Abstract
Estrogen and progesterone receptors (ERs and PRs) are known for their prognostic as well as treatment predictive value in breast cancer. Although these receptors are differentially expressed in some other malignancies, and likely participate in the biology of those cancer types, the relevance to outcome and therapy is not well established. The use of ER as a highly effective therapeutic target in oncology was pioneered in breast cancer, and the lessons learned from its success could potentially benefit patients with several other malignancies in which hormone receptors are highly expressed. Indeed, there are several potent drugs available that target hormone receptors. These agents show incontrovertible evidence of benefit in patients with hormone receptor-positive breast cancer. It is conceivable that these drugs may have salutary effects in a variety of cancers other than those originating in the breast, based on the overexpression of hormone receptors in some patients, and the preclinical and clinical reports showing responses to these drugs in diverse cancers, albeit in small series or anecdotally. We therefore undertook a literature review in order to summarize the current data regarding the biologic and clinical implications of expression of estrogen and progesterone receptors in various malignancies and the possibilities for deployment of hormone manipulation beyond breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Defining estrogen and progesterone receptor (ER and PR) status in tumor tissue is standard in patients suffering from breast carcinoma. Indeed, the expression of these receptors informs prognosis, and patients with breast cancer have shown excellent benefit from interrogating ER and PR status as a determinant of therapy. The exploration of ER and PR status and its relevance in other tumor types has, nonetheless, lagged behind breast cancer. Yet, ERs and/or PRSs have been documented in non-neoplastic tissues such as the skin [1–3], and multiple tumors have also been demonstrated to variably express ER and/or PR, including but not limited to the skin; cerebral meningioma; renal cell carcinoma, hepatocellular carcinoma, non-small cell lung carcinoma, and thyroid carcinoma; and breast, pancreatic, prostate, colon, and gastric adenocarcinoma (Table 1). The evidence is, however, not consistent, an issue perhaps attributable to a variety of factors: heterogeneous methods utilized to probe steroid hormonal status, differences between primary and metastatic tumors, intra-tumor heterogeneity, technical exigencies, and variability of interpretation by different pathologists [4, 5] (Table 2, Fig. 1). These issues have also been problematic for the breast cancer literature, but, despite the limitations, knowing ER and PR status has proven invaluable. Herein, we provide an overview of the literature on ER and PR expression in diverse tumors beyond breast cancer and implications for therapy.
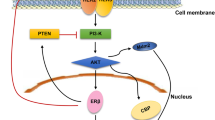
Sampling issues and ER-α/ER-β pathways. Center: sampling pitfalls regarding interpretation of IHC for hormonal estrogen receptors (ER) in solid tumors. Right: diagram representing the imbalance between ER-α and ER-β that results in estrogen-dependent malignant progression. ER-β has been designated as “the brake” of estrogen-mediated proliferation. Left: after estrogen binding, ER will function as a ligand-activated transcription factor that translocates to the nucleus and triggers gene expression. These hormone-attached receptors form dimers and bind to specific DNA sequences named estrogen response elements, removing DNA co = repressors, and enlisting co-activator proteins, with subsequent gene expression. ER will also be activated via a ligand-independent path as growth factor receptors may activate specific kinases that will directly phosphorylate ER triggering gene expression. Finally, a third signaling path involves a subpopulation of cell membrane-associated ER that, after activation by estrogen, forms a signaling complex that results in rapid activation of specific kinases. Possible relationship between ER and the PI3K pathway is also shown. Upon estrogen binding, signaling complexes assemble and sequentially activate tyrosine kinases (i.e., src), the serine/threonine kinase PI3K that produces phosphatidylinositol (3,4,5)-triphosphate (PIP3), and the subsequent interaction between Akt and mTOR
1.1 The duality of estrogen receptors—ER-alpha and ER-beta
One of the first descriptions of the fate of steroidal estrogen in target tissues was reported in 1960 [6]. Since then, ERs have been discerned in a wide range of tumors (Table 1). When ERs were first cloned in 1986 [7], it was believed that only one receptor existed. However, a second gene coding for an ER was cloned in 1996 [8]. It was given the name of ER-beta (ER-β) due to the striking similarity of its sequence with the classic ER [8], which was then denoted as ER-alpha (ER-α) or “classic” ER (Fig. 1). Since that time, several ER-β isoforms have been identified in diverse malignancies.
ER-α and ER-β are distinct genes/proteins. They are encoded by separate genes known as ESR1 and ESR2, respectively. ER-α is localized on chromosome 6 and ER-β on chromosome 14. These genes have significant sequence homology. ERs function as ligand-activated transcription factors. Hormone-activated ERs form dimers, including ER-αα or ER-ββ homodimers, or heterodimers. At least three ER-α and five ER-β isoforms have been identified.
The potent tumor growth-promoting activities of estrogens in target tissues are well established and are achieved in part by increased transcription of cell cycle genes via ER-α. Although ER-α activates transcription at the activator protein-1 site, ER-β might also inhibit gene transcription induced by ER-α. It is thus believed that ER-α is pro-proliferative, whereas ER-β is anti-proliferative, and that these antagonist forces co-exist in a finely tuned homeostatic balance [9]. Based on this concept, several studies suggested that an increase in the ER-α / ER-β ratio (Fig. 1) might have a possible role in tumor growth [10, 11]. Thus, it has been suggested that ER may have a dual role in cancer proliferation [12]. In a paradoxical fashion, however, estrogens and ER can also inhibit cancer cell invasiveness in experimental models [12]. Estrogens are proliferative signals in ER-positive cells, whereas anti-estrogens are effective weapons in our armamentarium against such hormone-fueled tumors. Nonetheless, estrogens can also apparently protect against cancer cell invasiveness through different mechanisms, which might explain why ER-driven tumors are usually well differentiated and less invasive than their ER-negative counterparts [12].
1.2 The good, the bad, and the ugly: ER-beta subtypes 1, 2, and 5
ER-β has a putative overall protective role against carcinogenesis and, in the world of hormonal receptors, has been named “the brake pedal” of tumor progression [13]; nevertheless, current identification of several other ER subtypes complicates this matter. Recently, Leung et al. [14] identified the ER-β isoforms, particularly in prostatic cancer samples, as well as distinct roles for ER-β1, ER-β2, and ER-β5. ER-β2 was the most common isoform, followed by ER-β1. The least common of the three isoforms was ER-β5. ER-β2 and ER-β5 have been observed most frequently in the cytoplasm, whereas ER-β1 is located primarily in the nucleus. The seminal contribution of this report stems from the fact that metastasis-promoting ER-β2 and ER-β5 were associated with a poor prognosis in prostate cancer [14]. Both isoforms, separately or together, enhanced cancer invasiveness, whereas ER-β5 promoted migration. Shaaban et al. [15] found that different forms of ERβ were relevant in breast cancer; specifically, their report showed that nuclear and cytoplasmic expression of ERβ1, ERβ2, and ERβ5 were associated with distinct prognostic outcomes for breast cancer patients. Most studies have however concentrated primarily on the “classical” wild-type ER-β (now called ER-β1), as the presence and roles of the new isoforms have yet to be validated across different malignancies.
1.3 The duality of progesterone receptors, PR-A and PR-B
A dichotomy similar to that discovered for ERs exists for PRs [16]. Indeed, there are two distinct isoforms of PR: PR-A and PR-B [17]. PR-B contains an additional 164 amino acids at the amino-terminus, possibly due to transcription from alternate promoters within the same gene [18]. PR-A seems to be a robust repressor of PR-B-mediated transcription in a hormone-dependent manner, which would suggest a specific role for the PR-A isoform as a regulator against uncontrolled stimulation by its PR-B counterpart. In the search for a biologic rationale for the existence of two almost identical forms of receptors, which nonetheless behave in an antagonistic fashion, the duality of the roles of PR as an activator or repressor of transcription serves to explicate the potential mechanism by which cells can signal opposite responses after being challenged by a single hormone [19].
1.4 Localization of hormonal receptors: plasma membrane, cytoplasm, or nuclear compartments
Immunohistochemistry (IHC), despite its shortcomings, remains the method of choice for the assessment of ER expression. The method has certain advantages in that the number of cancer cells and the presence of non-malignant cells in the specimen can be co-ascertained. Moreover, low-grade ER-positive (currently known in breast cancer literature as luminal A) cancer cells must be differentiated from high-grade ER-positive cancer cells as they might respond to therapy in a different way. These characteristics differentiate IHC from tissue-grinding methods that delineate receptor-binding activity or determination of messenger RNAs [20].
Hormonal receptors may be localized in the nucleus, plasma membrane, cytoplasm, and mitochondria, where they mediate the distinct physiological actions of estrogens. The subcellular localization of ERs determines the particular functionality of the ER, which is disturbed in several malignancies. The classical hormonal receptors, in addition to having a well-documented transcriptional potential, can also mediate the activation of intracellular signaling pathways (Fig. 1), including the rapid effects of estrogen on vasodilation and protection of endothelial cells against injury [21]. However, in spite of overlapping expression and functionality in multiple tissues, the precise mechanisms and consequences of their subcellular localization remain largely unclear.
