Abstract
Regeneration of dentin tissues in the pulp space of teeth serves the ultimate goal of preserving teeth via endodontic approaches. In recent times, many studies suggested that human dentin scaffolds combined with dental stem cells was a potential strategy for the complete dentin tissue regeneration. In this study, human dental pulp stem cells (DPSCs) were isolated and cultured. Dentin specimens were prepared from human third molars and treated with ethylene diamine tetra-acetic acid and citric acid to remove the smear layer. Then, DPSCs were cultured onto human treated dentin (hTD) and implanted in mouse model for 4, 6 and 8 weeks. The resulting grafts were assessed by hematoxylin and eosin stain and immunohistochemical stains. As a result, DPSCs were supported and induced to regenerate of dentin-like tissues which expressed specific dentin markers such as dentin sialophosphoprotein and dentin matrix protein 1 by combination with hTD in vivo. Furthermore, cells existed in the newly-formed dentin-like tissues also expressed typical human mitochondria antibodies, demonstrated that new tissues originated from human. In conclusion, the obtain results extend hopefully newly-established therapy to apply in endodontics and traumatic dental hard tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stem cell research has become a promising field for tissue regeneration and implementation of regenerative medicine. Since the discovery and characterization of multipotent mesenchymal stem cells (MSCs) from bone marrow, similar MSCs populations derived from other tissues have now been characterized. Postnatal stem cells have been isolated from a variety of tissues including bone marrow, brain, skin, skeletal muscle and the gastrointestinal tract (Baroffio et al. 1996; Slack 2000; Campagnoli et al. 2001; Gronthos et al. 2003; Zannettino et al. 2008). Recent studies have revealed the presence of adult stem cells in dental tissues (Handa et al. 2002; Huang et al. 2008; Rodríguez-Lozano et al. 2011). They are described as multipotent stem cells, capable of self-renewal and differentiation into various cell types, such as osteocytes, adipocytes, chondrocytes, cementoblasts, odontoblasts and neural cells (Gronthos et al. 2000, 2002; Batouli et al. 2003; Kamata et al. 2004).
Adult dental pulp contains a heterogeneous population of cells, including fibroblasts, nerve cells, undifferentiated mesenchymal cells or dental pulp stem cells (DPSCs), vascular cells. DPSCs are often found in highly vascularized sites and collected from the pulp tissue of clinically extracted human teeth by various isolation methods (Huang et al. 2006). These cells are expanded and expressed a heterogeneous assortment of markers associated with MSCs. Besides, DPSCs also express nestin and GFAP, which are molecules related to the neural crest-cell origin of the dental pulp (Lovschall et al. 2007). DPSCs are capable of differentiating into neuron-like cells, odontoblast-like cells, osteoblasts, chondrocytes, adipocytes, smooth and skeletal muscle cells (d’Aquino et al. 2007; Graziano et al. 2008). In particular, the importance of DPSCs is able to differentiate and secrete a matrix that produces osteogenesis and dentinogenesis in vitro and in vivo. Furthermore, DPSCs are potentially superior to other types of adult stem cell are extraction routinely throughout life. Even of more importance is the ability to secure DPSCs at a young age and store it for the future usage. A personalized stem cell can then be created from DPSC without using procedures that may cause ethical concerns (Batouli et al. 2003; Shi and Gronthos 2003; Miura et al. 2003).
Many kinds of synthetic and natural polymers have been utilized for dentin tissue engineering. However, few were able to regenerate the complete dentin tissue. Dentin, which is a mineral tissue, is included approximately 70 % hydroxyapatite and collagenous and noncollagenous organic matrix components. Dentin is formed by odontoblasts which establish a continuous single layer along the peripheral dental pulp tissue and deposit new layers of dentin throughout human life (Thesleff and Vaahtokari 1992; Stern et al. 2009). Dentin is an essential component for dental tissue engineering because makes up most of the tooth structure. Moreover, the soluble proteins of human dentin are bioactive proteins considered to be necessary for dentinogenesis (Chun et al. 2011; Li et al. 2011). Scaffolds from natural dentin, which were collected freely and eliminated as medical waste, have been suggested to be useful in dentin regeneration (Lluch et al. 2009).
