Abstract
The major goal of dental pulp tissue engineering is to enable the healing of inflamed tissue or to replace necrotic pulp tissue with newly formed dental pulp tissue. Here, we report a protocol for pulp tissue engineering in vivo in pulpotomized rat teeth using constructs of rat bone marrow mesenchymal stem cells, preformed biodegradable scaffolds, and hydrogel. The constructs were implanted into pulpotomized pulp chambers for 3, 7, or 14 days. At 3 days, cells were located mainly along the preformed scaffolds. At 7 days, pulp tissue regeneration was observed in almost the entire implanted region. At 14 days, pulp tissue regeneration further progressed throughout the implanted region. In immunohistochemistry, at 3 days, a number of small and round macrophages immunoreactive to CD68 were predominantly distributed around the scaffolds. The density of CD68+ macrophages decreased until 14 days. On the other hand, nestin-expressing odontoblast-like cells beneath the dentin at the border of implanted region increased until 14 days. Quantitative gene expression analysis revealed that odontoblast differentiation marker dentin sialophosphoprotein mRNA in the implanted region gradually increased until 14 days. Together, the results suggested that regeneration of dental pulp tissue had occurred. Thus, our study provides a novel experimental rat model of dental pulp regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental pulp tissue plays a crucial role in tooth homeostasis by maintaining tooth vitality and controlling pulp defensive functions [1]; thus, conservation of the pulp is critical [2]. Dental caries involving irreversible pulpitis is one of the largest obstacles to pulp preservation, and in most cases, root canal treatment is carried out to remove the whole pulp tissue even though a large proportion of the pulp remains healthy [3]. This treatment causes dentin loss and might increase the risk of tooth fracture, which inevitably results in extraction. If the lost pulp tissue could be regenerated, extraction may be prevented. Thus, dental pulp regeneration using stem cells and/or biomaterials is considered an important strategy for tooth retention. Preclinical studies on experimental animals are required to confirm the bioavailability, safety, and effectiveness of such treatments.
In the late 1980s, the concept of tissue engineering on the basis of biocompatible/biodegradable scaffolds was advocated [4]. Based on this concept, mesenchymal stem-like cells isolated from human dental pulp have been tested in animal models such as immunocompromised mice [5, 6]. However, the clinical application of cell-based therapies will require the development of sufficiently strong scaffolds for trapping stem cells, to allow their transplantation into the dental pulp [7, 8]. Hydrogels can be injected in situ to form a scaffold that adapts to the dentin surface of the pulp chamber [9]. However, hydrogels are soft, weak, and malleable; thus, when they are used for pulp regeneration, the pulp space is filled with restorative materials during the subsequent sealing of the cavity [10]. On the other hand, preformed scaffolds exhibit relatively high mechanical strength, thus preserving an appropriate pulp-chamber space; however, they do not easily adapt to the shape of the pulpal cavity [11]. A scaffold combining moderate strength and flexibility is desirable for filling spaces that are encased within rigid structures such as the pulp cavity.
The purpose of this study was to develop a protocol for dental pulp tissue engineering in vivo in pulpotomized teeth using rat bone marrow mesenchymal stem cells (RBMMSC) and combinations of hydrogel and preformed biodegradable scaffolds. Histological analysis and histochemical analysis of nestin—a marker for differentiated odontoblasts [12, 13]—as well as a quantitative gene-expression assay for dentin sialophosphoprotein (DSPP) were used for monitoring regeneration.
Materials and methods
Ethics statement
All experiments were approved by the animal care committee of Niigata University and conducted in accordance with the guidelines of the committee. Female Wistar rats, each weighing ~200 g, were used at 7 weeks of age (n = 48, Charles River Laboratories, Yokohama, Japan). The animals were housed at 22 °C on a standard light/dark schedule with access to food and water.
Administration of antibiotics and immunosuppressants
On days 3 and 1 before implantation, the rats were intraperitoneally injected with a mixture of cyclosporine (15 mg/kg; Novartis Pharma, Basel, Switzerland), methylprednisolone (10 mg/kg; Sawai Pharmaceutical, Osaka, Japan), ciprofloxacin (15 mg/kg; Meiji Seika Pharma, Tokyo, Japan), fluconazole (10 mg/kg; Pfizer, New York, NY), sulbactam/ampicillin (600 mg/kg; Pfizer), and vancomycin (10 mg/kg; Kobayashi Kako, Tokyo, Japan). On days 1–14 after the implantation procedure, the same medications were given once daily.
