Abstract
Coral is an osteoconductive material used as a bone graft extender and human platelet rich plasma has been used as a source of osteoinductive factor. A combination of human platelet rich plasma and coral is expected to create a composite with both osteoconductive and osteoinductive properties. This study examined the effect of a combination of human platelet rich plasma and coral on osteogenesis in vivo using rabbit model of bone healing. A critical size defect of 10 mm elongation was created in the radial diaphysis of 36 rabbit and either supplied with coral-human PRP, or coral alone or left empty (control group). The platelets in the PRP were about 10.1 fold compared to normal blood. Radiographs of each forelimb was taken postoperatively on 1st day and then at the 2nd, 4th, 6th and 8th weeks post injury to evaluate bone formation, union and remodeling of the defect. The operated radiuses were removed on 56th postoperative day and were grossly and histopathologically evaluated. In addition, biomechanical test was conducted on the operated and normal forearms of the rabbits. This study demonstrated that coral-human PRP (hPRP), could promote bone regeneration in critical size defects with a high regenerative capacity. The results of the present study demonstrated that coral-hPRP could be an attractive alternative for reconstruction of the major diaphyseal defects of the long bones in animal models.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Bone grafting is used to enhance healing in large bone defects resulting from trauma, tumors, osteitis, delayed unions, non unions, ostoectomies, arthrodesis, and multifragmentary fractures [1–3]. Various bone graft substitutes including autografts, allografts, xenografts, polymers, ceramics and some metal have been employed to promote bone reunion [4, 5]. They may provide a source of osteoprogenitor cells (osteogenesis), induce formation of osteoprogenitor cells from the surrounding tissues (osteoinduction), and provide mechanical support for vascular and bone ingrowth (osteoconduction) [6]. Autogenous bone still remains the “gold standard” of bone graft material in all facets of orthopedic surgery and is commonly used as a standard to which allografts and graft substitutes are compared [7–12]. While application of the autografts in the bone defects are effective in diminishing the risk of the infectious disease transmission, they have also optimal osteoconductive, osteoinductive, and osteogenic properties. Moreover, there is no immune response after their implantation, and this criterion enhanes their ability to incorporate into the new sites [13, 14]. As a graft, autogenous bone is ideal, but harvest of the autografts may be associated with severe donor site pain and morbidity even with new trapdoor harvesting techniques [15, 16]. In procedures requiring large amounts of graft, there may not be adequate quantities of autogenous bone available [16]. Because of the significant shortcomings of autogenous bone graft, a current understanding of available grafting alternatives is necessary. An allograft, by definition, is any tissue harvested from one individual and implanted into another of the same species [15]. In a search for an adequate substitute for autogenous bone, cadaveric allograft has been a viable option and structural and morselized forms are available and prepared as either fresh-frozen or freeze-dried [16]. These grafts provide a structural framework or scaffold for host tissue to grow, hence making allograft osteoconductive. Conversely, its osteoinductive properties are mediocre at best. Upon implantation, the host is expected to experience an intricate immune response [16, 17]. According to the above explanations, transplantation of autografts or allografts, mineral bone substitutes [18–20] and callus distraction are the most commonly used techniques for skeletal reconstruction. However, each of these procedures have its own significant limitations such as lack of availability or it may not be appropriate because of biological or biomechanical reasons [1, 21]. Therefore, osteoinductive stimulation of bone formation has received increasing interest.
Both demineralized bone matrix and growth factors have been used in numerous experimental and clinical defect situations [22]. A number of growth factors are present in PRP, including platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), transforming growth factor-β1 (TGF-β1), and insulin like growth factor (IGF) and they have a stimulating effect on healing of the bone defects. This stimulating effect is resulted due to chemotaxis induction as well as proliferation and differentiation of osteoblasts and their precursors [22, 23]. An easy and more physiological way of application of growth factors to bone defects is via the use of platelet-rich plasma (PRP), a thrombocyte concentrate made up of autogenous blood [23, 24].
Several investigations previously demonstrated a positive effect of PRP on wound healing [25–27]. A lot of studies have been conducted to investigate the effects of PRP upon regeneration of the bone defects [24, 28–34]. However, the results of these studies are controversial. Marx et al. [24] used PRP for reconstruction of the maxillofacial defects in humans and found that PRP resulted in a quicker maturation of autogenous bone transplants and resulted a higher bone density. Another prospective study also reported a positive effect of PRP in a similar defect situation [29]. Further clinical investigations suggested an osteogenic potential of PRP but did not include control groups [30, 32, 33] or could not identify any positive effect [35]. It should be stated that not only the clinical data are contradictory, but in vivo experimental findings are also inconsistent. In a bone defect in the iliac crest of dogs, PRP combined with demineralized bone powder enhanced bone formation around the titanium implants [36]. In a rabbit skull model, however, PRP did not influence bone healing [28]. In a similar study in pigs, PRP enhanced bone density temporarily when applied together with autograft but it was not effective when used in conjunction with a collagen scaffold containing additional osteoinductive proteins [34].
