Abstract
Purpose
The most important challenges in tissue engineering are to find an effective growth/differentiating factor for stem cell induction while retaining high cell vitality and convert to correct end cell linage. In this study, for the first time, self-administered dentin matrix derivatives were used as a growth/differentiation factor for dentin regeneration.
Methods
In the fourth passage, colonies of human dental pulp stem cells (hDPSCs) were extracted; then, the purified cells were evaluated based on the percentage of vital cells by the MTT method. Afterward, hDPSCs in the first group were cultured with self-administered dentin derivative signal (DDS), while those in the second group with demineralized freeze-dried bone allograft (DFDBA) as a signal for 3, 7, and 10 days, and then compared with group 3 (negative control) that contained only hDPSC colonies. Three groups were evaluated based on cell differentiation and expression of osteoblast and odontoblast cell markers by immunocytochemistry (ICC) staining.
Results
In the first group, hDPSCs were differentiated into odontoblast and in the second group into osteoblast. The mean expression percentage of the dentin sialophosphoprotein (DSPP) marker in differentiated cells in the first group was significant at days 3, 7, and 10 (p ≤ 0.0001). Also, the mean expression of the bone sialoprotein (BSP) marker was significant in the second group on days 3, 7, and 10 (p ≤ 0.0001).
Conclusion
hDPSCs in scaffold-free culture enable to differentiate to odontoblasts and osteoblasts through DDS and DFDBA. Both the DSPP and BSP markers were increasingly expressed with time and effective in cell induction. Future in vivo studies can recommend to investigate the role of such natural growth factors in regenerative endodontic and dentistry in clinics.
Lay Summary
This study evaluated the differentiation capability of dental pulp stem cells by two bioactive materials, self-administered dentin derivative signal (DDS) and demineralized freeze-dried bone allograft (DFDBA), as induction signals. The challenge in this regard is to find a simple, suitable, and efficient method of DDS preparation, retaining proteins undamaged and ensuring enough disinfected particles for cell exposure. For this purpose, we investigated freeze-dried methods that are commonly applied in material preparation, and tested DDS with the scaffold-free stem cell’s culture comparing with DFDBA that exhibits great induction potential to be used for tissue engineering in dentistry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Due to the humidity and darkness of the oral cavity, it is the best environment that facilitates the growth of microorganisms, so almost everyone is at risk of tooth decay, periodontal disease, and eventually tooth loss. Conventional or gold standard treatments include restorations, root canal therapy, replacement of teeth with a variety of prostheses, and implants, which each have their advantages and disadvantages, so do not provide the ideal function and esthetic like a natural tooth [1]. For example, endodontic therapy in addition to complications that may occur during operation has limitations such as reducing the strength of the tooth and making it prone to fracture [2].
Treating various diseases using stem cells has created a new era in tissue engineering. Several studies are performed on the identification, isolation, proliferation, and application of dental stem cells, and some successes have been achieved in this field. The emergence of stem cell science stimulated dental researchers to find a way to rebuild the lost tooth tissue [3, 4]. Stem cells are multi-potential cells that can differentiate into different types of cells and replace damaged and lost tissues in different parts of the body [5].
Almost all human tissues contain stem cells and (or) progenitor cells that are activated in response to growth and development or during tissue repair following injury or trauma. Currently, dental tissues containing periodontal ligament (PDL), dental papillae, and dental follicles of deciduous and permanent teeth buds have been identified as available sources of undifferentiated cells [6]. Because teeth and their associated structures are made up of a variety of tissues, stem cells extracted from teeth have the potential to produce other types of tissues such as bone, cartilage, nerve, blood vessels, connective tissue, muscle, and dentin-pulp complex.
Since the structure of the teeth and their surroundings includes various tissues, stem cells extracted from the tooth have the potential to produce other types of tissues such as bone, cartilage, nerve, blood vessels, connective tissue, muscles, and dentin-pulp complex. Therefore, it can be argued that dental stem cells are applicable for the regeneration of the whole body. For example, these cells can be used to regenerate knee cartilage in a person who has lost cartilage as a result of exercise. Thus, since the pulp of removed teeth contains undifferentiated cells, it can be used for tissue engineering purposes [7].
