Abstract
Fibrin-platelet glue (FPG) is a blood derivative, in which platelets and fibrinogen are concentrated in a small plasma volume, by differential centrifugation and precipitation. It can form a three-dimensional and biocompatible fibrin scaffold with a myriad of growth factors and proteins that are released progressively to the local environment and contribute to the accelerated postoperative bone healing. Gelatin (Gel) is a derivative of collagen and can promote cell adhesion and proliferation due to its unique sequence of amino acids, so it is suitable for bone tissue applications. This study examined the effects of Gel, FPG and their combinations as bone scaffold on the healing of surgically created critical-size defects in rat radius. Fifty critical size defects of 5 mm long were bilaterally created in the radial diaphysis of 25 rats. The animals were randomly divided into five equal groups as empty defect, autograft, Gel, FPG and Gel–FPG groups (n = 10 in each group). Radiographs of each forelimb were taken postoperatively on the 1st day and then at the 28th and 56th days post injury to evaluate bone formation, union and remodeling of the defect. After 56 days, the rats were euthanized and their harvested healing bone samples were evaluated by histopathology, scanning electron microscopy (SEM) and biomechanical testing. The results of present study showed that the Gel alone did not significantly affect bone healing and regeneration; however, the Gel treated defects promoted healing more than those that were left untreated (negative control). Furthermore, the FPG-enhanced grafts provided a good scaffold containing numerous growth factors for proliferation of osteoinduction and was effective in improving the structural and functional properties of the newly formed bone more than that of the untreated and also the Gel treated groups. Incorporation of Gel into the FPG scaffold improved healing potential of the FPG scaffold; however, it was still inferior to the autograft (positive control). Although the Gel–FPG scaffolds had best effectiveness during bone regeneration, it still needs to be further enhanced by incorporation of the ceramic and osteoinductive biomaterials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Various bone graft substitutes including autografts, allografts, xenografts, polymers, ceramics and some metals have been employed to promote bone reunion (Oryan et al. 2016a; Parizi et al. 2012). To succeed, any substitute should be biocompatible, biodegradable, bioactive, non-toxic, non-antigenic, microporous, provide scaffolding for angiogenesis and new bone outgrowth, and could be easily handled (Anitua et al. 2006; Meimandi-Parizi et al. 2013; Oryan et al. 2016a).
Since biodegradable natural polymers such as collagen, gelatin (Gel), fibrin, chitosan, alginate, chondroitin sulphate, and hyaluronic acid, as the native extra cellular matrix (ECM), have similar structure, they have been widely applied in scaffolds for tissue engineering (Khan et al. 2012; Oryan et al. 2016a; Yazdimamaghani et al. 2014). Gel has been extensively utilized in pharmaceutical, medical and bioengineering purposes because of its excellent biocompatibility, hydrophilicity, and biodegradability (Oryan et al. 2016a; Takahashi et al. 2005; Usta et al. 2003). Gel can promote cell adhesion and proliferation due to its unique sequence of amino acids such as glycine, proline and hydroxyproline, so it is suitable for bone tissue applications (Oryan et al. 2016a; Takahashi et al. 2005; Yazdimamaghani et al. 2014).
Engineered biomaterials combined with growth factors have emerged as a new treatment alternative in bone repair and regeneration (Liu et al. 2009b). Fibrin-platelet glue (FPG) is a blood derivative, generated by differential centrifugation and precipitation, in which platelets and fibrinogen are concentrated in a small plasma volume (Burnouf et al. 2013, 2009; Thorn et al. 2004). Application of FPG as a scaffold in tissue-engineered bone seems attractive because the fibrin part contains high concentrations of fibrinogen, which can produce a dense clot with sufficient adhesive strength to facilitates the application of scaffold into the recipient defect and maintain a required configuration (Liao et al. 2011; Thorn et al. 2004; Zhu et al. 2006). In addition to the physical benefits, the incorporation of platelets into the fibrin glue also accelerates the bone graft healing process through the release of numerous different growth factors from the platelets upon activation with thrombin (Lee et al. 2007; Ross et al. 1986; Thorn et al. 2004). Simple encapsulation of growth factors in three-dimensional fibrin network of FPG may contribute to a prolonged retention and sequestration of the platelet growth factors and of their chemotactic and mitogenic activities for a longer period of time (Chen et al. 2008; Liao et al. 2011; Zhu et al. 2006).
Based on the current state of bone tissue engineering, both FPG and Gel have wide tissue engineering applications so that they are used as basic biomaterials in fabrication of various bone scaffolds. These are both used in combination with other polymeric and ceramic materials in order to fabricate composite bone scaffolds. Regarding the current literature and to the knowledge of the authors, although FPG and Gel have extensively been used in bone regeneration, the pure effects of either FPG, Gel or their combination have not been investigated in the experimental studies. Given the above explanations, this study was designed to investigate the role of FPG, Gel and their combinations as Gel–FPG scaffold on surgically critical-size defects (CSD) radial bone defect in a rat model. We hypothesized that both the FPG and Gel may have some beneficial effects on bone regeneration but each of such biomaterials may have different potency. In addition, combination of these biomaterials may preserve the beneficial effects of both biomaterials, while this strategy may reduce the limitations of the individuals.
Materials and methods
Preparation of gelatin, fibrin-platelet glue and gelatin-fibrin-platelet glue scaffolds
The Gel scaffold was prepared by chemical cross-linking of the gelatin powder (G9391, Sigma-Aldrich) with glutaraldehyde (G6403, Sigma-Aldrich). Briefly, 4.29 wt% aqueous solution of gelatin (7 ml) was mixed at 5000 rpm at 37 °C for 3 min, using a homogenizer (Sealed Unit, Silverson Machines Ltd., UK). After the addition of glutaraldehyde aqueous solution, the resulting solution was cast into a polypropylene dish at 4 °C for 12 h for gelatin cross-linking. The cross-linked gelatin hydrogels were then placed into 100 mM aqueous glycine solution at 37 °C for 1 h to block the residual aldehyde groups of glutaraldehyde. Following a complete washing with double distilled water, the hydrogels were freeze-dried at − 20 °C for 48 h (Alpha 2–4 LD Plus, Christ, Germany). Finally, the Gel scaffolds were sterilized under 60 Co γ-ray irradiation at a dose of 15 kGy and kept in vacuumed packs until further use (Liu et al. 2009b; Oryan et al. 2016a).
