 112.07, 100.68, 60.58, 55.51, 18.98, 14.39. MS (EI, m/z): 285.3 (M+), 256.2, 212.1, 183.1.
3.3.6 Ethyl 4-(4-methoxyphenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (13f)
Yellow solid. 1H NMR (500 MHz, CDCl3) δ 8.01 (brs, 1H, NH), 7.23 (d, J = 8.7 Hz, 2H), 6.83 (d, J = 8.7 Hz, 2H), 5.66 (brs, 1H, NH), 5.35 (d, J = 2.8 Hz, 1H), 4.07 (q, J = 7.1, 2H), 3.78 (s, 3H), 2.33 (s, 3H), 1.17 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, CDCl3) δ 165.92, 159.42, 153.71, 146.24, 136.36, 128.01, 114.18, 101.78, 60.18, 55.46, 55.34, 18.81, 14.39. MS (EI, m/z): 290.87 (M+); 261.64; 217.59; 183.54; 155.39.
3.3.7 Ethyl 4-(3-bromophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (13g)
Pale yellow solid. 1H NMR (400 MHz, DMSO-d6) δ 9.26 (brs, 1H, NH), 7.78 (brs, 1H, NH), 7.43 (d, J = 7.7 Hz, 1H), 7.37 (s, 1H), 7.29 (t, J = 7.7 Hz, 1H), 7.22 (d, J = 7.5 Hz, 1H), 5.12 (d, J = 2.6 Hz, 1H), 3.98 (q, J = 7.0 Hz, 2H), 2.23 (s, 3H), 1.08 (t, J = 7.0 Hz, 3H). 13C NMR (100 MHz, DMSO-d6) δ 165.58, 152.32, 149.40, 147.92, 131.25, 130.58, 129.60, 125.70, 121.95, 99.01, 59.74, 54.02, 18.27, 14.49. MS (EI, m/z): 339.1 (M+); 309.1; 294.0; 265.0; 183.1.
3.3.8 Ethyl 4-(3-fluorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (13h)
White solid. 1H NMR (400 MHz, CDCl3) δ 8.33 (s, 1H), 7.20 (td, J = 8.0, 5.9 Hz, 1H), 7.04 (m, 1H), 6.96 (m, 1H), 6.87 (m, 1H), 6.23 (s, 1H), 5.30 (d, J = 3.0 Hz, 1H), 4.01 (q, J = 7.1 Hz, 2H), 2.28 (s, 3H), 1.10 (t, J = 7.1 Hz, 3H).13C NMR (100 MHz, CDCl3) δ 165.72, 162.95 (d, 1 J CF = 246.3 Hz), 153.01, 147.5, 146.72 (d, 3 J CF = 6.1 Hz), 130.21 (d, 3 J CF = 8.1 Hz), 122.34 (d, 4 J CF = 2.8 Hz), 114.70 (d, 2 J CF = 21.2 Hz), 113.71 (d, 2 J CF = 21.8 Hz), 100.53, 60.07, 55.17 (d, 4 J CF = 1.7 Hz), 18.73, 14.27. 19F NMR (376 MHz, CDCl3) δ -113.52. MS (EI, m/z): 278.86 (M+); 249.77; 232.73; 183.61; 155.51.
3.3.9 Methyl 4-(4-chlorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (13i)
Pale yellow solid. 1H NMR (500 MHz, DMSO-d6) δ 9.11 (brs, 1H, NH), 7.51 (brs, 1H, NH), 7.26 (m, 4H), 5.26 (d, J = 3.3 Hz 1H), 3.59 (s, 3H), 2.31 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 165.65, 152.46, 148.13, 142.95, 132.18, 128.28, 128.03, 98.98, 53.57, 50.38, 18.19. MS (EI, m/z): 280.1 (M +); 265.1; 221.1; 169.0.
3.3.10 t-Butyl 4-(4-chlorophenyl)-6-methyl-2-oxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (13j)
White solid. 1H NMR (500 MHz, DMSO-d6) δ 9.17 (brs, 1H, NH), 7.74 (brs, 1H, NH), 7.46 (m, 2H), 7.29 (m, 2H), 5.14 (d, J = 3.2 Hz, 1H), 2.28 (s, 3H), 1.35 (s, 9H).13C NMR (126 MHz, DMSO-d6) 13C NMR (101 MHz, DMSO-d6) δ 164.64, 151.88, 147.74, 143.88, 131.67, 128.30, 128.17, 100.03, 79.27, 53.74, 27.83, 17.68. MS (EI, m/z):322.2 (M+), 265.1, 231.2, 155.1.