Dental pulp stem cells (DPSCs) have been used in new dentistry methods such as periodontal and dental repair, encourage dental pulp repair, craniofacial bone regeneration, and tooth bud production. DPSCs are easily available, and unlike the mesenchymal bone marrow stem cells, have a higher association with dental tissues, and they are better to be used for dental engineering [8]. The most prominent feature of DPSCs is their ability to regenerate the dentin-pulp-like complex, which is made of mineralized matrix and odontoblast-coated tubules and fibrosis tissue containing blood vessels that is similar to the natural dentin-pulp complex found in humans [8].
Unlike tooth epithelial stem cells, which are the precursors of tooth enamel tissue, undifferentiated ectomesenchyme cells do not completely disappear after tooth eruption in humans. Today, even at age 90, it is possible to isolate precursor stem cells from the permanent dental pulp. In response to injury, these cells become active and regenerate lost tissue [9]. Therefore, the use of DPSCs has become popular among dental scientists as a stem cell–based therapy by tissue engineering, because of simple extraction and minimal immunogenicity. So, bone tissue engineering can be a critical element in treating periodontal and maxillofacial bone defects [10].
Tissue engineering based on allogeneic or autogenic stem cells has been progressively developed, because of no ethical controversy or immunogenicity, no delay in finding a donor, and isolation in adulthood. Unlike conventional transplanted methods, it has low antigenicity, an ideal shape, and plastic properties, among other advantages [11, 12].
To date, researchers have been able to produce bone tissue, pulp-dentin complex, cementum, and periodontal fibers in the laboratory using tooth stem cells, but regeneration of enamel, dentin, and cementum has not yet been possible [13, 14].
Various signals and scaffolds have been tested by different studies for dental tissue engineering. The aim of the present study was, in the first step, to expose DPSCs to DFDBA, which contains matrix proteins and bone growth factors, to stimulate their differentiation into osteoblasts and bone tissue structure formation, and secondly, for the first time in dental engineering, to investigate the demineralized dentin powder (dentin matrix derivatives) that contained dentinal protein and stored growth factors for the differentiation of DPSCs into odontoblasts and form the dentin.
Materials and Methods
This experimental in vitro study was conducted in the Research Center of Neural System Stem Cells of Semnan University of Medical Sciences after approval by the Ethics Committee of Semnan University of Medical Sciences (IR.SEMUMS.REC.1396.246). hDPSCs from the DPS-13 cell line (with C10896 IBRC cell code) were purchased from the Cellular and Molecular Laboratory of the Iranian Biological and Genetic Resource National Center in a specific culture medium. This company proved and guarantied the quality and validation of their human dental pulp stem cells.
Cell Subculture
Since the fourth cell subculture is usually used to induce differentiation of stem cells, after preparing the cells, they were proliferated and subcultured. In the first subculture, the cells were placed in a 25-mL flask containing α-MEM (Sigma M0644) and ES-FSC (Gibco 16,141–079) and placed in an incubator at 37 °C, 95% humidity, and 5% CO2. The cell culture media were exchanged every 2–3 days for about 2 weeks (until achieving appropriate density).
After filling the flask floor, the cell subculture was performed using Try/EDTA (Gibco 25,300–054), and after four consecutive subcultures and purification of the cells, the cells were isolated from the flask floor and counted using Trypan Blue and Nicobar slide. In the following, 200,000 cells were placed in a 25-mL flask for each group. For further experiments, the fourth passage cells were placed in the medium containing ES-FCS 50% (Gibco 16,141–07), DMSO 10% (Sigma D2650), and 40% of mesenchymal stem cell culture medium, and were transferred to a nitrogen tank.
Study Groups
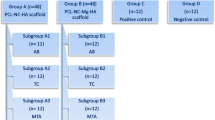
Three culture groups were used in this study, as follows:
-
First group: flasks containing hDPSC colonies (200,000 cells) treated with dentin matrix derivatives which contained proteins and growth factors of dentin derivative signal (DDS) with our patent number 14005060003038752 (0.7 mL from 100 μg/mL) [15].
-
Second group: flasks containing hDPSCs (200,000 cells) treated with DFDBA, which contained proteins and growth factors of bone matrix (0.7 mL from 100 μg/mL) [15].
-
Third group (or negative control): flasks that contained only hDPSC colonies (200,000 cells).