Certain coral species form a structure that resembles the matrix of bone. Each species builds a structurally and geometrically typical calcium carbonate skeleton. Choice of the appropriate species therefore enables a desired and constant implant structure to be achieved. More than 2000 coral species have been described from the intertropical area and, of these, fourteen corals have been studied as possible bone substitutes. The following genera have already been used as bone grafts: Pocillopora, Acropora, Montipora, Porites, Goniopora, Fungia, Polyphyllia, Favites, Acanthastrea, Lobophyllia and Turbinaria [37]. The most prominent species in terms of cover and frequency are Porites lutea and P. compressa in Persian Gulf and Kish Island [38, 39].
Calcium carbonate (CaCO3) resembles hydroxyapatite in many respects. The material is biocompatible and osteoconductive but, similar to hydroxyapatite, has no osteoinductive properties [40]. The main difference of CaCO3 with hydroxyapatite is the resorption. Resorption seems to be clinically unimportant with hydroxyapatite, but the animal experiments have shown resorption rates of only a few weeks, when calcium carbonate is used [41].
No assessment has been made as yet of a combination of calcium carbonate and human plasma rich platelet (hPRP) in healing of the bone defects. Therefore, the present study was designed to determine whether hPRP in combination with coral could regenerate a large segmental bone defect which would otherwise not repair. Coral was selected as a scaffold because of its interconnected porous architecture, high compressive breaking stress, good biocompatibility and resorbability. The experiment was devised to compare the healing potential of hPRP delivered on a porous coral biomatrix with that of the biomatrix alone, or a defect left empty.
2 Materials and methods
2.1 Animals and operative procedures
Thirty-six New Zealand white rabbits, 12 months old, of both sexes, weighing 2.0 ± 0.5 kg, were kept in separate cages, fed a standard diet and allowed to move freely during the study. The animals were randomly divided into three equal groups as coral group (n = 12), coral-hPRP group (n = 12) and empty group (n = 12, control group). All the animals were anesthetized by intramuscular administration of 40 mg/kg ketamine hydrochloride and 5 mg/kg xylazine. The right forelimb of all the animals was prepared aseptically for operation. A 5 cm incision was made craniomedially over the skin of fore limb and the radius was exposed by dissecting the surrounding muscles. A 10 mm segmental defect was then created in the mid portion of each radius as a critical size bone defect. In the coral group and coral-hPRP group, the bone defect was filled with Persian Gulf coral segments. Four days after operation 1 ml hprp was injected percutaneously into the defect of bones in the animals of the coral-hPRP group while defects of the rabbits of the control group was left empy. Normally surgical procedures lead to inflammatory reactions and the acute phase of the inflammatory processes usually lasts for about 4 days. The authors proposed that if the hPRP was injected intraoperatively during the surgical operation, the injected hPRP could be destroyed by the inflammatory mediators and it possibly was not effective in the later stages of healing. In addition, the authors let the fibrin clot present in the hemaetoma to be removed from the defected area and then the hPRP was injected in the organized tissue to exert its effect in a more longer period of fibroplasia phase of healing. The animals were housed in compliance with our institution’s guiding principles ‘‘in the care and use of animals’’. The local Ethics Committee for animal experiments approved the design of the experiment.
2.2 PRP preparation
Human PRP was prepared and supplied by the Shiraz Blood bank Center. About 500 ml blood from a healthy donor was collected in 70 ml of anticoagulants (citrate–phosphate–dextrose [CPD]) and cooled to about 22°C. Within 24 h of extraction, the blood was separated through centrifugation into erythrocytes, buffy coat (leukocytes and thrombocytes) and plasma. From the buffy coat the leukocytes were removed through filtration, and the isolated fraction of platelets was human PRP. To obtain information on the increase in platelet concentration and the final concentration of platelets in the PRP of the blood, both whole blood and prepared PRP were subjected to platelet counts. Platelet counts were performed using a hematology analyzer (Advia 120, Bayer B.V., Mijdrecht, Netherlands). Number of platelets in whole blood and PRP was 239 × 109/l and 2422 × 109/l respectively.
2.3 Preparation of coral implants
Coral exoskeleton from Porites sp. (Persian Gulf, Kish Island, Iran) was used in the form of cylindrical blocks of 4 mm in diameter and 10 mm long. The coral implants were sterilized by autoclaving so that the composition remained intact [42]. The implants were prepared as segmented cone shape to allow them to fill the created defects.