Preparation of the RBMMSC and preformed scaffolds
RBMMSC (Lonza, Basel, Switzerland) were cultured in stem cell growth medium (Lonza). Porous poly(l-lactic acid) (PLLA) scaffolds with a mean pore diameter of 180 μm were produced as described previously [14]. The scaffolds were dried, cut into pieces of ~0.5 × 0.5 × 0.5 mm under a microscope, sterilized twice by exposure to ultraviolet light for 1 h and soaking in 70% ethanol for 1 day, and washed overnight in DNase/RNase-free distilled water at 4 °C. Three hours before the implantation procedure, the scaffolds were dried and sterilized with ultraviolet radiation. RBMMSC (2 × 105) were resuspended in 400 µl of 1:1 DMEM/F12 (Gibco/Invitrogen)/Matrigel hydrogel (Corning Inc., Corning, NY) and allowed to absorb into the scaffold sponges in a CO2 incubator for 30 min prior to implantation.
Implantation of the RBMMSC, preformed scaffolds, and hydrogel constructs into the upper first molars
The rats were anesthetized with 8% chloral hydrate (350 mg/kg, intraperitoneally). The maxillary molars and gingival tissue were disinfected with 2.5% hydrogen peroxide and the occlusal surface of the maxillary first molar was disinfected with 2.5% sodium hypochlorite. Subsequently, the dental pulp of the upper first molars was exposed and pulpotomized using a No. 1/2 round bur. The exposed area was rinsed with 1.5% sodium hypochlorite followed by 15% EDTA. After 1 µL of Matrigel with or without RBMMSC was injected, RBMMSC/PLLA/Matrigel constructs were implanted into the cavity for 3, 7, or 14 days (n = 8 in each group). The cavity was sealed with temporary filling material (Cavit™, 3M ESPE Dental AG, Seefeld, Germany). Then, a dentin and enamel-bonding agent (Clearfil Tri-S Bond ND; Kuraray Medical, Tokyo, Japan) was applied to the tooth according to the manufacturer’s protocol. The occlusal cavity was covered with an adhesive restorative material (flowable resin composite; Shofu, Kyoto, Japan). PLLA/Matrigel constructs without cells implanted for 14 days, pulpotomized teeth without implantation for 14 days (sealed with Cavit), and normal teeth from rats treated with antibiotics and immunosuppressants served as controls (n = 8 in each group).
Sample preparation
At 3, 7, or 14 days after implantation, the animals (n = 4 in each group) were sacrificed by transcardiac perfusion with 3% paraformaldehyde and 0.2% glutaraldehyde. The maxillary first molars were retrieved and soaked in 3% paraformaldehyde for 24 h. Following the demineralization of the samples with 10% EDTA, they were embedded in an embedding medium (OCT compound; Sakura Finetek, Tokyo, Japan) and cut into 6-μm-thick cryosections in a cryostat. Hematoxylin and eosin staining were performed. Images were captured using the software NIS-Elements (Nikon, Tokyo, Japan), and the cellularity of the normal and engineered coronal pulp tissues was assessed.
The remainder of maxillary left first molars (n = 4 teeth in each group) was retrieved and demineralized with a mixture of EDTA and a storage medium (RNAlater; Thermo Fisher Scientific, Waltham, MA) [15]. The samples were cut into 10-μm-thick sections and mounted on glass foiled PEN slides for laser capture microdissection (Leica Microsystems, Wetzlar, Germany). Then, the coronal pulp area was retrieved using a microsurgery knife under a microscope for quantitative real-time (q)PCR. For teeth in which constructs without cells were implanted or pulpotomized teeth without implantation (sealed with Cavit), qPCR analysis was not performed as the pulp was very poorly regenerated.