1.5 Co-expression of and interaction with androgen receptors
The literature suggests that androgen receptors (AR) expression is widespread among malignancies. Furthermore, some tumors, such as salivary gland ductal tumors, have AR positivity rates approaching 100 % in some subtypes [22]. Of interest, breast [23] and gynecologic tumors also have high rates of AR positivity [24]. The latter may have special clinical relevance since agents such as anastrozole, an aromatase inhibitor used to suppress estrogen levels in patients with ER-positive breast tumors, can elevate androgen levels [25]. The interaction of estrogen or progesterone or their inhibitors with androgen receptors may also be important. For instance, Mobbs et al. [26] studied the concentration of free and total androgen receptors (ARs) in prostate neoplasia. In untreated carcinoma samples, the occupancy of cytoplasmic AR by endogenous androgens was high. Orchiectomized patients had AR levels consistent with androgen deprivation as total cellular AR was depleted. However, samples obtained from patients who had received chronic diethylstilbestrol (DES) (a synthetic nonsteroidal estrogen) showed high total cellular AR levels, and most of the AR was present as free cytoplasmic AR [26]. The interaction of estrogens, progesterone, and androgens and their receptors may therefore be of importance to protean malignancies that co-express these receptors and for the potential of effective hormonal manipulation (Fig. 2).
Simplified mechanism of action of estrogen receptor (ER) antagonists, selective estrogen receptor modulators (SERM), progesterone receptor (PR) agonists, and selective progesterone receptor modulators (SPRM). SERMs are a class of compounds that act on the estrogen receptor; they are distinguished from pure estrogen receptor agonists or antagonists in that their action is different in various tissues, thereby permitting selective inhibition or stimulation of estrogen-like action in various tissues. Examples include raloxifene, which acts as an estrogen antagonist in uterine and breast tissue and as an agonist in bone (see also Table 3). Similarly, SPRMs are agents that act of the progesterone receptor in a selective fashion, being agonist in some tissues and antagonists in others. An example of an SPRM is ulipristal. In contrast, progesterone is a full progesterone receptor agonist, and aglepristone is a full antagonist
1.6 Expression and biology of hormone receptors in diverse cancers
A subset of patients with multiple different tumor types can express ER and/or PR receptors (Table 1). Furthermore, both preclinical and clinical evidence suggests that these receptors participate in the biology of some of these malignancies. Indeed, patients with cancers other than those derived from the breast that are hormone receptor-positive have been shown to respond to anti-estrogen agents, though the number of patients reported is generally small.
2 Head and neck malignancies
2.1 Thyroid carcinoma
Thyroid cancer is the most common endocrine neoplasm. Premenopausal women have the highest risk for developing papillary and follicular thyroid carcinoma, which suggests a hormonal influence [27]. In the thyroid microenvironment, estrogen seems to be anti-apoptotic via Bcl expression [28] and pro-angiogenic via ER and vascular endothelial growth factor signaling [29]. Preclinical data show that there is evidence of cross talk between estrogen and the PI3K [30] and/or ERK1/2 pathway [31].
In earlier studies that did not distinguish between ER-α and ER-β [32], the incidence of ER-positive cases was 24 % (31/130) for nodular goiters, 22 % (8/37) for follicular adenomas, 11 % (2/18) for follicular carcinomas, 31 % (37/119) for papillary carcinomas, 0 % (0/35) for medullary carcinomas, and 0 % (0/12) for undifferentiated carcinomas, suggesting that the incidence of ER reactivity is higher in well-differentiated thyroid lesions. Similar findings have been reported by others [33, 34] though, when isoforms of ER were examined, there was more inconsistency in results [35–37] [38] [39].
In regard to PR, Kansakar et al. [40] found significantly higher PR in tumors compared to normal tissue (N = 104 patients), while in a smaller study, no PR positivity was found [41]. Bléchet et al. [42] reported that 7 % of medullary thyroid cancers (2/28) expressed PR.
The clinical implications of these results merit investigation. As an example, albeit an anecdotal one, Khalil et al. [43] reported a 29-year-old woman with non-resectable papillary thyroid cancer who had a dramatic response to anti-estrogen therapy with tamoxifen.
2.2 Thymoma and thymic carcinoma
Early studies that did not distinguish between ER-α and ER-β showed ER positivity in 37.5 % (3/8) cases of thymoma; PR was not detected in any of the cases (0/8) [44]. Ishibashi et al. [45] found positive nuclear immunoreactivity for ER-α in 66 % of cases, for ER-β in 7 %, for PR-A in 4 %, and for PR-B in 49 %. Mimae et al. [46] found a high rate of ER-β expression in thymomas (82.9 %) and thymic carcinomas (76.4 %), whereas the expression rates for other hormonal receptors were low, including ERα (13.6 %) and PR-A (0.71 %). Interestingly, intra-tumoral estradiol apparently abrogates cell proliferation via ER-α in human thymoma epithelial cells, leading some authors to speculate that estradiol could be an effective therapy for thymoma [47].
2.3 Nasopharyngeal carcinoma and juvenile nasopharyngeal angiofibroma
Juvenile nasopharyngeal angiofibroma is a benign hormone-driven tumor that primarily afflicts adolescent males and expresses ER (0–25 %) and PR (0–58 %) [48, 49]. Looking at malignant tumors, particularly nasopharyngeal carcinomas, Xu et al. [50] found ER in 94.3 % (67/71) and PR in 43.6 % (31/71). A retrospective study by Mo et al. [51] suggested that positive expression, particularly strong reactivity, of ER and PR correlated with poor prognosis and development of metastasis. We were unable to find studies of hormonal manipulation in nasopharyngeal carcinoma despite the high degree of ER/PR expression in this tumor.
2.4 Salivary gland tumors
Salivary gland tumors seem to be hormonally driven [22, 52] in a similar fashion to that of breast cancer, and some salivary tumors such as adenoid cystic carcinomas and salivary ductal cancers portray microscopic similarities to primary lesions of the breast [53].
Nasser et al. [22] reported a series of 78 formalin-fixed, paraffin-embedded salivary gland tumors: ER and PR reactivity were seen in only a few cases of salivary gland tumors, and all 26 benign salivary gland tumors were negative for ER and PR. AR reactivity is also seen in 20 % (2/10) of acinic cell carcinomas, 20 % (2/10) of mucoepidermoid carcinomas, and 20 % (2/10) of adenoid cystic carcinomas [22]. Fan et al. [54] reported that salivary duct carcinoma expresses AR in nearly all cases. Therefore, receptor reactivity might differ depending on the type of salivary gland tumor [55]. As another example, patients with recurrent pleomorphic adenomas of the parotid gland showed a high PR expression (96 %) compared to a control group (61 %) with primary pleomorphic adenoma, whereas ER expression was present but relatively low in those groups (19 and 17 %, respectively) [55].
Despite the morphologic similarity of adenoid cystic salivary gland tumors and breast cancer, the former have been shown to either not express ERs [52] or to express only ER-β as in the case of salivary adenocarcinoma cells [56]. Even so, an anecdotal report by Shadaba and colleagues [57] described treatment with tamoxifen in a patient with adenoid cystic carcinoma of the parotid gland with an 18 + -month partial remission achieved.
2.5 Laryngeal cancer
ERs were present in 37 % (17/46) and PRs in 48 % (22/46) of patients with laryngeal squamous cell carcinoma [58]. A different study evaluated samples from 15 laryngeal carcinomas assayed both by mRNA and protein levels for ER (53.3 %) and PR (73.3 %), finding greater immunopositivity in malignant cells than in normal adjacent tissue [59]. However, Urba et al. [60] treated 12 patients with recurrent laryngeal carcinoma with tamoxifen, reporting no clinical responses, although only two samples were assayed for ER and PR. In other squamous head and neck tumors, functional ER may be present in 40.3 % of cases [61], while PR positivity may be low [62].
3 Cutaneous malignancies
3.1 Melanoma
Hormone receptors have been implicated in melanoma [63]. ER positivity has been found in 46 % (16/35) [63] and PR in 18 % of 45 cases of malignant melanoma [64]. Even so, Creagan et al. [65] showed no responses to the anti-estrogen tamoxifen in 25 patients. In contrast, however, Karakousis et al. [66] showed that three women had objective responses (of 17 patients with melanoma treated with tamoxifen). Quencez et al. [67] also showed responses in two of four patients treated with tamoxifen for cutaneous ER-positive metastases from melanoma. Cocconi et al. [68] reported that dacarbazine plus tamoxifen is more effective than dacarbazine alone (response rate of 28 % versus 12 %, P = 0.03, and median survival of 48 versus 29 weeks, P = 0.02).
4 Genitourinary malignancies
4.1 Testicular germ cell tumors
Preclinical data suggest that estrogens might fuel human testicular germ cell cancer proliferation via membrane-mediated activation of extracellular regulated kinase (ERK)1/2 and cAMP-dependent protein kinase A (PKA) [69]. The expression of hormone receptors differs by tumor type [70]. ER-α was absent in all of the testicular germ cell cancers studied by Pais et al. [70]; ER-β expression was markedly diminished in seminomas, embryonal cell carcinomas, and in mixed germ cell tumors [70].
4.2 Bladder cancer
Urothelial carcinoma of the urinary bladder, unlike prostate and breast cancer, is currently not considered as a hormone-driven malignancy [71]. Wei et al. [72] pooled data from five different studies (N = 651 patients) regarding ER expression in bladder urothelial carcinoma, finding it in 10.9 % (71/651) of cases. None of the specimens containing urothelial carcinoma of the bladder exhibited PR expression [73]. However, Shen et al. [74] documented that only 0.8 % (2/224) of human bladder cancers weakly expressed ER-α, whereas ER-β was expressed in 63 % (141/224) of samples. Estradiol modestly enhanced growth in cancer cell lines, while anti-estrogens, such as hydroxytamoxifen and raloxifene, halted the growth of cancer cell lines [74]. Treatment with tamoxifen leading to resolution of cutaneous metastatic implants from transitional cell carcinoma has been documented in a case report [75].
