Abstract
Hydroxyapatite is an osteoconductive material used as a bone graft extender and exhibits excellent biocompatibility with soft tissues such as skin, muscle and gums, making it an ideal candidate for orthopedic and dental implants or components of implants. Synthetic hydroxyapatite has been widely used in repair of hard tissues, and common uses include bone repair, bone augmentation, as well as coating of implants or acting as fillers in bone or teeth. On the other hand, human platelet rich plasma (hPRP) has been used as a source of osteoinductive factor. A combination of hPRP and hydroxyapatite is expected to create a composite with both osteoconductive and osteoinductive properties. This study examined the effect of a combination of hydroxyapatite and hPRP on osteogenesis in vivo, using rabbit model bone healing. A critical size defect of 10 mm long was created in the radial diaphysis of 36 rabbit and either supplied with hydroxyapatite-human PRP or hydroxyapatite or was left empty (control group). Radiographs of each forelimb were taken postoperatively on 1st day and then at the 2nd, 4th, 6th and 8th weeks post injury to evaluate bone formation, union and remodeling of the defect. The operated radiuses of half of the animals in each group were removed on 56th postoperative day and were grossly and histopathologically evaluated. In addition, biomechanical test was conducted on the operated and normal forearms of the other half of the animals of each group. This study demonstrated that hydroxyapatite-humanPRP, could promote bone regeneration in critical size defects with a high regenerative capacity. The results of the present study demonstrated that hydroxyapatite-hPRP could be an attractive alternative for reconstruction of the major diaphyseal defects of the long bones in animal models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evaluation of biomaterials for bone healing promotion is a key issue in orthopedic research surgery. Autogenous bone still remains the “gold standard” of bone graft material in all facets of orthopedic surgery and is commonly used as a standard to which allografts and graft substitutes are compared (Alexander 1985; Alexander 1987; Brinker et al. 1997; Fitch et al. 1997; Fox 1984; McLaughlin and Roush 1998; Bigham et al. 2009). While application of the autografts in the bone defects is effective in diminishing the risk of the infectious disease transmission, they have also optimal osteoconductive, osteoinductive, and osteogenic properties. Moreover, there is no immune response after their implantation, and this criterion enhances their ability to incorporate into the new sites (Lohmann et al. 2001; Pokorny et al. 2003; Shafiei et al. 2009). However, a number of disadvantages such as morbidity in the donor site, the need for general anesthesia or sedation, and occasional requirement for more than one surgical field have also been described in utilization of the autogenous bone grafts. In addition, the graft survival is unpredictable, its resorption cannot be foretold and its availability is limited (Bauer and Muschler 2000; Keating and McQueen 2001; Dehghani et al. 2008). Therefore, several biocompatible materials have emerged as the substitutes of the autologous bone in the recent years. These can be classified into two major organic and synthetic groups. The biological biomaterials can be of allogeneic or homologous (human cortical bone and demineralised bone matrix or demineralized freeze-dried bone), heterologous, or xenogeneic (organic bovine, porcine, caprine, coral-derived hydroxyapatite) and replicating origin (morphogenetic proteins). The artificial or synthetic hydroxyapatite, bioglass and bioceramics are the most popular synthetic biomaterials used in Human and Veterinary Orthopedics (Esposito et al. 2006).
A number of growth factors, including platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), transforming growth factor-β1 (TGF-β1), and insulin like growth factor (IGF) are available in PRP that have stimulating effect on healing of the bone defects. This stimulating effect is resulted due to chemotaxis induction as well as proliferation and differentiation of the osteoblasts and their precursors (Bostrom et al. 1999; Weibrich et al. 2002). An easy and more physiological way of application these growth factors to bone defects is via the use of platelet-rich plasma (PRP), a thrombocyte concentrate made up of autogenous blood (Marx et al. 1998; Weibrich et al. 2002).
Several investigations have previously demonstrated the positive effect of PRP on wound healing (McClain et al. 1996; Mustoe et al. 1991; Saba et al. 2002). A lot of studies have been conducted to investigate the effects of PRP upon regeneration of the bone defects (Marx et al. 1998; Aghaloo et al. 2002; Anitua 1999; Kassolis et al. 2000; Nash et al. 1994; Robiony et al. 2002; Rodriguez et al. 2003; Schlegel et al. 2004). However, the results of these studies are controversial. Marx et al. (1998) and Anitua (1999) used PRP for reconstruction of the maxillofacial defects in humans and found that PRP resulted in a quicker maturation of autogenous bone transplants and resulted to a higher bone density(Marx et al. 1998).