Previous researches demonstrated that hDPSCs cultured on mechanically and chemically treated dentin appeared to establish an odontoblast-like morphology, with cytoplasmic processes extending into dentinal tubules. Their results suggest that isolated hDPSCs may differentiate into odontoblasts on dentin in vitro (Huang et al. 2006). However, it is not clear whether dentin scaffolds induce hDPSCs to regenerate the complete dentin tissue. Therefore, in this study we combined human treated dentin (hTD) and cultured DPSCs to assess regeneration of human dentin-like tissue in vivo.
Materials and methods
Isolation and culture of human dental pulp stem cells (DPSCs)
DPSCs were isolated and cultured followed protocol described previously (Tran et al. 2011). In brief, the human third molars were obtained from healthy donors at the Maxillofacial Faculty, under the approval of the Ethical Committee of Ho Chi Minh City Medicine and Pharmacy University, and stored in Dulbecco’s modified Eagle’s medium (DMEM, Sigma, St. Louis, MO) supplemented with antibiotics (300 U/ml penicillin and 300 mg/ml streptomycin, Sigma). Small fragments of dental pulp tissues were seeded onto 35-mm dishes (NUNC, Roskilde, Denmark) with Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 Ham medium (DMEM/F12, Sigma) supplemented with 10 % fetal bovine serum (FBS, Sigma) and maintained in 5 % CO2 at 37 °C. The fourth passage cells were used for following studies.
Characterization of cell proliferation
The DPSCs at the fourth passage were seeded with a density of 103 cells/well of 96-well culture dish (NUNC) with culture medium. Cell density was identified everyday, continuously in 10 days. Growth curve was determined from counted cell quantity. To assess the time of duplication or doubling time (DT), the following equation was applied: DT = CT/log N/N0 × 3.31, where CT = culture time, N = final number of cells, N0 = initial number of cells (Piera-Velazquez et al. 2002).
Cell-surface-marker characterization
The DPSCs at the fourth passage were detached and identified specific surface antigen expression by flow cytometry. After the cells were harvested and transferred, they were fixed for 15 min in 4 % paraformaldehyde. The cells were incubated with 3 % bovine serum albumin and then with primary antibodies (BD Biosciences) raised against CD44, CD90, CD73, CD34, CD45, and HLA-DR for 1 h. The cells were washed with wash buffer, and the secondary antibody was added for 45 min at room temperature. Finally, the cells were washed three times and analyzed with a flow cytometer (FACSCalibur, BD Biosciences).
Multilineage differentiation
The fourth passage cells were seeded into 35 mm dishes and cultured until they reached subconfluence. After that, cells were cultured in the adipogenesis medium [DMEM/F12 medium containing 10 % FBS, 0.2 mM indomethacin, 0.5 mM isobutyl-methylxanthine, 100 nM dexamethasone, 10 mM insulin, 100 U/ml penicillin, and 100 mg/ml streptomycin (Sigma)] and osteogenesis medium [DMEM/F12 medium containing 10 % FBS, 100 nM dexamethasone, 100 mM β-glycerol phosphate and 50 µg/ml α-ascorbic acid 2-phosphate, 100 U/ml penicillin, and 100 mg/ml streptomycin (Sigma)]. After 2–3 weeks of induction, the cells were stained with oil red O or alizarin red to evaluate adipogenesis and osteogenesis, respectively.
Fabrication of human treated dentin
Dentin tissues were prepared from collected human third molars after the pulp tissue had been removed to obtain DPSCs. Periodontal tissues were completely eliminated by using mechanical method. Dentin tissues were cut into a cube shape (approximately 3 mm long × 2 mm wide × 1 mm thick). Dentin specimens were treated with 19 % citric acid (Sigma) for 1 min and 17 % diamine tetra-acetic acid (EDTA) (Sigma) for 10 min to remove the smear layer (Tran et al. 2011). hTDs were then sterilized by gamma radiation. Mineral trioxide aggregate (MTA, Tulsa Dental Products, Tulsa, OK) scaffolds were used as a control group.