After 3, 7, and 10 days, purified cells were isolated, and the vitality percentages of cells by the MTT method and cell differentiation and expression of osteoblast and odontoblast cell markers were evaluated by the ICC method.
Preparation of Demineralized Dentin Matrix Derivatives
Dentin matrix derivatives were exploited from extracted teeth by the following method. The teeth were disinfected using sodium hypochlorite solution. First of all, the enamel and cement tissues were removed by diamond fissure bur (008) with water spray, and pulp tissue was removed by an excavator. Then, the dentin tissue was divided into 1 × 1 mm blocks and placed in acetic acid (5%) for 14 days until full demineralization and the dentin protein matrix has remained. Then, a freeze dryer device (Christ Co, Germany, Model: Alpha 1–2 LD Plus) was used to dry and prepare the powder from the obtained dentin blocks and preserve its proteins. Therefore, the obtained material was frozen, followed by conversion of the water from solid to gas via a sublimation process that preserved the chemical and physical characteristics of the material.
MTT Test to Determine the Proliferation Rate and the Vitality of Cells
In each group, treated cells after 24 h were evaluated using MTT as follows. The culture medium of the plates’ surface was removed and washed with PBS twice. Then, 100 μL culture medium without serum and 10 μL MTT solution were added to each well, followed by 2 h of incubation at 37 °C, 5% CO2, and 90% humidity for 4 h. Then, 100 μL isopropanol acid 0.4% was added to each well, followed by incubation for 10 min. Optical absorption (OD) of each well was recorded at 492 nm wavelength with ELISA Reader (Awareness Technology Inc., Palm City, FL 34,990, USA). In addition, a standard graph (p < 0.05) was drawn to determine the percentage of living cells using the following formula for each group:
Cell Differentiation Stage
For evaluating the differentiation of hDPSC cells into osteoblast and odontoblast cells, first stem cells were cultured (100 cells/cm2) in plates containing 6 wells. Then, when the dish floor was filled by 70–80%, the medium was replaced by DMEM differentiation medium (containing 15% ES-FCS, 50 μg/mL ascorbic acid-2 phosphate, 10−8 MD dexamethasone, and 10 mM 6-glycerol phosphate). The cells were cultured for 10 days, and the medium was replaced every 3 days. Eventually, the differentiation of hDPSCs to osteoblasts and odontoblasts and reproduced tissues was evaluated by using a light microscope using an immunocytochemistry staining method.
Immunocytochemistry Staining Method
This method was used to evaluate the quality of developed dentin and bone tissues made by differentiated odontoblasts and osteoblasts from dental pulp stem cells using anti-DSPP and anti-BSP. Then, magnification was performed using an optical invert microscope (10 × and 100 ×). For the negative control group, all stages were performed, except for incubation of the primary antibody.
Statistical Analysis
Data were analyzed using SPSS version 24 by the Shapiro–Wilk test, one-way variance analysis, and Tukey test. Statistical significance was considered when p value < 0.05.
Results
The Vitality of Cells—MTT
Mean ± standard deviation (SD) of the vitality of cells for groups one, two, and three was 0.13 ± 0.03, 0.12 ± 0.01, and 0.10 ± 0.02, respectively, which their difference was not statistically significant (p = 0.465). Therefore, in all three groups, the cells had a similar vitality rate (Fig. 1).
Comparison of the three groups’ vitality after 24 h. Mean ± standard deviation (SD) of the vitality of cells for groups one, two, and three was 0.13 ± 0.03, 0.12 ± 0.01, and 0.10 ± 0.02, respectively, which their difference was not statistically significant (p = 0.465). Therefore, in all three groups, the cells had a similar vitality rate
Immunocytochemistry
The expression percentage of the DSPP marker was measured in group one (dentin matrix derivatives), on days 3, 7, and 10, and in the negative control group, which only contained hDPSC colony. The results are provided in the following figures, which indicate increased expression of DSPP on days 7 and 10, which were exposed to dentin matrix derivatives for a longer period (Fig. 2).
The mean expression percentage of the DSPP marker in group one and negative control between days 3, 7, and 10 was statistically significant (p < 0.001) (Fig. 3). So, in all binary comparisons between groups, the mean expression percentage of the DSPP marker was significantly different (in all cases p < 0.001).
The immunocytochemistry showed that the expression percentage of the BSP marker in group two on days 3, 7, and 10, as well as the negative control group, was increased on days 7 and 10, which were exposed to dentin matrix derivatives for a longer period (Fig. 4).