3 Post operative evaluations
3.1 Radiological evaluation
To evaluate bone formation, proximal and distal union and remodeling of the defect, radiographs of each forelimb was taken postoperatively on 1st day and then at the 2nd, 4th, 6th and 8th weeks post injury. Any bone formation between coral and proximal segment of the radius bone was considered as the proximal union and any bone formation between the coral with the distal segment of the radius bone was considered as the distal union. The results were scored using the modified Lane and Sandhu scoring system [43] (Table 1).
3.2 Gross evaluation
The operated radial bones of rabbits were removed on 56th postoperative day; at this time the operated radius was evaluated for gross signs of healing. Examination and blinded scoring of specimens included presence of bridging bone, indicating a complete union (+3 score), presence of cartilage, soft tissue or cracks within the defect indicating a possible unstable union (+1 or +2 score), or complete instability at the defect site indicating no union (0 score).
4 Histopathological evaluation
Eight weeks after operation the rabbits were euthanized for histopathological and biomechanical evaluation. Histopathological evaluation was carried out on six rabbits of each group randomly. Right forelimb were harvested and dissected free of soft tissues. Sagital sections containing the defect were cut with a slow speed saw. Each slice was then fixed in 10% formalin. The formalin-fixed bone samples were decalcified in 15% buffered formic acid solution and processed for routine histological examination. Two 5 micron thick sections were cut from the centers of each specimen and were stained with Hematoxylin and Eosin. The sections were blindly evaluated and scored by two pathologists according to Emery’s scoring system [44] and based on this scoring system the defects were evaluated as follows: When the gap was empty (score = 0), if the gap was filled with fibrous tissue only (score = 1), with more fibrous tissue than fibrocartilage (score = 2), more fibrocartilage than fibrous tissue (score = 3), fibrocartilage only (score = 4), more fibrocartilage than bone (score = 5), more bone than fibrocartilage (score = 6) and filled only with bone (score = 7).
4.1 Biomechanical evaluation
The biomechanical test was conducted on the injured treated and untreated and their normal contralateral bones of each rabbit. The tests were performed using a universal tensile testing machine (Instron, London, UK). The three-point bending test was performed to determine the mechanical properties of bones. The bones were placed horizontally on two rounded supporting bars located at a distance of 30 mm, and were loaded at the midpoint of the diaphysis by lowering the third bar so that the defect was in the middle and had an equal distance from each grip. The bones were loaded at a rate of 10 mm/min until fracturing occurred. Changes in the length (ΔL) and toleration of ultimate (maximum) load were detected from the graph sketched by the machine.
The bending stiffness was derived using the following equation:
Bending stiffness (or bending rigidity) S = EI in Nmm2, which is the product of the Elastic modulus E and the axial second moment of inertia I. This is calculated by the formula: S = EI = (L3/48) × (delta F/delta w), where L is the distance between the supporting bars, F is the force, and w is the deformation. Delta F/delta w is taken from the (most) linear part of the load-deformation curve. The data derived from the load-deformation curves such as ultimate load and calculated bending stiffness were expressed as mean ± SEM for each group.
5 Statistical analysis
The radiological, clinical and histopathological data were compared by Kruskal–Wallis, non- parametric ANOVA, when P-values were found to be less than 0.05, then pair wise group comparisons was performed by Mann–Whitney U test. The biomechanical data were compared by a Paired t test between the treated and utreated bone of the contralateral data and one way ANOVA test used for biomechanical analysis between treated bones of groups. All biomechanical data passed normally distribution test and Bonferroni’s method used for multiple testing and results presented as means ± SEM (SPSS version 17 for windows, SPSS Inc, Chicago, USA).
6 Results
6.1 Radiological findings
There was a significant difference in bone formation between control with coral and coral-platelet group on the 56th day post injury (P < 0.05). By day 56, there had been 75–100% bone formation in the animals of the coral and coral-hPRP group and 50–75% bone formation in those of the control group (Table 2) (Fig. 1).
Bone union had occurred in coral-hPRP rabbits by day 14th, 28th and 42nd post-surgery, but not in the controls and coral group. In addition, bone union in the animals of the coral group by day 42nd post-surgery was more prominent than the control ones. This trend continued with less union occurring in controls (Tables 3, 4; Fig. 1).
Remodeling was not found in either group on 14th, 28th, 42nd and 56th days post surgery (Table 5; Fig. 1).