The FPG was prepared from platelet rich plasma (PRP) of rat by Thorn et al. method (Thorn et al. 2004). Briefly, the blood was withdrawn via heart aspiration and mixed with citrate phosphate dextrose at a ratio of 1 ml citrate phosphate dextrose to 5 ml blood. The PRP was separated from the blood by centrifugation (IEC PR-J centrifuge, Damon/IEC Division, USA) for 15 min at 327×g and at ambient temperature. The fibrinogen in the glue was precipitated from the PRP by ethanol precipitation at low temperature. The precipitated fibrinogen was separated by centrifugation at 3000×g for 8 min at 0–4 °C. The separated fibrinogen together with the modified PRP was used to enrich the fibrinogen with growth factors. Thorn et al. (2004) reported that the autologous fibrin glue prepared by this technique contains high platelet and fibrinogen concentrations. Finally, the platelet enrich fibrinogen solution was mixed with a combination of calcium chloride (Merck, Cat. No. 102382)/topical bovine thrombin (T-4648, Sigma-Aldrich) (10 CC of 10% calcium chloride mixed with 10,000 units of topical bovine thrombin) (Butterfield et al. 2005; Findikcioglu et al. 2009) for preparation of FPG prior to operation.
The hybrid scaffolds were prepared as follows: the Gel scaffolds, sterilized previously with 60 Co γ-ray irradiation, were infused with 0.1 ml platelet enriched fibrinogen solution for 5 min, then with 0.1 ml calcium chloride/topical bovine thrombin. After complete reaction for 30 min at room temperature, the hybrid scaffolds were freeze-dried at − 20 °C for 48 h and kept in vacuumed packs until further use (Liu et al. 2009b).
Ethics
The animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH) (NRC 2011). The study was approved by the local Ethics Committee of “Regulations for Using Animals in Scientific Procedures” in the School of Veterinary Medicine of Shiraz University.
Animals and operative procedures
A total of 25 mature male Wistar Albino rats, weighing between 250 and 300 g, purchased from Shiraz University of Medical Sciences, Shiraz, Iran, were used in this study. The animals had full access to standard food and water ad libitum throughout the duration of the study. The rats were anesthetized with an intramuscular injection, using a combination of 75 mg/kg ketamine hydrochloride and 5 mg/kg xylazine (both from Alfasan; Woerden, Netherlands). The right and left forelimb of all the animals was prepared aseptically for operation. An incision was made craniomedially over the skin of forelimb and the radius was exposed by dissecting the surrounding muscles and tendons. A 5 mm segmental bone defects were created in the middle of the radial diaphysis as a critical size bone defect by an electrical bone saw at 150 rpm under saline irrigation to prevent thermal necrosis (Marathon, Escort-III, Daegu, South Korea). All bone debris and interosseous membrane in the defect site were washed and wiped away. As the radius and ulna are fused together by interosseous membrane, adequate stability was achieved by leaving the ulna intact without any fixation of the radius. A number of 50 radial bone defects were created in totally 25 rats. The bone defects were randomly divided into five equal groups (n = 10 for each). The defects in the 1st group (empty defect) remained intact and served as negative control for the test groups. The defects in the 2nd group (autograft) were filled with autologous bone graft to be considered as positive control group. In a group of animals containing both groups of empty defect (right side) and autograft treated defect (left side), the autografts were harvested from the contralateral radii of the group 1 so that the transected corticomedullary bone segment in the empty defect group (right side) was used as autograft to fill the defect area of the group 2 (left side). The defects in the 3rd, 4th and 5th group (test groups) were filled with Gel, FPG and Gel–FPG), having the size and shape similar to the radial defects (2 × 2 × 5 mm3), respectively. The muscles, fascia and skin were then approximated in a routine fashion, using 4-0 sutures (Ethicon; Somerville, New Jersey, USA). Post-surgically, analgesia and antibiotic therapy were performed by daily sub-cutaneous injections of 20 mg/kg tramadol chloride (Darou Pakhsh Pharmaceutical Mfg. Co., Tehran, Iran) for 3 days and 20 mg/kg enrofloxacin (Razak Laboratory Co., Tehran, Iran) for 5 days.
Clinical examination
After surgery and induction of bone defects, the rats were observed for physical activities including weight bearing on injured forearms, and post-surgical clinical changes such as edema and hyperemia at the defect areas, pain on palpation, and the appetite status.
Radiological evaluation
To evaluate bone formation, proximal and distal union and remodeling of the defect, radiographs of each forelimb was taken postoperatively on 1st day and then at the 28th and 56th days post injury, using X-ray machine (Soyee, BLD-31-C, Seoul, South Korea). The results were scored using the modified Lane and Sandhu scoring system by two veterinary radiologists (Lane and Sandhu 1987; Oryan et al. 2014b; Table 1).
Sample collection
Fifty-six days after operation the rats were euthanized (Parizi et al. 2013; Shafiei-Sarvestani et al. 2012). For such purpose, first, the animals were anesthetized by intramuscular injection of ketamine and xylazine. Then, the breathing of the anesthetized animals was stopped by intracardiac injection of 150 mg/kg potassium chloride (Pasteur Institute, Tehran, Iran). The right and left forelimbs were harvested and dissected free of soft tissues. The bone samples of each group (n = 10) were randomly divided into two equal subgroups as follow: Subgroup A (n = 5): these bone samples were used for histopathologic and scanning electron microscopic studies (SEM). These bone samples were cut to two pieces with a sagittal sections containing the defect for histopathologic and SEM studies. For this purpose, the bones were cut to two pieces containing the defect with a sagittal section made by a slow speed saw. Thus, the same tissue was investigated for both methods [histopathology (n = 5) and SEM (n = 5)]. Subgroup B (n = 5): these bone samples were used for biomechanical testing.