Further clinical investigations suggested an osteogenic potential of PRP but those experiments either did not include control groups (Kassolis et al. 2000; Robiony et al. 2002; Rodriguez et al. 2003) or could not identify any positive effect (Froum et al. 2002). It should be stated that not only the clinical data are contradictory, but in vivo experimental findings are also inconsistent. In a bone defect in the iliac crest of dogs, PRP combined with demineralized bone powder enhanced bone formation around the titanium implants (Kim et al. 2002). In a rabbit skull model, however, PRP did not influence bone healing (Aghaloo et al. 2002). In a similar study in pigs, PRP enhanced bone density temporarily when applied together with autograft but it was not effective when used in conjunction with a collagen scaffold containing additional osteoinductive proteins (Schlegel et al. 2004). Therefore, there are numerous biomaterials available for use to promote bone healing (Esposito et al. 2006), but the exact indication of each of them remains controversial.
No assessment has been made as yet of a combination of human plasma rich platelet (hPRP) with hydroxyapatite on the healing of the long bone defects. Our hypothesis is that the hPRP with other biomaterials such as synthetic hydroxyapatite can promote bone regeneration in radial bone defects in rabbit model. Therefore, hydroxyapatite was selected as a scaffold because of its interconnected porous architecture, high compressive breaking stress, proper biocompatibility and resorbability. The experiment was designed to compare the healing potential of hPRP delivered on a porous hydroxyapatite with that of the hydroxyapatite alone, whil in the animals of the third group the defects were left empty.
Materials and methods
Animals and operative procedures
Thirty six New Zealand white rabbits, 12 months old, of both sexes, weighing 2.00 ± 0.50 kg, were kept in separate cages, fed a standard diet and allowed to move freely during the study. The animals were randomly divided into 3 equal groups as hydroxyapatite group (n = 12), hydroxyapatite-hPRP group (n = 12) and empty group (n = 12, control group). All the animals were anesthetized by intramuscular administration of 40 mg/kg ketamine hydrochloride and 5 mg/kg xylazine. In all animals the right forelimb was prepared aseptically for operation. A 5 cm incision was made craniomedially over the skin of the fore limb and the radius was exposed by dissecting the surrounding muscles. A 10 mm segmental defect was then created in the mid portion of each radius as a critical size bone defect. In the animals of the hydroxyapatite group and hydroxyapatite-hPRP group, the bone defect was filled with hydroxyapatite segments (OS Satura®, Isotis Co, Netherland). Four days after operation 1 ml hPRP was injected percutaneously into the defect of bones in the animals of the hydroxyapatite-hPRP group while the defects of the rabbits of the control group were left empty. The animals were housed in compliance with our institution’s guiding principles ‘‘in the care and use of animals’’. The local Ethics Committee for animal experiments approved the design of the experiment
Preparation of PRP
Human PRP was prepared and supplied by the Shiraz Blood Bank Center. About 500 ml blood from a healthy donor was collected in 70 ml of anticoagulants (citrate–phosphate–dextrose [CPD]) and cooled to about 22°C. Within 24 h of extraction, the blood was separated through centrifugation into erythrocytes, buffy coat (leukocytes and thrombocytes) and plasma. From the buffy coat the leukocytes were removed through filtration, and the isolated fraction of platelets was human PRP. To obtain information on the increase in platelet concentration and the final concentration of platelets in the PRP of the blood, both whole blood and prepared PRP were subjected to platelet counts. Platelet counts were performed using a hematology analyzer (Advia 120, Bayer B.V., Mijdrecht, Netherlands). Number of platelets in the whole blood and PRP was 239 × 109/l and 2,422 × 109/l respectively.
Post operative evaluations
Radiological evaluation
To evaluate bone formation, union and remodeling of the defect, radiographs of each forelimb was taken postoperatively on 1st day and then at the 2nd, 4th, 6th and 8th weeks post injury. The results were scored using the modified Lane and Sandhu scoring system (Lane and Sandhu 1987; Table 1).
Gross evaluation
The operated radial bones of rabbits were removed on 56th postoperative day; at this time the operated radius was evaluated for gross signs of healing. Examination and blinded scoring of the specimens included presence of bridging bone, indicating a complete union (+3 score), presence of cartilage, soft tissue or cracks within the defect indicating a possible unstable union (+1 or +2 score), or complete instability at the defect site indicating no union (0 score).