4.3 Prostate cancer
The roles of androgens in prostate cancer are well known. Even so, it may be the balance between androgens and estrogens that is critical to prostate health and disease. Serum testosterone decreases in aging men, while estradiol remains stable or even increases slightly with aging. The variation in the androgen to estrogen ratio has been hypothesized to be responsible for the transition from benign to malignant prostate tissue [76]. The normal stromal tissue in the prostate expresses some aromatase, whereas malignant epithelial tissue in prostate cancer induces aromatase levels, which ultimately alters the ratio between androgens and estrogen [76]. Estrogen, in addition to androgens, may be a candidate therapeutic target.
In this regard, epithelial ER-α immunostaining was present in 80 % of prostatic carcinoma cells, whereas approximately 15 % of stromal cells were positively immunostained for ER-α [77]. PR was found in 92 % (12/13) of prostate cancers [78]. On the other hand, Asgari et al. [79] found ER-β expression in 100 % (29/29) of low and intermediate prostatic carcinomas and in 83 % (19/23) of high-grade tumors. Leav et al. [80] also found that prostatic carcinogenesis was characterized by a loss of ER-β in high-grade dysplasia. Differences between studies may be in part accountable to the anatomic location of the specimens within the prostate (Table 2). For instance, the ratio of AR to PR in the central zone of the prostate is 1.5 to 2.0 while the same ratio in the peripheral zone of the prostate is 0.3 to 0.5 [81]. In a phase II trial of tamoxifen (41 evaluable patients), there was one durable complete remission (1+ year) [82].
5 Lung malignancies
5.1 Non-small cell lung cancer
Wei et al. [72] reported ER expression in 11.7 % (89/760; denominator from pooled data) of lung adenocarcinomas; PR expression was found in 4 % (19/479) of lung adenocarcinomas. A more recent study by Rades et al. [83] reported 20.6 % (12/58) ER-α and 8.4 % (5/59) PR positivity. ER-α was a harbinger of a negative prognosis for men and women, whereas PR status was not associated with outcome. Mauro et al. [84] found total cytoplasmic and/or nuclear immunostaining in 77.6 % (45/58) for ER-α and 75.9 % (44/58) for ER-β; lack of nuclear ER-β and loss of EGFR expression were independently associated with a worse prognosis [84]. Sun et al. [85] reported that EGFR mutation was independently associated with female gender, negative PR expression, and negative aromatase expression, all of which were statistically significant.
Shen et al. [86] illustrated the fact that dual exposure with tamoxifen, an anti-estrogen, and gefitinib, an EGFR tyrosine kinase inhibitor, in non-small cell lung cancer cell lines showed synergistic anti-proliferative effects likely due to functional cross talk between such pathways. Hormonal manipulation in non-small cell lung cancer has been paired with chemotherapy in the clinic with acceptable toxicity, although it remains unclear whether or not the addition of hormonal manipulation would be superior to chemotherapy alone. Perez et al. [87] showed anti-tumor activity in 4 out of 10 patients with non-small cell lung cancer in a phase I trial of high-dose tamoxifen plus cisplatin. Lara et al. [88] showed an overall response rate of 18 % with a median overall survival of 8.1 months in a phase II trial of high-dose toremifene (an oral selective estrogen receptor modulator) in combination with cisplatin. Chen et al. [89] showed that five out of 25 patients (20 %) had a partial response after 2 cycles with a median survival of 7.7 months in a phase II trial of tamoxifen added to a regimen that included ifosfamide, epirubicin, and cisplatin.
5.2 Small cell lung cancer
Figueredo et al. [90] reported an encouraging phase I/II study of verapamil and tamoxifen added to the initial chemotherapy of small cell lung cancer that documented 24 % complete and 34 % partial response rates with a median survival of 46 weeks. Chen et al. [91] assessed the feasibility of a regimen containing tamoxifen, ifosfamide, epirubicin, and cisplatin for patients with extensive-disease small cell lung cancer; nevertheless, median survival in chemotherapy-naive patients did not increase when compared to the results of a previous trial of ifosfamide plus etoposide. A phase III trial [92] testing a regimen of cisplatin, etoposide, and radiotherapy, with or without tamoxifen, in patients with limited-stage small cell lung cancer failed to show improved survival in the tamoxifen group (88 % for 154 patients treated without tamoxifen and 84 % for 153 patients treated with tamoxifen), although it was an unselected population in terms of ER status.
6 Gastrointestinal malignancies
6.1 Gastric and esophageal carcinomas
Data pooled from 19 previous studies evaluating ER expression and 10 studies evaluating PR expression, irrespective of the antibody used, documented ER expression in 15.9 % (155/972) of cases; however, that number dropped to 0.4 % (2/467) when studies utilizing seldom used older antibodies were excluded [72]. By the same token, when more stringent criteria was used to screen studies that did not specify the type of antibody used, PR expression was found only in 0.5 % (2/383) of cases. A randomized, controlled trial of adjuvant tamoxifen in gastric cancer, in which 55.8 % of tumors were ER positive by an immunohistologic method (ERD5), showed that tamoxifen did not influence survival, although ER receptor status was an independent prognostic factor [93]. Again, discrepancies between the rates of ER expression may be explained by different immunohistologic methods used in older studies (Table 2). Mifepristone, an anti-progestin, abrogates proliferation of PR-positive human gastric cancer cells in vitro and in vivo mice xenografts [94]. Matsuyama et al. [95] further divided ER into ER-α and ER-β, finding positivity in 0 % (0/29) and 100 % (29/29), respectively. Ryu et al. [96] obtained more conservative numbers, showing that 45.3 % (67/148) of gastric cancer patients displayed ER-β positivity.
6.2 Pancreatic adenocarcinomas
Wei et al. [72] found that ER was found in 0 % (0/276) cases, while PR expression was found in 3 % (2/64) (pooled data from nine studies). Of interest, Lamy et al. [97] reported a complete response to tamoxifen in a patient with metastatic pancreatic adenocarcinoma. Hormonal modulation in advanced pancreatic cancer has been added to chemotherapy too. Tomao et al. [98] showed a partial response in 11 % of patients while 48 % experienced stable disease lasting at least 8 weeks. Eckel et al. [99] showed similar results. Sun et al. [100] suggested that curcumin-induced upregulation of microRNA-22 expressions in pancreatic cells suppressed the expression of the target gene estrogen receptor 1 (ESR1).
6.3 Malignant peritoneal mesothelioma
Chua et al. [101] described ER and PR positivity in 80 and 100 %, respectively, in patients with malignant peritoneal mesothelioma. A phase III randomized trial of surgery with or without intraoperative photodynamic therapy and postoperative immunochemotherapy (cisplatin, interferon alpha-2b, and tamoxifen) showed that the addition of intraoperative photodynamic therapy failed to improve local control or survival in malignant pleural mesothelioma [102]. The role of tamoxifen was not clarified.
7 Gynecologic malignancies
7.1 Cervical cancer
Kwasniewska et al. [103] reported weak immunoreactivity (+/+++) across multiple hormonal receptors including ER expression in 5 % (10/200) of squamous cell carcinoma of the cervix and in 6 % (3/50) of adenocarcinoma of the cervix. In the same study, PR expression was seen in 3 % (6/200) of squamous cell carcinoma of the cervix and in 4 % (2/50) of adenocarcinoma of the cervix. A trial of tamoxifen in patients with recurrent non-squamous cell cancer of the cervix [104] revealed an objective response rate of 11.1 % (two partial and one complete response).
7.2 Uterine cancer
IHC analysis of 144 patients with primary endometrial cancer demonstrated ER positivity in 79.5 % (97/122) and PR positivity in 86.9 % (106/122) of patients [105]. A phase II study of fulvestrant in patients with recurrent endometrial cancer performed by the Gynecologic Oncology Group revealed that one (3 %) patient had a complete response and four (13 %) had partial responses out of 31 ER-positive patients [106]. Interestingly, Xie et al. [107] showed in vitro that metformin stimulated PR expression. Ramondetta et al. [108] led a phase II clinical trial of the anti-progesterone mifepristone (RU-486) in patients with PR-positive advanced endometrioid adenocarcinoma or low-grade endometrial stromal sarcoma; no partial or complete responses were observed [108].
7.3 Ovarian cancer
Sinn et al. [109] found PR immunostaining positivity in 31.5 % (45/143) of patients with ovarian carcinoma associated with a favorable prognosis. De Stefano et al. [110] demonstrated positive nuclear ER-α in 74 % of 58 primary advanced serous carcinomas, whereas there was ER-β positivity in 89 % of the same population. Of note, cytoplastic immunopositivity was the main pattern seen in malignant cases although nuclear staining was the norm when normal ovarian tissue was assessed. Farinola et al. [111] reported PR and ER-α positivity in 98 and 66 % of granulosa cell tumors, respectively, and in Sertoli-Leydig cell tumors of 86 and 79 %, respectively. Although epidemiologic studies as well as animal experiments and receptor studies demonstrate that many malignant ovarian tumors are endocrine related and hormone dependent, the place of hormonal therapy in the management of patients with ovarian cancer remains unsettled. Most trials of hormonal treatment in ovarian cancer have been retrospective, involved only limited numbers of patients, and utilized a variety of hormonal preparations with different degrees of potency at different dosages. Overall, in advanced disease, response to hormonal therapy is modest, with about an 8 % objective response rate, albeit with almost no side effects. In a similar patient setting, more toxic agents do not yield a better outcome [112].