Histopathological evaluation
The bone specimens were fixed in 10% neutral buffered formalin. The formalin-fixed bone samples were rinsed with water and then decalcified in 10% nitric acid solution and processed for routine histological examination. Next, two 5 μm thick sections were cut in a longitudinal direction from the centers of each specimen and stained with Hematoxylin and Eosin (H&E) for analysis by light microscopy (Olympus CX-41, Tokyo, Japan). Finally, the sections were blindly evaluated and scored by two pathologists according to Emery’s scoring system (Bigham-Sadegh et al. 2013; Emery et al. 1994; Table 2). Images of the histologic sections were captured by a digital camera (Olympus E-P1, Olympus Optical, Tokyo, Japan) connected to a light microscope. The volumes of the regenerated fibrous, cartilage and bone tissues in defected area were also calculated from the provided images (Moshiri et al. 2015; Oryan et al. 2016a).
Scanning electron microscopy
To visualize the surface and structure of the healing area, the bone specimens were fixed in cold 2.5% buffered glutaraldehyde. Dehydration was performed on the samples with an increasing graded ethanol series and let dry overnight in a freeze drier. The dried specimens were mounted on aluminum stubs using carbon double sided tape and sputter-coated with gold (SPI-Module Sputter Coater). The coated samples were degassed in vacuum and observed using high-resolution images obtained, using filed emission scanning electron microscopy (SEM) (Vega-3, Tescan, AS, Brno, Czech Republic) with accelerating voltage 20 kV at different magnifications.
Biomechanical testing
The bone samples were wrapped in a saline-soaked gauze bandage to prevent dehydration and stored at − 20 °C in small, sealed freezer bags. At the day of testing, the bones were slowly thawed to room temperature and kept wrapped in the saline-soaked gauzes except during measurements. Mechanical testing was performed on the fused radius and ulna complex as a unit. The three-point bending test was performed, using a universal tensile testing machine (Santam, STM-20, Tehran, Iran) to determine the mechanical properties of bones according to the previously described procedures (Järvinen et al. 1998; Leppänen et al. 2006; Oryan et al. 2012). Briefly, the bones were placed on their lateral surface on two rounded supporting bars (plates with rounded edges of 4.0 mm diameter) located at a distance of 16 mm, and were loaded at the midpoint of the diaphysis by lowering the third bar (a plate with rounded edges of 10 mm diameter) so that the defect was in the middle and had an equal distance from each grip. The bones were loaded at a rate of 1 mm/s until fracturing occurred. The behavior of each specimen under loading was characterized by determining the following parameters from the load-deformation to destruction curve:
-
1.
Ultimate strength or maximum load was determined as the highest point of the load-deformation curve (N).
-
2.
Yield strength was determined as the point in the stress–strain curve at which the curve levels off and plastic deformation begins to occur (N). Prior to the yield point the material will deform elastically and will return to its original shape when the applied stress is removed. Once the yield point is passed, some fraction of the deformation will be permanent and non-reversible.
-
3.
Stiffness is the coefficient of inclination for the linear portion of the load-deformation curve. It is easily calculated by measuring the slope of a line drawn as a tangent to the curve at any defined point in the linear portion of the curve. The slope gives the approximate stiffness of the preparation (N/mm).
-
4.
Strain is the specimen’s extension (the percentage of elongation) at the ultimate strength region. The term “strain” means the fractional increase in length of the material due to an applied load. It is calculated by dividing the extension by the original length of the specimen.
-
5.
Energy absorption was determined as the area under the load-deformation curve until the point of failure (N mm).
The data derived from the load deformation curves were expressed as Mean ± SEM for each group and the ultimate and yield strength, stiffness, ultimate and yield strain and absorbed energy were measured and recorded.
Statistical analysis
The radiological and histopathological data were compared by Kruskal–Wallis, non-parametric analysis of variance (ANOVA); when P values were found to be less than 0.05, then pair wise group comparisons was performed by Mann–Whitney U test. The biomechanical data were compared by one-way ANOVA with subsequent Tukey post hoc tests. A P < 0.05 was considered statistically significant. All biomechanical data passed normally distribution test and Bonferroni’s method used for multiple testing and the results were presented as mean ± SEM (SPSS version 23 for windows, SPSS Inc., Chicago, USA).
Results
Clinical evaluation
No death occurred among the animals during the course of the experiment and they had good physical activities, weight gain and appetite until euthanasia. The animals in all the groups used their forearms because of the ulna and its supportive role.
Radiological findings
The results of radiological evaluations at 28th and 56th days after bone surgery are presented in Table 3 and Fig. 1. The autograft group showed significantly the best bone formation, radiological union and remodeling scores in comparison to other groups on 28 and 56 days after the injury (P < 0.05). The only significant radiological differences in the sum of healing scores of the bone defect between the negative control (empty defect) group with all three treatment groups was observed on Gel–FPG group on day 56th post-injury (P = 0.020).
Radiographs at the 1st (a), 28th (b), and 56th (c) postoperative day. The empty defects are the most radiolucent amongst all groups, whereas autograft are the most radiopaque followed by the Gel–FPG, FPG and Gel groups at 28 and 56 days after bone surgery. Bone formation was 75–100% at 56 days after bone injury, in the autograft group. The FPG and Gel–FPG scaffolds had 25–50% and 50–75% bone formation, respectively, while it was 0–25% in the Gel and empty defect groups
There were significant differences in bone formation between the animals of the Gel–FPG group with those of the empty defect group on the 28th and 56th post-injury days (P = 0.036 and P = 0.036, respectively).