Histopathological evaluation
Eight weeks after operation the rabbits were euthanized for histopathological and biomechanical evaluation. The histopathological evaluation was carried out on six rabbits of each group randomly. The right forelimb of each animal was harvested and dissected free of soft tissues. Sagital sections containing the defect were cut with a slow speed saw. Each slice was then fixed in 10% neutral buffered formalin. The formalin-fixed bone samples were decalcified in 15% buffered formic acid solution and processed for routine histological examination. Two 5 micron in thickness sections were cut from the centers of each specimen and were stained with hematoxylin and eosin. The sections were blindly evaluated and scored by two pathologists according to the Emery’s scoring system (Emery et al. 1994) and based on this scoring system the defects were evaluated as follows: When the gap was empty (score = 0), if the gap was filled with fibrous connective tissue only (score = 1), with more fibrous tissue than fibrocartilage (score = 2), more fibrocartilage than fibrous tissue (score = 3), fibrocartilage only (score = 4), more fibrocartilage than bone (score = 5), more bone than fibrocartilage (score = 6) and filled only with bone (score = 7).
Biomechanical evaluation
The biomechanical test was conducted on the injured and normal contralateral bones of each rabbit. The tests were performed using a universal tensile testing machine (Instron, London, UK; Oryan et al. 2008, 2011; Oryan and Shoushtari 2009). The three-point bending test was performed to determine the mechanical properties of bones. The bones were placed horizontally on two rounded supporting bars located at a distance of 30 mm, and were loaded at the midpoint of the diaphysis by lowering the third bar so that the defect was in the middle and had an equal distance from each grip. The bones were loaded at a rate of 10 mm/min until fracturing occurred. The behavior of each specimen under loading was characterized by determining the following parameters from the load deformation to destruction curve.
-
1.
Tan-α: the coefficient of inclination for the linear portion of the load-deformation curve, represents the index of stiffness of the material and is expressed as N/mm. It is easily calculated by measuring the slope of a line drawn as a tangent to the curve at any defined point. The slope gives the approximate stiffness of the preparation.
-
2.
Ultimate strength: the highest registered load (N).
-
3.
The specimen’s extension at the ultimate strength region: the term “strain” means the fractional increase in length of the material due to an applied load. It is calculated by dividing the extension by the original length of the specimen. Strain is more useful than extension, because it minimizes the influence of length measurement error and does not depend on the specimen size.
-
4.
Stress: Ultimate strength proportion to cross sectional area
The data derived from the load deformation curves were expressed as Mean ± SEM for each group and maximum load, stiffness, stress and strain was measured and recorded.
Statistical analysis
The radiological, clinical and histopathological data were compared by Kruskal–Wallis, non-parametric ANOVA, when P values were found to be less than 0.05, then pair wise group comparisons was performed by Mann–Whitney U test. The biomechanical data were compared by a student’s t test between the treated and normal limb data and one way ANOVA test was used for biomechanical analysis between the treated bones of all groups (SPSS version 17 for windows, SPSS Inc, Chicago, USA).
Results
Radiological findings
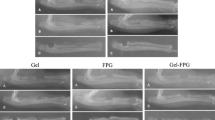
There was a significant difference in bone formation between the hydroxyapatite with hPRP-hydroxyapatite group on the 14th post injury days (P < 0.05). Bone healing in hPRP-hydroxyapatite group was superior to hydroxyapatite group. Superior bone formation was observed in hPRP-hydroxyapatite group in comparison with control group in 42nd post injury days (P < 0.05). There was a significant difference in bone formation between the control and hydroxyapatite with hPRP-hydroxyapatite group on the 56th post injury days (P < 0.05). By day 56, there was 75–100% bone formation in the animals of the hydroxyapatite and hPRP-hydroxyapatite group compared to those of the control group that showed 50–75% bone formation (Table 2; Figs. 1, 2, 3).
Bone union had occurred in the hydroxyapatite and hPRP-hydroxyapatite rabbits by day 28th, 42nd and 56th post-injury, but not in controls group. In addition, bone union in the animals of the hydroxyapatite group by day 42nd and 56 post-injury was more prominent than the control ones. This trend continued with less union occurring in the animals of the control group (Tables 3, 4; Figs. 1, 2, 3).