8 Lessons from erα-positive breast cancer
The use of ER as a highly effective therapeutic target in oncology was pioneered in breast cancer (Table 3), and the lessons learned from its success could potentially benefit patients across malignancies. The frequency of ER and PR positivity varies widely, from 0 % in triple-negative breast cancer to ~60 % in patients with other types of breast cancer [113]. Ng et al. [114] stratified subgroups of breast cancer patients depending on their hormonal receptor phenotype and found the following percentages: 46.8 % (1230/2629), ER+/PR+; 11.6 % (306/2629), ER+/PR-; 4.6 %, (122/2629), ER-/PR+; and 37 % (972/2629), ER-/PR-. The mechanism of action of drugs (Table 3, Fig. 2) used in the management of ER-positive metastatic breast cancer includes competitive antagonists of the ER, molecules that act as antagonists or agonists depending on the target tissue, receptor down-regulators, and aromatase inhibitors that block estrogen synthesis (Table 3) [115].
The most critical steps for initiation and progression of ERα-positive breast cancers are thought to be upregulation of ERα expression. There are several mechanisms implicated in ER upregulation: increased promoter activity of the ERα gene (ESR1) at the transcriptional level, changes in miRNAs that control ESR1 level, ESR1 gene amplification, and diminished degradation of ERα protein through ubiquitination and proteasomal pathways. Other mechanisms may also be operative, albeit incompletely elucidated. For instance, it has been suggested that ERα (ESR1) gene amplification is frequent in breast cancer [116] and identifies those individuals with high ERα expression. More recently, however, it has been posited that the clustered FISH signals interpreted as ESR1 amplification may be due to the accumulation of transcripts, rather than amplification [117].
Several different point mutations in the ligand-binding domain of ESR1 have also been identified in tumor samples from patients with ER-positive metastatic breast cancer. They are discerned after treatment with anti-estrogen therapy, but are rare in primary untreated cancers [118, 119]. Some of these mutations have also been seen in endometrial tumors. In functional modeling of molecular dynamics, these mutations confer constitutive ligand-independent activation of ER and may mediate anti-estrogen resistance.
In addition to deregulation of the components of the ER pathway itself, multiple other mechanisms responsible for endocrine resistance have been proposed: alterations in cell cycle and cell survival signaling molecules and the activation of escape pathways including, but not limited to, EGFR/HER2 (in breast cancer). Activation of the PI3K/AKT/mTOR pathway is also known to attenuate the effects of hormone therapy; combining aromatase inhibitors with mTOR inhibitors has shown efficacy in breast cancer [120] as well as ER-positive ovarian and uterine cancer [121].
9 Conclusions
The determination of ER and PR status in breast cancer proved to be crucial for predicting response to endocrine therapy. While high ER and PR expression have been found in a subgroup of patients with many other malignancies (Table 1), in the majority of cases, they are not being routinely tested as possible targets for therapy. Furthermore, there is a dearth of therapeutic studies using standardized ER or PR assessment and targeting outside of breast cancer, though anecdotal reports and small studies suggest efficacy with minimal toxicity in several cancers including, but not limited to, gynecologic, pancreatic, and lung neoplasms. The biology of ER and PR is however complex with several receptor subtypes that may act to either stimulate or inhibit cell growth. Co-expression of androgen receptors is also found in many malignancies, and these receptors and their ligands may be impacted by hormonal modulation targeted at ER or PR. The differential expression of ER and PR across tumors, and the availability of potent agents that can manipulate hormone status, suggests that interrogation and prosecution of these targets warrant more robust evaluation beyond breast cancer.
References
Molteni, A., Bahu, R. M., Battifora, H. A., Fors, E. M., Reddy, J. K., Rao, M. S., et al. (1979). Estradiol receptor assays in normal and neoplastic tissues. A possible diagnostic acid for tumor differentiation. Annals of Clinical and Laboratory Science, 9(2), 103–108.
Enmark, E., & Gustafsson, J. A. (1999). Oestrogen receptors—an overview. Journal of Internal Medicine, 246(2), 133–138.
Thornton, M. J. (2002). The biological actions of estrogens on skin. Experimental Dermatology, 11(6), 487–502.
Kumar, S. K., Gupta, N., Rajwanshi, A., Joshi, K., & Singh, G. (2012). Immunochemistry for oestrogen receptor, progesterone receptor and HER2 on cell blocks in primary breast carcinoma. Cytopathology, 23(3), 181–186. doi:10.1111/j.1365-2303.2011.00853.x.
Thompson, A. M., Jordan, L. B., Quinlan, P., Anderson, E., Skene, A., Dewar, J. A., et al. (2010). Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS). Breast Cancer Research, 12(6), R92. doi:10.1186/bcr2771.
Jensen, E. V., & Jacobson, H. I. (1960). Fate of steroidal estrogens in target tissues. In G. Pincus & E. P. Vollmer (Eds.), Biological activities of steroids in relation to cancer (pp. 161–174). New York: Academic Press.
Greene, G. L., Gilna, P., Waterfield, M., Baker, A., Hort, Y., & Shine, J. (1986). Sequence and expression of human estrogen receptor complementary DNA. Science, 231(4742), 1150–1154.
Mosselman, S., Polman, J., & Dijkema, R. (1996). ER beta: identification and characterization of a novel human estrogen receptor. FEBS Letters, 392(1), 49–53.
Chen, G. G., Vlantis, A. C., Zeng, Q., & van Hasselt, C. A. (2008). Regulation of cell growth by estrogen signaling and potential targets in thyroid cancer. Current Cancer Drug Targets, 8(5), 367–377.
Brandenberger, A. W., Tee, M. K., & Jaffe, R. B. (1998). Estrogen receptor alpha (ER-alpha) and beta (ER-beta) mRNAs in normal ovary, ovarian serous cystadenocarcinoma and ovarian cancer cell lines: down-regulation of ER-beta in neoplastic tissues. The Journal of Clinical Endocrinology and Metabolism, 83(3), 1025–1028. doi:10.1210/jcem.83.3.4788.
Bardin, A., Boulle, N., Lazennec, G., Vignon, F., & Pujol, P. (2004). Loss of ERbeta expression as a common step in estrogen-dependent tumor progression. Endocrine-Related Cancer, 11(3), 537–551.
Platet, N., Cathiard, A. M., Gleizes, M., & Garcia, M. (2004). Estrogens and their receptors in breast cancer progression: a dual role in cancer proliferation and invasion. Critical Reviews in Oncology/Hematology, 51(1), 55–67. doi:10.1016/j.critrevonc.2004.02.001.
Signoretti, S., & Loda, M. (2001). Estrogen receptor beta in prostate cancer: brake pedal or accelerator? The American Journal of Pathology, 159(1), 13–16.
Leung, Y. K., Lam, H. M., Wu, S., Song, D., Levin, L., Cheng, L., et al. (2010). Estrogen receptor beta2 and beta5 are associated with poor prognosis in prostate cancer, and promote cancer cell migration and invasion. Endocrine-Related Cancer, 17(3), 675–689. doi:10.1677/ERC-09-0294.
Shaaban, A. M., Green, A. R., Karthik, S., Alizadeh, Y., Hughes, T. A., Harkins, L., et al. (2008). Nuclear and cytoplasmic expression of ERbeta1, ERbeta2, and ERbeta5 identifies distinct prognostic outcome for breast cancer patients. Clinical Cancer Research, 14(16), 5228–5235. doi:10.1158/1078-0432.CCR-07-4528.
Horwitz, K. B., & Alexander, P. S. (1983). In situ photolinked nuclear progesterone receptors of human breast cancer cells: subunit molecular weights after transformation and translocation. Endocrinology, 113(6), 2195–2201. doi:10.1210/endo-113-6-2195.
O'Malley, B. W., & Schrader, W. T. (1972). Progesterone receptor components: identification of subunits binding to the target-cell genome. Journal of Steroid Biochemistry, 3(3), 617–629.
Kastner, P., Krust, A., Turcotte, B., Stropp, U., Tora, L., Gronemeyer, H., et al. (1990). Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. The EMBO Journal, 9(5), 1603–1614.
Vegeto, E., Shahbaz, M. M., Wen, D. X., Goldman, M. E., O'Malley, B. W., & McDonnell, D. P. (1993). Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Molecular Endocrinology, 7(10), 1244–1255. doi:10.1210/mend.7.10.8264658.
Badve, S., & Nakshatri, H. (2009). Oestrogen-receptor-positive breast cancer: towards bridging histopathological and molecular classifications. Journal of Clinical Pathology, 62(1), 6–12. doi:10.1136/jcp.2008.059899.
Boonyaratanakornkit, V., & Edwards, D. P. (2004). Receptor mechanisms of rapid extranuclear signalling initiated by steroid hormones. Essays in Biochemistry, 40, 105–120.
Nasser, S. M., Faquin, W. C., & Dayal, Y. (2003). Expression of androgen, estrogen, and progesterone receptors in salivary gland tumors. Frequent expression of androgen receptor in a subset of malignant salivary gland tumors. American Journal of Clinical Pathology, 119(6), 801–806. doi:10.1309/RVTP-1G0Q-727W-JUQD.
Hu, R., Dawood, S., Holmes, M. D., Collins, L. C., Schnitt, S. J., Cole, K., et al. (2011). Androgen receptor expression and breast cancer survival in postmenopausal women. Clinical Cancer Research, 17(7), 1867–1874. doi:10.1158/1078-0432.CCR-10-2021.
Ito, K., Suzuki, T., Akahira, J., Moriya, T., Kaneko, C., Utsunomiya, H., et al. (2002). Expression of androgen receptor and 5alpha-reductases in the human normal endometrium and its disorders. International Journal of Cancer, 99(5), 652–657. doi:10.1002/ijc.10394.