Regeneration of dentin-like tissue in vivo
In order to prove the role of hTD scaffolds in induction for regeneration of dentin tissue of DPSCs in vivo, we subcutaneously implanted samples into the dorsum of Nude mice. All in vivo experiments were carried out in accordance with the ethical guidelines for Animal Care and Use Committee of University of Medicine and Pharmacy at Ho Chi Minh City. Nude mice were divided into three groups including one experiment group (hTD combined with DPSCs) and two control groups (MTA combined with DPSCs and single hTD). hTD and MTA scaffolds were soaked in culture medium at 37 °C overnight. Then, DPSCs were seeded onto scaffolds at density of 3 × 104 cells/scaffold and incubated in 5 % CO2 at 37 °C for 3 days. Surgical operation was performed under deep anesthesia. After 4, 6 and 8 weeks, Nude mice were deeply anesthetized to collect all structures (one experiment group and two control groups). Structures were fixed with 10 % formaldehyde for 24 h at 4 °C and stained with hematoxylin and eosin (H&E) stain and immunohistochemical stains. Immunohistochemical antibodies included dentin matrix protein 1 (DMP-1), dentin sialophosphoprotein (DSPP) and human mitochondria (Sigma).
Results
Isolation and culture of human dental pulp stem cells (DPSCs)
Characterization of cell proliferation
After four times of subculturing, DPSCs strongly proliferated from the second day to the seventh day, peaked in the seventh day which was confluence, and gradually decreased in some days after that (Fig. 1).
Cell-surface-marker characterization
Surface markers expressed on the DPCs at the fourth passage were analyzed by flow cytometry. As a result, the DPSCs strongly expressed markers CD44 (99.84 %), CD73 (99.77 %) and CD90 (97.34 %) and lacked expression markers CD34 (0.42 %), CD45 (0.08 %), and HLA DR (0.18 %) (Fig. 2).
Differentiation into adipocytes and osteoblasts
The DPSCs at the fourth passage were differentiated into adipocytes and osteoblasts. The differentiation was achieved after 14–21 days. After 21 days, the result of oil red O and alizarin red staining showed that there were mineralized nodule (Fig. 3b) and lipid droplets (Fig. 4b) formation inside the differentiated cells.
Fabrication of human treated dentin
Based on results of SEM, dentin surface was readily visible in all specimens as evidenced by lack of smear layer and abundance of patent dentinal tubules (Fig. 5a). Furthermore, after three cultured days in vitro, DPSCs began spreading onto hTD surface (Fig. 5b).
Regeneration of dentin-like tissue in vivo
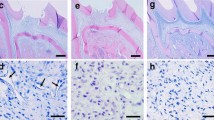
After surgical operation, all experimental mice rehabilitated quickly. Their normally activities were not affected by implanted materials. Based on results of H&E stain, in the experiment group, there was the formation of new tissues at 4, 6 and 8 weeks. Newly-formed tissue thickness and cell density increased from 4 to 6 weeks. However, cell density did not increase at 8 weeks. At 6 and 8 weeks, area adjacent to hTD took form concentrated matrix and expand over time. Furthermore, most cells were buried in concentrated matrix at 8 weeks (Fig. 6a–c). In the MTA control group, only a thin dentin-like matrix layer was formed on MTA scaffold surfaces at 8 weeks (no cells were observed) (Fig. 6d). On the other hand, no new tissue formed by using a hTD without DPSCs (single hTD control group) at 4, 6 and 8 weeks (Fig. 6e).