Remodeling was superior in hPRP-hydroxyapatite group in comparison with the hydroxyapatite and control groups on 42th and 56th days post surgery. However, the animals of the Hydroxyapatite group showed better remodeling criteria on day 56 than those of the control group (Table 5; Figs. 1, 2, 3).
Gross and histopathological findings
The defect areas of the rabbits in all groups showed various amounts of new bone formation; however, the defects of the animals of the control group left blank or generally contained the least amounts of new bone and were often filled with a mixture of fibrous connective tissue and cartilage. The union scores of the rabbits administered with hydroxyapatite or hydroxyapatite-hPRP were statistically superior to those of the animals of the control group (Table 6). The union scores at macroscopic level correlated closely with the radiographic union score on day 56 post injury.
At histopathologic level, the defectes of the animals of the hydroxyapatite and hydroxyapatite-hPRP group showed more advanced healing criteria than those of the control group (Table 6).
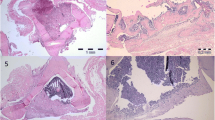
Fibrous nonunions or fibrocartilages in the defects of the animals of the control group were dominant and the lesions of these animals showed poor re-vascularization. Bridging callus or histological union did not develop in any of these defects. These criteria lead to very slow healing process in the animals of the control group (Fig. 4).
The defects of two rabbits of the hydroxyapatite group were filled with mature cortical bone and the lesion in the rest three from four rabbits was substituted with fibrocartilage tissues. However, the defects of the animals in the hydroxyapatite group showed some angiogenic activities but it was not as well as those of the hydroxyapatite-platelet group (Fig. 4).
Normal trabecular and woven bone were uniformly formed within the defects of the animals that were treated with hydroxyapatite-platelet regimen and the lesions of five from six animals of this group were filled with woven bone and showed proper maturation, however, the defect of the last rabbit contained more fibrocartilage than bone. The regenerated bone completely spanned the defect and most instantly produced full histologic union. Active endochondral ossification and secondary fracture repair took place in the middle of the defect of the animals of the hydroxyapatite-platelet group (Fig. 4). No significant inflammatory response was evident in the lesions of the animals of different groups at 8 weeks post injury, although it may have been present earlier (Figs. 5, 6, 7, 8, 9).
Biomechanical findings
There was statistical significant difference (P = 0.01) between the injured bone with normal bone of the control group in terms of ultimate strength and stiffness and the normal bones had superior ultimate strength and stiffness(P = 0.04) compared to their normal contralateral bones. However, the ultimate strength and stiffness of the treated animals of both hydroxyapatite and hydroxyapatite-hPRP groups showed more advanced values so that they were not statistically significant with those of their normal contralaterals. There was statistical significant difference between the treated bone of the hydroxyapatite-hPRP with those of the control group (P = 0.001) in terms of stress and the treated bones had superior stress compared to those of the injured bones of the animals of the control group (Table 7).
Discussion
To evaluate the bone healing potential of a combination of hydroxyapatite and human PRP a defect model was established in the radial bone of rabbits. This model has previously been reported suitable because there is no need for internal or external fixation which influences the healing process (An and Friedman 1999). The segmental defect was created in the middle portion of the radius as long as 10 mm for inducing nonunion defect and to prevent spontaneous and rapid healing (Bolander and Galian 1983).
The hypothesis was on the basis that addition of the hPRP to a mixture of the particulate hydroxyapatite in a critical size defect of the radial bone of rabbit could have a positive effect on bone formation.
The results of the radiological, macroscopical, histological and biomechanical examinations showed that bone healing were enhanced when hPRP was used concurrent with hydroxapatite. These results are not in agreement with the rare studies that have been conducted previously (Mooren et al. 2007).
This study was performed to provide an explanation for the existing confusion in the literature regarding the efficacy of PRP treatment in combination with other artificial bone graft substitutes, and to give more insights into the effect of PRP on bone regeneration. To the authors’ knowledge this is one of the first studies, which presents new data on the bone regenerative properties of human PRP as a xenogenic PRP concurrent with hydroxyapatite effects on bone healing in rabbit model.
