Abstract
Demineralized bone matrix (DBM) has been regarded as an ideal bone substitute as a native carrier of bone morphogenetic proteins (BMPs) and other growth factors. However, the osteoinductive properties diverse in different DBM products. We speculate that the harvest origin further contributing to variability of BMPs contents in DBM products besides the process technology. In the study, the cortical bone of femur, tibia, humerus, and ulna from a signal donor were prepared and followed demineralizd into DBM products. Proteins in bone martix were extracted using guanidine-HCl and collagenase, respectively, and BMP-2 content was detected by sandwich enzyme-linked immunosorbent assay (ELISA). Variability of BMP-2 content was found in 4 different DBM products. By guanidine-HCl extraction, the average concentration in DBMs harvested from ulna, humerus, tibia, and femur were 0.613 ± 0.053, 0.848 ± 0.051, 3.293 ± 0.268, and 21.763 ± 0.344, respectively (p < 0.05), while using collagenase, the levels were 0.089 ± 0.004, 0.097 ± 0.004, 0.330 ± 0.012, and 1.562 ± 0.008, respectively (p < 0.05). In general, the content of BMP-2 in long bones of Lower limb was higher than that in long bones of upper limb, and GuHCl had remarkably superior extracted efficiency for BMP-2 compared to collagenase. The results suggest that the origin of cortical bones harvested to fabricate DBM products contribute to the variability of native BMP-2 content, while the protein extracted method only changes the measured values of BMP-2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Repair of large bone defects is one of the major therapeutic goals in orthopaedic fields, resulting from trauma, infection and resection of tumors. Iliac crest bone graft which possesses three essential elements of bone regeneration, including osteogenesis, osteoinduction, and osteoconduction, is the gold standard for bone reconstruction (Zhang et al. 2019). However, some notable drawbacks, such as harvest-related complications in donor, prolonged operative time, limited volume, and poor bone quality in certain patients, limit the utility of autograft (Loeffler et al. 2012; Campana et al. 2014). Therefore, bone substitutes have been prepared by tissue engineering technology, mainly consisting of hydroxyapatite, calcium ceramics, bioactive glasses, recombinant bone morphogenetic proteins, and combinations of these. Unfortunately, none of them is the ideal alternatives (Campana et al. 2014; Greenwald et al. 2001; Bae et al 2013; Bhamb et al. 2019).
Demineralized bone matrix (DBM) derived from cortical bone, is prepared by decalcification process. The leaving collagenous interwoven structure provides the scaffold for bone ingrowth following implantation, and the non-collagen protein, referring to bone morphogenetic proteins (BMPs) and other growth factors render the osteoinductive potential. As the native carriers of BMPs, DBM is widely used in orthopaedic and dental applications (Huber et al. 2017). Many researchers deemed the BMP in DBM as the key role to exert the osteoinduction, and attempted to investigate the collection of content of BMP in DBM and the osteogenic phenomenon both in vitro and vivo (Murray et al. 2007; Oliveira Pinho et al. 2021; Katz et al. 2009; Honsawek et al. 2005). In general, BMP contents exhibit variability in different DBM products, considering these factors: donor age and gender (Schwartz et al. 1998), particle size (Pietrzak and Ali 2015), residual calcium content (Pietrzak et al. 2011), carriers (Bal et al. 2021), sterilization (Ku et al. 2022), and storage conditions such as moisture and temperature (Han et al. 2005). Due to heterogeneity in DBM formulation, protein extraction and BMP detection methods, these results cannot be compared directly.
In a vitro study, Bae et al (2006) detect BMP-2, 4, and 7 in 9 commercially available DBM prepares using 3 different manufacturer’s production lots of each DBM formulation. They found a higher variability in concentration of BMPs among 3 different lots of the same DBM formulation than that among different DBM formulations. To further verify the intravariability in the same DBM formulation, Bae assessed the BMP in ten lots of a single DBM product. The results depicted a significant lot-to-lot variability in terms of both BMP concentrations (BMP-2 range from 22 to110 pg/mg DBM and BMP-7 range from 44 to 125 pg/mg DBM) (Bae et al. 2010). If the formulation and process of DBM are constant, then the harvest site of cortical bone used for formulating DBM is defined as a clinical variable. Thus, we hypothesize that the native BMP content varies in different long bone.