Histological results of regeneration of dentin-like tissue in vivo. a–c Formation of new dentin-like tissues at 4, 6 and 8 weeks, d only dentin-like matrix were formed on MTA scaffold surfaces at 8 weeks, e no new tissue was formed on single hTDs at 8 weeks. Regenerated tissues on hTD were positive with DMP-1 and DSPP at 6 weeks (f, h) and 8 weeks (g, i). Regenerated tissues and its cells were positive with human mitochondria antibodies at 6 weeks (j) and 8 weeks (k)
Expression of DSPP and DMP-1 was detected in the new tissues formed in hTDs at 4 (data not shown), 6 and 8 weeks (Fig. 6f–i). These results demonstrated that the newly-formed tissues were dentin-like tissues. In order to demonstrate whether participation of the implanted DPSCs induced to regenerate dentin-like tissues, human mitochondria antibody, which only responded to a human source, was used. All cells in regenerated tissues were positive with human mitochondria antibody, proven that implanted DPSCs participated in human dentin-like tissues regeneration in vivo (Fig. 6j, k).
Discussion
Five types of human dental stem cells have been isolated and characterized: DPSCs (Gronthos et al. 2000), stem cells from human exfoliated deciduous teeth (SHED) (Miura et al. 2003), periodontal ligament stem cells (PDLSCs) (Seo et al. 2004), dental follicle progenitor cells (DFPCs) (Morsczeck et al. 2005) and stem cells from apical papilla (SCAP) (Sonoyama et al. 2008). Dental pulp, entrapped within the ‘sealed niche’ of the pulp chamber, is an extremely rich site for stem cells isolation. Dental pulp tissue has been reported to contain MSC-like population referred to as DPSCs (Gronthos et al. 2000). Several studies have reported that DPSCs express MSC markers such as CD10, CD13, CD29, CD44, CD59, CD73, CD90 and CD105, and do not express CD14, CD34, CD45, HLA-DR. Furthermore, DPSCs are capable of differentiation into odontoblast-like cells, osteoblasts, adipocytes, smooth and skeletal muscle cells (d’Aquino et al. 2007; Karaöz et al. 2009; Nakamura et al. 2009).
In this study, we demonstrated that isolation of human DPSCs by outgrowth method, allowed the recovery of a population of dental MSCs, which showed a notable proliferation potential, multipotency. Furthermore, DPSCs exhibited a DT of 54.38 h which revealed that DPSCs have higher proliferation capacities than bone marrow stem cells (BMSCs) (61.2 h) and slower proliferation capacities than umbilical cord stem cells (UCSCs) (24 h) and adipocyte stem cells (ADSCs) (45.2 h) (Hass et al. 2011). In addition, our data also showed that, DPSCs were expressed over 95 % positive for the CD44, CD73 and CD90 antigens, less than 2 % of the leucocyte marker CD45, the primitive haematopoietic progenitor and endothelial cell marker CD34, the B cell markers HLA-DR by flow cytometry analysis.
Until now, many materials have been used to create scaffolds for dentin regeneration. However, few have succeeded in obtaining complete dentin tissue (Li et al. 2011; Honda et al. 2007; Scheller et al. 2009). Previous studies have demonstrated that hTD could be a conformable scaffold for dental tissue engineering because of its non-immunogenicity, suitable mechanical properties, and releasing dentinogenetic factors. Previous studies also have shown that soluble proteins extracted from dentin, such as DSPP, DMP-1, proteoglycans (decorin and biglycan), as well as growth factors such as TGF-β, bone morphogenic protein (BMP), can regulate dentinogenesis and mineral formation (Chun et al. 2011; Li et al. 2011; Guo et al. 2009). In our experiment, we sterilized hTD by gamma radiation. Gamma radiation is mainly used for the sterilization of pharmaceuticals and biomaterials in graft. Results showed that hTD scaffolds sterilized by gamma radiation remained high level of bioactive protein such as TGF-β and DMP-1 (data not shown).
EDTA, which is widely used in endodontic therapy, is a chelating agent capable of removing inorganic material to enlarge root canals, removing the smear layer, and preparing the dentinal walls for better adhesion of filling materials. The combined use of EDTA and acid citric has been shown to be particularly effective for smear layer and debris removal (Tran et al. 2011; Nakashima and Terata 2005; Huang et al. 2006). The result of treatment removed completely smear layer and exposed dentin tubules on hTD surfaces. Besides, we demonstrated that DPSCs could adhere and proliferate on hTDs in vitro (Tran et al. 2011). This indicated that hTD had good biocompatibility and supported cell growth.