Immunostaining of ED1 and nestin
The sections were incubated with a mouse anti-rat CD68 monoclonal antibody reactive to pan-macrophages (ED1; AbD Serotec, Oxford, UK) or a monoclonal anti-nestin antibody (R&D Systems, Minneapolis, MN), followed by a biotinylated horse anti-mouse immunoglobulin G (rat adsorbed; Vector Laboratories, Burlingame, CA) and an avidin–biotin–peroxidase complex (Elite ABC kit; Vector). The sections were developed with a diaminobenzidine (DAB) peroxidase substrate kit (Vector). For quantitative analysis, three sections were chosen from each specimen (n = 4 in each experimental group), and ED1+ cells beneath the implantation site were enumerated under a light microscope (Nikon, objective lens: 60×) with the aid of a 10 × 10 mm ocular grid. To calculate the density of stained cells, digital pictures of the sections were taken, and stored as jpeg files. The area corresponding to each region in each specimen was determined using ImageJ software (Version 1.37v; NIH, Bethesda, MD) and results were expressed as the mean count of cells per 1.0 mm2 area.
qPCR analysis
TaqMan gene expression assay probe and primer sets for rat dentin sialophosphoprotein (DSPP, Rn02132391_s1*) and GAPDH (Rn99999916_s1) were obtained from Applied Biosystems. Total RNA was extracted using TRIzol (Invitrogen) and the RNeasy Mini Kit (Qiagen, Valencia, CA), as described previously [16]. cDNA was generated from 1 μg RNA using the High-Capacity cDNA Reverse Transcription Kit (Invitrogen) according to the manufacturer’s instructions. qPCR was performed on a StepOne thermocycler (Applied Biosystems) using TaqMan Universal Master Mix II (Applied Biosystems). Gene expression was normalized to the GAPDH expression level.
Results
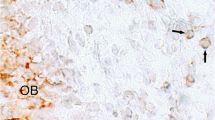
At 3 days after implantation, cells were located mainly along the PLLA scaffolds (Fig. 1a, b). No obvious infiltration of neutrophils was observed. Notably, at 7 days, pulp-like tissue regeneration was observed in the implanted region (Fig. 1c, d). At 14 days, the pulp-like tissue regeneration further progressed throughout the implanted region (Fig. 1e, f), and the engineered pulp-like tissue showed a similar cellularity as the control dental pulp (Fig. 1g, h). To assess the capacity of implanted RBMMSC to generate dental pulp, RBMMSC-loaded scaffolds were compared with scaffolds without cells at 14 days after implantation (Fig. 1i). The teeth transplanted with cell-less scaffolds showed incomplete hard tissue formation at the border between the implanted area and the remaining pulp tissue, and the implanted area showed poor tissue regeneration. PLLA scaffolds were not absorbed. The capacity of preformed PLLA scaffolds to generate dental pulp was assessed at 14 days after sealing the pulpotomized pulp chamber with Cavit without scaffold implantation; these teeth showed no pulp tissue regeneration and the pulp cavity was filled with restorative materials (Fig. 1j).
a–f Representative histological micrographs of the engineered pulp tissues at 3 days (a, b), 7 days (c, d), and 14 days (e, f) after implantation. g, h Normal pulp tissue. i Pulp tissue after implantation of scaffolds without RBBMSC at 14 days. j Pulp tissue after Cavit sealing without implantation of scaffolds at 14 days. Arrows indicate PLLA scaffolds. d dentin. Scale bar 200 µm (c, e, g), 100 µm (a, i, j), and 25 µm (b, d, f, h)
The CD68 antibody caused a patchy, granular intracytoplasmic staining pattern. At 3 days after implantation, a number of small and round cells immunoreactive to CD68 were predominantly distributed around the scaffolds (Fig. 2a, e). At 7 days, CD68+ cells showing variable staining intensity were predominantly distributed in the implanted region, and their density was significantly decreased (Fig. 2b, e). CD68+ cells with distinct phagosomes were predominantly found around the scaffolds (Fig. 2c). At 14 days, the density of CD68+ cells further decreased to a level similar to that in normal pulp (Fig. 2c, e).
Immunohistochemistry for CD68. CD68+ cells in the implanted region at 3 days (a), 7 days (b), and 14 days (c) after implantation, and in normal pulp (d). Scale bar 50 µm. e Density of CD68+ cells (mean ± SEM) in normal tissue and at 3, 7, and 14 days after implantation. *P < 0.05 (Mann–Whitney’s U test and Bonferroni’s correction)
Nestin-immunoreactive cells were not detected in the remaining pulp tissue near the implanted area at 3 days (Fig. 3a). At 7 days, nestin-positive odontoblast-like cells were often observed under the dentin near the implanted area (Fig. 3b). At 14 days, odontoblast-like cells arranged beneath the dentin near the implantation site, and most of these cells were immunoreactive to nestin (Fig. 3c). In teeth implanted with cell-less scaffolds, nestin immunoreactive odontoblast-like cells were virtually absent near the implanted area (Fig. 3d).