Baumgart, J., Nilsson, K., Stavreus Evers, A., Kunovac Kallak, T., Kushnir, M. M., Bergquist, J., et al. (2014). Androgen levels during adjuvant endocrine therapy in postmenopausal breast cancer patients. Climacteric, 17(1), 48–54. doi:10.3109/13697137.2013.800039.
Mobbs, B. G., Johnson, I. E., Connolly, J. G., & Thompson, J. (1983). Concentration and cellular distribution of androgen receptor in human prostatic neoplasia: can estrogen treatment increase androgen receptor content? Journal of Steroid Biochemistry, 19(3), 1279–1290.
Kumar, A., Klinge, C. M., & Goldstein, R. E. (2010). Estradiol-induced proliferation of papillary and follicular thyroid cancer cells is mediated by estrogen receptors alpha and beta. International Journal of Oncology, 36(5), 1067–1080.
Lee, M. L., Chen, G. G., Vlantis, A. C., Tse, G. M., Leung, B. C., & van Hasselt, C. A. (2005). Induction of thyroid papillary carcinoma cell proliferation by estrogen is associated with an altered expression of Bcl-xL. Cancer Journal, 11(2), 113–121.
Kamat, A., Rajoria, S., George, A., Suriano, R., Shanmugam, A., Megwalu, U., et al. (2011). Estrogen-mediated angiogenesis in thyroid tumor microenvironment is mediated through VEGF signaling pathways. Archives of Otolaryngology - Head and Neck Surgery, 137(11), 1146–1153. doi:10.1001/archoto.2011.194.
Antico-Arciuch, V. G., Dima, M., Liao, X. H., Refetoff, S., & Di Cristofano, A. (2010). Cross-talk between PI3K and estrogen in the mouse thyroid predisposes to the development of follicular carcinomas with a higher incidence in females. Oncogene, 29(42), 5678–5686. doi:10.1038/onc.2010.308.
Zeng, Q., Chen, G. G., Vlantis, A. C., & van Hasselt, C. A. (2007). Oestrogen mediates the growth of human thyroid carcinoma cells via an oestrogen receptor-ERK pathway. Cell Proliferation, 40(6), 921–935. doi:10.1111/j.1365-2184.2007.00471.x.
Tavangar, S. M., Monajemzadeh, M., Larijani, B., & Haghpanah, V. (2007). Immunohistochemical study of oestrogen receptors in 351 human thyroid glands. Singapore Medical Journal, 48(8), 744–747.
Lewy-Trenda, I. (1998). Estrogen receptors in the malignant and benign neoplasms of the thyroid. Polski Merkuriusz Lekarski, 5(26), 80–83.
Hiasa, Y., Nishioka, H., Kitahori, Y., Yane, K., Nakaoka, S., Ohshima, M., et al. (1993). Immunohistochemical analysis of estrogen receptors in 313 paraffin section cases of human thyroid tissue. Oncology, 50(2), 132–136.
Di Vito, M., De Santis, E., Perrone, G. A., Mari, E., Giordano, M. C., De Antoni, E., et al. (2011). Overexpression of estrogen receptor-alpha in human papillary thyroid carcinomas studied by laser-capture microdissection and molecular biology. Cancer Science, 102(10), 1921–1927. doi:10.1111/j.1349-7006.2011.02017.x.
Vaiman, M., Olevson, Y., Habler, L., Eviatar, E., Zehari, S., Sandbank, J., et al. (2010). The estrogen receptors in the papillary carcinoma of the thyroid gland. Oncology Research, 18(11–12), 537–540.
Vaiman, M., Olevson, Y., Habler, L., Kessler, A., Zehavi, S., & Sandbank, J. (2010). Diagnostic value of estrogen receptors in thyroid lesions. Medical Science Monitor, 16(7), BR203–BR207.
Ceresini, G., Morganti, S., Graiani, V., Saccani, M., Milli, B., Usberti, E., et al. (2006). Estrogen receptor (ER)-beta, but not ER-alpha, is present in thyroid vessels: immunohistochemical evaluations in multinodular goiter and papillary thyroid carcinoma. Thyroid, 16(12), 1215–1220. doi:10.1089/thy.2006.16.1215.
Vannucchi, G., Perrino, M., Rossi, S., Colombo, C., Vicentini, L., Dazzi, D., et al. (2010). Clinical and molecular features of differentiated thyroid cancer diagnosed during pregnancy. European Journal of Endocrinology, 162(1), 145–151. doi:10.1530/EJE-09-0761.
Kansakar, E., Chang, Y. J., Mehrabi, M., & Mittal, V. (2009). Expression of estrogen receptor, progesterone receptor, and vascular endothelial growth factor-A in thyroid cancer. The American Surgeon, 75(9), 785–789. discussion 789.
Memon, G. R., Arain, S. A., Jamal, Q., & Ansari, T. (2005). Immunohistochemical study of progesterone receptors in thyroid gland. The Journal of the Pakistan Medical Association, 55(8), 321–324.
Blechet, C., Lecomte, P., De Calan, L., Beutter, P., & Guyetant, S. (2007). Expression of sex steroid hormone receptors in C cell hyperplasia and medullary thyroid carcinoma. Virchows Archiv, 450(4), 433–439. doi:10.1007/s00428-007-0379-6.
Khalil, A. B., Trudel, M., & Creel, R. (2010). Papillary carcinoma of the thyroid with fibromatosislike stroma: case report and review of literature. Endocrine Practice, 16(4), 650–655. doi:10.4158/EP09061.CR.
Kobayashi, S., Tobioka, N., Samoto, T., Tanaka, H., & Masaoka, A. (1982). Studies on the estrogen receptor in human meningioma and thymoma. Nihon Naibunpi Gakkai Zasshi, 58(10), 1333–1340.
Ishibashi, H., Suzuki, T., Suzuki, S., Moriya, T., Kaneko, C., Takizawa, T., et al. (2003). Sex steroid hormone receptors in human thymoma. The Journal of Clinical Endocrinology and Metabolism, 88(5), 2309–2317. doi:10.1210/jc.2002-021353.
Mimae, T., Tsuta, K., Takahashi, F., Yoshida, A., Kondo, T., Murakami, Y., et al. (2011). Steroid receptor expression in thymomas and thymic carcinomas. Cancer, 117(19), 4396–4405. doi:10.1002/cncr.26061.
Ishibashi, H., Suzuki, T., Suzuki, S., Moriya, T., Kaneko, C., Nakata, T., et al. (2005). Estrogen inhibits cell proliferation through in situ production in human thymoma. Clinical Cancer Research, 11(18), 6495–6504. doi:10.1158/1078-0432.CCR-04-2495.
Brentani, M. M., Butugan, O., Oshima, C. T., Torloni, H., & Paiva, L. J. (1989). Multiple steroid receptors in nasopharyngeal angiofibromas. Laryngoscope, 99(4), 398–401. doi:10.1288/00005537-198904000-00007.
Montag, A. G., Tretiakova, M., & Richardson, M. (2006). Steroid hormone receptor expression in nasopharyngeal angiofibromas. Consistent expression of estrogen receptor beta. American Journal of Clinical Pathology, 125(6), 832–837. doi:10.1309/W5CM-3A3N-H7P4-F5P2.
Xu, B., Hu, P., Wu, Q., Hou, J., Zhang, B., Chen, X., et al. (1999). Relationship between expression of estrogen receptor progesterone receptor and the biological characteristics of nasopharyngeal carcinoma. Lin Chuang Er Bi Yan Hou Ke Za Zhi, 13(8), 347–349.
Mo, L., Kuang, G., Luo, Y., & Yang, R. (2006). Relationship between the expression of estrogen and progestogen receptors in distant metastasis of nasopharyngeal carcinoma. Lin Chuang Er Bi Yan Hou Ke Za Zhi, 20(11), 494–495.
Shick, P. C., Riordan, G. P., & Foss, R. D. (1995). Estrogen and progesterone receptors in salivary gland adenoid cystic carcinoma. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics, 80(4), 440–444.
Fahim, L., Weinreb, I., Alexander, C., & Perez Ordonez, B. (2008). Epithelial proliferation in small ducts of salivary cystadenoma resembling atypical ductal hyperplasia of breast. Head and Neck Pathology, 2(3), 213–217. doi:10.1007/s12105-008-0061-6.
Fan, C. Y., Melhem, M. F., Hosal, A. S., Grandis, J. R., & Barnes, E. L. (2001). Expression of androgen receptor, epidermal growth factor receptor, and transforming growth factor alpha in salivary duct carcinoma. Archives of Otolaryngology - Head and Neck Surgery, 127(9), 1075–1079.
Glas, A. S., Hollema, H., Nap, R. E., & Plukker, J. T. (2002). Expression of estrogen receptor, progesterone receptor, and insulin-like growth factor receptor-1 and of MIB-1 in patients with recurrent pleomorphic adenoma of the parotid gland. Cancer, 94(8), 2211–2216. doi:10.1002/cncr.10445.
Ohshiro, K., Rayala, S. K., Williams, M. D., Kumar, R., & El-Naggar, A. K. (2006). Biological role of estrogen receptor beta in salivary gland adenocarcinoma cells. Clinical Cancer Research, 12(20 Pt 1), 5994–5999. doi:10.1158/1078-0432.CCR-06-1251.
Shadaba, A., Gaze, M. N., & Grant, H. R. (1997). The response of adenoid cystic carcinoma to tamoxifen. The Journal of Laryngology and Otology, 111(12), 1186–1189.