3.3.11 5-Acetyl-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-one (13k)
Yellow solid. 1H NMR (500 MHz, DMSO-d6) δ 9.17 (s, 1H, NH), 7.81 (s, 1H. NH), 7.32 (m, 2H), 7.24 (m, 3H), 5.25 (d, J = 3.5 Hz, 1H), 2.29 (s, 3H), 2.10 (s, 3H).13C NMR (126 MHz, DMSO-d6) δ 194.68, 152.56, 148.60, 144.68, 128.96, 127.78, 126.86, 109.99, 54.21, 30.79, 19.37. MS (EI, m/z): 229.1 (M+); 215.1; 187.1; 153.1.
3.3.12 5-Acetyl-4-(4-chlorophenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (13l)
White solid. 1H NMR (500 MHz, DMSO-d6) δ 9.22 (brs, 1H, NH), 7.85 (brs, 1H, NH), 7.38 (m, 2H), 7.26 (m, 2H), 5.26 (d, J = 3.5 Hz, 1H), 2.29 (s, 3H), 2.12 (s, 3H). 13C NMR (126 MHz, DMSO-d6) δ 194.07, 152.02, 148.43, 143.18, 131.80, 128.44, 128.28, 109.48, 53.05, 30.40, 18.95 MS (EI, m/z):263.1; 249.1; 221.1; 153.1.
3.3.13 5-Acetyl-4-(4-bromophenyl)-6-methyl-3,4-dihydropyrimidin-2(1H)-one (13m)
Yellow solid.1H NMR (500 MHz, DMSO-d6) δ 9.22 (brs, 1H, NH), 7.85 (brs, 1H, NH), 7.52 (m, 2H), 7.19 (m, 2H), 5.24 (d, J = 3.5 Hz, 1H), 2.28 (s, 3H), 2.12 (s, 3H).13C NMR (126 MHz, DMSO-d6) δ 194.06, 152.01, 148.44, 143.59, 131.36, 128.64, 120.34, 109.44, 53.11, 30.41, 18.96. MS (EI, m/z): 309.96 (M+); 265.76; 153.54.
3.3.14 Ethyl 6-methyl-4-phenyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (13n)
Pale yellow solid. 1H NMR (500 MHz, DMSO-d6) δ 10.42 (s, 1H, NH), 9.74 (brs, 1H, NH), 7.44 (m, 2H), 7.37 (m, 1H), 7.32 (m, 2H), 5.27 (d, J = 3.7 Hz, 1H), 4.11 (q, J = 7.1 Hz, 2H), 2.39 (s, 3H), 1.19 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 174.22, 165.10, 145.00, 143.48, 128.53, 127.65, 126.36, 100.70, 59.56, 54.03, 17.15, 13.99. MS (EI, m/z): 276.69 (M+); 247.61; 199.52; 171.42; 153.43.
3.3.15 Ethyl 4-(4-chlorophenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate (13o)
Yellow solid. 1H NMR (500 MHz, DMSO-d6) δ 10.38 (s, 1H), 9.66 (s, J = 1.7 Hz, 1H), 7.42 (d, J = 8.5 Hz, 2H), 7.22 (d, J = 8.5 Hz, 2H), 5.17 (d, J = 3.7 Hz, 1H), 4.00 (q, J = 7.1, 2H), 2.29 (s, 3H), 1.10 (t, J = 7.1 Hz, 3H). 13C NMR (126 MHz, DMSO-d6) δ 174.22, 164.95, 145.34, 142.35, 132.23, 128.56, 128.28, 100.27, 59.63, 53.42, 17.16, 13.98. MS (EI, m/z): 310.97 (M+); 281.88; 237.76; 199.67; 171.58; 153.60.
3.3.16 Methyl 4-(4-chlorophenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxyl-ate (13p)
Yellow solid. 1H NMR (500 MHz, DMSO-d6) δ 10.40 (s, 1H, NH), 9.68 (brs, 1H, NH), 7.41 (d, J = 9.1 Hz, 2H), 7.23 (d, J = 9.1 Hz, 2H), 5.18 (d, J = 3.7 Hz, 1H), 3.55 (s, 3H), 2.30 (s, 3H). 13C NMR (126 MHz, DMSO-D6-d6) δ 174.30, 165.49, 145.62, 142.18, 132.31, 131.14, 129.34, 128.62, 128.24, 100.05, 53.33, 51.12, 17.24. MS (EI, m/z): 296.79 (M+);
4 Conclusion
Nafion-Ga, a new efficient heterogeneous catalyst system for organic synthetic transformations was developed. The new catalyst is environmentally safe and easily applicable. Biginelli reaction, one of the most important multicomponent reactions, was successfully carried out using the new catalyst system with high efficacy. Majority of the reactions were performed under solvent free conditions or using minimal solvent (for effective stirring). Reactions of aromatic aldehydes with the 1,3-dicarbonyl compounds (methyl/ethyl acetoacetate or pentan-2,4-dione) and urea or thiourea gave the correcsponding functionalized 3,4-dihydropyrimidin-2(1H)-one or thione products (DHPMS) in good to excellent yields. Simple reaction conditions, clean reaction, easy purification and high yields of the products are some of the salient features of this method.