The objectives of this study were 1) to extract proteins in DBM products derived from four different human long bone and compare the quantity of BMP-2 among them, 2) to Compare the extracted efficiency for BMP-2 of the GuHCl and collagenase methods. This would provide further insight into the native BMPs with bone as well as the diverse osteoinductive properties of DBM.
Materials and methods
Preparation of DBM
Samples were obtained from cortical sections of 4 different long bones (femur, tibia, humerus, and ulna) of a signal donor. Briefly, the bone was consequently cleaned of soft tissue, washed, defatted, lyophilized and milled into particles with size ranging from 315 to 710 μm. The particles were demineralized in 0.6 N hydrochloric acid for 25 min at room temperature, then rinsed in phosphate buffer solution (pH: 5.5–7.5), followed by lyophilized. The residual calcium concentration in the DBM was 2% (percent weight [w/w]) by atomic absorption spectroscopy. The DBM samples were sorted into fDBM, tDBM, hDBM, and uDBM according to their origins.
Protein extraction
300 mg DBM samples were placed into 5 mL of 4 M guanidine-HCI (GuHCI) in 0.05 M Tris–HCl (pH 7.4) and stirred at 4 °C for 24 h, followed by centrifuged at 20000 g speed for 15 min, and the supernatant was collected. The precipitate was then resuspended in 5 mL of fresh GuHCl/Tris–HCl solution and stirred for an additional 5 h, followed by centrifugation and retention of the supernatant. The two supernatants were combined and dialyzed against 500 mL of 0.05 M Tris–HCl with a 10 KDa molecular weight cutoff membrane for 15 h at 4 °C. The Tris–HCl was then replaced with an equivalent volume of fresh buffer, and dialysis continued for an additional 10 h. The liquid in the dialysis bag was extractable protein, which was transferred to a new centrifuge tube, and measured the volume. Three independent guanidine extractions were performed for each type of specimen.
The aliquots of each sample were extracted by collagenase digestion. 300 mg particles were placed in 6 ml 20 mM Tris–HCl (pH 8.0) solution contained with 0.1 mM NaCl, 50 mM CaCl2, 50 mM MgCl2, 1 mM N-ethylmaleimide, 0.1 mM benzosulfonyl fluoride, and 0.1 mM benzamidine-HCl. Commercial collagenase was added into solution for a final concentration of 25 collagenase digestion units (CDUs)/ml, and shacked continuously overnight at 37 °C. Then tubes were centrifuged at 20000 g speed for 15 min, and the supernatant was transferred to a bag with a 10 KDa molecular weight cutoff and dialyzed into 500 ml distilled water overnight at 4 °C. The sample volume was recorded followed by BMPs analysis. Each group particle was extracted for three times.
BMPs assays
Sandwich enzyme-linked immunosorbent assay (ELISA) was used to assess the amounts of BMP with Commercially available quantikine kits purchased from MULTI SCIENCES (LIANKE) BIOTECH, CO, LTD (human BMP-2 ELISA Kit, sensitivity, 1.73 pg/ml; BMP-7 ELISA Kit, sensitivity, 2.06 pg/ml). ELISA was run in strict accordance with the instructions and in duplicate for each extraction. The concentration of BMPs was defined as the measured value by ELISA multiplied by the volume of the extraction and then divided by the mass of the DBM, and was expressed as ng/g DBM.
Statistical analysis
The data were represented as means with standard errors. The significance of differences among multiple groups was determined by one way ANOVA and a post hoc test. Student t test was used for comparing the extracted efficiency of GuHCl and collagenase methods. A p value of less than 0.05 was considered to be statistically significant. The SPSS 25.00 software was utilized.
