Abstract
Long-term safety of second generation drug-eluting stents (DES) has not yet been evaluated. We sought to evaluate the very late phase (> 3 years) vascular response after second generation everolimus-eluting stent (EES) as compared with first generation sirolimus-eluting stent (SES) by using optical coherence tomography (OCT). We examined the vascular response in 39 patients with a total of 55 DESs [31 EESs (mean 54 months after stenting) and 24 first generation SES (mean 66 months after stenting)] by OCT. The frequency of lesions with any malapposed stent struts (19% vs. 46%, p = 0.035) and evagination (6% vs. 42%, p = 0.002) was significantly lower. Segments with malapposed stent struts were significantly shorter (0.4 ± 0.9 mm vs. 1.9 ± 3.5 mm, p = 0.024), maximal malapposition area and malapposition volume were significantly smaller (0.26 ± 0.38 mm2 vs. 0.95 ± 1.54 mm2, p = 0.019, and 0.78 ± 1.35 mm3 vs. 6.22 ± 15.76 mm3, p = 0.016, respectively) in EES. Compared with first generation SES, second generation EES showed more favourable vascular responses at the very late phase.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Drug-eluting stents (DES) have markedly reduced the mid-term (< 12 months) incidence of angiographic restenosis and target lesion revascularization (TLR) after stent implantation in comparison with bare-metal stents (BMS) [1, 2]. However, late-phase clinical events, including late stent thrombosis and delayed restenosis, termed as late DES failure, have been proposed as potential concerns after first generation DES implantation [3, 4]. Several pathological and optical coherence tomography (OCT) studies have demonstrated that delayed arterial healing with poor strut coverage and/or strut malapposition have been identified as major substrate responsible for late and very late stent thrombosis (LST/VLST) after first generation sirolimus-eluting stent (SES) [5,6,7,8]. Furthermore, previous studies have demonstrated that atherogenic changes within the neointima after stent implantation, termed as neoatherosclerosis (NA), is one of the major causes of late DES failure [9,10,11,12]. Several studies also suggested that chronic inflammation, which induced by polymers of DES, develops delayed arterial healing and NA within first generation DES-treated lesion and contributes toward late DES failure [6,7,8,9,10].
Compared with the first-generation DES, the second-generation DES, such as the everolimus-eluting stent (EES), have been developed to improve the safety profile by means of more biocompatible polymers, reduced drug dose with adapted release kinetics, and reduced strut thickness [13, 14]. These enhanced properties may diminish development of delayed arterial healing and NA. Some clinical trials demonstrated the superior efficacy and safety of EES within 3 years after stent implantation compared with first generation DES [13, 15]. However, long-term safety of EES has not yet been evaluated. We therefore evaluated the very late phase (> 3 years) vascular response after second-generation EES as compared with first generation SES by using OCT.
Methods
Study population
The Wakayama Medical University Hospital OCT registry is a single-center registry of consecutive patients who underwent OCT of the coronary arteries from January 2006. Among patients in this OCT registry, patients who met the following inclusion criteria were enrolled into the present study: (1) patients treated with SES (CYPHER™; Cordis, Johnson and Johnson, Miami Lakes, FL, USA), or EES (XIENCE V™/xpedition™/prime™; Abbott Vascular, Santa Clara, CA, USA, or Promus™/Promus Element™/Promus PREMIER™; Boston Scientific), (2) patients who underwent coronary angiography and OCT examination > 3 years after stent implantation, and (3) patients whose DES-treated lesions exhibit no stent failure such as stent thrombosis and binary restenosis (% diameter stenosis > 50%). During this period, OCT examination was performed for the following reasons: (1) planned follow-up coronary angiography and OCT due to other study protocols, regardless of symptoms, (2) evidence of myocardial ischemia, stable angina, or acute coronary syndrome, or (3) planned follow-up coronary angiography for other stent-treated lesions. Exclusion criteria are: (1) stent-in-stent lesion, (2) lesion requiring revascularization, or (3) lesion with insufficient OCT image quality. A total of 31 EESs from 22 patients and 24 first generation SES from 17 patients were identified for the analysis. Data on patient characteristics were collected by reviewing medical records.