Proximal and distal bone union in the animals of the treatment groups by days 28 and 56 post-injury were not significantly prominent than the empty defect ones (P > 0.05).
The animals of the treatment groups did not show better remodeling criteria on days 28th and 56th post operation than those of the defect group (P > 0.05).
Histopathological findings
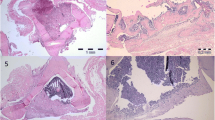
Histopathologic findings were assigned to each group on the basis of the volume of the observed tissues such as the fibrous, fibrocartilage, hyaline cartilage and bone tissues (Table 4, Figs. 2, 3). At histopathologic level, the lesions in the empty defect group showed significantly the lowest microscopic scores and had highest fibrous tissue volumes in comparison to other groups on 56 days after the injury (P < 0.05). The Gel and FPG treated defects had greater cartilage volume compared with the empty defect group (P = 0.027 and P = 0.041, respectively) and the Gel–FPG group had higher bone volume than the empty defect group (P = 0.044). Moreover, the FPG and Gel–FPG treated defects had also significantly superior microscopic scores than the empty defect group (P = 0.008 and 0.008, respectively). The bone volume of the autograft group was significantly superior to the other groups and these animals had the best microscopic scores compared to other groups (P < 0.05).
Tissue volumes of the healing bone defects after 56 days of bone injury. The volume of fibrous tissue in the empty defect group was significantly superior to other groups (P < 0.05). The defect treated with Gel and FPG groups showed significantly higher volume of cartilage tissue when compared to the empty defect groups (P = 0.027 and P = 0.041, respectively). The Gel–FPG group had greater bone tissue than the empty defect group (P = 0.044). The bone tissue volume of the autograft group was significantly superior to other groups (P < 0.05)
Histopathological sections of the radial bone defects after 56 days of injury; stained with H&E, longitudinal view. The lesion has been filled with a loose areolar connective tissue in the empty defect group and a fibrocartilage tissue was visible at the edges of the radial bone. Note the mild inflammatory response consisting of mononuclear cells in the defected area of this group. In the autograft group, a matrix composed of hyaline cartilage, calcified cartilage and osseous tissue are predominant in the defect site. Note the neovascularization in the grafted area of this group. The defects in the Gel group are filled with a non-homogenous matrix composed of fibrous connective, fibrocartilage and hyaline cartilage. A hyaline cartilaginous matrix at the middle part of the lesion and calcified cartilage at the edges of the lesion have filled the defected site in the FPG and Gel–FPG groups. Note to extensive endochondral ossification and development of the woven bone containing several osteons at the bone edges in these two groups. RBE radial bone edge, LAFT loose areolar fibrous tissue, CT cartilaginous tissue, DFT dense fibrous tissue, HCT hyaline cartilaginous tissue, FCT fibrocartilaginous tissue, IC inflammatory cells, RBCs red blood cells, NRM newly regenerated matrix, CCT calcified cartilaginous tissue, WB woven bone, PO primary osteon, EO endochondral ossification
Qualitatively, 56 days after bone surgery, the gap in the animals of the empty defect group was replaced with fibrous or fibrocartilages and the lesions showed poor re-vascularization (Fig. 2). Bridging callus or histological union did not develop in any of the defects of these animals. These criteria lead to very slow healing process in this group.
Both ends of the corticomedullary implanted autograft were connected to the edges of the old radial bones by a non-homogenous matrix composed of cartilaginous and osseous tissues. A number of blood vessels degrading the implanted graft were observed in the lesion of this group. The regenerated cartilage and bone spanned the defect and most instantly produced histologic union and the lesions showed some marrow formation.
The defect was filled with a mixed tissue consisting of fibrous connective, fibrocartilage and hyaline cartilage tissue, in the Gel treated group. The Gel scaffolds were totally degraded and no remnants were evident in the injured area.
The fibrous and cartilage tissues in the defects of the FPG and Gel–FPG groups, were gradually substituted with bone. The lesions in these animals were filled with a variable soft to hard tissue consisting of fibrocartilage, hyaline cartilage and woven bone particularly in both the distal and proximal ends of the defects. However, maturation was not as well as those of the autograft group. In the FPG and Gel–FPG groups, no remnants of the scaffold were seen in the injured area. Both the FPG and Gel–FPG groups showed hypertrophic bone edges and the newly formed cells were proliferating from the bone edges into the middle part of the defect area and more woven bone formation was seen in the lesions of these groups. In most cases, such feature was less evident in the Gel group. In general, no significant inflammatory response was evident in the lesions of the animals of different treatment groups at the 56th post injury day, although it may have been present earlier.
Scanning ultra-microscopy
Irregular collagen fibrils were seen in the defects of empty group after 56 days of bone injury (Fig. 4). An accumulation of hydroxyapatite crystals was visible along with the newly formed bone, in the lesions of the autograft group. There were cartilage and few hydroxyapatite crystals in the Gel treated defects. Calcified cartilaginous matrices containing a number of deposited hydroxyapatite crystals were observed as the newly regenerated tissue in the treated FPG and Gel–FPG lesions.
Scanning ultramicrographs of the bones after 56 days of surgery. Highly calcified bone matrix can be seen in the healthy bone. The defect has been filled with collagen fibrils in the empty defect group. Calcified bone matrix and hydroxyapatite crystals has filled the defect sites in the autograft group. A fibrocartilage matrix and few hydroxyapatite crystals are observed in the Gel treated defects. A calcified cartilage tissue and calcified bone matrix containing deposited hydroxyapatite crystals are present in the defect sites in the FPG and Gel–FPG groups
Biomechanical performance
The data obtained from the biomechanical testing have been shown in Table 5. The failure mechanism of the bones was very consistent; the failure took place exactly at the defected area with the fracture line being perpendicular to the long axis of the bone. The autograft group showed significantly higher ultimate strength and yield strength as compared with the defect, Gel, FPG and Gel–FPG treated groups at 56 days after the injury (P < 0.05). There was statistically significant difference between the empty defect group with the FPG (P = 0.017) and the Gel–FPG (P = 0.017) groups in terms of ultimate strength. The Gel–FPG group also had superior yield strength (P = 0.049) compared to the empty defect group. There were no statistically significant differences between the three treatment groups with those of the positive and negative control groups in terms of stiffness, strain and absorbed energy (P > 0.05).