6.2 Gross and histopathological findings
The defect areas of all rabbits contained new bone; however, the defects left blank or generally contained the least amount of new bone and were often filled with a mixture of fibrous connective tissue and cartilage. The union scores of the rabbits administered with coral or coral-hPRP were statistically superior to control group and their values were greater than the control animals (Table 6). The union score at macroscopic level correlated closely with the radiographic union score at day 56 (Figs. 2, 3).
In histological evaluation coral and coral-platelet group were superior to control group (Table 6). Fibrous nonunions or fibrocartilages were produced in the defects of the animals of the control group and the lesions of these animals were poorly re-vasculated. Bridging callus or histological union did not develop in any of these defects. These phonemes lead to very slow healing process in control group (Figs. 4, 5).
The defect of two rabbits of coral group were filled with mature cortical bone and the lesion in the rest four rabbits were substituted fibrocartilage tissues. However, the defects of the animals in the coral group showed some angiogenic activities but it was not as well as those of the coral-platelet group (Figs. 6, 7). Normal trabecular and woven bone were uniformly formed within the defects of the animals that were treated with coral-platelet and the lesions of three from six animals of this group were filled with woven bone and showed proper maturation. The regenerated bone completely spanned the defect and most instantly produced full histologic union. Active endochondral ossification and secondary fracture repair took place in the middle of the defect in coral-platelet group (Figs. 8, 9). No significant inflammatory response was evident in the lesions of the animals of different groups at 8 weeks, although it may have been present earlier (Fig. 10).
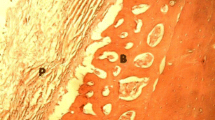
Photomicrograph of sagittal section of coral group, bone marrow was not forming in the grafted area (note to white arrow), although trabecular bone was observed in the grafted area (note to white arrow). Note to the old bone and bone marrow with old bone–coral integration (black arrows) (H & E stain 10×)
6.3 Biomechanical findings
There was statistical significant differences between the treated bone with normal bone of the control group in terms of ultimate load (P = 0.01) and the normal bone had superior ultimate load compared to those of the treated bone in the animals of control group. However, there was no significant difference (P > 0.05) between the treated with untreated bones of the animals of other both groups. In addition, there was significant difference between the treated bone of the coral-hPRP with the treated bone of the control group (P = 0.01). The treated bone of the coral-hPRP had superior ultimate load in comparison with treated bone in the control group. Bending stiffness in untreated bone of control group was significantly (P = 0.02) superior to treated bone of contralateral side. There was no significant difference (P > 0.05) between the treated bones of three groups (P > 0.05) (Table 7).
7 Discussion
To evaluate the bone healing potential of a combination of coral and human PRP a defect model was established in the radial bone of rabbits. This model has previously been reported suitable because there is no need for internal or external fixation which influences the healing process [45]. The segemental defect was created in the middle portion of the radius as long as 10 mm for inducing nonunion defect and to prevent spontaneous and rapid healing [46].
This study was performed to provide an explanation for the existing confusion in the literature regarding the efficacy of PRP treatment in combination with other artificial bone graft substitutes, and to give more insight into the effect of PRP on bone regeneration. To the authors’ knowledge this is one of the first studies, which presents new data on the bone regenerative properties of human PRP as a xenogenic PRP concurrent with coral effects on bone healing in rabbit model.
The clinical and experimental data in the literature regarding the osteogenic potential of PRP are controversial. The results of the present investigation confirm a number of clinical and experimental studies demonstrating a positive influence of PRP on bone regeneration [24, 29, 34, 47]. However, in human maxillofacial defects, neither autograft nor allograft or a mineral bone substitute material enhanced bone formation when augmented with PRP [35, 48, 49]. In a non-critical rabbit skull defect, PRP was not superior to the empty defect nor did PRP increased bone formation by autogenous bone [28].
This study demonstrates coral-PRP’s role in treating bone defects. From both quantitative and qualitative analyses conducted using the four outcome measurements described in this study, significant differences are apparent between the coral-PRP treated groups and the two other groups.
Platelet rich plasma was found to be effective only when used together with bone graft [50]. Artificial bone graft made from cultured corals was used as it has no osteoinductive potential. Its use with PRP provided the evidence required to demonstrate the tissue-enhancing ability of PRP. Overally, the results of this study correlate with the findings of other studies that supported the use of PRP in almost similar conditions. Papa et al. [51], used a mixture of aragonitic calcium carbonate and autologous platelet-rich plasma and compared it with that of a previously published study in which bovine bone and autologous bone were used in 50 sinus lift operations. They found that the newly formed bone showed morphologic and structural characteristics that were similar in all the grafting materials (bovine bone, autologous bone, and coral). Although all the grafting materials did yield good results of maturation of the newly formed bone, however the best results were achieved using autologous bone. In another study Zhang et al. [52] studied the growth-factor gene releasing coral composites as a regenerative material for periodontal regeneration. Their study, demonstrated the potential of coral scaffold combined PDGFB gene as a good substrate candidate in periodontal tissue regeneration. This has convinced many clinicians and scientists to support its use in clinical practice [35, 36, 53].