The mean expression percentage of the BSP marker in the second group and negative control between days 3, 7, and 10 was statistically significant (p < 0.0001) (Fig. 5). So, in all binary comparisons between groups, the mean expression percentage of the BSP marker was significantly different (in all cases p < 0.001).
Discussion
Cells, signals, and scaffolds are three key elements in tissue engineering [15]. A high number of mesenchymal stem cells are available in the pulp of primary teeth, but they reduce in adult teeth. According to some researchers, these cells are totipotent and when needed can be converted into odontoblast, fibroblasts, macrophages, and other cells, but with age progression, their number decreases. Nowadays, extensive efforts have been made to investigate the effect of signals on the differentiation of the DPSC cells both in vivo and in vitro.Even though conventional modalities deal with high levels of success for many dental diseases, an ideal therapy might be regenerative dentistry in which contaminated or necrotic dental tissues are removed and replaced with healthy vital tissue [9]. In the present study, the effect of DFDBA and demineralized dentin powder was evaluated on the proliferation and differentiation of the hDPSCs to osteoblast and odontoblast cells.
The results showed that bone and dentin matrix derivative materials significantly affected the proliferation of the hDPSCs and their differentiation into osteoblast and odontoblast cells in vitro. In the same way, Wang et al. (2013) reported that enamel matrix derivative materials stimulated on the proliferation and differentiation of the hDPSCs into odontoblasts [16]. Iohara K et al. (2011) also investigated complete regeneration of dental pulp in a dog tooth after pulpectomy with the transfer of CD105 + stem cell and other cells along with stromal cell–derived factor-1 (SDF-1) to induce tooth revitalization. The results showed that CD105 + stem cells expressed more angiogenic/neurotrophic factors compared to other cells. Besides, two-dimensional electrophoresis analysis, reverse transcription chain reaction, and chain polymerase reaction illustrated that quantitative and qualitative expression patterns of protein and mRNA of regenerated pulp are similar to the normal pulp [17]. However, in contrast to them using stem cells to complete dental pulp regeneration in vivo conditions, in the present study DPSCs were used for dentin regeneration in vitro. They did not mention whether the differentiation of investigated cells into odontoblast occurred or not. Since the results of both studies are significant, the use of stem cells in pulp and dentin regeneration using suitable signals is noticeable and can be considered in future studies.
As Peng Liu (2022) reviewed, DPSCs are more frequently used in dental regenerative research, because of the following: their allogeneic or autogenic sources are available, no ethical controversy, no immunogenicity, low antigenicity, and ideal cell line differentiation and plastic properties [10].
In the present study, differentiation of DPSCs to odontoblast was successful. Raoof et al. (2014), in a study on isolation and differentiation of DPSCs to odontoblast, investigated 20 impact wisdom teeth (human third molar) using a new method of culture of digested pulp tissue fragments to isolate stem cells. They also studied the expression of some stem cell marker genes in these cells. These cells were then induced for odontoblastic differentiation using dexamethasone and bone-shaping protein as signals, although, in contrast to this study, the results disclosed that odontoblastic markers such as DSPP and DMP-1 proteins were not expressed in induced cell culture. Hence, despite the successful isolation of stem cells with a new culture method, the induced cells did not differentiate into odontoblast, which can be due to the applied differentiation method, cell subculture during induction, induction materials, single layer culture, and specially type of signals [14].
In terms of various studies about enamel matrix derivative (EMD), E. Aurstad Riksen et al. (2014) investigate the effects of EMD on the induction of reactive dentin formation and compared the effect of EMD (5–50 μg/mL) on untreated human pulp cells and cells incubated with dexamethasone (8–10 mg) for 1, 2, 3, 7, and 14 days in the culture medium. Analysis of gene expression using Affymetrix microchips showed that 10 μg/mL EMD could not only regulate hundreds of genes but also stimulate the gene expression of proteins involved in mesenchymal cell proliferation and differentiation. Both EMD and dexamethasone associated with increased expression of amelogenin, dentinogenic markers of dentin sialophosphoprotein (DSSP), DMP1, dentin acidic phosphoprotein 1, and bone markers of osteocalcin, BGLAP, and collagen type 1 (COL1A1) [18]. That was in line with this study.
