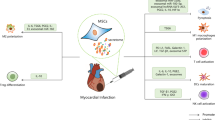
Abstract
Myocardial infarction triggers reparative inflammatory processes programmed to repair damaged tissue. However, often additional injury to the myocardium occurs through the course of this inflammatory process, which ultimately can lead to heart failure. The potential beneficial effects of cell therapy in treating cardiac ischemic disease, the number one cause of death worldwide, are being studied extensively, both in clinical trials using adult stem cells as well as in fundamental research on cardiac stem cells and regenerative biology. This review summarizes the current knowledge on molecular and cellular processes implicated in post-infarction inflammation and discusses the potential beneficial role cell therapy might play in this process. Due to its immunomodulatory properties, the mesenchymal stromal cell is a candidate to reverse the disease progression of the infarcted heart towards heart failure, and therefore is emphasized in this review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic heart disease including myocardial infarction (MI) is the number one cause of death worldwide [1]. MI typically results from a (thrombotic) occlusion of a coronary artery leading to myocardial ischemia [2]. Typically, after diagnosis of MI primary percutaneous coronary intervention (PCI) of the infarct related coronary artery is performed to achieve reperfusion, limit tissue necrosis and improve the clinical outcome. Additionally, reperfusion triggers the immune system to initiate an essentially regenerative signaling cascade programmed to repair the damaged tissue after removal of dead cells and matrix debris [3]. However, this immune-mediated response needs to be tightly regulated to prevent additional myocardial tissue damage which may invoke congestive heart failure [4, 5]. Although PCI limits tissue damage inflicted by myocardial ischemia, this intervention typically does not halt or even reverse the loss of functional myocardium [6].
To limit (additional) damage to the myocardium after MI, novel therapeutic interventions involving cell-based therapies have emerged in order to increase our arsenal for treating ischemic heart disease [7]. In this review we systematically summarize the current state of knowledge on the inflammatory response involved in post-myocardial infarct inflammation and discuss how cell therapy may attenuate certain deleterious aspects of this response and may improve cardiac function after MI.
The Post-infarction Inflammatory Response
Myocardial ischemia results in cell death, initiating an inflammatory response ultimately resulting in scar formation [8]. This process of myocardial infarct healing occurs through three successive phases: the inflammatory phase, the proliferative phase and finally the maturation phase [9, 10].
The Inflammatory Phase
The immune system comprises an innate and adaptive system. The innate immune system regulates the non-specific immediate response against invading pathogens and injury, whereas the adaptive immune system involves specific recognition of foreign antigens and progresses with a delay as it requires prior activation by innate immune cells. As a consequence, the first phase of the reparative process after MI is mediated by the innate immune system [10].
Initially, platelets are activated upon myocardial injury to prevent bleeding. Platelets aggregate locally to form a fibrin-rich matrix and release important growth factors such as platelet-derived growth factor (PDGF) and Platelet-Factor 4 that aid the repair process [11]. In parallel, platelets produce platelet activating factor thereby stimulating the influx and adhesion of neutrophilic granulocytes to the site of injury [12]. Neutrophils are among the first innate immune cells to enter the myocardium, which occurs within hours after the ischemic event. Their recruitment is stimulated by Reactive Oxygen Species (ROS) produced by activated cardiac myocytes and vascular endothelial cells [10]. ROS (including hydrogen peroxides, superoxide anions and hydroxy radicals) are formed by the incomplete reduction of molecular oxygen and activate the chemotactic cytokine interleukin (IL)-8 / chemokine (CXC motif) ligand 8 as well as the endothelial surface molecule intercellular adhesion molecule-1 (ICAM-1), together coordinating neutrophil recruitment.
Upon arrival, neutrophils secrete proteolytic enzymes that clear the infarct from dead cells and debris [10, 13]. However, the activated neutrophils also contribute to the production of ROS which react directly with cellular lipids, proteins and DNA released from the damaged cells. In this context ROS act as signaling intermediates that activate the transcription factor Nuclear Factor-κB (NF-κB) resulting in the production of pro-inflammatory cytokines and chemokines, but also of growth factors important for tissue repair such as Transforming Growth factor-beta (TGF-β) [10, 14, 15]. Tissue damage inflicted by ROS needs to be limited as early as possible as demonstrated in a study of MI in dogs using free radical scavenging catalase and the anti-oxidant enzyme superoxide dismutase-1. In this study it was shown that infarct size was reduced only when the treatment was given prior to coronary occlusion [16].
It is however difficult to denote the exact role of neutrophils in myocardial repair. Smaller infarcts were observed upon myocardial reperfusion in experimental animals depleted of neutrophils, suggesting that neutrophils have a deleterious effect in myocardial injury followed by reperfusion [17]. However, infarct sizes were not altered when neutrophil recruitment was prohibited in mice deficient for ICAM-1 and P-selectin, despite a reduction in neutrophil trafficking [18]. Initial neutrophil influx is followed by the recruitment of monocytes, which is mainly mediated by the chemokine monocyte chemo attractant protein-1 (MCP-1)/ chemokine (C-C motif) ligand 2. In a study in MCP-1 deficient mice, it was shown that the absence of MCP-1 did not alter infarct size, but attenuated ventricular remodeling, reduced and delayed monocyte/macrophage recruitment and delayed replacement of cardiomyocytes with granulation tissue and diminished myofibroblast accumulation [19]. Phenotypically monocytes can be distinguished in different subsets and numerous studies have tried to attribute different roles to distinct subsets as monocytes appear to be involved in both pathogenic as well as reparative inflammatory responses. In mice, monocytes that express high levels of the molecule lymphocyte antigen 6c (Ly-6C) are regarded as pro-inflammatory monocytes. In mouse MI studies these pro-inflammatory Ly-6Chigh monocytes are recruited from the bone marrow to the infarcted heart expressing the C-C chemokine receptor 2 (CCR2), where they remain in high numbers until 3 days after MI, scavenging debris and secreting inflammatory cytokines and matrix degrading proteases [20, 21].
The recruitment of neutrophils and monocytes is thus crucial for the initiation of the repair process, but their contribution is determined by the actual signaling cascades that are activated. Intracellular components released from necrotic cardiomyocytes are sensed by innate immune cells that become activated upon tissue entry [22]. The most prominent pathways by which the innate immune system initiates a post-infarction inflammatory response are: 1) the Toll-like receptor (TLR)-mediated pathway; 2) the complement cascade and; 3) the earlier mentioned ROS. These three pathways all converge to activate NF-κB, a transcription factor that drives the expression of numerous genes. In a resting cell the NF-κB dimer is sequestered in the cytoplasm as an inactive protein bound by the inhibitor of κB, IκB. Upon activation of the NF-κB pathway, the IκB protein is degraded, releasing the NF-κB dimer which then translocates to the nucleus where it regulates gene expression by binding specific promoter sequences. Since NF-κB regulates so many different genes ranging from pro-inflammatory cytokines, chemokines, matrix metalloproteinase (MMP) as well as genes involved in cell survival and proliferation, [23, 24] it is considered as one of the most important players throughout the whole process of tissue repair. A recent review summarizes several studies highlighting the participation of NF-κB in post-MI inflammation [24]. A reduction of myocardial infarct size was observed after ischemia/reperfusion in an experimental model where NF-κB activity was blocked by prohibiting DNA-binding using decoy oligodeoxynucleotides, whereas a recent report by Hamid et al. reported that prolonged activity of NF-κB in myocardial tissue results in a chronic inflammatory state with detrimental consequences for infarct healing [25]. Both studies underscore the role of NF-κB in post-MI inflammation [26].