Mineral trioxide aggregate (MTA) is biomaterial that has been investigated for endodontic applications since the early 1990s. MTA was first described in the dental scientific literature in 1993 and was given approval for endodontic use by the U.S. Food and Drug Administration in 1998. MTA has been shown to induce hard-tissue repair of exposed pulps in experimental animals and to generate a greater frequency of dentin bridge formation than earlier materials (Lee et al. 1993; Schmitt and Bogen 2001; Andelin et al. 2003).
hTD scaffolds seeded with DPSCs were implanted into nude mice at the dorsum where non-affected normal activities of experimental animals, non-potential mineralization and abundant vasculargenesis. Implantation immediately after seeding DPSCs to the hTD scaffolds did not perform because DPSCs needed more time to adhere and spread on scaffold surfaces. According to growth curve of DPSCs (Fig. 2), the lag phase tended to be about 2 days for culture meaning that DPSCs could start to proliferate 2 days after being seeded onto hTD scaffolds. Furthermore, less than 3 days in vitro culture of hTD/DPSCs also did not obtain satisfactory regenerated results; concurrently, 3 days in vitro culture were proposed because risk of contamination for in vitro culture likely increased with time (Li et al. 2011).
During the last years, several studies investigated that hDPSCs formed multilayer and secreted extracellular matrix onto the treated dentin surface. Furthermore, hTD potentially induced DPSCs to establish an odontoblast-like morphology with a cytoplasmic process extending into a dentinal tubule after 14 days (Huang et al. 2006), 10 days (Shao et al. 2011) or 28 days (Neunzehn et al. 2014) in vitro. Through an in vivo study, pieces of root canals of the extracted teeth, containing collagen or PLA scaffolds seeded with the autologous cryopreserved DPSCs, were implanted into the fresh post-extraction socket of the mini pig jaw. The results showed an odontoblast-like cell construct with an organic matrix formation on the root canal wall surface of porcine teeth after 6 and 10 weeks. More specifically, the newly formed odontoblastic-like cell layer lining along the existing canal walls stained positive for DMP-1 antibody (Kodonas et al. 2012).
In this study, implantable structures were evaluated at 4, 6 and 8 weeks that identified growth and change of newly-formed tissues. Mineralization of new tissues occurred at the junction with hTD and developed on the opposite side; similar to the natural formation of human dentin. Specially, all hTD scaffolds and DPSCs derived from human; therefore this study was step ahead in dental tissue engineering. Two control groups lacked one of important elements (hTD or DPSCs), therefore no regeneration of new tissues. This suggested that hTD and DPSCs played essential role in the regeneration of dentin-like tissues in vivo.
Histological examination of implanted DPSCs/hTD structures showed that newly-formed tissues were positive with two specific markers for dentin matrix (DMP-1 and DSPP). During dentin formation, odontoblasts synthesize several noncollagenous proteins and deposit into the dentin extracellular matrix. One of these proteins is DSPP, which is believed to play a regulatory role in the mineralization of reparative dentin; it also serves as a specific marker for the odontoblastic phenotype (Papagerakis et al. 2002). Besides, DMP-1 is a multifunctional protein, prominent member of one category of non-collagenous proteins and plays an essential role in biomineralization. More recently, DMP-1 was found to induce cytodifferentiation of DPSCs into odontoblast-like cells during dentinogenesis, indicating that it could act as a morphogen with the potential to regenerate dentin-like tissue and to form reparative dentin (Toyosawa et al. 2004).
Results of our study indicated that hTD scaffolds induced and supported DPSCs to secrete extracellular matrix and regenerate dentin-like tissue. In addition, cells of newly-formed tissues were positive with human mitochondria; concurrently, no new tissue formed by using single hTD, suggested that the seeded DPSCs were essential role to participate in the regenerated dentin-like tissues.