References
Trost B (1991) Science 254:1471
Sheldon RA (1992) Chem Ind 23:903
Anastas PT, Warner JC (1998) Green chemistry theory and practice. Oxford University Press, Oxford
Olah GA, Nojima M, Kerekes I (1973) Synthesis 779
Olah GA, Nojima M, Kerekes I (1973) Synthesis 780
Olah GA, Nojima M, Kerekes I (1975) J Am Chem Soc 97:208
Olah GA, Welch JT, Vankar YD, Nojima M, Kerekes I, Olah JA (1979) J Org Chem 44: 3872 and references cited there in
Olah GA (1991) US Patent 5073674
Olah GA, Wang Q, Li X-Y, Bucci I (1992) Synthesis 1085
Olah GA, Wang Q, Trivedi N, Prakash GKS (1992) Synthesis 465
Olah GA, Wang Q, Li X-Y, Prakash GKS (1993) Synlett 32
Olah GA, Wang Q, Li X-Y, Prakash GKS (1993) Synthesis 207
Prakash GKS, Mathew T, Hoole D, Esteves PM, Wang Q, Rasul G, Olah GA (2004) J Am Chem Soc 126:15770
Prakash GKS, Panja C, Mathew T, Olah GA (2007) Catal Lett 114:24
Prakash GKS, Paknia F, Chacko S, Mathew T, Olah GA (2008) Heterocycles 76:783
Prakash GKS, Paknia F, Narayanan A, Rasul G, Mathew T, Olah GA (2012) J Fluorine Chem 143:292
Prakash GKS, Paknia F, Vaghoo H, Rasul G, Mathew T, Olah GA (2010) J Org Chem 75:2219
Prakash GKS, Paknia F, Mathew T, Mloston G, Joschek JP, Olah GA (2011) Org Lett 13:4128
Olah GA, Iyer PS, Prakash GKS (1986) Synthesis 513 and references cited therein
Prakash GKS, Mathew T, Krishnaraj S, Marinez ER, Olah GA (1999) Appl Catal A 181:283
Yamato T, Hideshima C, Prakash GKS, Olah GA (1991) J Org Chem 56:2089l
Olah GA, Yamato T, Iyer PS, Prakash GKS (1986) J Org Chem 51:2826
Olah GA, Mathew T, Farnia M, Prakash GKS (1999) Synlett 1067
Olah GA, Mathew T, Prakash GKS (2001) Chem Commun 1696
Prakash GKS, Mathew T, Mandal M, Farnia M, Olah GA (2004) Arkivoc 103
Olah GA, Farooq O, Farnia SMF, Olah JA (1988) J Am Chem Soc 110:2560
Olah GA, Farooq O, Cheng LX, Farnia MAF, Aklonis JJ (1992) J Appl Polym Sci 45:1355
Prakash GKS, Yan P, Török B, Bucsi I, Tanaka M, Olah GA (2003) Catal Lett 85:1
Prakash GKS, Prakash GKS, Yan P, Török B, Olah GA (2003) Synlett 527
Prakash GKS, Yan P, Török B, Olah GA (2003) Catal Lett 87:109
Cui D, Kawamura M, Shimada S, Hayashi T, Tanaka M (2003) Tetrahedron Lett 44:4007
Yan P, Batamack P, Prakash GKS, Olah GA (2005) Catal Lett 101:141
Yan P, Batamack P, Prakash GKS, Olah GA (2005) Catal Lett 103:165
Deng X-M, Sun X-L, Tang Y (2005) J Org Chem 70:6537
Lacey JR, Anzalone PW, Duncan CM, Hackert MJ, Mohan RS (2005) Tetrahedron Lett 46:8507
Nguyen R-V, Li C (2005) J Am Chem Soc 127:17184
Prakash GKS, Mathew T, Panja C, Vaghoo H, Venkataraman K, Olah GA (2007) Org Lett 9:179
Prakash GKS, Mathew T, Panja C, Alconcel S, Vaghoo H, Olah GA (2007) Proc Natl Acad Sci USA 104:3703
Prakash GKS, Vaghoo H, Venkat A, Panja C, Chacko S, Mathew T, Olah GA (2009) Future Med Chem 1:909
Han X, Wu J (2010) Org Lett 12:5780
Prakash GKS, Do C, Mathew T, Olah GA (2010) Catal Lett 137:111
Prakash GKS, Do C, Mathew T, Olah GA (2011) Catal Lett 141:507