TLRs are a family of heterodimeric transmembrane pattern recognition receptors that recognize and bind antigens derived from pathogens or damaged tissues, the so called damage-associated molecular patterns (DAMPs). Upon ligand binding most TLRs activate NF-κB leading to the expression of pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), pro IL-1β and interferons. Among the ten human TLRs identified, TLR1, 2, 4–6 and 11 are expressed on the cell surface, whilst TLR3 and 7–9 are expressed in intracellular vesicles, mostly endosomes. TLRs are pre-assembled as low-affinity dimers which undergo a conformational change upon ligand binding. Although initially described as receptors that recognize pathogen-derived molecules, several non-pathogenic endogenous molecules have been identified to bind and activate TLRs. For instance TLR4 binds not only to lipopolysaccharide but also to certain heat shock proteins and extracellular matrix remnants such as hyaluronan and fibronectin [27] suggesting a broad role for TLR4 in tissue injury and repair. It has been observed that TLR4 is upregulated in injured myocardium of both humans and mice [28]. Also, in TLR4 deficient mice, MI induced hearts were characterized by reduced left ventricular remodeling with preserved systolic function, but without affecting the infarct size. The infarcted area showed increased collagen density with fewer macrophages and reduced cytokine levels and MMP activity, identifying TLR4 as an important component of the post-MI remodeling process [29]. Next to TLR activation, the release of DAMPs also triggers the complement cascade.
The complement system is a network of soluble and surface bound proteins able of recognizing, tagging and eliminating microbial intruders and foreign cells via initiation of the immune response. The complement cascade consists of three main pathways which are all involved in immunopathological diseases [30]. In a rat model of MI it was shown that ischemic myocardial injury activates the complement cascade, and mRNA and proteins of the complement pathway are upregulated in areas of MI [31–34]. The importance of complement pathway activation in mononuclear cell recruitment was shown in a canine model of cardiac ischemia in which upon cardiac reperfusion, the complement pathway induced migration of monocytes into the myocardium [35]. Studies have been performed in which certain elements of the complement cascade have been inhibited using cobra venom or soluble human complement receptor to antagonize complement signaling. These studies showed a reduction in myocardial necrosis and a decrease in infarct size suggesting a role for the complement pathways in myocardial injury [36, 37].
In conclusion, all actions combined result in recruitment of leucocytes to the infarcted area, the clearance of dead cells and debris and the activation of signaling cascades leading to the production of a variety of essential growth factors for repair of the infarcted area, and the transition towards the proliferative phase [38].
The Proliferative Phase
At this stage neutrophils, mononuclear cells, endothelial cells and pericytes all work together to resolve the initial inflammatory reaction and direct it towards a healing process. Short–lived neutrophils become apoptotic and release mediators such as annexin A1 and lactoferrin that suppress further neutrophil recruitment [39]. The Ly-6Chigh monocytes express the orphan nuclear hormone receptor, nuclear receptor subfamily 4, group a, member 1 (Nr4a1) which reduces the CCR2 dependent recruitment of Ly-6Chigh monocytes towards the infarct. In addition, Ly-6Chigh monocytes differentiate into Ly-6Clow macrophages in the local cardiac tissue. Ly-6Clow macrophages clear the apoptotic neutrophils and are associated with an increased presence of the anti-inflammatory factors IL-10, TGF-β and vascular endothelial growth factor (VEGF) countering the inflammatory response by recruitment of myofibroblasts for scar tissue formation and thereby contributing to infarct healing [20, 40]. A recent study performed by Hilgendorf et al. indicated another important anti-inflammatory role for Nr4a1, as cardiac macrophages in Nr4a1-deficient mice showed a more inflammatory profile and as a result these animals had a decreased cardiac function and increased cardiac remodeling in contrast to wildtype controls following MI [40]. Whilst Ly-6Chigh monocyte levels decrease, Ly-6Clow monocytes, resident in the cardiac tissue, peak 7 days after MI and afterwards also decrease. Ly-6Clow monocytes are also Nr4a1 dependent, as Nr4a1-deficient animals had no Ly-6Clow monocytes in either the cardiac tissue or the peripheral circulation. The role of Ly6Clow monocytes is still under investigation, but they are important in the inflammatory process by the clearance of endothelial necrotic cells via TLR-7 activation [41]. A recent study showed a similar monocyte pattern in post-mortem tissue of human MI patients as mainly CD14+CD16− monocytes were present in the cardiac infarct tissue in the inflammatory phase after MI, while in the proliferative phase both CD14+CD16− and CD14+CD16+ monocytes were observed [42]. Since CD14+CD16− monocytes in humans are comparable to Ly6Chigh monocytes in mice [21, 43], this indicates the monocyte response is comparable between species.
The uptake of apoptotic cells by macrophages induces the release of anti-inflammatory factors such as IL-10 and TGF-β, and lipid mediators such as lipoxins and resolvins which further stimulate the removal of inflammatory leukocytes [23, 44].
After MI, IL-10 becomes highly expressed, mainly by activated T lymphocytes and monocytes as described above. As IL-10 inhibits the secretion of IL-1α, IL-1β, TNF-α, IL-6 and IL-8, it suppresses the ongoing inflammation process [5, 23]. In addition, IL-10 induces the production of a group of peptidases involved in extracellular matrix (ECM) degradation called tissue inhibitor of metalloproteinases (TIMPs), thereby promoting ECM remodeling [10, 45, 46]. IL-10 deficient mice showed an increased mortality concomitant with an enhanced immune response during myocardial ischemia followed by reperfusion, as measured by a higher neutrophil recruitment, elevated plasma TNF-α and a higher expression of ICAM-1 [47]. In a similar study elevated mRNA levels of TNF-α and MCP-1 were also observed in the infarcted heart of IL-10 deficient mice. However, in this study mortality rates were similar to wild type mice due to the variable effects of IL-10, affecting the production of numerous cytokines such as IL-1 and IL-6 [48]. Both IL-1α and IL-1β are upregulated in experimental models of MI and promote the inflammatory reaction by the induction of cytokine and chemokine production [10]. In contrast, IL-6 appears to have a beneficial role in tissue repair [11]. IL-6 protects cardiomyocytes against apoptosis and induces cardiomyocyte hypertrophy. IL-6 expression is induced in the healing infarct, and can be produced by mononuclear cells, cardiomyocytes and fibroblasts within the ischemic myocardium [10, 49, 50].
TGF-β is upregulated in experimental models of MI and initiates the transition from inflammation to fibrosis by pro-inflammatory cytokine suppression [38]. The secretion of TGF-β will initiate fibroblast growth as well as angiogenesis, whereas MMPs and TIMPs produced by the activated macrophages aid in the extracellular remodeling of the regenerating cardiac tissue [5, 10]. Angiogenesis is crucial to provide oxygen to the injured tissue and maintain cell metabolism [10]. One of the most important angiogenic factors during the proliferative phase is hypoxia-inducible factor 1, expressed early after myocardial ischemia, which upregulates the chemokine stromal cell-derived factor 1-α (SDF-1) and its receptors CXCR4 and CXCR7 [51] and activates the release of VEGF, one of the key growth factors in neoangiogenesis [52]. After SDF-1 is expressed, hematopoietic stem cells and endothelial progenitor cells are recruited to the ischemic myocardium where they improve angiogenesis as has been demonstrated by several studies [51, 53–57]. PDGF signaling induces maturation of the neovessels via the formation of a mural coat of pericytes surrounding the vessel. Withdrawal of PDGF from this process leads to apoptosis of the endothelial cells [58].