Results
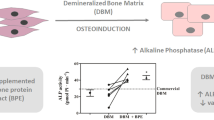
Variability of BMP-2 concentrations in 4 different DBM products was detected by ELISA method. When samples were extracted by GuHCl, the ranges spanned were considerable, from 0.6 to 22 ng of BMP-2 per gram of DBM. The average concentration (and standard deviation) in DBMs harvested from ulna, humerus, tibia, and femur were 0.613 ± 0.053, 0.848 ± 0.051, 3.293 ± 0.268, and 21.763 ± 0.344, respectively. The differences between each two groups were significant (p < 0.05). In general, the content of BMP-2 in long bones of Lower limb was higher than that in long bones of upper limb. Of note, the concentration of BMP-2 of group fDBM was highest, at a lever that was nearly 35.5fold greater than that in group uDBM.
Using collagenase extraction method, the values of BMP-2 in group uDBM, hDBM, tDBM, and fDBM were 0.089 ± 0.004, 0.097 ± 0.004, 0.330 ± 0.012, and 1.562 ± 0.008, respectively. Compared to GuHCl, the contents of BMP-2 extracted by collagenase were significantly reduced (p < 0.05). However, it should be emphasized that the trends of BMP-2 concentration either in all DBM samples or in the upper and lower limbs were stationary. The datas were showed in Table 1 and Fig. 1.
Discussion
Due to the excellent osteoinductivity, many DBM products are successfully used in clinical bone reconstruction, mainly including bone cyst, trauma, spinal and extremity, and maxillofacial surgery, and are verified safety and effective (Pacaccio and Stern 2005; Dinopoulos and Giannoudis 2006; Drosos et al. 2007, 2015). However, other in vivo studies reveal a low level of evidence concerning efficiency in critical sized defects (van Houdt et al. 2017; Alaee et al. 2014). Most possible explanation for a diverse osteoinductive capacity might be a variable amount of growth factors especially BMP-2 in the different DBM products.
Previous studies have evaluated the content of several growth factors in DBM products, such as BMP-2, BMP-4, BMP-7, vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), Insulin like growth factor (IGF), and fibroblast growth factor (FGF). Among these cytokines, BMP-2 is the hotpot as it considered by experts to be one of the most osteogenic elements. Doner gender and age, demineralized process, particle size, sterilization and storage condition, have been successively found to play pivotal role in the variability of BMP-2 in DBM formulations. However, to our knowledge, there is no direct evidence that the harvest site of cortical bone might influence the content of BMP-2 in DBM products.
In this work, we measured the content of BMP-2 in DBM products harvested from different long bones of single donor. In addition, the manufacturing process of DBM and biochemical assay methods were homogeneous. Regardless of GuHCl or collagenase extraction, the trend of BMP-2 concentration was fDBM > tDBM > hDBM > uDBM, indicating that the distribution of BMP-2 varies in different long bones, and the contents in lower limbs were higher than those in upper limbs. One possible explanation is that the content of BMP-2 is heterogeneous in different bones at embryonic stage, just like mesenchymal stem cells (MSCs). An animal experiment showed that the proliferative capacity and homing efficiency depended on the microanatomical location of stem cells (Ellis et al. 2011). Siclari et al. (2013) attempted to separately isolate endosteal bone marrow using a unique enzymatic digestion approach and to perform CFU-F assay, in his report, he found that endosteal bone marrow contained a higher concentration of mesenchymal progenitors than central bone marrow, even within the same long bone, and attributed this phenomenon to the different rates of bone remolding. Since BMP-2 can act on mesenchymal stem cells and induce differentiation into osteoblasts/osteocytes and can attract osteoclasts, key cell types that carry out bone remodeling, herein, we convinced that the contents of BMP-2 in different long bone were related to their distinct remodeling rates. Furthermore, the content of BMP-2 in long bone might change even after individual birth. An interesting founding was that the long bones of lower limbs contained more BMP-2 than the upper limbs. As we know, the long bones of the lower limbs are weight-bearing bones, while the long bones of the upper limbs are mainly connective bones, and in this sense, compared to the latter, the former has a special biomechanical environment. We speculate another possible explanation that postnatal biomechanics alter the remolding microenvironment of different long bones, resulting in the variable distributions of BMP-2 in the lower and upper limbs. Maybe this discrepancy only occurs in long bones of humans but not animals with quadruped locomotion, which need the evidence supported by basic animal experiments.