Conclusion
This research described the successful combination of hTD and human DPSCs. This is one of potential strategies for dentin-like tissue regeneration based on tissue engineering principle.
References
Andelin W, Shabahang S, Wright K (2003) Identification of hard tissue after experimental pulp capping using dentin sialoprotein (DSP) as a marker. J Endod 29:646–650
Baroffio A, Hamann M, Bernheim L, Bochaton-Piallat ML, Gabbiani G, Bader CR (1996) Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation 60:47–57
Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, Gronthos S, Robey PG, Shi S (2003) Comparison of stem cells mediated osteogenesis and dentinogenesis. J Dent Res 82:976–981
Campagnoli C, Roberts IA, Kumar S, Bennett PR, Bellantuono I, Fisk NM (2001) Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 98:2396–2402
Chun SY, Lee HJ, Choi YA, Kim KM, Baek SH, Park HS, Kim JY, Ahn JM, Cho JY, Cho DW, Shin HI, Park EK (2011) Analysis of the soluble human tooth proteome and its ability to induce dentin/tooth regeneration. Tissue Eng Part A 17:181–191
d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, Papaccio G (2007) Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ 4:1162–1171
Graziano A, d’Aquino R, Laino G, Proto A, Giuliano MT, Pirozzi G, De Rosa A, Di Napoli D, Papaccio G (2008) Human CD34+ stem cells produce bone nodules in vivo. Cell Prolif 41:1–11
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci 97:13625–13630
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S (2002) Stem cell properties of human dental pulp stem cells. J Dent Res 81:531–535
Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ (2003) Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci 116:1827–1835
Guo WH, He Y, Zhang XJ, Lu W, Wang CM, Yu H, Liu Y, Li Y, Zhou Y, Zhou J, Zhang M, Deng Z, Jin Y (2009) The use of dentin matrix scaffold and dental follicle cells for dentin regeneration. Biomaterials 30:6708–6723
Handa K, Saito M, Tsunoda A, Yamauchi M, Hattori S, Sato S, Toyoda M, Teranaka T, Narayanan AS (2002) Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect Tissue Res 43:406–408
Hass R, Kasper C, Böhm S, Jacobs R (2011) Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9:12
Honda MJ, Tsuchiya S, Sumita Y, Sagara H, Ueda M (2007) The sequential seeding of epithelial and mesenchymal cells for tissue-engineered tooth regeneration. Biomaterials 28:680–689
Huang GT, Sonoyama W, Chen J, Park SH (2006) In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res 324:225–236
Huang GT, Sonoyama W, Liu Y, Liu H, Wang S, Shi S (2008) The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod 34:645–651
Kamata N, Fujimoto R, Tomonari M, Taki M, Nagayama M, Yasumoto S (2004) Immortalization of human dental papilla, dental pulp, periodontal ligament cells and gingival fibroblasts by telomerase reverse transcriptase. J Oral Pathol Med 33:417–423
Karaöz E, Doğan BN, Aksoy A, Gacar G, Akyüz S, Ayhan S, Genç ZS, Yürüker S, Duruksu G, Demircan PC, Sariboyaci AE (2009) Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol 133:95–122
Kodonas K, Gogos C, Papadimitriou S, Kouzi-Koliakou K, Tziafas D (2012) Experimental formation of dentin-like structure in the root canal implant model using cryopreserved swine dental pulp progenitor cells. J Endod 38:913–919
Lee SJ, Monsef M, Torabinejad M (1993) Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod 19:541–544
Li R, Guo W, Yang B, Guo L, Sheng L, Chen G, Li Y, Zou Q, Xie D, An X, Chen Y, Tian W (2011) Human treated dentin matrix as a natural scaffold for complete human dentin tissue regeneration. Biomaterials 32:4525–4538
Lluch AV, Fernandez AC, Ferrer GG, Pradas MM (2009) Bioactive scaffolds mimicking natural dentin structure. J Biomed Mater Res B Appl Biomater 90:182–194