To evaluate the regeneration status of the pulp tissue, we assessed the mRNA expression of the odontoblast differentiation marker DDSP in the coronal pulp tissue (Fig. 4a). Although the expression level of DSPP in the engineered pulp was lower than that in normal pulp, it gradually increased until 14 days (Fig. 4).
Discussion
In this study, a novel method for dental pulp tissue engineering of pulpotomized rat molars with RBMMSC, biodegradable scaffolds, and hydrogels was successfully developed. The pulpotomized region of the pulp was filled with regenerated tissue with no obvious inflammation for 7 days. These results indicate that the rat pulpotomy model is useful for dental pulp regeneration research and may contribute to the development of therapeutic approaches.
This study used preformed, porous, biodegradable PLLA scaffolds [17] because PLLA is approved by the US Food and Drug Administration for human clinical applications. Scaffolds with a mean pore diameter of 180 μm were prepared, as scaffolds of this diameter previously allowed the generation of well-vascularized tumors in immunodeficient mice [16]. In another study, dental pulp stem cells (DPSC) were seeded into PLLA scaffolds set within the pulp-chamber space in human tooth slices, and the tooth slices were implanted subcutaneously in immunodeficient mice [18]. The resulting tissue exhibited an architecture and cellularity closely resembling those of dental pulp tissue; however, the regenerated tissue did not fill the entire pulp space [18].
Although MSC are present in various tissues [19], BMMSC possess the fundamental features of primary MSC while having a relatively higher proliferation capacity [20]. BMMSC have functional characteristics that make them useful for clinical application in regenerative medicine [21]. In addition, they can be easily isolated from the human body and stored for several years [22]. Autologous DPSC, which can be obtained from a diseased tooth and can even be transplanted back into the same tooth, are preferable for dental pulp regeneration; however, in some patients, DPSC might not be available for tissue engineering due to infection, inflammation, and/or necrosis of the pulp. In these cases, allogeneic stem cells such as BMMSC might be a suitable alternative. BMMSC could be implanted into defect sites containing scaffolds to promote the regeneration of damaged pulp tissue. However, when allogeneic cells are used for transplants, adjunctive immunosuppressive therapy should be considered [23].
This study tested the possibility of inducing pulp regeneration in pulpotomized rat teeth using a combination of engineering and restorative techniques. The strategy worked well, and at 7 days after implantation, the pulpotomized region was occupied by regenerated tissue with a cell density similar to that of normal, healthy dental pulp. Notably, mRNA expression of the odontoblast differentiation marker DSPP was upregulated at the implanted region at 14 days. Moreover, odontoblast-like cells expressing nestin, a marker for differentiated odontoblasts, were observed along the dentin–pulp border near the implanted region [24, 25]. These results suggest that the implantation of RBMMSC-loaded PLLA/Matrigel constructs promote the odontoblastic differentiation of cells in the implanted region and the surrounding tissue. In contrast, when cell-less constructs were implanted into the pulpotomized space, minimally regenerated and poorly organized tissue was observed, suggesting that RBMMSC are a useful source of stem cells for dental pulp tissue engineering and are essential for promoting rapid regeneration of dental pulp tissue.
Small and round CD68+ cells, probably representing newly recruited macrophages, were detected in the implanted region at 3 days. At 7 and 14 days, the scaffolds were gradually resorbed, and the number of CD68+ macrophage-like cells gradually decreased to a density similar to that in normal pulp. The application of graphene oxide scaffolds is known to enhance tissue repair via macrophage recruitment [24]. In addition, it has been reported that vascularization of polymeric scaffolds is entirely dependent on the action of recruited macrophages, and that the continued presence of M1 and M2 macrophages is essential for neovascularization of scaffolds [25]. Taken together, the present results suggest that macrophages are recruited in response to scaffold implantation and may play an essential role in promoting dental pulp tissue regeneration.
In conclusion, this study showed that implantation of RBMMSC in combination with the use of preformed scaffolds and hydrogel results in pulp tissue regeneration in pulpotomized pulp chambers in rats. The pulp tissue formed under the present experimental conditions did not exhibit any signs of inflammation. These findings will significantly aid the development of techniques for promoting dental pulp regeneration.