The clinical and experimental data in the literature regarding the osteogenic potential of PRP are controversial. The results of the present investigation confirm a number of clinical and experimental studies demonstrating a positive influence of hPRP on bone regeneration (Marx et al. 1998; Anitua 1999; Schlegel et al. 2004; Thorwarth et al. 2005). However, in human maxillofacial defects, neither autograft nor allograft or a mineral bone substitute material enhanced bone formation when augmented with PRP (Froum et al. 2002; Shanaman et al. 2001; Raghoebar et al. 2005). In a non-critical rabbit skull defect, autogenously PRP was not superior to the empty defect nor did PRP increased bone formation by autogenous bone (Aghaloo et al. 2002).
This study demonstrates the hydroxyapatite-hPRP’s role in treating bone defects. From both quantitative and qualitative analyses conducted using the four outcome measurements described in this study, significant differences were present between the defects of the animals of the hydroxyapatite-hPRP treated group and those of the two other groups.
Platelet rich plasma was found to be effective only when it was used together with bone graft. This has been similarly observed in the previous studies (Grageda 2004). Hydroxyapatite as a artificial bone graft was used as it has no osteoinductive potential. Its use with PRP provided the evidence required to demonstrate the tissue-enhancing ability of PRP. Overall, the results of this study correlate with the findings of other studies that supported the use of PRP in almost similar conditions. This has convinced many clinicians and scientists to support its use in clinical practice (Froum et al. 2002; Kim et al. 2002; Marx 2004).
The results of the present study indicate that hPRP in combination with hydroxyapatite stimulates a favorable reaction in the injured area of the long bones. The radiographic evaluation showed that the bone gap in the hydroxyapatite-hPRP group was healed before that of the control group and it was also already in the remodeling stage. While the defects of the control animals even at the end of 8 weeks post-injury were still in the fibroplasia stage. This fact was corroborated by macroscopic, microscopic and biomechanical data analysis, which showed that osteogenesis in the defects of the animals of hydroxyapatite-hPRP group was stronger than those of the two other groups at 56 days post-injury.
Hydroxyapatite, a crystalline phase of calcium phosphate found naturally in bone minerals, has shown tremendous promise as a graft material. It exhibits initial mechanical rigidity and structure, and demonstrates osteoconductive as well as angiogenic properties in vivo (Kilian et al. 2008; Appleford et al. 2009; Yoshikawa et al. 2000). In osteoperiosteal gaps bridged with hydroxyapatite only, the porosities were invaded with fibrous tissue or fibrocartilage tissues were more than bone tissues. Occasionally, bone formation was observed in direct contact with hydroxyapatite, confirming its osteoconductive ability, but it was insufficient to allow union. These findings are similar to those reported using hydroxyapatite. When the gap reaches a critical size the osteoconductive properties of the material are insufficient to fill the gap with formation of new bone (Ohgushi et al. 1989). Therefore this model proved to be adequate for evaluation of hydroxyapatite as a scaffold for human platelet.
More unexpected is formation of the cortex and medullary canal together with mature lamellar bone observed in most of the cases. The previous in vitro studies have shown that artificial bone graft materials supports the attachment, growth and differentiation of the bone-marrow stromal cells (Petite et al. 1996). The findings of the present study suggest that hydroxyapatite is a suitable resorbable carrier for platelet in vivo. It serves as a substrate to promote the stem cells of the bone marrow to attach and grow, and as a template, to guide bone morphogenesis in a clinically relevant volume.
The platelet rich plasma contains several growth factors including isomers of platelet drived growth factor (PDGF), transforming growth factor-X1 (TGF-X 1), transforming growth factor-2 (TGF-2), Insulin like growth factor-I (IGF-I), Insulin like growth factor-II (IGF-II) and vascular endothelial growth factor (VEGF). All these growth factors are promotors of bone regeneration. Platelet derived growth factor has been shown to be mitogenic for osteoblasts (Assoian et al. 1984) and stimulates migration of the mesenchymal progenitor cells (Fiedler et al. 2002). It has been stated that PDGF was able to induce callus formation in the bone defects of the animal models (Nash et al. 1994). TGF-X also has a stimulative effect on osteogenesis and inhibits bone resorption (Baylink et al. 1993). In addition, it has been reported that IGF-I and the angiogenic factor VEGF induced bone formation in rats (Spencer et al. 1991) and in rabbits (Street et al. 2002), respectively. The findings of the present study suggest that superiority of the angiogenesis in the defects of the animals of group hydroxyapatite-hPRP was possibly due to the presence of VEGF in human platelet. These growth factors support bone regeneration primarily via their chemotactic and mitogenic effects on preosteoblastic and osteoblastic cells. Due to this phenomenon, an enhanced bone formation criteria in the defects of the animals of the hydroxyapatite-hPRP group compared to those of the control ones was observed. However, hPRP does not contain BMPs, the most potent osteoinductive proteins, that are the only growth factors known to induce ectopic bone formation which promote stem cells to differentiate into the osteoblastic lineage (Cook 1999).