Lovschall H, Mitsiadis TA, Poulsen K, Jensen KH, Kjeldsen AL (2007) Coexpression of Notch3 and Rgs5 in the pericyte-vascular smooth muscle cell axis in response to pulp injury. Int J Dev Biol 51:715–721
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci 100:5807–5812
Morsczeck C, Gotz W, Schierholz J, Zeilhofer F, Kuhn U, Mohl C (2005) Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol 24:155–165
Nakamura S, Yamada Y, Katagiri W, Sugito T, Ito K, Ueda M (2009) Stem cell proliferation pathways comparison between human exfoliated deciduous teeth and dental pulp stem cells by gene expression profile from promising dental pulp. J Endod 35:1536–1542
Nakashima K, Terata R (2005) Effect of pH modified EDTA solution to the properties of dentin. J Endod 31:47–49
Neunzehn J, Weber MT, Wittenburg G, Lauer G, Hannig C, Wiesmann HP (2014) Dentin-like tissue formation and biomineralization by multicellular human pulp cell spheres in vitro. Head Face Med 10:25
Papagerakis P, Berdal A, Mesbah M, Peuchmaur M, Malaval L, Nydegger J (2002) Investigation of osteocalcin, osteonectin, and dentin sialophosphoprotein in developing human teeth. Bone 30:377–385
Piera-Velazquez S, Jimenez SA, Stokes D (2002) Increased life span of human osteoarthritic chondrocytes by exogenous expression of telomerase. Arthritis Rheum 46:683–693
Rodríguez-Lozano FJ, Bueno C, Insausti CL, Meseguer L, Ramírez MC, Blanquer M, Marín N, Martínez S, Moraleda JM (2011) Mesenchymal stem cells derived from dental tissues. Int Endod J 44:800–806
Scheller EL, Krebsbach PH, Kohn DH (2009) Tissue engineering: state of the art in oral rehabilitation. J Oral Rehabil 36:368–389
Schmitt D, Bogen G (2001) Multifaceted use of ProRoot MTA root canal repair material. Pediatr Dent 23:326–330
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155
Shao MY, Fu ZS, Cheng R, Yang H, Cheng L, Wang FM, Hu T (2011) The presence of open dentinal tubules affects the biological properties of dental pulp cells ex vivo. Mol Cells 31:65–71
Shi S, Gronthos S (2003) Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 18:696–704
Slack JM (2000) Stem cells in epithelial tissues. Science 287:1431–1433
Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S (2008) Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34:166–171
Stern MM, Myers RL, Hammam N, Stern KA, Eberli D, Kritchevsky SB, Soker S, Van Dyke M (2009) The influence of extracellular matrix derived from skeletal muscle tissue on the proliferation and differentiation of myogenic progenitor cells ex vivo. Biomaterials 30:2393–2399
Thesleff I, Vaahtokari A (1992) The role of growth factors in determination and differentiation of the odontoblastic cell lineage. Proc Finn Dent Soc 88:357–368
Toyosawa S, Okabayashi K, Komori T, Ijuhin N (2004) mRNA expression and protein localization of dentin matrix protein 1 during dental root formation. Bone 34:124–133
Tran LBH, Doan NV, To MQ, Phan KN, Hoang TH, Hoang DBT, Dang VNM, Nguyen TT (2011) Study on culture of human dental pulp stem cells to apply in tissue engineering. J Biomim Biomater Tissue Eng 11:13–20
Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, Gronthos S (2008) Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol 214:413–421
Acknowledgments
This research is funded by Vietnam National University-Ho Chi Minh city under Grant number B2012-18-11TĐ.
Conflict of interest
The authors declare that there is no personal or financial conflict of interests in the current research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tran, H.L.B., Doan, V.N. Human dental pulp stem cells cultured onto dentin derived scaffold can regenerate dentin-like tissue in vivo. Cell Tissue Bank 16, 559–568 (2015). https://doi.org/10.1007/s10561-015-9503-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10561-015-9503-z