Discussion
To evaluate the bone healing potential of Gel, FPG and their combinations a defect model was established in the radial bone of rat. This model has previously been reported suitable because there is no need for internal or external fixation which influences the healing process (An and Freidman 1998; Oryan et al. 2016a). The segmental defect was created in the middle portion of the radius as long as 5 mm to induce a nonunion defect as a critical size defect and to prevent spontaneous and rapid healing (Oryan et al. 2016a; Öztürk et al. 2006). The hypothesis was on the basis that both the Gel and FPG may have some beneficial effects on bone regeneration but each of such biomaterials may have different potency. In addition, combination of these biomaterials may preserve the beneficial effects of both biomaterials, while this strategy may reduce the limitations of the individuals. The results of the radiological, histological, ultrastructural and biomechanical analysis showed that regardless of the autograft group, among the scaffolds used in this investigation, the best results were obtained from the Gel–FPG treated defects followed by FPG.
The bone defects treated with Gel alone were associated with minor superiority healing to the empty defect group so that the difference between them was significant only in percent of cartilaginous tissue. In fact, the healing process in the empty defect group remained in the initial stages and the fibrous connective tissue was the main constituent in the lesions of the animals of this group, while the healing stage was more advanced in the Gel treated group and there was some evidence of cartilage cells in the latter group. Although a huge background exists in the literature that have suggested application of Gel as an acceptable biomaterial in bone tissue engineering (Fukui et al. 2012; Liu et al. 2009b; Takahashi et al. 2005; Yazdimamaghani et al. 2014), here we showed that Gel has limited value in bone regeneration in vivo so that its potential in promoting bone healing was inferior to the FPG and Gel–FPG biomaterial. Based on the previous reports, Gel has been suggested as a suitable biomaterial for scaffold fabrication in vitro and its composites have been suggested to promote bone repair (Khan et al. 2012; Liu et al. 2009a; Mozafari et al. 2010). This is a fact that the in vitro characterizations and results such as MTT assay and cell differentiation tests does not guarantee the real in vivo role of Gel (Oryan et al. 2016a). Therefore, our in vivo results does not support the in vitro results of the previously published studies. On the other hand, Gel has been used as a biomaterial to enhance in vitro characteristics of the composite scaffolds and thus its combined effects with polymeric and ceramic materials have been used in vivo. This is the major difference explaining why the Gel based composites were able to significantly promote bone regeneration in vivo while pure Gel scaffold did not considerably promote bone regeneration. In fact, the pure effect of Gel regarding the bone regenerative ability of this bio-implant has not been in the focus of the previous studies. In opposite with our findings, Sohn et al. (2010) evaluated the efficacy of absorbable gelatin sponge on new bone formation in the maxillary sinus of nine patients for a 6-month period. They observed new bone consolidation in the sinus on radiographs and suggested gelatin sponge as a proper treatment strategy for sinus augmentation even without any additional bone graft. However, it should be stated that healing of critical size defects in weight bearing bones is a more complicated process than the skull bones (e.g. sinus bones), because the skull bones have superior blood circulation and tolerate less mechanical forces than the long weight bearing bones (Oryan et al. 2014a, 2016a). In another study, Oryan et al. (2016a) investigate the role of gelatin and their combinations as chitosan–gelatin scaffold on healing and regeneration of critical sized radial bone defects. They concluded that incorporation of gelatin into the chitosan scaffold improved healing potential of the chitosan; however, it was still inferior to the gelatin scaffold alone. This study was performed in circumstances similar to our study on experimentally induced radial bone defect in a rat model. The difference between our results and the result of Oryan et al. study may be because of the different protocol for preparation of Gel scaffold and also more concentration of their Gel scaffold compared with our scaffold (10% vs. 4.29 wt%).
The present experiment showed that Gel had a more positive effect in bone regeneration when it was used together with FPG. This improvement might be attributed to the higher bioactivity properties of the FPG than the Gel and thus the FPG improved the effectiveness of the Gel. Fifty-six days after implantation of the scaffolds in the bone defects, FPG and Gel–FPG scaffolds were replaced with the newly formed tissues consisting of fibrocartilage, hyaline cartilage and osseous tissues. Compared with the empty defect group with spontaneous healing process and Gel within initial stages of bone healing, the FPG and Gel–FPG treated defects had higher cartilaginous and bone cells and the healing was more advanced. In line with our findings, a huge background exists in the literature that have suggested FPG to be applied as an acceptable biomaterial in bone tissue engineering (Lee et al. 2007; Thorn et al. 2004; Zhu et al. 2006). In addition to the physical benefit of fibrin glue in bone surgery, as the fibrin network has been known to act as a scaffold for the invasion of cells and as a carrier for bone induction, the incorporation of platelets into the fibrin glue also accelerates the bone healing process through the release of numerous different growth factors from the platelets upon activation with thrombin (Chen et al. 2008; Lee et al. 2007; Thorn et al. 2004). Once the platelet concentrate of FPG is activated, a three-dimensional and biocompatible fibrin scaffold is formed, a myriad of growth factors and proteins are released progressively to the local environment and contributing to the accelerated postoperative bone healing (Anitua et al. 2006).