The present study was approved by the institutional review board, and written informed consent was obtained from all patients.
Patient clinical data
Clinical data of the patients included age, sex, hypertension [defined as systolic blood pressure (BP) ≥ 140 mmHg, diastolic BP ≥ 90 mmHg, or antihypertensive medication use], diabetes mellitus (defined as hemoglobin A1c ≥ 6.5% or antidiabetic medication use), dyslipidemia (defined as low-density lipoprotein cholesterol ≥ 140 mg/dl or antilipidemic medication use), and current smoker (defined as having smoked at least 100 cigarettes lifetime and smoking currently).
Angiographic analysis
Coronary angiography analysis was carried out in the standard manner. Quantitative coronary angiographic analysis was performed using a validated automated edge detection algorithm (CAAS-5, Pie Medical, Maastricht, Netherlands) by experienced investigators (M.T or Y.S) who were blinded to the clinical information and OCT findings. Reference vessel diameter, minimum lumen diameter and percent diameter stenosis [(1 − minimum lumen diameter/reference lumen diameter) × 100] were measured, both at the index procedure at pre and post intervention and at follow up, and acute lumen gain (minimum lumen diameter at post intervention − minimum lumen diameter at pre intervention).
OCT image acquisition
OCT imaging was performed by using C7-XR (St. Jude Medical, St. Paul, Minnesota, USA), ILUMIEN OPTIS (Abbott Vascular, Santa Clara, California, USA) or LUNAWAVE (Terumo, Tokyo, Japan). OCT imaging catheter was advanced distally to the target portion over a 0.014-inch conventional angioplasty guidewire. Preheated contrast media at 37 °C was flushed through the guiding catheter at a rate of 2–4 ml/s for approximately 3–6 s using an injector pump. When a blood-free image was observed, the OCT imaging core was retracted at a rate of 18–40 mm/s using an automatic pullback device. The OCT images were stored digitally for subsequent analysis.
OCT analysis
OCT analysis was performed using a dedicated off-line review system with semi-automated contour-detection software (Abbott Vascular, Santa Clara, California, USA or Terumo, Tokyo, Japan), based on previous reports [14, 16, 17]. The Z-offset was adjusted again before FD-OCT analysis. All cross-sectional images (frames) were initially screened to assess quality. Cross-sections with inadequate images, including poor quality caused by residual blood or artifact, non-circumferential stent visualization caused by imaging wire bias, side branches, or overlapping segments, were excluded from the analyses [14, 16]. All OCT images were analyzed by experienced investigators (Y.I and K.S) who were blinded to the angiographic findings and clinical information.
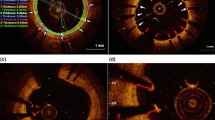
The criteria for the diagnosis of NA were lesions with lipid-laden neointima, neointima with calcification, thin-cap fibroatheroma-like neointima or neointimal rupture [7, 10, 12, 16]. Lipid-laden neointima was defined as a signal-poor region with diffuse borders, and neointima with calcification was defined as a well-delineated, signal-poor region with sharp borders [7, 10, 12, 16]. A thin-cap fibroatheroma-like neointima was defined as neointima with a fibrous cap thickness at the thinnest part < 65 μm and an angle of lipid-laden neointima > 180° [7, 10,11,12, 16]. Neointimal rupture was defined as a discontinuity of the fibrous cap overlying a lipid-laden neointima [7, 10,11,12, 16]. Coronary evagination was defined as the presence of an outward bulge in the luminal vessel contour between apposed struts where the maximum depth of the bulge exceeded the actual strut thickness, as measured semiautomatically from the deepest point in the bulge to the stent area trace [7, 16]. Microvessels were defined as well-delineated low back scattering structures < 200 μm in diameter showing a trajectory within the vessel [10, 11, 17]. Intracoronary thrombi were defined as signal-rich, low-back scattering protrusions or high-backscattering protrusions within the lumen showing signal-free shadowing in OCT images (dimension ≥ 250 μm) [14, 16] (Fig. 1).