The enhanced healing effects of the human PRP after combination with human bone graft material, compared to a combination with a synthetic bone substitute, can also be explained by the mechanism of action of PRP. According to Marx et al. (Marx 2004), PRP is thought to exert its effects on living cells. Consequently, when PRP is used together with synthetic, non-cellular bone substitutes less promotion of bone formation could be expected compared to its application with the bone graft material. The beneficial effects of PRP applied in combination with a synthetic bone substitute, depend on the number of resident osteoprogenitor cells at the bone defect site. Occasionally, the osteoconductive materials can obscure the true effects of PRP. However, in the present study, combination of hPRP with hydroxyapatite lead to superior bone healing in comparison with the hydroxyapatite alone and the control group. Therefore, based on the findings of the present study, it could be concluded that high concentrations of platelets is effective and lead to superior and faster bone formation. While Schlegel et al. (2004) and Thorwarth et al. (2005) got better results by administering higher doses of hPRP (6.5-fold compared to normal blood) than with lower platelet concentrations (4.1-fold) on bone regeneration in skull defects of minipigs (Schlegel et al. 2004; Thorwarth et al. 2005), some other experimental studies found no correlation between the platelet concentration and the observed biological effects (Aghaloo et al. 2002; Kim et al. 2002).
In overall, possibly one reason in demonstrating such conflicting findings regarding use of the platelet rich plasma in the treatment of a wide variety of “wounds” has to do with the variability in concentrations of growth and differentiation factors in differing preparations of platelet rich plasma. Therefore, an estimate of the concentration of at least one growth and differentiation factor in such hPRP preparations should be performed in the future studies. Unfortunately, in the present study the authors did not quantify either existence or concentration of main growth factors of the hPRP used in their experiment. In fact, it is not the platelet rich plasma, after all, that represents the “healing properties” of what is being used, but rather the combined growth and differentiation factors that the platelet rich plasma presents to some wound site. However, it is suggested in future some of the confusion in the prior literatures.
Conclusions
In conclusion this study demonstrated that hydroxyapatite-hPRP could promote bone regeneration in critical size defects with a high regenerative capacity. This finding will nominate hydroxyapatite-hPRP as an attractive alternative for reconstruction of the major diaphyseal defects in the long bones in animal models. Combination of functional biomaterials or autografts, precursor cells or osteoinductive growth factors with hydroxyapatite in animal models, in the future studies, may introduce more effective therapeutic regimes in regeneration and bone formation of the long bone injuries.
References
Aghaloo TL, Moy PK, Freymiller EG (2002) Investigation of platelet-rich plasma in rabbit cranial defects: a pilot study. J Oral Maxillofac Surg 60:1176–1181
Alexander JW (1985) Leonard’s orthopedic surgery of the dog and cat, 3rd edn. WB Sounders Company, Florida
Alexander JW (1987) Bone grafting. Vet Clin North Am Small Anim Pract 17(4):811–819
An YH, Friedman RJ (1999) Animal models in orthopedic research, 1st edn. CRC Press Inc., Boca Raton
Anitua E (1999) Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants 14:529–535
Appleford MR, Oh S, Oh N, Ong JL (2009) In vivo study on hydroxyapatite scaffolds with trabecular architecture for bone repair. J Biomed Mater Res A 89(4):1019–1027
Assoian RK, Grotendorst GR, Miller DM, Sporn MB (1984) Cellular transformation by coordinated action of three peptide growth factors from human platelets. Nature 309:804–806
Bauer TW, Muschler GF (2000) Bone graft materials: an overview of the basic science. Clin Orthop Rel Res 371:10–27