Inhibition of TGF-β during the early inflammatory phase after myocardial injury results in a significant increase in mortality and an exacerbated left ventricular dilatation via enhanced cytokine synthesis in mice [59] Moreover, TGF-β inhibits immune cell proliferation and stimulates fibroblasts to produce ECM proteins such as collagens, fibronectin, tenascin and proteoglycans and ultimately suppresses matrix degradation via inhibition of proteinases such as plasminogen activators and collagenases while stimulating production of proteinase inhibitors such as plasminogen activator inhibitor-1 and TIMPs [60–62]. Resident cardiac fibroblasts entering the infarcted tissue differentiate to myofibroblasts that express contractile proteins such as α-smooth muscle actin. Myofibroblast differentiation is induced by mechanical stress, TGFβ/Smad3 signaling and alterations in the composition of the ECM such as up regulation of ED-A fibronectin [63, 64]. These myofibroblasts are predominantly present in the infarct border zone and have a high proliferative capacity [10, 65]. They are the main source of ECM proteins needed to generate a collagen scar [66]. Induction of the pro-inflammatory cytokine TNF-α diminishes ECM collagen synthesis followed by an increase of the MMP activity of cardiac fibroblasts [10, 67]. However, TNF-α deficient mice are protected from cardiac rupture and chronic dysfunction following infarction [68], indicating the pleiotropic role of the cytokine.
One of the important ECM constituents is hyaluronan, a high molecular weight polymer under physiologic conditions, which becomes degraded upon tissue injury. Hyaluronan fragments stimulate endothelial cells and macrophages to secrete pro-inflammatory cytokines and chemokines and clearance of these fragments precedes the resolution of the inflammatory phase [10, 69, 70]. Finally, there is an accumulation of mast cells during cell proliferation and fibrosis [71]. The exact role of mast cells in the process of cardiac inflammation and repair is still under investigation, but one function of mast cells might be the regulation of fibrosis by the secretion of MMPs [72], inducing tissue remodeling. The summation of these processes finally leads to the formation of highly vascularized granulation tissue and abolition of the pro-inflammatory environment enabling repair.
The Maturation Phase
The formation of the scar, initiated during the proliferative phase, is followed by its maturation when endothelial cells have proliferated to form an extensive microvascular network. Only a part of these vessels mature through the mural wall formation by pericytes and myofibroblasts. These mature vessels aid scar stabilization by providing oxygen and nutrients [23]. However, the remainder of neovessels do not mature and undergo apoptosis together with the remaining myofibroblasts [63]. The highly-vascularized granulation tissue formed during the inflammatory phase, is finally replaced by a collagen-rich scar, completing the process of infarct healing [10]. The site of coronary occlusion, duration of ischemia and timing of reperfusion all influence the inflammatory process and therefore the time course of infarct healing will vary between individuals.
After completion of the reparative response, some fibroblasts remain in the non-infarcted myocardium and may become activated via increased wall stress where they contribute to ventricular remodeling and ventricular dysfunction by producing matrix proteins and proteases [63]. Increasing the number of myofibroblasts as well as the number of capillaries by blocking frizzled signaling via Wnt3a and Wnt5a antagonizing peptides reduced infarct size and increased infarct thickness in a mouse model of MI, suggesting that preservation of cardiac function after MI can (amongst others) be influenced by modulation of myofibroblasts [73].
In conclusion, inflammatory processes play a crucial role initially clearing the debris of apoptotic cells but also regulating essential repair mechanisms to form mature scar tissue. However, an elaborate immune response clearing as much damaged cellular tissue as possible also induces undesirable collateral damage to surrounding healthy tissue.
Therapeutic Approaches Targeting Cardiac Inflammation and Ischemia–Reperfusion Injury After Myocardial Ischemia
The progress made in understanding cardiac inflammation initiated experimental studies aiming to modulate the unwanted cardiac tissue injury induced by post-MI inflammation and reperfusion therapy. Initial studies targeting pathways of oxidation, inflammation, sodium-hydrogen exchange, nitric oxide metabolism and metabolic pathways showed positive results on clinical parameters such as reduction of infarct size; however these results need confirmation in large trials [74, 75]. The purine analogue acadesine, which increases adenosine levels in energy-deprived tissues, has been studied as a pharmalogical intervention in an ischemia-reperfusion setting [76]. A meta-analysis summarizing all studies that have tested acadesine in 4043 patients undergoing coronary artery bypass grafting (CABG) surgery, suggested a 27 % reduction of the perioperative occurrence of MI (3.6 vs 4.9 %, P = 0.02) and a 26 % decrease in the combined outcome of stroke/MI/cardiac death (7.6 vs 4.6 %, P = 0.04) [77]. However, the largest trial performed called the Reduction in cardiovascular Events by acaDesine trial in subjects undergoing CABG surgery (RED-CABG), was stopped after 3080 of the originally projected 7500 study participants were randomized because of a low expectancy to obtain statistically significant differences. This underscores that beneficial effects are variable.
One of the earliest results of pharmacological intervention to inhibit the inflammatory response after MI was described by Roberts et al. who infused multiple doses of the anti-inflammatory drug methylprednisolone in patients with MI and reported an augmentation of the infarct size and accentuation of malignant arrhythmias. These catastrophic results of the methylprednisolone study made clear that an absolute suppression of the immune system after MI is not desirable for it also interferes with the reparative aspects of the immune response [23, 78].
A growing number of alternative promising therapeutic interventions targeting the cardiac inflammation process, including ischemic pre- or post-ischemic conditioning, has been proposed and in part already investigated in patients or is about to be examined in clinical trials [79–81]. Recently, Padfield et al. determined the effects of etanercept, a TNF-α antagonist, in patients after MI. Whereas they observed a modest anti-inflammatory effect possibly through a decrease in neutrophil recruitment and IL-6 concentrations, TNF-α levels were increased as were platelet activators and aggregators, making it less suitable as a therapeutic candidate to treat MI [82]. In another study, patients were treated with intravenous immunoglobulin after PCI, however without any beneficial effect on either cardiac function or remodeling [83]. A large trial investigating the effects of pexelizumab, an antibody binding the C5 component of complement, did not influence mortality or development of heart failure in cardiac patients [84].
Other promising therapeutic interventions showed contrasting results. The immunosuppressive drug cyclosporine that inhibits the opening of mitochondrial permeability-transition pores caused smaller infarct sizes and attenuated left ventricular remodeling in initial clinical trials when administered after primary PCI [85, 86]. However this was not reproduced in a more recent trial where cyclosporine was injected before thromobolytic treatment [87]. Blockade of the IL-1 receptor by anakinra attenuated cardiac remodeling in a first small pilot study in MI patients [88]. A second study however, did not confirm these results [89].
So far, the effects of different anti-inflammatory therapies are incongruent and their clinical applicability remains unclear. More importantly, this therapeutic approach will only attenuate the results of the inflammation process itself, among which the remodeling process. Here lies a role for the still emerging field of cell-based therapy, as this may influence the post-MI inflammation process, but also potentially regenerate the infarcted tissue [90].
Cell-Based therapy
While the amount of therapeutic strategies to treat ischemic events has increased dramatically the past decade, patients are often still prone to develop heart failure, since there are no therapeutic options available to reverse the loss of functional myocardium. Therapeutic cell therapy has the advantage that it can be delivered locally into infarcted tissue, either as a cell suspension or on a supportive scaffold. Additionally, genetic modification allows for cells to be custom-tailored to improve results.