Prakash GKS, Mathew T, Olah GA (2012) Acc Chem Res 45:565
Han X, Li H, Hughes RP, Wu J (2012) Angew Chem Int Ed Engl 51:10390
Prakash GKS, Munoz SB, Papp A, Masood K, Bychinskaya I, Mathew T, Olah GA (2012) Asian J Org Chem 1:146
Prakash GKS, Bychinskaya I, Marinez ER, Mathew T, Olah GA (2013) Catal Lett 143:303
Leavy H, Anvir D (2005) Adv Funct Mater 15:1141
Hantzsch A (1881) Chem Ber 14:1637
Hantzsch A (1882) Just Leib Ann Chem 215:1
Singh K, Arora D, Singh K, Sing S (2009) Mini-Rev Med Chem 9:95
Triggle DJ, Janis RA (1987) Ann Rev Pharmacol Toxicol 27:347
Rovnyak GC, Kimball SD, Baeyer B, Cuicinotta G, Dimarco JD, Gougoutas J, Hedberg A, Malley M, McCarthy JP, Zhang R, Moreland S (1995) JMedChem 38:119
Cho H, Ueda M, Shima K, Mizuno A, Hayashimatsu M, Ohnaka Y, Takeuchi Y, Hamaguchi M, Aisaka K, Hidaka T, Kawai M, Takeda M, Ishihara T, Funahashi K, Satoh F, Morita M, Noguchi T (1989) J Med Chem 32:2399
Grover GJ, Dzwonczyk S, McMullen DM, Normansdin DE, Parham CS, Selph PG, Moreland S (1995) J Cardiovasc Pharmacol 26(2):289
Biginelli P (1893) Gazz Chim Ital 23:360
Biginelli P (1891) Chem Ber 24:2962
Biginelli P (1891) Chem Ber 24:1317
Biginelli P (1889) Gazz Chim Ital 19:212
Ramos LM, Guido BC, Nobrega CC, José RC, Silva RG, de Oliveira HCB, Gomes AF, Gozzo FC, Neto BAD (2013) Chem Eur J 19:4156
Peng J, Deng Y (2001) Tetrahedron Lett 42:5917
Karthikeyan P, Aswar SA, Muskawar PN, Bhagat PM, Kumar SS (2013) J Organomet Chem 723:154
Rashmi SV, Sandhya NC, Raghava B, Kumara MN, Mantelingu K, Rangappa KS (2012) Synth Commun 42:424
Kappe CO, Kuman D, Varma RS (1999) Synthesis 1799
Ramalinga K, Vijayalakshmi P, Kaimal TNB, Ramalinga L (2001) Synlett 863
Saha S, Moorthy JN (2011) J Org Chem 76:396
Xin J, Chang L, Hou Z, Shang D, Liu X, Feng X (2008) Chem Eur J 14:3177
Wu Y-Y, Chai Z, Liu X-Y, Zhao G, Wang S-W (2009) Eur J Org Chem 904
Wang Y, Yang H, Yu J, Miao Z, Chen R (2009) Adv Synth Catal 351:3057
Harmer MA, Farneth WE, Sun Q (1996) J Am Chem Soc 118:7708
Verma S, Jain SL, Sain B (2010) Tetrahedron Lett 51:6897
Pasunooti KK, Chai H, Jensen CN, Gorityala BK, Wang S, Liu X-W (2011) Tetrahedron Lett 52:80
Cepenec I, Litvić M, Filipan-Litvić M, Grüngold I (2007) Tetrahedron 63:11822
Cepenec I, Litvić M, Bartolinčić A, Lovrić M (2007) Tetrahedron 63:4275
de Vasconcelos A, Oliveria PS, Ritter M, Freitag AR, Romano RL, Quina FH, Pizzuti L, Pereira CMP, Stefanello FM, Barschak AG (2012) J Biochem Mol Toxicol 26:155
Ma Y, Qian C, Wang L, Yang M (2000) J Org Chem 65:3864
Acknowledgments
Financial Support by the Loker Hydrocarbon Research Institute is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Prakash, G.K.S., Lau, H., Panja, C. et al. Synthesis of Dihydropyrimidinones/Thiopyrimidinones: Nafion-Ga, an Efficient “Green” Lewis Acid Catalyst for the Biginelli Reaction. Catal Lett 144, 2012–2020 (2014). https://doi.org/10.1007/s10562-014-1364-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-014-1364-8