The results of the present study indicate that hPRP in combination with coral stimulates a favorable reaction in the injured area of the long bones. The radiographic evaluation showed that the bone gap in the coral-hPRP group was healed before that of the control group and it was also already in the remodeling stage. While the defects of the control animals even at the end of eight weeks post-injury were still in the fibroplasia stage. This fact was corroborated by macroscopic, microscopic and biomechanical data analysis, which showed that osteogenesis in the defects of the animals of coral-hPRP group, was stronger than those of the two other groups at 56 days post-injury. Clinically, coral is used with success in spinal fusion [54, 55], cranial surgery [56] and dentistry [57]. It is osteoconductive but is not osteogenic.
In osteoperiosteal gaps bridged with blocks of coral only, the porosities were invaded with fibrous tissue or fibrocartilage tissues and bone tissues were formed very rarely [58]. Occasionally, bone formation was observed in direct contact with coral confirming its osteoconductivity, but it was insufficient to allow union [58]. In another study, bone formation in the porous calcium carbonate and porous hydroxyapatite in ectopic sites were determined and the results indicated that bone formation in the calcium carbonate derived from marine corals was comparable to the bioactive hydroxyapatite [59]. Therefore, this model proved to be adequate for the evaluation of coral as a scaffold for human platelet.
Ohgushi et al. [59] and Vuola et al. [60] demonstrated that supplementation of coral with whole marrow increased osteogenesis in the ectopic model. We have shown that addition of hPRP to coral led to an enhancement in osteogenesis in large bone defects in rabbits. Bone formation occurred in sufficient amounts to allow union of the defects.
More unexpected is the formation of cortex and of a medullary canal with mature lamellar bone observed in the most favourable cases. The previous in vitro studies have shown that coral supports attachment, growth and differentiation of the bone-marrow stromal cells [61]. The findings of the present study suggest that coral is a suitable resorbable carrier for platelet in vivo. It serves as a substrate to promote the stem cells of the bone marrow to attach and grow, and as a template guides bone morphogenesis in a clinically relevant volume.
Platelet rich plasma contains several growth factors including isomers of PDGF, TGF-X 1, TGF-2, IGF-I, IGF-II and VEGF that all of them are promotors of bone regeneration. PDGF has been shown to be mitogenic for osteoblasts [62] and stimulates migration of the mesenchymal progenitor cells [63]. It has been stated that PDGF was able to induce callus formation in the bone defects of the animal models [31]. TGF-X also has a stimulative effect on osteogenesis and inhibits bone resorption [64]. In addition, it is reported that IGF-I and the angiogenic factor VEGF induced bone formation in rats [65] and in rabbits [66], respectively. In our study angiogenesis was superior in group coral-hPRP due to presence of VEGF in human platelet. In summary, these growth factors support bone regeneration primarily via their chemotactic and mitogenic effects on the preosteoblastic and osteoblastic cells. Due to this phenomenon, an enhanced bone formation criteria in the animals of the coral-hPRP group compared to those of the control ones was observed. However, hPRP does not contain BMPs, the most potent osteoinductive proteins, that are the only growth factors known to induce ectopic bone formation which promote stem cell differentiation into the osteoblastic lineage [67].
The enhanced healing effects of human PRP after combining it with human bone graft material compared to a combination with a synthetic bone substitute, can also be explained by the mechanism of action of PRP. According to Marx et al. [53], PRP is thought to exert its effect on living cells. Consequently, when PRP is used together with synthetic, non-cellular bone substitutes less promotion of bone formation could be expected compared to its application with the bone graft material. The beneficial effects of PRP applied in combination with a synthetic bone substitute, depend on the number of resident osteoprogenitor cells at the bone defect site. Occasionally, the osteoconductive materials can obscure the true effect of PRP. However in the present study, combination of hPRP with coral lead to superior bone healing in comparison with the coral or control group. Therefore, based on the findings of the present study, it could be concluded that high concentrations of platelets is effective and lead to superior and faster bone formation. Because, Schlegel et al. [34] and Thorwarth et al. [47] got better results by administering higher doses of hPRP (6.5-fold compared to normal blood) than with lower platelet concentrations (4.1-fold) on bone regeneration in skull defects of minipigs while some other experimental studies found no correlation between the platelet concentration and the observed biological effects [28, 36]. In a recent study it has been shown that hPRP had superior osteogenic capacity in comparison with those of the rat PRP and goat PRP in rat bone healing model [68]. In our study hPRP were used instead of rabbit PRP because, several studies have indicated presence of several growth factors in the hPRP [23, 62, 63, 69] but to the author knowledge, there is no publication as yet about the growth factors of rabbit PRP in the literature. In addition, preparation of PRP from the animal blood is not a standardized procedure such as PRP preparation from human blood. The critical effective amounts of platelets in PRP for different animal species, levels of GFs in different animal species and similarities or differences in their mechanisms of action with PRP of humans have still to be defined. Until then, the animal PRP preparations/studies should be interpreted carefully [68]. Production of xenoreactive antibodies against hPRP could not be excluded, which might have affected the results, however, in the current model, no histological signs of acute or chronic inflammatory response in hPRP xenograft was observed, although it may have been present earlier.