Maurizi, M., Ferrandina, G., Almadori, G., Scambia, G., Cadoni, G., D'Agostino, G., et al. (1998). Prognostic significance of methyl-p-hydroxy-phenyllactate-esterase activity in laryngeal squamous cell carcinoma. British Journal of Cancer, 77(8), 1253–1259.
Bianchini, C., Pastore, A., Pelucchi, S., Torreggiani, E., Lambertini, E., Marchesi, E., et al. (2008). Sex hormone receptor levels in laryngeal carcinoma: a comparison between protein and RNA evaluations. European Archives of Oto-Rhino-Laryngology, 265(9), 1089–1094. doi:10.1007/s00405-008-0589-9.
Urba, S. G., Carey, T. E., Kudla-Hatch, V., Wolf, G. T., & Forastiere, A. A. (1990). Tamoxifen therapy in patients with recurrent laryngeal squamous carcinoma. Laryngoscope, 100(1), 76–78. doi:10.1288/00005537-199001000-00015.
Lukits, J. (2009). The effect of the microenvironment of head and neck cancers on tumor progression. Magyar Onkologia, 53(1), 51–59. doi:10.1556/MOnkol.53.2009.1.8.
Schuller, D. E., Abou-Issa, H., & Parrish, R. (1984). Estrogen and progesterone receptors in head and neck cancer. Archives of Otolaryngology, 110(11), 725–727.
Fisher, R. I., Neifeld, J. P., & Lippman, M. E. (1976). Oestrogen receptors in human malignant melanoma. Lancet, 2(7981), 337–339.
Avril, M. F., Delarue, J. C., & Prade, M. (1985). Estrogen and progesterone receptors in malignant melanoma, benign pigmented nevi and basocellular epithelioma. Pathologie Biologie (Paris), 33(1), 39–44.
Creagan, E. T., Ingle, J. N., Green, S. J., Ahmann, D. L., & Jiang, N. S. (1980). Phase II study of tamoxifen in patients with disseminated malignant melanoma. Cancer Treatment Reports, 64(2–3), 199–201.
Karakousis, C. P., Lopez, R. E., Bhakoo, H. S., Rosen, F., Moore, R., & Carlson, M. (1980). Estrogen and progesterone receptors and tamoxifen in malignant melanoma. Cancer Treatment Reports, 64(6–7), 819–827.
Quencez, E., Blanc, D., Adessi, G., Agache, P., & Laurent, R. (1985). Role of an anti-estrogen, tamoxifen, in the treatment of metastatic malignant melanoma. Annales de Dermatologie et de Vénéréologie, 112(4), 341–346. 349.
Cocconi, G., Bella, M., Calabresi, F., Tonato, M., Canaletti, R., Boni, C., et al. (1992). Treatment of metastatic malignant melanoma with dacarbazine plus tamoxifen. New England Journal of Medicine, 327(8), 516–523. doi:10.1056/NEJM199208203270803.
Bouskine, A., Nebout, M., Mograbi, B., Brucker-Davis, F., Roger, C., & Fenichel, P. (2008). Estrogens promote human testicular germ cell cancer through a membrane-mediated activation of extracellular regulated kinase and protein kinase A. Endocrinology, 149(2), 565–573. doi:10.1210/en.2007-1318.
Pais, V., Leav, I., Lau, K. M., Jiang, Z., & Ho, S. M. (2003). Estrogen receptor-beta expression in human testicular germ cell tumors. Clinical Cancer Research, 9(12), 4475–4482.
Miyamoto, H., Zheng, Y., & Izumi, K. (2012). Nuclear hormone receptor signals as new therapeutic targets for urothelial carcinoma. Current Cancer Drug Targets, 12(1), 14–22.
Wei, S., Said-Al-Naief, N., & Hameed, O. (2009). Estrogen and progesterone receptor expression is not always specific for mammary and gynecologic carcinomas: a tissue microarray and pooled literature review study. Applied Immunohistochemistry and Molecular Morphology, 17(5), 393–402. doi:10.1097/PAI.0b013e31819faa07.
Bolenz, C., Lotan, Y., Ashfaq, R., & Shariat, S. F. (2009). Estrogen and progesterone hormonal receptor expression in urothelial carcinoma of the bladder. European Urology, 56(6), 1093–1095. doi:10.1016/j.eururo.2009.06.032.
Shen, S. S., Smith, C. L., Hsieh, J. T., Yu, J., Kim, I. Y., Jian, W., et al. (2006). Expression of estrogen receptors-alpha and -beta in bladder cancer cell lines and human bladder tumor tissue. Cancer, 106(12), 2610–2616. doi:10.1002/cncr.21945.
Dellagrammaticas, D., Bryden, A. A., & Collins, G. N. (2001). Regression of metastatic transitional cell carcinoma in response to tamoxifen. The Journal of Urology, 165(5), 1631.
Ellem, S. J., & Risbridger, G. P. (2010). Aromatase and regulating the estrogen:androgen ratio in the prostate gland. The Journal of Steroid Biochemistry and Molecular Biology, 118(4–5), 246–251. doi:10.1016/j.jsbmb.2009.10.015.
Royuela, M., de Miguel, M. P., Bethencourt, F. R., Sanchez-Chapado, M., Fraile, B., Arenas, M. I., et al. (2001). Estrogen receptors alpha and beta in the normal, hyperplastic and carcinomatous human prostate. The Journal of Endocrinology, 168(3), 447–454.
Wolf, R. M., Schneider, S. L., Pontes, J. E., Englander, L., Karr, J. P., Murphy, G. P., et al. (1985). Estrogen and progestin receptors in human prostatic carcinoma. Cancer, 55(10), 2477–2481.
Asgari, M., & Morakabati, A. (2011). Estrogen receptor beta expression in prostate adenocarcinoma. Diagnostic Pathology, 6, 61. doi:10.1186/1746-1596-6-61.
Leav, I., Lau, K. M., Adams, J. Y., McNeal, J. E., Taplin, M. E., Wang, J., et al. (2001). Comparative studies of the estrogen receptors beta and alpha and the androgen receptor in normal human prostate glands, dysplasia, and in primary and metastatic carcinoma. The American Journal of Pathology, 159(1), 79–92.
Bashirelahi, N., Young, J. D., Shida, K., Yamanaka, H., Ito, Y., & Harada, M. (1983). Androgen, estrogen, and progesterone receptors in peripheral and central zones of human prostate with adenocarcinoma. Urology, 21(5), 530–535.
Spremulli, E., DeSimone, P., & Durant, J. (1982). A phase II study Nolvadex: tamoxifen citrate in the treatment of advanced prostatic adenocarcinoma. American Journal of Clinical Oncology, 5(2), 149–153.
Rades, D., Setter, C., Dahl, O., Schild, S. E., & Noack, F. (2012). The prognostic impact of tumor cell expression of estrogen receptor-alpha, progesterone receptor, and androgen receptor in patients irradiated for nonsmall cell lung cancer. Cancer, 118(1), 157–163. doi:10.1002/cncr.26282.
Mauro, L. V., Dalurzo, M., Carlini, M. J., Smith, D., Nunez, M., Simian, M., et al. (2010). Estrogen receptor beta and epidermal growth factor receptor as early-stage prognostic biomarkers of non-small cell lung cancer. Oncology Reports, 24(5), 1331–1338.
Sun, H. B., Zheng, Y., Ou, W., Fang, Q., Li, P., Ye, X., et al. (2011). Association between hormone receptor expression and epidermal growth factor receptor mutation in patients operated on for non-small cell lung cancer. The Annals of Thoracic Surgery, 91(5), 1562–1567. doi:10.1016/j.athoracsur.2011.02.001.
Shen, H., Yuan, Y., Sun, J., Gao, W., & Shu, Y. Q. (2010). Combined tamoxifen and gefitinib in non-small cell lung cancer shows antiproliferative effects. Biomedicine and Pharmacotherapy, 64(2), 88–92. doi:10.1016/j.biopha.2009.06.010.
Perez, E. A., Gandara, D. R., Edelman, M. J., O'Donnell, R., Lauder, I. J., & DeGregorio, M. (2003). Phase I trial of high-dose tamoxifen in combination with cisplatin in patients with lung cancer and other advanced malignancies. Cancer Investigation, 21(1), 1–6.
Lara, P. N., Jr., Gandara, D. R., Longmate, J., Gumerlock, P. H., Lau, D. H., Edelman, M. J., et al. (2001). Activity of high-dose toremifene plus cisplatin in platinum-treated non-small-cell lung cancer: a phase II California Cancer Consortium Trial. Cancer Chemotherapy and Pharmacology, 48(1), 22–28.
Chen, Y., Perng, R. P., Yang, K. Y., Lin, W. C., Wu, H. W., Tsai, C. M., et al. (2000). Phase II study of tamoxifen, ifosfamide, epirubicin and cisplatin combination chemotherapy in patients with non-small cell lung cancer failing previous chemotherapy. Lung Cancer, 29(2), 139–146.
Figueredo, A., Arnold, A., Goodyear, M., Findlay, B., Neville, A., Normandeau, R., et al. (1990). Addition of verapamil and tamoxifen to the initial chemotherapy of small cell lung cancer. A phase I/II study. Cancer, 65(9), 1895–1902.
Chen, Y. M., Perng, R. P., Yang, K. Y., Wu, H. W., Lin, W. C., Liu, J. M., et al. (2000). Combination chemotherapy with tamoxifen, ifosfamide, epirubicin and cisplatin in extensive-disease small-cell lung cancer. Zhonghua Yi Xue Za Zhi (Taipei), 63(8), 605–611.