Moreover, certain stem cell populations such as mesenchymal stromal cells (MSCs) have the additional advantage of diminishing the deleterious effects of the inflammatory response that accompanies repair by secretion of different paracrine factors acting on several immune cell populations [91, 92]. However, the potential of cell therapy to influence post-MI inflammation has not been studied extensively yet, leaving for the moment a gap in our knowledge about the effect and capacity cell therapy might have in modulating post-MI inflammation. The field of stem cell transplantation was accelerated a decade ago by a preclinical study that reported improved cardiac regeneration upon infusion of bone marrow-derived cells into a cardiac ischemic mouse heart [93]. These results initiated a new area of research, exploring the potential of cell therapy to regenerate the diseased heart and clinical trials quickly followed.
The ideal cardiac regenerative therapy involves a cell type that is easily accessible, produces the optimal combination of paracrine factors, is able to engraft in the injured cardiac tissue niche, can possibly even differentiate into a cardiomyocyte or other desired cardiac cell types, and can be delivered via a safe and minimally invasive procedure. In search for this cell type, a variety of cell populations are being studied, all initially aimed toward regenerating cardiac tissues, each having their own advantages and limitations [94].
Transplantation of various cell types such as hematopoietic and non-hematopoietic bone marrow-derived stem cells as well as MSCs and other adult stem cells has been performed in experimental and clinical studies with the purpose to stimulate neoangiogenesis [95]. It is reported that therapeutic cell therapies can regulate tissue inflammation through paracrine mechanisms acting on angiogenesis, apoptosis and scar formation and are able to potentiate recruitment of endogenous stem cells to the site of injury [90, 92]. In addition, there are cell types that have proven to be able to form de novo cardiomyocytes, such as embryonic stem cells, induced pluripotent stem cells and cardiac progenitor cells (CPCs) [96]. The CPCs can be isolated from the adult heart and show spontaneous electrical activity and action potentials upon appropriate in vitro differentiation [97].
In the cardiac field, the effect of cell therapy has been studied in different animal models, but studying inflammation has not been a main focus in these studies (Table 1).
Mesenchymal Stromal Cells
Over the last years many studies have focused on the therapeutic potential of MSCs in different diseases in animals and humans, due to their versatile nature which includes their immunomodulatory capacities. This cell type was first described by Friedenstein et al. in 1968 and has already been studied in clinical trials [98]. The MSC is a rare population of multipotent cells, present in bone marrow and other mesenchymal tissues like adipose tissue. MSCs are poorly defined but ex vivo expanded MSC populations are traditionally characterized by the presence of surface antigens CD90, CD73, CD105 and MHC-I and the absence of characteristic hematopoietic cell surface antigens such as CD45, CD34, CD80 and MHC-II. MSCs are capable of differentiating into multiple mature cell lineages including chondrocytes, osteoblasts and adipocytes. Due to its limited plasticity and restricted lifespan the MSC has a major theoretic advantage regarding safety compared to the ES and IPS cell, with a reduced risk of tumorigenicity, a major concern of therapeutic cell products. Whilst most cell populations are studied for their potential to regenerate damaged tissues, the MSC is additionally capable of dampening deleterious aspects of the immune response that accompanies injury. Inhibition of undesired immune responses by MSC infusion has been observed in experimental animal models for various diseases and underscores the potential of MSCs for clinical immune regulation [99]. The clinical applicability of MSCs for immunological disease was initially shown in patients with graft-versus-host disease (GvHD) after bone marrow transplantation [100] . In a successive phase II study it was found that MSC administration improved the manifestations of GvHD in the majority of patients [101]. These positive results of MSC therapy led to MSCs entering various clinical trials. Notwithstanding the positive effects of MSCs, the cellular and molecular mechanisms responsible are complex, probably multifactorial in nature and poorly understood.
MSCs are immunosuppressive in vitro, evidenced by their ability to suppress the proliferation of T-cells and their effect on cytokine profiles [102–104]. Furthermore, MSCs are able to induce the formation of CD4+CD25+FOXP3+ regulatory T cells [105], and interfere with the differentiation, maturation and function of antigen presenting dendritic cells, thereby directly affecting processes such as immunity and tolerance [106]. Huang et al. showed that neither infusion of allogenic nor syngeneic MSCs after MI in rats elicited a significant immune response, confirming the lack of immunogenic surface antigen expression or expression of antigens in an immunoregulatory fashion on such MSCs. Syngeneic MSC therapy improved cardiac function up to 6 months after infusion when compared to controls, whereas allogeneic MSC therapy improved cardiac function up to 3 months only. However, in vitro treatment before infusion of MSCs with 5-azacytidine, VEGF or TGF-β in an effort to stimulate differentiation towards myogenesis, endothelial cells or smooth muscle cells respectively, altered the immunogenic surface antigen expression profile of these cells, potentially triggering an immune response in vivo after allogeneic MSC infusion [107].
We recently demonstrated that MSCs act on monocyte differentiation, promoting the formation of anti-inflammatory IL-10 producing cells with low antigen presenting capacity [108]. MSCs have also been reported to inhibit the proliferation of B lymphocytes upon anti-Ig antibody, soluble CD40 ligand or cytokine-mediated activation [109] and have been suggested to inhibit IL-2- and IL-15-induced natural killer-cell proliferation [110]. In summary, these studies demonstrate the immunomodulatory capacities of MSCs in vitro, however the biological relevance of these findings in vivo is still largely unknown [111].
The first in vivo results were obtained in an experimental model of GvHD in which systemically infused MSC improved survival of mice transplanted with haplo-identical hematopoietic stem cell grafts [112, 113]. However, in another study injection of a single dose of MSCs did not ameliorate GvHD [114]. In the cardiac field, MSC infusion has been studied in different animal models. MSC transplantation after MI in a rat model showed an attenuation of the decline in cardiac function and the remodeling process, which may be explained by the anti-inflammatory properties of MSCs as the expression of TNF-α, IL-1β and IL-6 was reduced in these animals [115]. Infusion of MSCs in a rat MI model using a Langendorff apparatus also resulted in the highest preservation of cardiac function when compared to controls, most likely by a decrease of the pro-inflammatory cytokines TNF-α, IL-1 and IL-6. In addition, apoptosis was reduced, suggesting a beneficial role for MSC in apoptotic signaling, possibly via a signal transducer and activator of transcription 3 pathway [116]. This decrease in the pro-inflammatory cytokines TNF-α, IL-1 and IL-6 was also observed after injecting MSCs combined with either atorvastatin [117] or simvastatin, in a porcine MI model [118]. Herrmann et al. showed that infusion of MSCs, both naïve cells and cells pretreated with TGF-α decreased infarct size and preserved cardiac function, possibly through lowering of the TNF-α, IL-1β and IL-6 expression and increasing VEGF expression in a rat MI model [119]. The increased expression of VEGF by MSC therapy was also demonstrated after application of MSC/silk fibroin/hyaluronic acid patches in an MI model in rats, in addition to a decreased inflammatory response as demonstrated by reduced CD 68 expression [120]. Kim et al. showed preservation of cardiac function by infusion MSCs as well, with enhanced MSC engraftment and cardiac function preservation after TNF-α stimulation [121]. Lee et al. infused MSCs in an experimental MI mouse model where cells were afterwards cells entrapped in the lungs forming micro-emboli [122]. Subsequently, signals from the injured heart induced MSCs to secrete the anti-inflammatory protein tumor necrosis factor-inducible gene (TSG) 6 protein which suppresses the excessive and thereby deleterious inflammatory response involved in cardiac ischemia. This limited the protease release by macrophages and neutrophils, decreasing the damage to cardiomyocytes. Ultimately, an improvement of cardiac function and a decrease in scar formation of the left ventricle was observed. TSG-6, secreted by MSCs, has been shown to be a key anti-inflammatory factor in many other experimental disease models such as bleomyocin-induced lung injury, sterile cornea injury, and zymosan-induced peritonitis [123–126].