8 Conclusions
In conclusion this study demonstrated that coral-hPRP could promote bone regeneration in critical size defects better than coral alone in rabbit model. This finding will nominate coral-hPRP as an attractive alternative for reconstruction of the major diaphyseal defects in the long bones in rabbit models.
References
Arrington ED, Smith WJ, Chambers HG, Bucknell AL, Davino NA. Complications of iliac crest bone graft harvesting. Clin Orthop. 1996;329:300–9.
Damien C, Parsons R. Bone graft and bone graft substitutes: review of current technology and applications. J Appl Biomater. 1991;2:187–208.
Van heest A, Swiontkowski M. Bone-graft substitutes. Lancet. 1999;353:28–9.
Friedlaender GE. Bone grafts: the basic science rationale for clinical applications. J Bone Joint Surg Am. 1987;69:786–90.
Inoue K, Ohgushi H, Yoshikawa T, Okumura M, Sempuku T, Tamai S, et al. The effect of aging on bone formation in porous hydroxyapatite: biochemical and histologic analysis. J Bone Miner Res. 1997;12:989–94.
Albrek T, Johansson C. Osteoinduction, osteoconduction and osteointegration. Eur Spine J. 2001;10:S96–101.
Alexander JW. Leonard’s orthopedic surgery of the dog and cat. 3rd ed. Florida: WB Sounders Company; 1985.
Alexander JW. Bone grafting. Vet Clin of North Am Small Anim Pract. 1987;17(4):811–9.
Brinker WO, Piermattei DL, Flo GL. Bone grafting. Small animal orthopedics and fracture repair. 3rd ed. Florida: WB Saunders Company; 1997. p. 147–53.
Fitch R, Kerwin S, Newman-Gage H, Sinibaldi KR. Bone autografts and allografts in dogs. Comp Vet Cont Ed. 1997;19(5):558–75.
Fox SM. Cancellous bone grafting in the dog: an overview. J Am Anim Hosp Assoc. 1984;20:840–8.
McLaughlin RM, Roush JK. Autogenous cancellous and cortico-cancellous bone grafting. Vet Med. 1998;93(12):1071–4.
Lohmann CH, Andreacchio D, Koster G, Carnes DL, Dean BD, Schwartz Z. Tissue response and osteoinduction of human bone grafts in vivo. Arch Orthop Trauma Surg. 2001;121:583–90.
Pokorny JJ, Davids H, Moneim M. Vascularized bone graft for scaphoid nonunion. Tech Hand Up Extrem Surg. 2003;7:32–6.
Bauer TW, Muschler GF. Bone graft materials: an overview of the basic science. Clin Orthop Rel Res. 2000;371:10–27.
Keating JF, McQueen MM. Substitutes for autologous bone graft in orthopaedic trauma. J Bone Joint Surg Am. 2001;83-B:3–8.
Kim DH, Jenis L, Berta SC, Vaccaro AR. Bone graft alternatives in spinal fusion surgery. Cur Opin in Orthop. 2003;14:127–37.
Emami MJ, Oryan A, Saeidinasab H, Meimandi Parizi A. The effect of bone marrow graft on bone healing: a radiological and biomechanical study. Iran J Med Sci. 2002;27:63–6.
Meimandi Parizi A, Jelodar G, Moslemi H, KT A, Emami MJ. Influence of hydroxyapatite on fracture healing in diabetic rats: biomechanical and radiographic studies. Veterinarski Arhiv. 2010;80:113–20.
Meimandi Parizi A, Zeidabadi nejad GR. Biomechanical and radiographical evaluation of the effects of constant direct current on the fracture healing of the radius in the rabbits. J Fac Vet Med Univ Tehran. 1997;52:1–10.