McClay, E. F., Bogart, J., Herndon, J. E., Watson, D., 2nd, Evans, L., Seagren, S. L., et al. (2005). A phase III trial evaluating the combination of cisplatin, etoposide, and radiation therapy with or without tamoxifen in patients with limited-stage small cell lung cancer: Cancer and Leukemia Group B study (9235). American Journal of Clinical Oncology, 28(1), 81–90.
Harrison, J. D., Morris, D. L., Ellis, I. O., Jones, J. A., & Jackson, I. (1989). The effect of tamoxifen and estrogen receptor status on survival in gastric carcinoma. Cancer, 64(5), 1007–1010.
Li, D. Q., Pan, L. H., & Shao, Z. M. (2004). Inhibitory effects of mifepristone on the growth of human gastric cancer cell line MKN-45 in vitro and in vivo. Chinese Medical Sciences Journal, 19(4), 237–242.
Matsuyama, S., Ohkura, Y., Eguchi, H., Kobayashi, Y., Akagi, K., Uchida, K., et al. (2002). Estrogen receptor beta is expressed in human stomach adenocarcinoma. Journal of Cancer Research and Clinical Oncology, 128(6), 319–324. doi:10.1007/s00432-002-0336-3.
Ryu, W. S., Kim, J. H., Jang, Y. J., Park, S. S., Um, J. W., Park, S. H., et al. (2012). Expression of estrogen receptors in gastric cancer and their clinical significance. Journal of Surgical Oncology, 106(4), 456–461. doi:10.1002/jso.23097.
Lamy, R., Conroy, T., Brunaud, L., & Bresler, L. (2001). Tamoxifen for metastatic pancreatic adenocarcinoma: a complete response. Gastroentérologie Clinique et Biologique, 25(10), 912–913.
Tomao, S., Romiti, A., Massidda, B., Ionta, M. T., Farris, A., Zullo, A., et al. (2002). A phase II study of gemcitabine and tamoxifen in advanced pancreatic cancer. Anticancer Research, 22(4), 2361–2364.
Eckel, F., Lersch, C., Lippl, F., Assmann, G., & Schulte-Frohlinde, E. (2000). Phase II trial of cyclophosphamide, leucovorin, 5-fluorouracil 24-hour infusion and tamoxifen in pancreatic cancer. Journal of Experimental & Clinical Cancer Research, 19(3), 295–300.
Sun, M., Estrov, Z., Ji, Y., Coombes, K. R., Harris, D. H., & Kurzrock, R. (2008). Curcumin (diferuloylmethane) alters the expression profiles of microRNAs in human pancreatic cancer cells. Molecular Cancer Therapeutics, 7(3), 464–473. doi:10.1158/1535-7163.MCT-07-2272.
Chua, T. C., Yao, P., Akther, J., Young, L., Bao, S., Samaraweera, U., et al. (2009). Differential expression of Ki-67 and sex steroid hormone receptors between genders in peritoneal mesothelioma. Pathology and Oncology Research, 15(4), 671–678. doi:10.1007/s12253-009-9170-0.
Pass, H. I., Temeck, B. K., Kranda, K., Thomas, G., Russo, A., Smith, P., et al. (1997). Phase III randomized trial of surgery with or without intraoperative photodynamic therapy and postoperative immunochemotherapy for malignant pleural mesothelioma. Annals of Surgical Oncology, 4(8), 628–633.
Kwasniewska, A., Postawski, K., Gozdzicka-Jozefiak, A., Kwasniewski, W., Grywalska, E., Zdunek, M., et al. (2011). Estrogen and progesterone receptor expression in HPV-positive and HPV-negative cervical carcinomas. Oncology Reports, 26(1), 153–160. doi:10.3892/or.2011.1256.
Bigler, L. R., Tate Thigpen, J., Blessing, J. A., Fiorica, J., & Monk, B. J. (2004). Evaluation of tamoxifen in persistent or recurrent nonsquamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. International Journal of Gynecological Cancer, 14(5), 871–874. doi:10.1111/j.1048-891X.2004.14523.x.
Markova, I., Pilka, R., Duskova, M., Zapletalova, J., & Kudela, M. (2010). Prognostic significance of clinic pathological and selected immunohistochemical factors in endometrial cancer. Ceská Gynekologie, 75(3), 193–199.
Covens, A. L., Filiaci, V., Gersell, D., Lutman, C. V., Bonebrake, A., & Lee, Y. C. (2011). Phase II study of fulvestrant in recurrent/metastatic endometrial carcinoma: a Gynecologic Oncology Group study. Gynecologic Oncology, 120(2), 185–188. doi:10.1016/j.ygyno.2010.10.015.
Xie, Y., Wang, Y. L., Yu, L., Hu, Q., Ji, L., Zhang, Y., et al. (2011). Metformin promotes progesterone receptor expression via inhibition of mammalian target of rapamycin (mTOR) in endometrial cancer cells. The Journal of Steroid Biochemistry and Molecular Biology, 126(3–5), 113–120. doi:10.1016/j.jsbmb.2010.12.006.
Ramondetta, L. M., Johnson, A. J., Sun, C. C., Atkinson, N., Smith, J. A., Jung, M. S., et al. (2009). Phase 2 trial of mifepristone (RU-486) in advanced or recurrent endometrioid adenocarcinoma or low-grade endometrial stromal sarcoma. Cancer, 115(9), 1867–1874. doi:10.1002/cncr.24197.
Sinn, B. V., Darb-Esfahani, S., Wirtz, R. M., Budczies, J., Sehouli, J., Chekerov, R., et al. (2011). Evaluation of a hormone receptor-positive ovarian carcinoma subtype with a favourable prognosis by determination of progesterone receptor and oestrogen receptor 1 mRNA expression in formalin-fixed paraffin-embedded tissue. Histopathology, 59(5), 918–927. doi:10.1111/j.1365-2559.2011.04028.x.
De Stefano, I., Zannoni, G. F., Prisco, M. G., Fagotti, A., Tortorella, L., Vizzielli, G., et al. (2011). Cytoplasmic expression of estrogen receptor beta (ERbeta) predicts poor clinical outcome in advanced serous ovarian cancer. Gynecologic Oncology, 122(3), 573–579. doi:10.1016/j.ygyno.2011.05.025.
Farinola, M. A., Gown, A. M., Judson, K., Ronnett, B. M., Barry, T. S., Movahedi-Lankarani, S., et al. (2007). Estrogen receptor alpha and progesterone receptor expression in ovarian adult granulosa cell tumors and Sertoli-Leydig cell tumors. International Journal of Gynecological Pathology, 26(4), 375–382. doi:10.1097/pgp.0b013e31805c0d99.
Makar, A. P. (2000). Hormone therapy in epithelial ovarian cancer. Endocrine-Related Cancer, 7(2), 85–93.
Qi, J. P., Yang, Y. L., Zhu, H., Wang, J., Jia, Y., Liu, N., et al. (2012). Expression of the androgen receptor and its correlation with molecular subtypes in 980 chinese breast cancer patients. Breast Cancer (Auckl.), 6, 1–8. doi:10.4137/BCBCR.S8323.
Ng, C. H., Pathy, N. B., Taib, N. A., Mun, K. S., Rhodes, A., & Yip, C. H. (2012). The estrogen receptor negative-progesterone receptor positive breast carcinoma is a biological entity and not a technical artifact. Asian Pacific Journal of Cancer Prevention, 13(4), 1111–1113.
Smith, I. E., & Dowsett, M. (2003). Aromatase inhibitors in breast cancer. New England Journal of Medicine, 348(24), 2431–2442. doi:10.1056/NEJMra023246.
Holst, F., Stahl, P. R., Ruiz, C., Hellwinkel, O., Jehan, Z., Wendland, M., et al. (2007). Estrogen receptor alpha (ESR1) gene amplification is frequent in breast cancer. Nature Genetics, 39(5), 655–660. doi:10.1038/ng2006.
Ooi, A., Inokuchi, M., Harada, S., Inazawa, J., Tajiri, R., Kitamura, S. S., et al. (2012). Gene amplification of ESR1 in breast cancers—fact or fiction? A fluorescence in situ hybridization and multiplex ligation-dependent probe amplification study. The Journal of Pathology, 227(1), 8–16. doi:10.1002/path.3974.
Robinson, D. R., Wu, Y. M., Vats, P., Su, F., Lonigro, R. J., Cao, X., et al. (2013). Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nature Genetics, 45(12), 1446–1451. doi:10.1038/ng.2823.
Toy, W., Shen, Y., Won, H., Green, B., Sakr, R. A., Will, M., et al. (2013). ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. Nature Genetics, 45(12), 1439–1445. doi:10.1038/ng.2822.
Baselga, J., Campone, M., Piccart, M., Burris, H. A., 3rd, Rugo, H. S., Sahmoud, T., et al. (2012). Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. New England Journal of Medicine, 366(6), 520–529. doi:10.1056/NEJMoa1109653.
Wheler, J. J., Moulder, S. L., Naing, A., Janku, F., Piha-Paul, S. A., Falchook, G. S., et al. (2014). Anastrozole and everolimus in advanced gynecologic and breast malignancies: activity and molecular alterations in the PI3K/AKT/mTOR pathway. Oncotarget, 5(10), 3029–3038.
Shen, X. C., Gu, C. X., Qiu, Y. Q., Du, C. J., Fu, Y. B., & Wu, J. J. (2009). Estrogen receptor expression in adrenocortical carcinoma. Journal of Zhejiang University. Science. B, 10(1), 1–6. doi:10.1631/jzus.B0820072.