In the previous research of various growth factors in bone materials, the commonly used protein extractants mainly include guanidine hydrochloride, urea, sodium dodecyl sulfate, and collagenase. In this study, we extracted the proteins in DBM with both GuHCl and collagenase, and found that GuHCl had significantly superior extracted efficiency for BMP-2 compared to collagenase. Three possible explanations could account for this difference. First, as a protein dissociating agent, GuHCl can extracted the soluble bone morphogenetic proteins which associate with the collagen matrix through noncovalent bonds such as ionic or hydrophobic. While collagenase destroys the substrate to which the BMPs are associated, allowing their release and detection by following ELISA method (Pietrzak et al. 2006). It is possible that these methods differ in their efficiency of BMP extraction. Second, the water bath temperature maintains at 37 °C, compared to 4 °C in the GuHCl extracted process. The former is more conducive to the digestion of endogenous protease, resulting in reduction of absolute amount of BMPs. Finally, the purity of commercial collagenase maybe also a reason for the low efficiency of BMPs extraction.
The scope of BMP-2 concentration in DBM products ranges greatly, and direct comparison among the measured value in previous study is unrealistic, due to the heterogeneity of DBM samples. However, a general accepted viewpoint is that BMPs concentration is in nanogram quantities per gram of DBM, in accordance with our findings in the present study. Also of note, this work merely demonstrated the trends of BMP-2 concentration in different long bones, not the defined value, because neither GuHCl nor collagenase can extract all native content of BMP-2 in bones. That is to say, only extractable proteins can be measured and quantified. Pietrzak et al. (2012) found bovine DBM still actively eluting BMP-7 after 84 days with the amount eluted equivalent to 6 times the baseline level, indicating that BMP should be continuously released with the time of water bath. Pietrzak and Ali (2017) investigated the release of BMPs 2, 4, and 7 extracted from a clinical human DBM putty with GuHCl. The results showed that the baseline of BMP 2, 4, 7 concentrations were 28.1 ± 1.3 ng/g DBM, 0.577 ± 0.056 ng/g DBM, and 92.9 ± 7.5 ng/g DBM, and relative to baseline, the proportions released by 7 days were 11.1%, 3.9%, and 29.3%, respectively. This is strong evidence of 2 compartments in DBM differing by their affinity for BMPs (Houdt et al. 2017) and limitations of GuHCl extraction method, suggesting that published concentrations of BMPs in DBM are underestimates of the true values.
This study had some limitations. First, in order to maintain the absolute homogenization of DBM included in the study, a single donor was selected. Subject to the limitation of materials, the sample size is relatively small. Second, only BMP-2 was detected in the work, other growth factors concentrations in DBM harvested from different long bones were not be disclosed.
Conclusion
The distribution of BMP-2 in different long bones are variable. The origin of cortical bones harvested to fabricate DBM products significantly affects the BMP-2 content, and long bones of lower limbs contain greater BMP-2 concentration than that of upper limbs. Its influence on the osteoinductivity of DBM urgent to be proved by ongoing vitro and vivo studies.