The importance of the SDF-1 release by MSCs in the process of cardiac repair of MI was recently demonstrated in a model in conditional cardiac myocyte CXCR4 null mice [127]. In the absence of CXCR4, the SDF-1 receptor, preservation of cardiac function by MSCs is no longer observed, possibly due to a decrease in the recruitment of stem cells or an increase in apoptosis. An earlier study injected MSCs that over-expressed SDF-1, which resulted in increased angiogenesis through VEGF expression and subsequently preservation of cardiac function [128].
Dayan et al. showed that MSC therapy after MI in a mouse model decreased the number of monocytes and pro-inflammatory M1 phenotype macrophages. Also, in vitro and in vivo data demonstrated that the amount of M2 phenotype macrophages, which are associated with an anti-inflammatory phenotype, was increased, which was thought to be mediated by MSC secretion of the anti-inflammatory factor IL-10 [129]. This MSC-mediated switch from M1 phenotype to M2 phenotype macrophages was recently confirmed by another group [130]. In vitro experiments proposed that the modulation of macrophages may be dependent on cell-to-cell contact, as the secretion of reparative cytokines was highest in cultures of MSCs mixed with macrophages [130].
While the therapeutic effectiveness of MSCs has been shown in a number of studies as described above, the mechanisms through which MSCs act remain still unknown. Purported beneficial immunomodulatory factors derived from MSCs in addition to TSG-6, include inducible nitric oxide synthase, indoleamine dioxygenase, CCL2, SDF-1, IL-10 and prostaglandin E2. In addition, immunomodulatory effects may rely on pathways acting on specific immune cell populations or via cell-cell contact with dendritic cells, macrophages or other cells of the immune system [90, 91, 111, 130–132]. Clearly this must be studied more intensively and much progress will be made when the in vivo fate of MSCs can be determined to clarify the cellular interactions that are made during the initiation and ongoing process of repair.
Clinical Trials of MSCs
MSC therapy is at present being studied in various clinical trials for their efficacy in inflammatory and degenerative disorders. However, when entering the clinical arena potential risks have to be taken into account: the immunogenicity of the cells, the biosafety of medium components, the risk of ectopic tissue formation and potential in vitro transformation of cells during expansion [133].
The ClinicalTrials.gov web-based resource has summarized a large number of clinical trials that involve MSC therapy targeted against various diseases. One of the key clinical trials performed is a phase II trial in which 55 patients with steroid resistant acute GvHD were treated with MSCs [101]. In the 60 months follow up, infusion of in vitro expanded MSCs was considered a possibly effective therapy for this specific patient group. The mode of action of MSCs in GvHD seems highly related to their immune modulatory properties.
In the cardiac field MSC therapy has also been evaluated in numerous studies [134]. In 2004, a study of autologous bone-marrow-derived MSC infusion in patients with acute MI was performed [135]. In 69 patients undergoing PCI after acute MI significant improvements in left ventricular function were found, which were assessed by echocardiographic monitoring. The first phase-I, randomized, double blind, placebo-controlled, dose-escalation study of intravenous allogeneic adult MSCs in patients with acute MI was completed in 2009, suggesting it was safe to use allogeneic MSCs in patients after acute MI [136]. The same group reported in 2012 a direct comparison of autologous versus allogeneic bone marrow-derived MSCs in ischemic cardiomyopathy patients showing low rates of treatment-emergent serious adverse events, including immunologic reactions. A recent trial in ischemic cardiomyopathy patients showed no adverse effects of MSC injection and encouraging beneficial results, though the study size was small [137]. Injection of MSCs in chronic ischemic cardiomyopathy patients during CABG surgery showed a promising improvement of cardiac function and decreased scar size, however due to lack of placebo and small study size results are not conclusive [138]. Our group recently reported that intramyocardial injection of autologous MSCs using the NOGA injection system in acute MI patients was safe up to 5 years after injection, and was associated with improved cardiac function as compared to baseline [139]. In aggregate, the MSC injection favorably affected patient functional capacity, quality of life, and ventricular remodeling [140].
The current experimental and clinical data available indicate that MSC therapy is feasible and safe, and neither early toxicity nor later side effects have been found to date. However, long-term follow up studies in larger patient cohorts are warranted to give definitive answers whether long-term adverse events may occur [141]. The latest findings suggest that patients receiving cell therapy mainly experience beneficial results on clinical outcomes instead of objective parameters regarding cardiac function [134]. At present it is not clear whether the beneficial effect of MSCs in cardiac patients is also caused by a beneficial effect on post-MI inflammation or by other mechanisms. More research is needed to address this issue.
Summary and Future Perspectives
This review describes the role of the immune system in the healing processes following an acute ischemic event. The inflammatory response that occurs after MI is a precarious balance, since it is indispensable in the clearance of cell debris and ultimately the formation of a collagen scar but the pathways necessary for a timely initiation, suppression, resolution, and containment of the inflammatory response can also cause additional injury to the heart. When certain aspects of this inflammatory process triggered by cardiac injury are excessive, ultimately infarct expansion and adverse remodeling of the infarcted heart can occur [142, 143]. However, it is not fully known if suppression of the detrimental part of the inflammatory response would prevent the adverse remodeling and concomitant worse outcome in patients with MI and if this therapeutic goal can be reached clinically. Modulating the immune response after myocardial damage is a road less travelled that might be a promising therapeutic option for cardiac disease. Of all cell types, the MSC currently seems a suitable candidate for this specific goal, based on the proven immunomodulatory properties, in addition to the ability to secrete angiogenic factors such as VEGF, important for neoangiogenesis [144, 145]. Infusing MSCs in the ischemic myocardium therefore might not only improve cardiac function by dampening excessive immune responses but also induce growth of new vasculature. Recapitulating the studies on the physiologic function of MSCs in regulating the immune system in the hematopoietic niche and their ability to modulate immunity in cardiac disease might be a feasible option to move forward [146–148].
References
World Health Organization. Global atlas on cardiovascular disease prevention and control. Geneva, 2011.
Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95.
Jiang B, Liao R. The paradoxical role of inflammation in cardiac repair and regeneration. J Cardiovasc Transl Res. 2010;3:410–6.
Entman ML, Michael L, Rossen RD, Dreyer WJ, Anderson DC, Taylor AA, et al. Inflammation in the course of early myocardial ischemia. FASEB J. 1991;5:2529–37.
Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res. 2002;53:31–47.
Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44.
Tongers J, Losordo DW, Landmesser U. Stem and progenitor cell-based therapy in ischaemic heart disease: promise, uncertainties, and challenges. Eur Heart J. 2011;32:1197–206.
Mann DL. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res. 2002;91:988–98.
Frangogiannis NG. The mechanistic basis of infarct healing. Antioxid Redox Signal. 2006;8:1907–39.
Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111.
Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–84.
Kulkarni S, Woollard KJ, Thomas S, Oxley D, Jackson SP. Conversion of platelets from a proaggregatory to a proinflammatory adhesive phenotype: role of PAF in spatially regulating neutrophil adhesion and spreading. Blood. 2007;110:1879–86.
Liehn EA, Postea O, Curaj A, Marx N. Repair after myocardial infarction, between fantasy and reality: the role of chemokines. J Am Coll Cardiol. 2011;58:2357–62.
Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–6.
Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988;255:H1269–75.
Jolly SR, Kane WJ, Bailie MB, Abrams GD, Lucchesi BR. Canine myocardial reperfusion injury. Its reduction by the combined administration of superoxide dismutase and catalase. Circ Res. 1984;54:277–85.
Romson JL, Hook BG, Kunkel SL, Abrams GD, Schork MA, Lucchesi BR. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983;67:1016–23.