However, the clinical and experimental data in the literature regarding the osteogenic potential of platelet-derived products are controversial (Anitua et al. 2006; Findikcioglu et al. 2009; Trouillas et al. 2013). Although there are few controlled studies regarding application of fibrinogen and growth factors in wound healing, but variations in the type of grafts, anatomic sites, biological and surgical techniques, diversity of preparation protocols in different experiments makes the conclusion difficult and there is still significant disagreement as to whether or not the platelet-derived products enhances the healing of bone grafts (Anitua et al. 2006; Nagata et al. 2009; Trouillas et al. 2013). Variations in some key properties, including the platelet concentration, the type of clot activator, the leukocyte content and the time after clotting that the fibrin scaffold should be implanted in the injured area can markedly influence different biological effects (Anitua et al. 2006).
This study was performed to give more insights into the effect of the pure FPG and their combination with Gel on bone regeneration and also to provide an explanation for the existing confusion in the literature regarding the efficacy of the FPG treatment in combination with other artificial bone graft substitutes. The results of the present investigation confirm a number of clinical and experimental studies demonstrating a positive influence of FPG in bone substitute materials on bone regeneration (Ito et al. 2006; Trouillas et al. 2013; You et al. 2007). However, in some other studies the graft materials did not enhance bone healing when augmented with the platelet-derived products (Giovanini et al. 2010; Ranly et al. 2007; Sánchez et al. 2003). In our study, although the FPG and Gel–FPG treated defects had higher cartilaginous and bone cells and the healing was more advanced; however, they were not as good as the autograft group.
Osteoconductivity of a biomaterial refers to the ability of a biomaterial to guide new bone formation toward the normal anatomic line of bone and establishes the bone continuity. On the other hand, osteoinductivity of a biomaterial explains its ability to regenerate a new bony matrix in which the progenitor cells differentiate into osteoblasts and osteocytes and these latter specialized cell types synthetize new bone matrix (Oryan et al. 2014a, 2016b). In addition to other findings, we could claim that both the FPG and Gel–FPG scaffolds have some osteoinductive properties because in both groups some evidences of new bone formation were seen after 56 days of injury. However, new bone formation was limited to both defect edges and these scaffolds were not able to induce bone formation in the middle of the defect area and thus, they displayed low osteoconductive properties.
Nonetheless, these characteristics were more remarkable in the Gel–FPG group in comparison with the FPG alone so that the Gel–FPG promoted bone regeneration into the later stages of the healing process. Whereas the differences were not significant in most cases, the new bone formation and the volume of bone tissue present in the defect sites of the Gel–FPG group were more than the FPG group and were significantly higher than that in the empty defect group. These criteria might be attributed to the combination of biocompatible properties of the Gel and bioactive properties of the FPG. Gel as a good cell adhesiveness vehicle can evoke mesenchymal and osteochondral progenitor cells into the defect site (Kakkar et al. 2014; Moshiri et al. 2015; Zhang et al. 2009), while FPG as a reservoir of critical GFs can promote cell proliferation, cell differentiation and matrix synthesis (Burnouf et al. 2013, 2009; Zhu et al. 2006). On the other hand, addition of FPG into the Gel facilitates the clinical application of scaffold into the recipient defect and the shaping and placement of the graft much easier and maintains a required configuration (Liao et al. 2011; Thorn et al. 2004; Zhu et al. 2006).
Although combination of Gel and FPG could improve the efficacy of the scaffold in bone healing, it was still inferior to the autograft. In fact, radiological, histological, ultrastructural and biomechanical properties of the defects treated with Gel–FPG scaffolds still remained a problem in this study. It seems the osteoconductive and osteoinductive properties of the Gel–FPG scaffold should further be enhanced by incorporation of the ceramic materials particularly biphasic calcium phosphate, strontium salts and bioactive glasses in order to improve the functional and mechanical properties of the healing bone more than that we observed in the present investigation (Peter et al. 2010; Sellgren and Ma 2012). Moreover, based on the results of the present study, FPG has more beneficial bone regenerative features than Gel to be considered as a bio-implant in bone tissue engineering.
Based on the results of the present investigation and regarding the previously published results from the in vivo studies, it seems Gel and FPG have different in vivo behavior on bone regeneration related to the anatomic region. Regarding the literature, both the Gel and FPG showed beneficial effects on bone formation particularly in the head region (skull and maxillofacial bones) (Chen et al. 2008; Findikcioglu et al. 2009; Sohn et al. 2010). Compared with many of the previously published investigations, we showed Gel and FPG cannot restore the lost bone in the critical size large weight bearing bone defects such as radius, either alone or in combination. Thus, non-union or partial union is expected to occur if Gel, FPG and Gel–FPG scaffolds used in long bone reconstructive surgery. We showed that the FPG bioimplant has more beneficial effects on bone regeneration than the Gel. Therefore, FPG may be included in any composite scaffolds in order to improve scaffold biocompatibility, biodegradability and healing efficacy, more than Gel. If Gel is planned to be used for composite scaffold fabrication, to improve scaffold porosity, combination of Gel with FPG is a more advantageous option with better in vivo outcome. Therefore, researchers working in the field of bone tissue engineering should improve the in vivo effectiveness of Gel scaffolds to benefit from it in the clinical setting by incorporating more bioactive biomaterials and FPG could be an acceptable candidate in this regard.
The anatomic variation between the radial bone in human and each animal model should be considered when the results of such studies are aimed to be translated into clinical practice. Although the animal models may closely represent the physiological and mechanical characteristics of the human clinical situation, it must be emphasized that it is only an approximation and each animal model have its own unique advantages and disadvantages.
Conclusions
In conclusion, this study demonstrated that the Gel alone did not significantly affect bone healing and regeneration; however, Gel treated defects promoted healing more than those that were left untreated (negative control). Furthermore, the FPG-enhanced grafts provide a good scaffold containing numerous growth factors for proliferation of osteoinduction and was effective in improving the structural and functional properties of the newly formed bone more than that of the untreated and also the Gel treated groups. Incorporation of Gel into the FPG scaffold improved healing potential of the FPG scaffold; however, it was still inferior to the autograft (positive control). Although Gel–FPG scaffolds had advantageous effectiveness during bone regeneration, the scaffolds still need to be further enhanced possibly by incorporation of the ceramic and osteoinductive biomaterials.