Montanaro, D., Maggiolini, M., Recchia, A. G., Sirianni, R., Aquila, S., Barzon, L., et al. (2005). Antiestrogens upregulate estrogen receptor beta expression and inhibit adrenocortical H295R cell proliferation. Journal of Molecular Endocrinology, 35(2), 245–256. doi:10.1677/jme.1.01806.
Assimakopoulou, M., Sotiropoulou-Bonikou, G., Maraziotis, T., & Varakis, J. (1998). Does sex steroid receptor status have any prognostic or predictive significance in brain astrocytic tumors? Clinical Neuropathology, 17(1), 27–34.
Rogers, G. S., Flowers, J. L., Pollack, S. V., & McCarty, K. S., Jr. (1988). Determination of sex steroid receptor in human basal cell carcinoma. Journal of the American Academy of Dermatology, 18(5 Pt 1), 1039–1043.
Santos, G. A., Cunha, I. W., Rocha, R. M., Mello, C. A., Guimaraes, G. C., Fregnani, J. H., et al. (2010). Evaluation of estrogen receptor alpha, estrogen receptor beta, progesterone receptor, and cKIT expression in desmoids tumors and their role in determining treatment options. Bioscience Trends, 4(1), 25–30.
Ishizuka, M., Hatori, M., Dohi, O., Suzuki, T., Miki, Y., Tazawa, C., et al. (2006). Expression profiles of sex steroid receptors in desmoid tumors. Tohoku Journal of Experimental Medicine, 210(3), 189–198.
Halevy, A., Samuk, I., Halpern, Z., Copel, L., Sandbank, J., & Ziv, Y. (2010). Mifepristone (RU486), a pure antiprogesterone drug, in combination with vinblastine for the treatment of progesterone receptor-positive desmoid tumor. Techniques in Coloproctology, 14(3), 265–267. doi:10.1007/s10151-010-0591-2.
Bocale, D., Rotelli, M. T., Cavallini, A., & Altomare, D. F. (2011). Anti-oestrogen therapy in the treatment of desmoid tumours: a systematic review. Colorectal Disease, 13(12), e388–e395. doi:10.1111/j.1463-1318.2011.02758.x.
Debled, M., Le Loarer, F., Callonnec, F., Soubeyran, I., Cambon-Michot, C., Dujardin, F., et al. (2012). Complete response to exemestane in a patient with a desmoid tumor. Future Oncology, 8(4), 483–486. doi:10.2217/fon.12.24.
Brodsky, S. V., Gimenez, C., Ghosh, C., Melamed, M., & Ramaswamy, G. (2006). Estrogen and progesterone receptors expression in gastrointestinal stromal tumors and intramural gastrointestinal leiomyomas. International Journal of Gastrointestinal Cancer, 37(4), 129–132. doi:10.1007/s12029-007-9003-x.
Patel, S., DiBiase, S., Meisenberg, B., Flannery, T., Patel, A., Dhople, A., et al. (2012). Phase I clinical trial assessing temozolomide and tamoxifen with concomitant radiotherapy for treatment of high-grade glioma. International Journal of Radiation Oncology, Biology, and Physics, 82(2), 739–742. doi:10.1016/j.ijrobp.2010.12.053.
Christeff, N., Winter, C., Gharakhanian, S., Thobie, N., Wirbel, E., Costagliola, D., et al. (1995). Differences in androgens of HIV positive patients with and without Kaposi sarcoma. Journal of Clinical Pathology, 48(6), 513–518.
Ziegler, J. L., Katongole-Mbidde, E., Wabinga, H., & Dollbaum, C. M. (1995). Absence of sex-hormone receptors in Kaposi's sarcoma. Lancet, 345(8954), 925.
Lunardi-Iskandar, Y., Bryant, J. L., Zeman, R. A., Lam, V. H., Samaniego, F., Besnier, J. M., et al. (1995). Tumorigenesis and metastasis of neoplastic Kaposi's sarcoma cell line in immunodeficient mice blocked by a human pregnancy hormone. Nature, 375(6526), 64–68. doi:10.1038/375064a0.
Gill, P. S., Lunardi-Ishkandar, Y., Louie, S., Tulpule, A., Zheng, T., Espina, B. M., et al. (1996). The effects of preparations of human chorionic gonadotropin on AIDS-related Kaposi's sarcoma. New England Journal of Medicine, 335(17), 1261–1269. doi:10.1056/NEJM199610243351702.
Hong, A., & Leigh, B. R. (2002). Antiproliferative properties of toremifene on AIDS-related Kaposi's sarcoma cells. Chemotherapy, 48(5), 238–243.
Leitao, M. M., Soslow, R. A., Nonaka, D., Olshen, A. B., Aghajanian, C., Sabbatini, P., et al. (2004). Tissue microarray immunohistochemical expression of estrogen, progesterone, and androgen receptors in uterine leiomyomata and leiomyosarcoma. Cancer, 101(6), 1455–1462. doi:10.1002/cncr.20521.
Donnez, J., Tatarchuk, T. F., Bouchard, P., Puscasiu, L., Zakharenko, N. F., Ivanova, T., et al. (2012). Ulipristal acetate versus placebo for fibroid treatment before surgery. New England Journal of Medicine, 366(5), 409–420. doi:10.1056/NEJMoa1103182.
Baytur, Y. B., Ozbilgin, K., Cilaker, S., Lacin, S., Kurtul, O., Oruc, S., et al. (2007). A comparative study of the effect of raloxifene and gosereline on uterine leiomyoma volume changes and estrogen receptor, progesterone receptor, bcl-2 and p53 expression immunohistochemically in premenopausal women. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 135(1), 94–103. doi:10.1016/j.ejogrb.2006.07.042.
Check, J. H., Dix, E., Cohen, R., Check, D., & Wilson, C. (2010). Efficacy of the progesterone receptor antagonist mifepristone for palliative therapy of patients with a variety of advanced cancer types. Anticancer Research, 30(2), 623–628.
Vaquero, J., Marcos, M. L., Martinez, R., & Bravo, G. (1983). Estrogen- and progesterone-receptor proteins in intracranial tumors. Surgical Neurology, 19(1), 11–13.
Liu, J., Le, X., & Li, Y. (1998). Pathological study of ER and PR in osteosarcoma. Zhonghua Wai Ke Za Zhi, 36(4), 245–246. 245.
Slootweg, M. C., Swolin, D., Netelenbos, J. C., Isaksson, O. G., & Ohlsson, C. (1997). Estrogen enhances growth hormone receptor expression and growth hormone action in rat osteosarcoma cells and human osteoblast-like cells. The Journal of Endocrinology, 155(1), 159–164.
Dohi, O., Hatori, M., Suzuki, T., Ono, K., Hosaka, M., Akahira, J., et al. (2008). Sex steroid receptors expression and hormone-induced cell proliferation in human osteosarcoma. Cancer Science, 99(3), 518–523. doi:10.1111/j.1349-7006.2007.00673.x.
Rajabalian, S., Hajializadeh, Z., Pooraboli, I., Jangi-Aghdam, H., & Badinloo, M. (2010). Establishment, characterization, and drug sensitivity of a new Ewing sarcoma cell line (SS-ES-1). Journal of Pediatric Hematology/Oncology, 32(8), e331–e337. doi:10.1097/MPH.0b013e3181ee4d16.
DuBois, S. G., Perez-Atayde, A. R., McLean, T. W., & Grier, H. E. (2008). Late recurrence of ewing sarcoma during pregnancy: a report of 2 cases. Journal of Pediatric Hematology/Oncology, 30(9), 716–718. doi:10.1097/MPH.0b013e318175895f.
Hurteau, J. A., Brady, M. F., Darcy, K. M., McGuire, W. P., Edmonds, P., Pearl, M. L., et al. (2010). Randomized phase III trial of tamoxifen versus thalidomide in women with biochemical-recurrent-only epithelial ovarian, fallopian tube or primary peritoneal carcinoma after a complete response to first-line platinum/taxane chemotherapy with an evaluation of serum vascular endothelial growth factor (VEGF): A Gynecologic Oncology Group Study. Gynecologic Oncology, 119(3), 444–450. doi:10.1016/j.ygyno.2010.08.002.
Freeman, S. A., & Modesitt, S. C. (2006). Anastrozole therapy in recurrent ovarian adult granulosa cell tumors: a report of 2 cases. Gynecologic Oncology, 103(2), 755–758. doi:10.1016/j.ygyno.2006.06.022.
Singh, P. B., Matanhelia, S. S., & Martin, F. L. (2008). A potential paradox in prostate adenocarcinoma progression: oestrogen as the initiating driver. European Journal of Cancer, 44(7), 928–936. doi:10.1016/j.ejca.2008.02.051.
Langner, C., Ratschek, M., Rehak, P., Schips, L., & Zigeuner, R. (2004). Steroid hormone receptor expression in renal cell carcinoma: an immunohistochemical analysis of 182 tumors. The Journal of Urology, 171(2 Pt 1), 611–614. doi:10.1097/01.ju.0000108040.14303.c2.
Zeng, Q., Chen, G., Vlantis, A., Tse, G., & van Hasselt, C. (2008). The contributions of oestrogen receptor isoforms to the development of papillary and anaplastic thyroid carcinomas. The Journal of Pathology, 214(4), 425–433. doi:10.1002/path.2297.
Author information
Authors and Affiliations
Corresponding author
Additional information
JM and JW share first authorship.
Rights and permissions
About this article
Cite this article
Munoz, J., Wheler, J. & Kurzrock, R. Expression of estrogen and progesterone receptors across human malignancies: new therapeutic opportunities. Cancer Metastasis Rev 34, 547–561 (2015). https://doi.org/10.1007/s10555-014-9543-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-014-9543-z