References
Tatullo M, Marrelli M, Shakesheff KM, White LJ. Dental pulp stem cells: function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med. 2015;9:205–16.
Caplan DJ, Cai J, Yin G, White BA. Root canal filled versus non-root canal filled teeth: a retrospective comparison of survival times. J Public Health Dent. 2005;65:90–9.
Barthel CR, Rosenkranz B, Leuenberg A, Roulet JF. Pulp capping of carious exposures: treatment outcome after 5 and 10 years: a retrospective study. J Endod. 2000;26:525–8.
Vacanti CA. The history of tissue engineering. J Cell Mol Med. 2006;10:569–76.
Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, Shi S. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A. 2010;16:605–15.
Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, Gronthos S, Robey PG, Shi S. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res. 2003;82:976–81.
Galler KM, Hartgerink JD, Cavender AC, Schmalz G, D’Souza RN. A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue Eng Part A. 2012;18:176–84.
Aulisa L, Dong H, Hartgerink JD. Self-assembly of multidomain peptides: sequence variation allows control over cross-linking and viscoelasticity. Biomacromolecules. 2009;10:2694–8.
Abou Neel EA, Chrzanowski W, Salih VM, Kim HW, Knowles JC. Tissue engineering in dentistry. J Dent. 2014;42:915–28.
Yu N, Plachokova A, Yang F. Engineering of dental tissues: scaffolds and preclinical models. In: Huang GTJ, Thesleff I, editors. Stem cells in craniofacial development and regeneration. NJ: Wiley; 2013. p. 409–29.
Rosa V, Zhang Z, Grande RH, Nör JE. Dental pulp tissue engineering in full-length human root canals. J Dent Res. 2013;92:970–5.
Goldberg M, Smith AJ. Cells and extracellular matrices of dentin and pulp: a biological basis for repair and tissue engineering. Crit Rev Oral Biol Med. 2004;15:13–27.
Terling C, Rass A, Mitsiadis TA, Fried K, Lendahl U, Wroblewski J. Expression of the intermediate filament nestin during rodent tooth development. Int J Dev Biol. 1995;39:947–56.
Mooney DJ, Sano K, Kaufmann PM, Majahod K, Schloo B, Vacanti JP, Langer R. Long-term engraftment of hepatocytes transplanted on biodegradable polymer sponges. J Biomed Mater Res. 1997;37:413–20.
Belluoccio D, Rowley L, Little CB, Bateman JF. Maintaining mRNA integrity during decalcification of mineralized tissues. PLoS One. 2013;8:e58154.
Kaneko T, Zhang Z, Mantellini MG, Karl E, Zeitlin B, Verhaegen M, Soengas MS, Lingen M, Strieter RM, Nunez G, Nör JE. Bcl-2 orchestrates a cross-talk between endothelial and tumor cells that promotes tumor growth. Cancer Res. 2007;15:9685–93.
Ifkovits JL, Burdick JA. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13:2369–85.
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith AJ, Nör JE. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod. 2008;34:962–9.
Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13.
Sotiropoulou PA, Perez SA, Salagianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006;24:462–71.
Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–92.
Kotobuki N, Hirose M, Machida H, Katou Y, Muraki K, Takakura Y, Ohgushi H. Viability and osteogenic potential of cryopreserved human bone marrow-derived mesenchymal cells. Tissue Eng. 2005;11:663–73.
Nauta AJ, Westerhuis G, Kruisselbrink AB, Lurvink EG, Willemze R, Fibbe WE. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–20.
Nishida E, Miyaji H, Kato A, Takita H, Iwanaga T, Momose T, Ogawa K, Murakami S, Sugaya T, Kawanami M. Graphene oxide scaffold accelerates cellular proliferative response and alveolar bone healing of tooth extraction socket. Int J Nanomed. 2016;11:2265–77.
Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35:4477–88.
Acknowledgements
Grants-in-Aid for Scientific Research was received from the Japan Society for the Promotion of Science [Nos. 26293405, 25670808 (T. Okiji), 24592862, and 15K11110 (T. Kaneko)].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Ito, T., Kaneko, T., Sueyama, Y. et al. Dental pulp tissue engineering of pulpotomized rat molars with bone marrow mesenchymal stem cells. Odontology 105, 392–397 (2017). https://doi.org/10.1007/s10266-016-0283-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-016-0283-0