Representative images of OCT findings. a Covered stent strut, b uncovered stent strut (white arrows), c malapposed stent strut (white arrows), d lipid laden neointima (asterisks) and thin-cap fibroatheroma-like neointima, e Microvessel (white arrow). f Calcification within neointima (white arrowheads), g intracoronary thrombus (white arrow), h neointimal rupture (white arrow), i evagination (white arrowheads)
Quantitative strut level analysis was performed at 1-mm intervals along the entire stented segment, depending on the pullback speed used in each OCT pullback. Neointimal coverage was assessed on each individual strut. An uncovered strut was defined as a strut with a measured neointimal thickness equal to 0 μm [7, 8, 14, 16]. The maximum length of segment with uncovered struts was estimated as the number of consecutive frames with uncovered struts, and translated into mm-length according to the pullback speed used. A malapposed strut was defined as a strut with a distance between the center of the strut blooming and the adjacent vessel surface was more than 100 μm in EES, 170 μm in SES [7, 8, 14, 16, 18]. This criterion was determined by adding the actual strut thickness and polymer thickness to the OCT resolution limit. The maximum length of segment with malapposed struts was estimated as the number of consecutive frames with malapposed struts, and translated into mm-length according to the pullback speed used. Cross-sectional areas of stent, lumen (defined as intra-stent lumen plus extra-stent lumen), neointima (defined as stent minus intra-stent lumen), and malapposition (defined as extra-stent lumen) were also measured at intervals of 1 mm within the entire stented segment. The volume was calculated with the use of trapezoid rule.
Statistical analysis
Statistical analysis was performed using JMP 12.0 (SAS Institute, Cary, North Carolina, USA). Categorical variables were presented as counts and proportions, and comparison was performed using chi-square statistics or Fisher's exact test (if the expected cell value was < 5). Continuous variables were presented as the mean ± standard deviation and were compared using unpaired Student’s t tests. A p- value < 0.05 was considered statistically significant.
Results
Patient clinical characteristics
A total of 55 DESs [31 EESs and 24 first generation SES] were analyzed. The patient clinical characteristics are summarized in Table 1. There were no significant differences in clinical characteristics including coronary risk factors between the two groups. More than 80% of patients took aspirin and about 50% of patients took aspirin and thienopyridine at follow-up. There were no differences in medications and laboratory data at follow up between the two groups.
Lesion characteristics, stent profiles, procedural characteristics and angiographic findings
Baseline lesion characteristics, stent profiles, procedural characteristics, and angiographic findings both at index procedure and at follow up are summarized in Table 2. Lesion characteristics before intervention were mostly type B2 or C in both groups. There were no significant differences in baseline lesion characteristics, stent profiles and procedural characteristics, and QCA data at the index procedure between EES and first generation SES. In QCA data at follow-up, percent diameter stenosis was smaller in EES compared with first generation DES (14 ± 8% vs. 20 ± 10%, p = 0.037).
OCT findings at follow-up
OCT findings at follow-up are summarized in Table 3. In the stent-treated lesion level analysis, the frequency of lesions with any uncovered struts (39% vs. 46%, p = 0.595) and the maximum length of segments with uncovered struts (1.0 ± 1.6 mm vs. 2.0 ± 3.8 mm, p = 0.208) were not different between EES and first generation SES. The frequency of lesions with any malapposed struts (19% vs. 46%, p = 0.035) was significantly lower, and the maximum length of segments with malapposed struts (0.4 ± 0.9 mm vs. 1.9 ± 3.5 mm, p = 0.024) was shorter in EES compared with first generation DES. Although the frequency of lipid laden neointima, calcification within neointima, thin-cap fibroatheroma-like neointima, the frequency of intraluminal thrombus, neointimal rupture and neoatherosclerosis were not different between the two groups, the frequency of evagination was significantly lower (6% vs. 42%, p = 0.002), in EES compared with first generation SES. In morphometric analysis, although the minimum stent area (5.33 ± 1.80 mm2 vs. 4.74 ± 1.24 mm2, p = 0.171) and minimum lumen area (3.85 ± 1.74 mm2 vs. 3.12 ± 1.41 mm2, p = 0.101) were not different between the two groups, maximal neointimal area was significantly smaller in EES compared with first generation SES (2.16 ± 0.59 mm2 vs. 2.87 ± 1.70 mm2, p = 0.034) and neointimal volume there were a statistical trend toward smaller in EES compared to first SES (26.51 ± 10.11 mm3 vs 35.13 ± 19.41 mm3, p = 0.065). Furthermore, maximal malapposition area and malapposition volume were significantly smaller in EES compared with first generation SES (0.26 ± 0.38 mm2 vs. 0.95 ± 1.54 mm2, p = 0.019, and 0.78 ± 1.35 mm3 vs. 6.22 ± 15.76 mm3, p = 0.016, respectively). Representative OCT images of EES and first generation DES are shown in Figs. 2 and 3.