Baylink DJ, Finkelman RD, Mohan S (1993) Growth factors to stimulate bone formation. J Bone Miner Res 8:565–572
Bigham AS, Dehghani SN, Shafiei Z, Torabi Nezhad S (2009) Experimental bone defect healing with xenogenic demineralized bone matrix and bovine fetal growth plate as a new xenograft: radiological, histopathological and biomechanical evaluation. Cell Tissue Banking 10(1):33–41
Bolander ME, Galian G (1983) The use of demineralize bone matrix in the repair of segmental defect. J Bone Jt Surg 68A:1264–1274
Bostrom MP, Saleh KJ, Einhorn TA (1999) Osteoinductive growth factors in preclinical fracture and long bone defects models. Orthop Clin North Am 30:647–658
Brinker WO, Piermattei DL, Flo GL (1997) Bone grafting. Small animal orthopedics and fracture repair, 3rd edn. WB Saunders Company, Florida
Cook SD (1999) Preclinical and clinical evaluation of osteogenic protein-1 (BMP-7) in bony sites. Orthopedics 22:669–671
Dehghani SN, Bigham AS, Torabi Nezhad S, Shafiei Z (2008) Effect of bovine fetal growth plate as a new xenograft in experimental bone defect healing: radiological, histopathological and biomechanical evaluation. Cell Tissue Banking 9(2):91–99
Emery SE, Brazinski MS, Koka A, Bensusan JS, Stevenson S (1994) The biological and biomechanical effects of irradiation on anterior spinal bone grafts in a canine model. J Bone Jt Surg 76(4):540
Esposito M, Grusovin MG, Coulthard P, Worthington HV (2006) The efficacy of various bone augmentation procedures for dental implants: a Cochrane systematic review of randomized controlled clinical trials. Int J Oral Maxillofac Implants 21:696–710
Fiedler J, Roderer G, Gunther KP, Brenner RE (2002) BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem 87:305–312
Fitch R, Kerwin S, Newman-Gage H, Sinibaldi KR (1997) Bone autografts and allografts in dogs. Comp Vet Cont Ed 19(5):558–575
Fox SM (1984) Cancellous bone grafting in the dog: an overview. J Am Anim Hosp Assoc 20:840–848
Froum SJ, Wallace SS, Tarnow DP, Cho SC (2002) Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int J Periodont Restor Dent 22:45–53
Grageda E (2004) Platelet-rich plasma and bone graft materials: a review and a standardized research protocol. Implant Dentistry 13(4):301–309
Kassolis JD, Rosen PS, Reynolds MA (2000) Alveolar ridge and sinus augmentation utilizing platelet-rich plasma in combination with freeze-dried bone allograft: case series. J Periodontol 71:1654–1661
Keating JF, McQueen MM (2001) Substitutes for autologous bone graft in orthopaedic trauma. J Bone Joint Surg Am 83-B:3–8
Kilian O, Wenisch S, Karnati S, Baumgart-Vogt E, Hild A, Fu-hrmann R (2008) Observations on the microvasculature of bone defects filled with biodegradable nanoparticulate hydroxyapatite. Biomaterials 29(24–25):3429–3437
Kim SG, Kim WK, Park JC, Kim HJ (2002) A comparative study of osseointegration of Avana implants in a demineralized freeze-dried bone alone or with platelet-rich plasma. J Oral Maxillofac Surg 60:1018–1025
Lane JM, Sandhu HS (1987) Current approach to experimental bone grafting. Orthop Clin North Am 18:213–225
Lohmann CH, Andreacchio D, Koster G, Carnes DL, Dean BD, Schwartz Z (2001) Tissue response and osteoinduction of human bone grafts in vivo. Arch Orthop Trauma Surg 121:583–590
Marx RE (2004) Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 62(4):489–496
Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR (1998) Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 85:638–646
McClain SA, Simon M, Jones E, Nandi A, Gailit JO, Tonnesen MG (1996) Mesenchymal cell activation is the rate-limiting step of granulation tissue induction. Am J Pathol 149:1257–1270
McLaughlin RM, Roush JK (1998) Autogenous cancellous and cortico-cancellous bone grafting. Vet Med 93(12):1071–1074
Mooren RECM, Merkx MAW, Bronkhorst EM, Jansen JA, Stoelinga PJW (2007) The effect of platelet-rich plasma on early and late bone healing: an experimental study in goats. Int J Oral Maxillofac Implants Surg 36:626–631
Mustoe TA, Pierce GF, Morishima C, Deuel TF (1991) Growth factorinduced acceleration of tissue repair through direct and inductive activities in a rabbit dermal ulcer model. J Clin Invest 87:694–703
Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, Hicklin DJ (1994) Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone 15:203–208
Ohgushi H, Goldberg VM, Caplan AI (1989) Repair of bone defects with marrow cells and porous ceramic: experiments in rats. Acta Orthop Scand 60:334–339
Oryan A, Shoushtari AH (2009) Biomechanical properties and dry weight content of the developing superficial digital flexor tendon. Comp Clin Pathol 18:131–137
Oryan A, Goodship AE, Silver IA (2008) Response of a collagenase-induced tendon injury to treatment with a polysulphated glycosaminoglycan (Adequan). Connect Tissue Res 49:351–360
Oryan A, Moshiri A, Meimandiparizi A (2011) Effects of sodium-hyaluronate and glucosamine-chondroitin sulfate on remodeling stage of tenotomized superficial digital flexor tendon in rabbits: a clinical, histopathological, ultrastructural, and biomechanical study. Connect Tissue Res 52(4):329–339
Petite H, Kacem K, Triffitt JT (1996) Adhesion, growth and differentiation of human bone marrow stromal cells on non-porous calcium carbonate and plastic substrata: effects of dexamethasone and 1, 25 dihydroxyvitamin D3. J Mater Sci Mater in Med 7:665–671
Pokorny JJ, Davids H, Moneim M (2003) Vascularized bone graft for scaphoid nonunion. Tech Hand Up Extrem Surg 7:32–36
Raghoebar GM, Schortinghuis J, Liem RS, Ruben JL, van der Wal JE, Vissink A (2005) Does platelet-rich plasma promote remodeling of autologous bone grafts used for augmentation of the maxillary sinus floor? Clin Oral Implants Res 16:349–356
Robiony M, Polini F, Costa F, Politi M (2002) Osteogenesis distraction and platelet-rich plasma for bone restoration of the severely atrophic mandible: preliminary results. J Oral Maxillofac Surg 60:630–635
Rodriguez A, Anastassov GE, Lee H, Buchbinder D, Wettan H (2003) Maxillary sinus augmentation with deproteinated bovine bone and platelet rich plasma with simultaneous insertion of endosseous implants. J Oral Maxillofac Surg 61:157–163
Saba AA, Freedman BM, Gaffield JW, Mackay DR, Ehrlich HP (2002) Topical platelet-derived growth factor enhances wound closure in the absence of wound contraction: an experimental and clinical study. Ann Plast Surg 49:62–66
Schlegel KA, Donath K, Rupprecht S, Falk S, Zimmermann R, Felszeghy E (2004) De novo bone formation using bovine collagen and platelet-rich plasma. Biomaterials 25:5387–5393
Shafiei Z, Bigham AS, Dehghani SN, Torabi NS (2009) Fresh cortical autograft versus fresh cortical allograft effects on experimental bone healing in rabbits: radiological, histopathological and biomechanical evaluation. Cell Tissue Banking 10:19–26
Shanaman R, Filstein MR, Danesh-Meyer MJ (2001) Localized ridge augmentation using GBR and platelet-rich plasma: case reports. Int J Oral Maxillofac Implants Periodont Restor Dent 21:345–355
Spencer EM, Liu CC, Si EC, Howard GA (1991) In vivo actions of insulinlike growth factor-I (IGF-I) on bone formation and resorption in rats. Bone 12:21–26
Street J, Bao M, deGuzman L, Bunting S, Peale JF, Ferrara N (2002) Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA 99:9656–9661
Thorwarth M, Rupprecht S, Falk S, Felszeghy E, Wiltfang J, Schlegel KA (2005) Expression of bone matrix proteins during de novo bone formation using a bovine collagen and platelet-rich plasma (prp)-an immunohistochemical analysis. Biomaterials 26:2575–2584
Weibrich G, Kleis WK, Hafner G, Hitzler WE (2002) Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg 30:97–102
Yoshikawa T, Ohgushi H, Nakajima H, Yamada E, Ichijima K, Tamai S (2000) In vivo osteogenic durability of cultured bone in porous ceramics: a novel method for autogenous bone graft substitution. Transplantation 69(1):128–134
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oryan, A., Meimandi Parizi, A., Shafiei-Sarvestani, Z. et al. Effects of combined hydroxyapatite and human platelet rich plasma on bone healing in rabbit model: radiological, macroscopical, hidtopathological and biomechanical evaluation. Cell Tissue Bank 13, 639–651 (2012). https://doi.org/10.1007/s10561-011-9285-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-011-9285-x