Considering the remineralization of dentin, Han M. et al. (2017) examined the remineralization induction of dentin using an agar hydrogel in the biological imitator mineralization system in rabbits; as a result, they reported the partial remineralization of the dentin. They confirmed the effectiveness of this method in potentially improving dentin-related diseases such as erosion, abrasion, and hypersensitivity, but, contrary to the present study, new odontoblast cells were not developed, and the reactive dentin did not show optimum quality [19].
Fonto et al. (2005) conducted a study on rats to evaluate the power of EMD in combination with DFDBA to increase bone construction and the ability of these compounds to hold EMD at the operation location. They concluded that there was a significant difference between the groups, considering the formation of new bone in the second week, and DFDBA could cause new bone formation. They proposed that DFDBA alone could not induce osteoblast differentiation, and the combination of EMD and DFDBA was effective [20], but this study displayed that DFDBA alone had a great ability to induce osteogenesis.
Zhong et al. (2020) evaluated the effect of lncRNA-H19 on odontoblastic differentiation of human pulp stem cells in in vivo and in vitro environments and concluded that H19 expression could induce odontoblastic differentiation in human dental pulp stem cells [21]. Yang and Peern (2019) studied the effect of lipopolysaccharide (LPS) as a signal on proliferation and migration, as well as odontoblastic differentiation in NG2 + human pulp cells in vitro, and eventually reported positive results. In contrast to the present study’s findings, they evaluated NG2 + nerve cells of the pulp and concluded that the LPS stimulated the migration, proliferation, and odontoblastic differentiation in NG2+ cell culture, and bone morphogenetic proteins may be involved in their odontoblastic differentiation pathway. Their results indicated the distinct roles of tissue engineering in tooth decay repair and potential application in regenerative dentistry [22].
Ahangari Z. (2012) studied the stimulation of stem cells by propolis as a signal in vital pulp therapy in guinea pigs and for the first time documented that propolis (a herbal product extracted from bee hives) stimulated the pulp stem cells. Additionally, they claim that propolis provided potential advantages over calcium hydroxide as a capping agent in vital pulp therapy [23]. Therefore, seeking for more effective and expeditiously signals has been implemented by dental scientists until the most beneficial materials are reached.
The present study demonstrated the odontoblastic differentiation of hDPSCs using DDS a signal take out from the natural dentin of the extracted teeth. This signal is available everywhere and does not depend on expensive preparing processes as well as effective on stem cell induction without any known side effects which are its other advantages.
In this study, due to facility limitations, only MTT and ICC techniques were applied to evaluate vitality and differentiation of cells; thus, the use of more precious modalities is recommended for future studies.
Conclusion
Considering the positive findings of the present study to the conversion of dental pulp stem cells into odontoblast and osteoblast using natural growth factors derived from bone tissues and dentin in vitro conditions, the effect of these substances in regenerative endodontic and regeneration of tooth surrounding bone can be investigated in future in vivo studies.
Data Availability
Data generated or analyzed data during this study are included in this manuscript and are available from the corresponding author upon reasonable request.
Abbreviations
- hDPSCs:
-
Human dental pulp stem cells
- DFDBA:
-
Demineralized freeze-dried bone allograft
- ICC:
-
Immunocytochemistry
- DSPP:
-
Dentin sialophosphoprotein
- BSP:
-
Bon sialoprotein
- PDL:
-
Periodontal ligament
- SD:
-
Standard deviation
- SDF-1:
-
Stromal cell-derived factor-1
- EMD:
-
Enamel matrix derivative
- COL1A1:
-
Collagen type 1
References
Tziafas D, Kodonas K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J Endodontics. 2010;36(5):781–9.
Pettiette MT, et al. Endodontic complications of root canal therapy performed by dental students with stainless-steel K-files and nickel-titanium hand files. J Endodontics. 1999;25(4):230–4.
Nejati K, et al. GDNF gene-engineered adipose-derived stem cells seeded Emu oil-loaded electrospun nanofibers for axonal regeneration following spinal cord injury. J Drug Delivery Sci Technol. 2020;60:102095.
Dadashpour M, et al. Emerging importance of phytochemicals in regulation of stem cells fate via signaling pathways. Phytother Res. 2017;31(11):1651–68.
Strem BM, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54(3):132–41.