Hollinger JO, Brekke J, Gruskin E, Lee D. Role of bone substitutes. Clin Orthop Relat Res. 1996;324:55–65.
Bostrom MP, Saleh KJ, Einhorn TA. Osteoinductive growth factors in preclinical fracture and long bone defects models. Orthop Clin North Am. 1999;30:647–58.
Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30:97–102.
Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46.
McClain SA, Simon M, Jones E, Nandi A, Gailit JO, Tonnesen MG. Mesenchymal cell activation is the rate-limiting step of granulation tissue induction. Am J Pathol. 1996;149:1257–70.
Mustoe TA, Pierce GF, Morishima C, Deuel TF. Growth factorinduced acceleration of tissue repair through direct and inductive activities in a rabbit dermal ulcer model. J Clin Invest. 1991;87:694–703.
Saba AA, Freedman BM, Gaffield JW, Mackay DR, Ehrlich HP. Topical platelet-derived growth factor enhances wound closure in the absence of wound contraction: an experimental and clinical study. Ann Plast Surg. 2002;49:62–6.
Aghaloo TL, Moy PK, Freymiller EG. Investigation of platelet-rich plasma in rabbit cranial defects: a pilot study. J Oral Maxillofac Surg. 2002;60:1176–81.
Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14:529–35.
Kassolis JD, Rosen PS, Reynolds MA. Alveolar ridge and sinus augmentation utilizing platelet-rich plasma in combination with freeze-dried bone allograft: case series. J Periodontol. 2000;71:1654–61.
Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, Hicklin DJ. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone. 1994;15:203–8.
Robiony M, Polini F, Costa F, Politi M. Osteogenesis distraction and platelet-rich plasma for bone restoration of the severely atrophic mandible: preliminary results. J Oral Maxillofac Surg. 2002;60:630–5.
Rodriguez A, Anastassov GE, Lee H, Buchbinder D, Wettan H. Maxillary sinus augmentation with deproteinated bovine bone and platelet rich plasma with simultaneous insertion of endosseous implants. J Oral Maxillofac Surg. 2003;61:157–63.
Schlegel KA, Donath K, Rupprecht S, Falk S, Zimmermann R, Felszeghy E. De novo bone formation using bovine collagen and platelet-rich plasma. Biomaterials. 2004;25:5387–93.
Froum SJ, Wallace SS, Tarnow DP, Cho SC. Effect of platelet-rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int J Periodont Restor Dent. 2002;22:45–53.
Kim SG, Kim WK, Park JC, Kim HJ. A comparative study of osseointegration of Avana implants in a demineralized freeze-dried bone alone or with platelet-rich plasma. J Oral Maxillofac Surg. 2002;60:1018–25.
Bouchon C, Lebrun T, Rouvillain JL, Roudier M. The Caribbean Scleractinian corals used for surgical implants. Bull Inst Océanogr. 1995;14(3):111–22.
Kavousi J, Seyfabadi J, Rezai H, Fenner D. Coral reefs and communities of Qeshm Island, the Persian Gulf. Zool Stud. 2011;50(3):276–83.
Ghavam Mostafavi P, Fatemi SMR, Shahhosseiny MH, Hoegh-Guldberg O, WLW K. Predominance of clade D Symbiodinium in shallow-water reef-building corals of Kish and Larak Islands (Persian Gulf, Iran). Mar Biol. 2007;153:25–34.
Guillemin G, Patat JL, Fournie J, Chetail M. The use of coral as a bone graft substitute. J Biomed Mater Res. 1987;21(5):557–67.
Guillemin G, Meunier A, Dallant P, Christel P, Pouliquen J. Comparison of coral resorption and bone apposition with two natural corals of different porosities. J Biomed Mater Res. 1989;23(7):765–79.
Irigaray JL, Oudadesse H, El FH. Effet de la température sur la structure cristalline d’un Biocorail. J Therm Anal. 1993;39:3–14.
Lane JM, Sandhu HS. Current approach to experimental bone grafting. Orthop Clin North Am. 1987;18:213–25.
Emery SE, Brazinski MS, Koka A, Bensusan JS, Stevenson S. The biological and biomechanical effects of irradiation on anterior spinal bone grafts in a canine model. J Bone Jt Surg. 1994;76(4):540.
An YH, Friedman RJ. Animal models in orthopedic research. 1st ed. Boca Raton: CRC Press Inc.; 1999.
Bolander ME, Galian G. The use of demineralize bone matrix in the repair of segmental defect. J Bone Jt Surg. 1983;68A:1264–74.
Thorwarth M, Rupprecht S, Falk S, Felszeghy E, Wiltfang J, Schlegel KA. Expression of bone matrix proteins during de novo bone formation using a bovine collagen and platelet-rich plasma (prp)-an immunohistochemical analysis. Biomaterials. 2005;26:2575–84.