References
An YH, Freidman RJ (1998) Animal models in orthopaedic research. CRC Press, Boca Raton
Anitua E, Sánchez M, Nurden AT, Nurden P, Orive G, Andía I (2006) New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol 24:227–234
Bigham-Sadegh A, Oryan A, Mirshokraei P, Shadkhast M, Basiri E (2013) Bone tissue engineering with periosteal-free graft and pedicle omentum. ANZ J Surg 83:255–261
Burnouf T, Su CY, Radosevich M, Goubran H, El-Ekiaby M (2009) Blood-derived biomaterials: fibrin sealant, platelet gel and platelet fibrin glue. ISBT Sci Ser 4:136–142
Burnouf T, Goubran HA, Chen T-M, Ou K-L, El-Ekiaby M, Radosevic M (2013) Blood-derived biomaterials and platelet growth factors in regenerative medicine. Blood Rev 27:77–89
Butterfield KJ, Bennett J, Gronowicz G, Adams D (2005) Effect of platelet-rich plasma with autogenous bone graft for maxillary sinus augmentation in a rabbit model. J Oral Maxillofac Surg 63:370–376
Chen TM, Tsai J-C, Burnouf T (2008) Cranioplasty using osteoconductive scaffold and platelet glue. J Trauma Acute Care Surg 65:1321–1327
Emery SE, Brazinski MS, Koka A, Bensusan JS, Stevenson S (1994) The biological and biomechanical effects of irradiation on anterior spinal bone grafts in a canine model. J Bone Jt Surg Am 76:540–548
Findikcioglu K, Findikcioglu F, Yavuzer R, Elmas C, Atabay K (2009) Effect of platelet-rich plasma and fibrin glue on healing of critical-size calvarial bone defects. J Craniofac Surg 20:34–40
Fukui T, Ii M, Shoji T, Matsumoto T, Mifune Y, Kawakami Y, Akimaru H, Kawamoto A, Kuroda T, Saito T (2012) Therapeutic effect of local administration of low-dose simvastatin-conjugated gelatin hydrogel for fracture healing. J Bone Miner Res 27:1118–1131
Giovanini AF, Deliberador TM, Gonzaga CC, de Oliveira Filho MA, Göhringer I, Kuczera J, Zielak JC, de Andrade Urban C (2010) Platelet-rich plasma diminishes calvarial bone repair associated with alterations in collagen matrix composition and elevated CD34 + cell prevalence. Bone 46:1597–1603
Ito K, Yamada Y, Naiki T, Ueda M (2006) Simultaneous implant placement and bone regeneration around dental implants using tissue-engineered bone with fibrin glue, mesenchymal stem cells and platelet-rich plasma. Clin Oral Implant Res 17:579–586
Järvinen T, Sievänen H, Kannus P, Järvinen M (1998) Dual-energy X-ray absorptiometry in predicting mechanical characteristics of rat femur. Bone 22:551–558
Kakkar P, Verma S, Manjubala I, Madhan B (2014) Development of keratin–chitosan–gelatin composite scaffold for soft tissue engineering. Mater Sci Eng C 45:343–347
Khan MN, Islam JM, Khan MA (2012) Fabrication and characterization of gelatin-based biocompatible porous composite scaffold for bone tissue engineering. J Biomed Mater Res A 100:3020–3028
Lane JM, Sandhu H (1987) Current approaches to experimental bone grafting. Orthop Clin N Am 18:213–225
Lee H-J, Choi B-H, Jung J-H, Zhu S-J, Lee S-H, Huh J-Y, You T-M, Li J (2007) Maxillary sinus floor augmentation using autogenous bone grafts and platelet-enriched fibrin glue with simultaneous implant placement. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 103:329–333
Leppänen O, Sievänen H, Jokihaara J, Pajamäki I, Järvinen TL (2006) Three-point bending of rat femur in the mediolateral direction: introduction and validation of a novel biomechanical testing protocol. J Bone Miner Res 21:1231–1237
Liao H-T, Chen C-T, Chen C-H, Chen J-P, Tsai J-C (2011) Combination of guided osteogenesis with autologous platelet-rich fibrin glue and mesenchymal stem cell for mandibular reconstruction. J Trauma Acute Care Surg 70:228–237
Liu X, Smith LA, Hu J, Ma PX (2009a) Biomimetic nanofibrous gelatin/apatite composite scaffolds for bone tissue engineering. Biomaterials 30:2252–2258
Liu Y, Lu Y, Tian X, Cui G, Zhao Y, Yang Q, Yu S, Xing G, Zhang B (2009b) Segmental bone regeneration using an rhBMP-2-loaded gelatin/nanohydroxyapatite/fibrin scaffold in a rabbit model. Biomaterials 30:6276–6285
Meimandi-Parizi A, Oryan A, Moshiri A (2013) Role of tissue engineered collagen based tridimensional implant on the healing response of the experimentally induced large Achilles tendon defect model in rabbits: a long term study with high clinical relevance. J Biomed Sci 20:1
Moshiri A, Shahrezaee M, Shekarchi B, Oryan A, Azma K (2015) Three-dimensional porous gelapin–simvastatin scaffolds promoted bone defect healing in rabbits. Calcif Tissue Int 96:552–564
Mozafari M, Rabiee M, Azami M, Maleknia S (2010) Biomimetic formation of apatite on the surface of porous gelatin/bioactive glass nanocomposite scaffolds. Appl Surf Sci 257:1740–1749
Nagata MJ, Melo L, Messora MR, Bomfim SR, Fucini SE, Garcia VG, Bosco AF, Okamoto T (2009) Effect of platelet-rich plasma on bone healing of autogenous bone grafts in critical-size defects. J Clin Periodontol 36:775–783
NRC (2011) Guide for the care and use of laboratory animals. National Academies Press, Washington