Briaud SA, Ding ZM, Michael LH, Entman ML, Daniel S, Ballantyne CM. Leukocyte trafficking and myocardial reperfusion injury in ICAM-1/P-selectin-knockout mice. Am J Physiol Heart Circ Physiol. 2001;280:H60–7.
Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, et al. CCL2/Monocyte chemoattractant protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–9.
Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–47.
Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res. 2014;102:240–8.
Timmers L, Pasterkamp G, de Hoog VC, Arslan F, Appelman Y, de Kleijn DP. The innate immune response in reperfused myocardium. Cardiovasc Res 2012;94:276–83.
Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110:159–73.
Gordon JW, Shaw JA, Kirshenbaum LA. Multiple facets of NF-kappaB in the heart: to be or not to NF-kappaB. Circ Res. 2011;108:1122–32.
Hamid T, Guo SZ, Kingery JR, Xiang X, Dawn B, Prabhu SD. Cardiomyocyte NF-kappaB p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovasc Res. 2011;89:129–38.
Morishita R, Sugimoto T, Aoki M, Kida I, Tomita N, Moriguchi A, et al. In vivo transfection of cis element “decoy” against nuclear factor-kappaB binding site prevents myocardial infarction. Nat Med. 1997;3:894–9.
Hori M, Nishida K. Toll-like receptor signaling: defensive or offensive for the heart? Circ Res. 2008;102:137–9.
Frantz S, Kobzik L, Kim YD, Fukazawa R, Medzhitov R, Lee RT, et al. Toll4 (TLR4) expression in cardiac myocytes in normal and failing myocardium. J Clin Invest. 1999;104:271–80.
Timmers L, Sluijter JP, van Keulen JK, Hoefer IE, Nederhoff MG, Goumans MJ, et al. Toll-like receptor 4 mediates maladaptive left ventricular remodeling and impairs cardiac function after myocardial infarction. Circ Res. 2008;102:257–64.
Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–97.
Hill JH, Ward PA. The phlogistic role of C3 leukotactic fragments in myocardial infarcts of rats. J Exp Med. 1971;133:885–900.
Vakeva AP, Agah A, Rollins SA, Matis LA, Li L, Stahl GL. Myocardial infarction and apoptosis after myocardial ischemia and reperfusion: role of the terminal complement components and inhibition by anti-C5 therapy. Circulation. 1998;97:2259–67.
Yasojima K, Kilgore KS, Washington RA, Lucchesi BR, McGeer PL. Complement gene expression by rabbit heart: upregulation by ischemia and reperfusion. Circ Res. 1998;82:1224–30.
Rossen RD, Michael LH, Kagiyama A, Savage HE, Hanson G, Reisberg MA, et al. Mechanism of complement activation after coronary artery occlusion: evidence that myocardial ischemia in dogs causes release of constituents of myocardial subcellular origin that complex with human C1q in vivo. Circ Res. 1988;62:572–84.
Birdsall HH, Green DM, Trial J, Youker KA, Burns AR, MacKay CR, et al. Complement C5a, TGF-beta 1, and MCP-1, in sequence, induce migration of monocytes into ischemic canine myocardium within the first one to five hours after reperfusion. Circulation. 1997;95:684–92.
Maroko PR, Carpenter CB, Chiariello M, Fishbein MC, Radvany P, Knostman JD, et al. Reduction by cobra venom factor of myocardial necrosis after coronary artery occlusion. J Clin Invest. 1978;61:661–70.
Weisman HF, Bartow T, Leppo MK, Marsh Jr HC, Carson GR, Concino MF, et al. Soluble human complement receptor type 1: in vivo inhibitor of complement suppressing post-ischemic myocardial inflammation and necrosis. Science. 1990;249:146–51.
Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–6.
Bournazou I, Mackenzie KJ, Duffin R, Rossi AG, Gregory CD. Inhibition of eosinophil migration by lactoferrin. Immunol Cell Biol. 2010;88:220–3.
Hilgendorf I, Gerhardt LM, Tan TC, Winter C, Holderried TA, Chousterman BG, et al. Ly-6Chigh monocytes depend on Nr4a1 to balance both inflammatory and reparative phases in the infarcted myocardium. Circ Res. 2014;114:1611–22.
Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, et al. Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153:362–75.
van der Laan AM, Ter Horst EN, Delewi R, Begieneman MP, Krijnen PA, Hirsch A, et al. Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur Heart J. 2014;35:376–85.
Dutta P, Nahrendorf M. Regulation and consequences of monocytosis. Immunol Rev. 2014;262:167–78.
Lambert JM, Lopez EF, Lindsey ML. Macrophage roles following myocardial infarction. Int J Cardiol. 2008;130:147–58.
Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM. IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest. 1995;96:2304–10.
Frangogiannis NG, Mendoza LH, Lindsey ML, Ballantyne CM, Michael LH, Smith CW, et al. IL-10 is induced in the reperfused myocardium and may modulate the reaction to injury. J Immunol. 2000;165:2798–808.
Yang Z, Zingarelli B, Szabo C. Crucial role of endogenous interleukin-10 production in myocardial ischemia/reperfusion injury. Circulation. 2000;101:1019–26.
Zymek P, Nah DY, Bujak M, Ren G, Koerting A, Leucker T, et al. Interleukin-10 is not a critical regulator of infarct healing and left ventricular remodeling. Cardiovasc Res. 2007;74:313–22.
Wollert KC, Drexler H. The role of interleukin-6 in the failing heart. Heart Fail Rev. 2001;6:95–103.
Gallucci RM, Simeonova PP, Matheson JM, Kommineni C, Guriel JL, Sugawara T, et al. Impaired cutaneous wound healing in interleukin-6-deficient and immunosuppressed mice. FASEB J. 2000;14:2525–31.
Bromage DI, Davidson SM, Yellon DM. Stromal derived factor 1alpha: a chemokine that delivers a two-pronged defence of the myocardium. Pharmacol Ther. 2014;143:305–15.
Lee SH, Wolf PL, Escudero R, Deutsch R, Jamieson SW, Thistlethwaite PA. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342:626–33.
Mirshahi F, Pourtau J, Li H, Muraine M, Trochon V, Legrand E, et al. SDF-1 activity on microvascular endothelial cells: consequences on angiogenesis in in vitro and in vivo models. Thromb Res. 2000;99:587–94.
Elmadbouh I, Haider HK, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2007;42:792–803.
Hiasa K, Ishibashi M, Ohtani K, Inoue S, Zhao Q, Kitamoto S, et al. Gene transfer of stromal cell-derived factor-1alpha enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–61.
MacArthur Jr JW, Purcell BP, Shudo Y, Cohen JE, Fairman A, Trubelja A, et al. Sustained release of engineered stromal cell-derived factor 1-alpha from injectable hydrogels effectively recruits endothelial progenitor cells and preserves ventricular function after myocardial infarction. Circulation. 2013;128:S79–86.
Kanki S, Segers VF, Wu W, Kakkar R, Gannon J, Sys SU, et al. Stromal cell-derived factor-1 retention and cardioprotection for ischemic myocardium. Circ Heart Fail. 2011;4:509–18.
Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, et al. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol. 2006;48:2315–23.
Ikeuchi M, Tsutsui H, Shiomi T, Matsusaka H, Matsushima S, Wen J, et al. Inhibition of TGF-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc Res. 2004;64:526–35.
Bassols A, Massague J. Transforming growth factor beta regulates the expression and structure of extracellular matrix chondroitin/dermatan sulfate proteoglycans. J Biol Chem. 1988;263:3039–45.