References
Alaee F, Hong SH, Dukas AG, Pensak MJ, Rowe DW, Lieberman JR (2014) Evaluation of osteogenic cell differentiation in response to bone morphogenetic protein or demineralized bone matrix in a critical sized defect model using GFP reporter mice. J Orthop Res 32:1120–1128. https://doi.org/10.1002/jor.22657
Bae HW, Zhao L, Kanim LE, Wong P, Delamarter RB, Dawson EG (2006) Intervariability and intravariability of bone morphogenetic proteins in commercially available demineralized bone matrix products. Spine (Phila Pa 1976) 31:1299–1308. https://doi.org/10.1097/01.brs.0000218581.92992.b7
Bae H, Zhao L, Zhu D, Kanim LE, Wang JC, Delamarter RB (2010) Variability across ten production lots of a single demineralized bone matrix product. J Bone Jt Surg Am 92:427–435. https://doi.org/10.2106/JBJS.H.01400
Bae HW, Zhao L, Kanim LE, Wong P, Marshall D, Delamarter RB (2013) Bone marrow enhances the performance of rhBMP-2 in spinal fusion: a rodent model. J Bone Jt Surg Am 95:338–347. https://doi.org/10.2106/JBJS.K.01118
Bal Z, Korkusuz F, Ishiguro H, Okada R, Kushioka J, Chijimatsu R et al (2021) A novel nano-hydroxyapatite/synthetic polymer/bone morphogenetic protein-2 composite for efficient bone regeneration. Spine J 21:865–873. https://doi.org/10.1016/j.spinee.2021.01.019
Bhamb N, Kanim LEA, Drapeau S, Mohan S, Vasquez E, Shimko D et al (2019) Comparative efficacy of commonly available human bone graft substitutes as tested for posterolateral fusion in an athymic rat model. Int J Spine Surg 13:437–458
Campana V, Milano G, Pagano E, Barba M, Cicione C, Salonna G et al (2014) Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med 25:2445–2461. https://doi.org/10.1007/s10856-014-5240-2
Dinopoulos HT, Giannoudis PV (2006) Safety and efficacy of use of demineralised bone matrix in orthopaedic and trauma surgery. Expert Opin Drug Saf 5:847–866. https://doi.org/10.1517/14740338.5.6.847
Drosos GI, Kazakos KI, Kouzoumpasis P, Verettas DA (2007) Safety and efficacy of commercially available demineralised bone matrix preparations: a critical review of clinical studies. Injury 38(Suppl 4):13–21. https://doi.org/10.1016/s0020-1383(08)70005-6
Drosos GI, Touzopoulos P, Ververidis A, Tilkeridis K, Kazakos K (2015) Use of demineralized bone matrix in the extremities. World J Orthop 6:269–277. https://doi.org/10.5312/wjo.v6.i2.269
Ellis SL, Grassinger J, Jones A, Borg J, Camenisch T, Haylock D et al (2011) The relationship between bone, hemopoietic stem cells, and vasculature. Blood 118:1516–1524. https://doi.org/10.1182/blood-2010-08-303800
Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN (2001) Bone-graft substitutes: facts, fictions, and applications. J Bone Jt Surg Am 83:98–103. https://doi.org/10.2106/00004623-200100022-00007
Han B, Yang Z, Nimni M (2005) Effects of moisture and temperature on the osteoinductivity of demineralized bone matrix. J Orthop Res 23:855–861. https://doi.org/10.1016/j.orthres.2004.11.007
Honsawek S, Powers RM, Wolfinbarger L (2005) Extractable bone morphogenetic protein and correlation with induced new bone formation in an in vivo assay in the athymic mouse model. Cell Tissue Bank 6:13–23. https://doi.org/10.1007/s10561-005-1445-4
Huber E, Pobloth AM, Bormann N, Kolarczik N, Schmidt-Bleek K, Schell H et al (2017) Demineralized bone matrix as a carrier for bone morphogenetic protein-2: burst release combined with long-term binding and osteoinductive activity evaluated in vitro and in vivo. Tissue Eng Part 23:1321–1330. https://doi.org/10.1089/ten.TEA.2017.0005
Katz JM, Nataraj C, Jaw R, Deigl E, Bursac P (2009) Demineralized bone matrix as an osteoinductive biomaterial and in vitro predictors of its biological potential. J Biomed Mater Res B Appl Biomater 89:127–134. https://doi.org/10.1002/jbm.b.31195