Oryan A, Parizi AM, Shafiei-Sarvestani Z, Bigham A (2012) Effects of combined hydroxyapatite and human platelet rich plasma on bone healing in rabbit model: radiological, macroscopical, hidtopathological and biomechanical evaluation. Cell Tissue Bank 13:639–651
Oryan A, Alidadi S, Moshiri A, Maffulli N (2014a) Bone regenerative medicine: classic options, novel strategies, and future directions. J Orthop Surg Res 9:1
Oryan A, Bigham-Sadegh A, Abbasi-Teshnizi F (2014b) Effects of osteogenic medium on healing of the experimental critical bone defect in a rabbit model. Bone 63:53–60
Oryan A, Alidadi S, Bigham-Sadegh A, Moshiri A (2016a) Comparative study on the role of gelatin, chitosan and their combination as tissue engineered scaffolds on healing and regeneration of critical sized bone defects: an in vivo study. J Mater Sci Mater Med 27:155
Oryan A, Alidadi S, Moshiri A (2016b) Platelet-rich plasma for bone healing and regeneration. Expert Opin Biol Ther 16:213–232
Öztürk A, Yetkin H, Memis L, Cila E, Bolukbasi S, Gemalmaz C (2006) Demineralized bone matrix and hydroxyapatite/tri-calcium phosphate mixture for bone healing in rats. Int Orthop 30:147–152
Parizi AM, Oryan A, Shafiei-Sarvestani Z, Bigham A (2012) Human platelet rich plasma plus Persian Gulf coral effects on experimental bone healing in rabbit model: radiological, histological, macroscopical and biomechanical evaluation. J Mater Sci Mater Med 23:473–483
Parizi AM, Oryan A, Shafiei-Sarvestani Z, Bigham-Sadegh A (2013) Effectiveness of synthetic hydroxyapatite versus Persian Gulf coral in an animal model of long bone defect reconstruction. J Orthop Traumatol 14:259–268
Peter M, Binulal N, Nair S, Selvamurugan N, Tamura H, Jayakumar R (2010) Novel biodegradable chitosan–gelatin/nano-bioactive glass ceramic composite scaffolds for alveolar bone tissue engineering. Chem Eng J 158:353–361
Ranly DM, Lohmann CH, Andreacchio D, Boyan BD, Schwartz Z (2007) Platelet-rich plasma inhibits demineralized bone matrix-induced bone formation in nude mice. J Bone Jt Surg 89:139–147
Ross R, Raines EW, Bowen-Pope DF (1986) The biology of platelet-derived growth factor. Cell 46:155–169
Sánchez AR, Sheridan PJ, Kupp LI (2003) Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants 18:1
Sellgren KL, Ma T (2012) Perfusion conditioning of hydroxyapatite–chitosan–gelatin scaffolds for bone tissue regeneration from human mesenchymal stem cells. J Tissue Eng Regen Med 6:49–59
Shafiei-Sarvestani Z, Oryan A, Bigham AS, Meimandi-Parizi A (2012) The effect of hydroxyapatite-hPRP, and coral-hPRP on bone healing in rabbits: radiological, biomechanical, macroscopic and histopathologic evaluation. Int J Surg 10:96–101
Sohn D-S, Moon J-W, Moon K-N, Cho S-C, Kang P-S (2010) New bone formation in the maxillary sinus using only absorbable gelatin sponge. J Oral Maxillofac Surg 68:1327–1333
Takahashi Y, Yamamoto M, Tabata Y (2005) Enhanced osteoinduction by controlled release of bone morphogenetic protein-2 from biodegradable sponge composed of gelatin and β-tricalcium phosphate. Biomaterials 26:4856–4865
Thorn J, Sørensen H, Weis-Fogh U, Andersen M (2004) Autologous fibrin glue with growth factors in reconstructive maxillofacial surgery. Int J Oral Maxillofac Surg 33:95–100
Trouillas M, Prat M, Doucet C, Ernou I, Laplace-Builhé C, Saint Blancard P, Holy X, Lataillade J-J (2013) A new platelet cryoprecipitate glue promoting bone formation after ectopic mesenchymal stromal cell-loaded biomaterial implantation in nude mice. Stem Cell Res Ther 4:1
Usta M, Piech D, MacCrone R, Hillig W (2003) Behavior and properties of neat and filled gelatins. Biomaterials 24:165–172
Yazdimamaghani M, Vashaee D, Assefa S, Walker K, Madihally S, Köhler G, Tayebi L (2014) Hybrid macroporous gelatin/bioactive-glass/nanosilver scaffolds with controlled degradation behavior and antimicrobial activity for bone tissue engineering. J Biomed Nanotechnol 10:911–931
You T-M, Choi B-H, Zhu S-J, Jung J-H, Lee S-H, Huh J-Y, Lee H-J, Li J (2007) Platelet-enriched fibrin glue and platelet-rich plasma in the repair of bone defects adjacent to titanium dental implants. Int J Oral Maxillofac Implants 22:1
Zhang S, Huang Y, Yang X, Mei F, Ma Q, Chen G, Ryu S, Deng X (2009) Gelatin nanofibrous membrane fabricated by electrospinning of aqueous gelatin solution for guided tissue regeneration. J Biomed Mater Res A 90:671–679
Zhu S-J, Choi B-H, Jung J-H, Lee S-H, Huh J-Y, You T-M, Lee H-J, Li J (2006) A comparative histologic analysis of tissue-engineered bone using platelet-rich plasma and platelet-enriched fibrin glue. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol 102:175–179
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gholipour, H., Meimandi-Parizi, A., Oryan, A. et al. The effects of gelatin, fibrin-platelet glue and their combination on healing of the experimental critical bone defect in a rat model: radiological, histological, scanning ultrastructural and biomechanical evaluation. Cell Tissue Bank 19, 341–356 (2018). https://doi.org/10.1007/s10561-017-9679-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-017-9679-5