Laiho M, Saksela O, Andreasen PA, Keski-Oja J. Enhanced production and extracellular deposition of the endothelial-type plasminogen activator inhibitor in cultured human lung fibroblasts by transforming growth factor-beta. J Cell Biol. 1986;103:2403–10.
Edwards DR, Murphy G, Reynolds JJ, Whitham SE, Docherty AJ, Angel P, et al. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987;6:1899–904.
Chen W, Frangogiannis NG. Fibroblasts in post-infarction inflammation and cardiac repair. Biochim Biophys Acta 2012;1833:945–53.
Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–16.
Virag JI, Murry CE. Myofibroblast and endothelial cell proliferation during murine myocardial infarct repair. Am J Pathol. 2003;163:2433–40.
Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–11.
Siwik DA, Chang DL, Colucci WS. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res. 2000;86:1259–65.
Sun M, Dawood F, Wen WH, Chen M, Dixon I, Kirshenbaum LA, et al. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation. 2004;110:3221–8.
Taylor KR, Trowbridge JM, Rudisill JA, Termeer CC, Simon JC, Gallo RL. Hyaluronan fragments stimulate endothelial recognition of injury through TLR4. J Biol Chem. 2004;279:17079–84.
Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, et al. Resolution of lung inflammation by CD44. Science. 2002;296:155–8.
Frangogiannis NG, Perrard JL, Mendoza LH, Burns AR, Lindsey ML, Ballantyne CM, et al. Stem cell factor induction is associated with mast cell accumulation after canine myocardial ischemia and reperfusion. Circulation. 1998;98:687–98.
Levick SP, Melendez GC, Plante E, McLarty JL, Brower GL, Janicki JS. Cardiac mast cells: the centrepiece in adverse myocardial remodelling. Cardiovasc Res. 2011;89:12–9.
Laeremans H, Hackeng TM, van Zandvoort MA, Thijssen VL, Janssen BJ, Ottenheijm HC, et al. Blocking of frizzled signaling with a homologous peptide fragment of wnt3a/wnt5a reduces infarct expansion and prevents the development of heart failure after myocardial infarction. Circulation. 2011;124:1626–35.
Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106:360–8.
Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35.
Galinanes M, Bullough D, Mullane KM, Hearse DJ. Sustained protection by acadesine against ischemia- and reperfusion-induced injury. Studies in the transplanted rat heart. Circulation. 1992;86:589–97.
Mangano DT. Effects of acadesine on myocardial infarction, stroke, and death following surgery. A meta-analysis of the 5 international randomized trials. The multicenter study of perioperative ischemia (McSPI) research group. JAMA. 1997;277:325–32.
Roberts R, DeMello V, Sobel BE. Deleterious effects of methylprednisolone in patients with myocardial infarction. Circulation. 1976;53:I204–6.
Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med. 2011;17:1391–401.
Moreira DM, da Silva RL, Vieira JL, Fattah T, Lueneberg ME, Gottschall CA. Role of vascular inflammation in coronary artery disease: potential of anti-inflammatory drugs in the prevention of atherothrombosis : inflammation and anti-inflammatory drugs in coronary artery Disease. Am J Cardiovasc Drugs 2014. [Epub ahead of print]
Christia P, Frangogiannis NG. Targeting inflammatory pathways in myocardial infarction. Eur J Clin Invest. 2013;43:986–95.
Padfield GJ, Din JN, Koushiappi E, Mills NL, Robinson SD, Cruden NM, et al. Cardiovascular effects of tumour necrosis factor alpha antagonism in patients with acute myocardial infarction: a first in human study. Heart. 2013;99:1330–5.
Gullestad L, Orn S, Dickstein K, Eek C, Edvardsen T, Aakhus S, et al. Intravenous immunoglobulin does not reduce left ventricular remodeling in patients with myocardial dysfunction during hospitalization after acute myocardial infarction. Int J Cardiol. 2013;168:212–8.
Armstrong PW, Granger CB, Adams PX, Hamm C, Holmes Jr D, O’Neill WW, et al. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007;297:43–51.
Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–81.
Mewton N, Croisille P, Gahide G, Rioufol G, Bonnefoy E, Sanchez I, et al. Effect of cyclosporine on left ventricular remodeling after reperfused myocardial infarction. J Am Coll Cardiol. 2010;55:1200–5.
Ghaffari S, Kazemi B, Toluey M, Sepehrvand N. The effect of prethrombolytic cyclosporine-A injection on clinical outcome of acute anterior ST-elevation myocardial infarction. Cardiovasc Ther. 2013;31:e34–9.
Abbate A, Kontos MC, Grizzard JD, Biondi-Zoccai GG, Van Tassell BW, Robati R, et al. Interleukin-1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU-ART] Pilot study). Am J Cardiol. 2010;105:1371–7.
Abbate A, Van Tassell BW, Biondi-Zoccai G, Kontos MC, Grizzard JD, Spillman DW, et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia commonwealth University-Anakinra remodeling trial (2) (VCU-ART2) pilot study]. Am J Cardiol. 2013;111:1394–400.
Zamilpa R, Navarro MM, Flores I, Griffey S. Stem cell mechanisms during left ventricular remodeling post-myocardial infarction: repair and regeneration. World J Cardiol. 2014;6:610–20.
van den Akker F, de Jager SC, Sluijter JP. Mesenchymal stem cell therapy for cardiac inflammation: immunomodulatory properties and the influence of toll-like receptors. Mediat Inflamm. 2013;2013:181020.
Thakker R, Yang P. Mesenchymal stem cell therapy for cardiac repair. Curr Treat Options Cardiovasc Med. 2014;16:323.
Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–5.
Ptaszek LM, Mansour M, Ruskin JN, Chien KR. Towards regenerative therapy for cardiac disease. Lancet. 2012;379:933–42.
Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97.
Mummery CL, Zhang J, Ng ES, Elliott DA, Elefanty AG, Kamp TJ. Differentiation of human embryonic stem cells and induced pluripotent stem cells to cardiomyocytes: a methods overview. Circ Res. 2012;111:344–58.
Goumans MJ, de Boer TP, Smits AM, van Laake LW, van Vliet P, Metz CH, et al. TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 2007;1:138–49.
Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230–47.
Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14–20.
Le BK, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–41.
Le BK, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–86.
Rasmusson I, Ringden O, Sundberg B, Le BK. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005;305:33–41.
Sato K, Ozaki K, Oh I, Meguro A, Hatanaka K, Nagai T, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109:228–34.
Di NM, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–43.
Crop MJ, Baan CC, Korevaar SS, Ijzermans JN, Weimar W, Hoogduijn MJ. Human adipose tissue-derived mesenchymal stem cells induce explosive T-cell proliferation. Stem Cells Dev. 2010;19:1843–53.
Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105:4120–6.
Huang XP, Sun Z, Miyagi Y, McDonald KH, Zhang L, Weisel RD, et al. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–29.
Melief SM, Geutskens SB, Fibbe WE, Roelofs H. Multipotent stromal cells skew monocytes towards an anti-inflammatory interleukin-10-producing phenotype by production of interleukin-6. Haematologica. 2013;98:888–95.
Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–72.
Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74–85.
Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499–506.
Maitra B, Szekely E, Gjini K, Laughlin MJ, Dennis J, Haynesworth SE, et al. Human mesenchymal stem cells support unrelated donor hematopoietic stem cells and suppress T-cell activation. Bone Marrow Transplant. 2004;33:597–604.
Polchert D, Sobinsky J, Douglas G, Kidd M, Moadsiri A, Reina E, et al. IFN-gamma activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur J Immunol. 2008;38:1745–55.
Sudres M, Norol F, Trenado A, Gregoire S, Charlotte F, Levacher B, et al. Bone marrow mesenchymal stem cells suppress lymphocyte proliferation in vitro but fail to prevent graft-versus-host disease in mice. J Immunol. 2006;176:7761–7.