Shanaman R, Filstein MR, Danesh-Meyer MJ. Localized ridge augmentation using GBR and platelet-rich plasma: case reports. Int J Oral Maxillofac Implants Periodont Restor Dent. 2001;21:345–55.
Raghoebar GM, Schortinghuis J, Liem RS, Ruben JL, van der Wal JE, Vissink A. Does platelet-rich plasma promote remodeling of autologous bone grafts used for augmentation of the maxillary sinus floor? Clin Oral Implants Res. 2005;16:349–56.
Grageda E. Platelet-rich plasma and bone graft materials:a review and a standardized research protocol. Implant Dent. 2004;13(4):301–9.
Papa F, Cortese A, Sagliocco R, Farella M, Banzi C, Maltarello MC, et al. Outcome of 47 consecutive sinus lift operations using aragonitic calcium carbonate associated with autologous platelet-rich plasma: clinical, histologic, and histomorphometrical evaluations. J Craniofac Surg. 2009;20(6):2067.
Zhang Y, Wang Y, Shi B, Cheng X. A platelet-derived growth factor releasing chitosan/coral composite scaffold for periodontal tissue engineering. Biomaterials. 2007;28(8):1515–22.
Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489–96.
Pouliquen JC, Noat M, Verneret C, Guillemin G, Patat J. Coral as a substitute for bone graft in posterior spine fusion in childhood. Fr J Orthop Surg. 1989;3:272–80.
Zajour W, Dehoux E, Deprey F, Segal P. Use of coral as a bone graft substitute for anterior fusion of lower spine. Orthop Prod News. 1992:38–9.
Roux FX, Brasnu D, Loty B, George B, Guillemin G. Madreporic coral: a new bone graft substitute for cranial surgery. J Neurosurg. 1988;69(4):510–3.
Yukna RA. Clinical evaluation of coralline calcium carbonate as a bone replacement graft material in human periodontal osseous defects. J Periodontol. 1994;65:177–85.
Ohgushi H, Goldberg VM, Caplan AI. Repair of bone defects with marrow cells and porous ceramic: experiments in rats. Acta Orthop Scand. 1989;60:334–9.
Ohgushi H, Okumura M, Yoshikawa T, Inboue K, Senpuku N, Tamai S, et al. Bone formation processin porous calcium carbonate and hydroxyapatite. J Biomed Mater Res. 1992;26(7):885–95.
Vuola J, Goransson H, Bohling T, Asko-Seljavaara S. Bone marrow induced osteogenesis in hydroxyapatite and calcium carbonate implants. Biomaterials. 1996;17(18):1761–6.
Petite H, Kacem K, Triffitt JT. Adhesion, growth and differentiation of human bone marrow stromal cells on non-porous calcium carbonate and plastic substrata: effects of dexamethasone and 1, 25 dihydroxyvitamin D3. J Mater Sci Mater in Med. 1996;7:665–71.
Assoian RK, Grotendorst GR, Miller DM, Sporn MB. Cellular transformation by coordinated action of three peptide growth factors from human platelets. Nature. 1984;309:804–6.
Fiedler J, Roderer G, Gunther KP, Brenner RE. BMP-2, BMP-4, and PDGF-bb stimulate chemotactic migration of primary human mesenchymal progenitor cells. J Cell Biochem. 2002;87:305–12.
Baylink DJ, Finkelman RD, Mohan S. Growth factors to stimulate bone formation. J Bone Miner Res. 1993;8:565–72.
Spencer EM, Liu CC, Si EC, Howard GA. In vivo actions of insulinlike growth factor-I (IGF-I) on bone formation and resorption in rats. Bone. 1991;12:21–6.
Street J, Bao M, deGuzman L, Bunting S, Peale JF, Ferrara N. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–61.
Cook SD. Preclinical and clinical evaluation of osteogenic protein-1 (BMP-7) in bony sites. Orthopedics. 1999;22:669–71.
Plachokova AS, van den Dolder J, van den Beucken JJJP, Jansen JA. Bone regenerative properties of rat, goat and human platelet-rich plasma. Int J Oral Maxillofac Implants. 2009;38(8):861–9.
Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, Hicklin DJ. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone. 1994;15(2):203–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parizi, A.M., Oryan, A., Shafiei-Sarvestani, Z. et al. Human platelet rich plasma plus Persian Gulf coral effects on experimental bone healing in rabbit model: radiological, histological, macroscopical and biomechanical evaluation. J Mater Sci: Mater Med 23, 473–483 (2012). https://doi.org/10.1007/s10856-011-4478-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4478-1