Ku JK, Kim IH, Um IW, Kim BH, Yun PY (2022) Effect of gamma irradiation on the osteoinductivity of demineralized dentin matrix for allografts: a preliminary study. J Funct Biomater 13:14. https://doi.org/10.3390/jfb13010014
Loeffler BJ, Kellam JF, Sims SH, Bosse MJ (2012) Prospective observational study of donor-site morbidity following anterior iliac crest bone-grafting in orthopaedic trauma reconstruction patients. J Bone Joint Surg Am 94:1649–1654. https://doi.org/10.2106/JBJS.K.00961
Murray SS, Brochmann EJ, Harker JO, King E, Lollis RJ, Khaliq SA (2007) A statistical model to allow the phasing out of the animal testing of demineralised bone matrix products. Altern Lab Anim 35:405–409. https://doi.org/10.1177/026119290703500412
Oliveira Pinho F, Pinto Joazeiro P, Santos AR Jr (2021) Evaluation of the growth and differentiation of human fetal osteoblasts (hFOB) cells on demineralized bone matrix (DBM). Organogenesis 17:136–149. https://doi.org/10.1080/15476278.2021.2003134
Pacaccio DJ, Stern SF (2005) Demineralized bone matrix: basic science and clinical applications. Clin Podiatr Med Surg 22:599–606. https://doi.org/10.1016/j.cpm.2005.07.001
Pietrzak WS, Ali SN (2015) The extraction and measurement of bone morphogenetic protein 7 from bovine cortical bone as a function of particle size. J Craniofac Surg 26:296–299. https://doi.org/10.1097/SCS.0000000000001301
Pietrzak WS, Ali SN (2017) The elution kinetics of BMP-2, BMP-4, and BMP-7 from a commercial human demineralized bone matrix putty. J Craniofac Surg 28:2183–2188. https://doi.org/10.1097/SCS.0000000000004016
Pietrzak WS, Woodell-May J, McDonald N (2006) Assay of bone morphogenetic protein-2, -4, and -7 in human demineralized bone matrix. J Craniofac Surg 17:84–90. https://doi.org/10.1097/01.scs.0000179745.91165.73
Pietrzak WS, Ali SN, Chitturi D, Jacob M, Woodell-May JE (2011) BMP depletion occurs during prolonged acid demineralization of bone: characterization and implications for graft preparation. Cell Tissue Bank 12:81–88. https://doi.org/10.1007/s10561-009-9168-6
Pietrzak WS, Dow M, Gomez J, Soulvie M, Tsiagalis G (2012) The in vitro elution of BMP-7 from demineralized bone matrix. Cell Tissue Bank 13:653–661. https://doi.org/10.1007/s10561-011-9286-9
Schwartz Z, Somers A, Mellonig JT, Carnes DL Jr, Dean DD, Cochran DL et al (1998) Ability of commercial demineralized freeze-dried bone allograft to induce new bone formation is dependent on donor age but not gender. J Periodontol 69:470–478. https://doi.org/10.1902/jop.1998.69.4.470
Siclari VA, Zhu J, Akiyama K, Liu F, Zhang X, Chandra A et al (2013) Mesenchymal progenitors residing close to the bone surface are functionally distinct from those in the central bone marrow. Bone 53:575–586. https://doi.org/10.1016/j.bone.2012.12.013
van Houdt CIA, Cardoso DA, van Oirschot B, Ulrich DJO, Jansen JA, Leeuwenburgh SCG et al (2017) Porous titanium scaffolds with injectable hyaluronic acid-DBM gel for bone substitution in a rat critical-sized calvarial defect model. J Tissue Eng Regen Med 11:2537–2548. https://doi.org/10.1002/term.2151
Zhang H, Yang L, Yang XG, Wang F, Feng JT, Hua KC et al (2019) Demineralized bone matrix carriers and their clinical applications: an overview. Orthop Surg 11:725–737. https://doi.org/10.1111/os.12509
Acknowledgements
We thank all the participants in this study.
Funding
This study was funded entirely by the Spine Research Foundation. No funding was received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Yong-jie Zhao has received research support from Beijing Wonderful Biomaterials Company. Other authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Yj., Yin, G., Liu, B. et al. Variability of BMP-2 content in DBM products derived from different long bone. Cell Tissue Bank 25, 697–703 (2024). https://doi.org/10.1007/s10561-024-10132-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-024-10132-5