Morsczeck C, et al. Somatic stem cells for regenerative dentistry. Clin Oral Invest. 2008;12(2):113–8.
Josefson, Deborah. "Stem cells in tooth pulp could be used in research." BMJ 326.7396 (2003):950.
Gronthos S, Brahim J, Li W. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81(8):531–5.
Murray PE, Garcia-Godoy F, Hargreaves KM. Regenerative endodontics: a review of current status and a call for action. J Endodontics. 2007;33(4):377–90.
Liu P, et al. Application of dental pulp stem cells in oral maxillofacial tissue engineering. Int J Med Sci. 2022;19(2):310.
Khachatryan L, Khachatryan G, Hakobyan G. The treatment of lower jaw defects using vascularized fibula graft and dental implants. J Craniofacial Surg. 2018;29(8):2214–7.
Liang F, et al. Alternatives to autologous bone graft in alveolar cleft reconstruction: the state of alveolar tissue engineering. J Craniofacial Surg. 2018;29(3):584–93.
Karanxha L, et al. Combined effects of simvastatin and enamel matrix derivative on odontoblastic differentiation of human dental pulp cells. J Endodontics. 2013;39(1):76–82.
Homayounfar N, et al. Isolation, characterization, and differentiation of dental pulp stem cells in ferrets. J Endodontics. 2016;42(3):418–24.
Min K-S, Yang S-H, Kim E-C. The combined effect of mineral trioxide aggregate and enamel matrix derivative on odontoblastic differentiation in human dental pulp cells. J Endodontics. 2009;35(6):847–51.
Zhang L, et al. Review scaffold design and stem cells for tooth regeneration. Japanese Dental Sci Rev. 2013;49(1):14–26.
Iohara K, et al. Complete pulp regeneration after pulpectomy by transplantation of CD105+ stem cells with stromal cell-derived factor-1. Tissue Eng Part A. 2011;17(15–16):1911–20.
Riksen EA, et al. Enamel matrix derivative promote primary human pulp cell differentiation and mineralization. Int J Mol Sci. 2014;15(5):7731–49.
Han M, et al. In vivo remineralization of dentin using an agarose hydrogel biomimetic mineralization system. Sci Rep. 2017;7(1):1–9.
Font, Kerri. Comparison of Demineralized Dentin and Demineralized Freeze Dried Bone as Carriers for Enamel Matrix Proteins in a Rat Critical Size Defect. Air force inst of tech wright-pattersonafb oh, 2005.
Zhong J, et al. LncRNA H19 promotes odontoblastic differentiation of human dental pulp stem cells by regulating miR-140-5p and BMP-2/FGF9. Stem Cell Res Ther. 2020;11(1):1–13.
Yang G, et al. Lipopolysaccharide upregulates the proliferation, migration, and odontoblastic differentiation of NG2+ cells from human dental pulp in vitro. Cell Biol Int. 2019;43(11):1276–85.
Ahangari Z, et al. Effect of propolis on dentin regeneration and the potential role of dental pulp stem cell in guinea pigs. Cell Journal (Yakhteh). 2012;13(4):223.
Funding
In this research, we used the financial support of Semnan University of Medical Sciences Research and Technology Vice-Chancellor with the code 1359, as well as the executive support of the Nervous System Stem Cell Research Center.
Author information
Authors and Affiliations
Contributions
M.J. designed the study, performed and wrote the manuscript draft, and edited and submitted it. H.R. S. assisted in research design, performed and supervised the laboratory process, and edited the final manuscript. S.Z. supervised the laboratory process and edited the final manuscript. R.G.H. analyzed the data and kindly adjusted the manuscript. A.N. and A.J. searched the literature, gathered data, and cooperated in writing the manuscript. Moreover, all authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This research project (ref no: IR.SEMUMS.REC.1396.246) was approved by the ethics committee of Semnan University of Medical Science, Semnan, Iran.
Consent to Participate
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jalili Sadrabad, M., Sameni, HR., Zarbakhsh, S. et al. The Effect of Bone and Dentin Matrix Derivatives on the Differentiation of Human Dental Pulp Stem Cells for Osteogenesis and Dentinogenesis in a Scaffold-Free Culture. Regen. Eng. Transl. Med. 9, 416–423 (2023). https://doi.org/10.1007/s40883-022-00291-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-022-00291-w