Guo J, Lin GS, Bao CY, Hu ZM, Hu MY. Anti-inflammation role for mesenchymal stem cells transplantation in myocardial infarction. Inflammation. 2007;30:97–104.
Poynter JA, Herrmann JL, Manukyan MC, Wang Y, Abarbanell AM, Weil BR, et al. Intracoronary mesenchymal stem cells promote postischemic myocardial functional recovery, decrease inflammation, and reduce apoptosis via a signal transducer and activator of transcription 3 mechanism. J Am Coll Surg. 2011;213:253–60.
Yang YJ, Qian HY, Huang J, Geng YJ, Gao RL, Dou KF, et al. Atorvastatin treatment improves survival and effects of implanted mesenchymal stem cells in post-infarct swine hearts. Eur Heart J. 2008;29:1578–90.
Yang YJ, Qian HY, Huang J, Li JJ, Gao RL, Dou KF, et al. Combined therapy with simvastatin and bone marrow-derived mesenchymal stem cells increases benefits in infarcted swine hearts. Arterioscler Thromb Vasc Biol. 2009;29:2076–82.
Herrmann JL, Abarbanell AM, Wang Y, Weil BR, Poynter JA, Manukyan MC, et al. Transforming growth factor-alpha enhances stem cell-mediated postischemic myocardial protection. Ann Thorac Surg. 2011;92:1719–25.
Chi NH, Yang MC, Chung TW, Chen JY, Chou NK, Wang SS. Cardiac repair achieved by bone marrow mesenchymal stem cells/silk fibroin/hyaluronic acid patches in a rat of myocardial infarction model. Biomaterials. 2012;33:5541–51.
Kim YS, Park HJ, Hong MH, Kang PM, Morgan JP, Jeong MH, et al. TNF-alpha enhances engraftment of mesenchymal stem cells into infarcted myocardium. Front Biosci (Landmark Ed). 2009;14:2845–56.
Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63.
Ortiz LA, Dutreil M, Fattman C, Pandey AC, Torres G, Go K, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci U S A. 2007;104:11002–7.
Oh JY, Lee RH, Yu JM, Ko JH, Lee HJ, Ko AY, et al. Intravenous mesenchymal stem cells prevented rejection of allogeneic corneal transplants by aborting the early inflammatory response. Mol Ther. 2012;20:2143–52.
Roddy GW, Oh JY, Lee RH, Bartosh TJ, Ylostalo J, Coble K, et al. Action at a distance: systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells. 2011;29:1572–9.
Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood. 2011;118:330–8.
Dong F, Harvey J, Finan A, Weber K, Agarwal U, Penn MS. Myocardial CXCR4 expression is required for mesenchymal stem cell mediated repair following acute myocardial infarction. Circulation. 2012;126:314–24.
Tang J, Wang J, Yang J, Kong X, Zheng F, Guo L, et al. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur J Cardiothorac Surg. 2009;36:644–50.
Dayan V, Yannarelli G, Billia F, Filomeno P, Wang XH, Davies JE, et al. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol. 2011;106:1299–310.
Ben-Mordechai T, Holbova R, Landa-Rouben N, Harel-Adar T, Feinberg MS, Abd Elrahman I. I, et al. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J Am Coll Cardiol. 2013;62:1890–901.
Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141–50.
Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–36.
Bernardo ME, Fibbe WE. Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Ann N Y Acad Sci. 2012;1266:107–17.
Telukuntla KS, Suncion VY, Schulman IH, Hare JM. The advancing field of cell-based therapy: insights and lessons from clinical trials. J Am Heart Assoc. 2013;2:e000338.
Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–5.
Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–86.
Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, et al. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73.
Karantalis V, DiFede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, et al. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the prospective randomized study of mesenchymal stem cell therapy in patients undergoing cardiac surgery (PROMETHEUS) trial. Circ Res. 2014;114:1302–10.
Rodrigo SF, van Ramshorst RJ, Hoogslag GE, Boden H, Velders MA, Cannegieter SC, et al. Intramyocardial injection of autologous bone marrow-derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow-up. J Cardiovasc Transl Res. 2013;6:816–25.
Hare JM, Fishman JE, Gerstenblith G, Difede Velazquez DL, Zambrano JP, Suncion VY, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA 2012; 1–11.
Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59:1662–9.
Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol. 2010;176:2177–87.
Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, et al. CD44 is critically involved in infarct healing by regulating the inflammatory and fibrotic response. J Immunol. 2008;180:2625–33.
Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7:e35685.
Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Jung S, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–89.
Mendez-Ferrer S, Ellison GM, Torella D, Nadal-Ginard B. Resident progenitors and bone marrow stem cells in myocardial renewal and repair. Nat Clin Pract Cardiovasc Med. 2006;3 Suppl 1:S83–9.
Mendez-Ferrer S, Frenette PS. Hematopoietic stem cell trafficking: regulated adhesion and attraction to bone marrow microenvironment. Ann N Y Acad Sci. 2007;1116:392–413.
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–34.
Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, et al. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–201.
Zhang S, Ge J, Sun A, Xu D, Qian J, Lin J, et al. Comparison of various kinds of bone marrow stem cells for the repair of infarcted myocardium: single clonally purified non-hematopoietic mesenchymal stem cells serve as a superior source. J Cell Biochem. 2006;99:1132–47.
Henning RJ, Shariff M, Eadula U, Alvarado F, Vasko M, Sanberg PR, et al. Human cord blood mononuclear cells decrease cytokines and inflammatory cells in acute myocardial infarction. Stem Cells Dev. 2008;17:1207–19.
Ciulla MM, Montelatici E, Ferrero S, Braidotti P, Paliotti R, Annoni G, et al. Potential advantages of cell administration on the inflammatory response compared to standard ACE inhibitor treatment in experimental myocardial infarction. J Transl Med. 2008;6:30.
Xu S, Xu X, Guo Y, Gao W. Inflammatory responses after intracoronary mononuclear BM cell therapy in swine. Bone Marrow Transplant. 2009;44:427–31.
Tavares AM, da Rosa Araujo AS, Baldo G, Matte U, Khaper N, Bello-Klein A, et al. Bone marrow derived cells decrease inflammation but not oxidative stress in an experimental model of acute myocardial infarction. Life Sci. 2010;87:699–706.
Premaratne GU, Ma LP, Fujita M, Lin X, Bollano E, Fu M. Stromal vascular fraction transplantation as an alternative therapy for ischemic heart failure: anti-inflammatory role. J Cardiothorac Surg. 2011;6:43.
van Dijk A, Naaijkens BA, Jurgens WJ, Nalliah K, Sairras S, van der Pijl RJ, et al. Reduction of infarct size by intravenous injection of uncultured adipose derived stromal cells in a rat model is dependent on the time point of application. Stem Cell Res. 2011;7:219–29.
Acknowledgments
This work was supported by the Translational Regenerative Medicine (TeRM-Smart Mix SSM06004), the BioMedical Materials (SMARTCARE) program grants and the TAS research program 11.600.1016, which is financed by the Netherlands Organisation for Health Research and Development (ZonMW), the Netherlands
Conflict of interest
The authors confirm that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Vanessa-leigh van Zuylen and Melina C. den Haan contributed equally
Rights and permissions
About this article
Cite this article
van Zuylen, Vl., den Haan, M.C., Geutskens, S.B. et al. Post-myocardial Infarct Inflammation and the Potential Role of Cell Therapy. Cardiovasc Drugs Ther 29, 59–73 (2015). https://doi.org/10.1007/s10557-014-6568-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10557-014-6568-z