A representative case of EES. Everolimus-eluting stent (EES) (2.5*28 mm) was implanted in the proximal portion of high lateral branch. Angiography and OCT was performed at 49 months after EES implantation Angiography showed no stenosis in the high lateral branch (arrow), a cross sectional OCT images showed complete covered stent struts with homogeneous neointima (b–d)
A representative case of SES. Sirolimus-eluting stent (SES) (3.5*18 mm) was implanted in the proximal portion of left anterior descending coronary artery (LAD) Angiography and OCT was performed at 52 months after SES implantation Angiography showed no stenosis in the LAD (arrow), a Cross sectional OCT images showed lipid laden neointima (asterisks) (b), malapposed stent strut (white arrow heads), and evagination (white arrows) (c and d)
Discussion
The main findings of the present OCT study were the following: (1) lesions with any malapposed stent struts were less often seen and segments with malapposed stent struts were shorter in EES compared with first generation SES; (2) maximal malapposition area and malapposition volume were smaller in EES; (3) the frequency of evagination was lower in EES; and (4) maximal neointimal area was significantly smaller in EES, a and neointimal volume was tending to be smaller in EES. Our results suggest that EES has more favorable vascular response at very late phase (> 3 years) and probably safer course following stent implantation compared to first generation SES.
VLST following DES
VLST is a quite rare but serious complication that often results in myocardial infarction or cardiac death, which remains an important concern after DES implantation [3, 5]. Previous studies using OCT have reported that the mechanisms promoting VLST after DES implantation vary. In one multicenter OCT study addressing the possible mechanisms of VLST, Taniwaki et al. concluded that the leading associated findings of VLST in descending order were malapposition (34.5%), neoatherosclerosis (27.6%), uncovered struts (12.1%), stent under-expansion (6.9%), and evagination (5.2%); and the longitudinal extension of malapposed and uncovered stent was the most important correlate of thrombus formation in VLST [7]. Human autopsy studies have demonstrated that hypersensitivity to the polymer of first generation DES causes the delayed arterial healing with persistent inflammation characterized by strut malapposition with positive arterial remodeling [6]. Another autopsy study reported that first generation DES implantation in atherosclerotic lesions with lipid-rich plaques might delay arterial healing and impair stent endothelialization [5]. Because sirolimus is highly lipophilic, it is likely that this agent has high affinity for lipid-rich plaques, dwell there for long periods of time, and influence healing by retarding smooth muscle cell proliferation and endothelial regrowth. This delayed arterial healing such as strut malapposition and incomplete strut coverage are identified as the major pathologic substrate responsible for VLST after first generation DES implantation. This is in keeping with a prior report by Guagliumi et al. showing higher rates of both uncovered and malapposed struts in patients with VLST in comparison with control patients [8]. One long-term observational study by Galløe et al. showed that VLST occurred in 13.3% of patients who underwent first generation SES implantation, with a steady annual rate of 1.2% after the first year [19]. Serial OCT observations study by Takano et al. demonstrated that frequency of malapposed struts and area of evagination after first generation SES implantation increased from 2 to 4 years [20]. In the present study, the frequency of lesions with any malapposed struts and evagination was significantly higher, the maximum length of segments with malapposed struts was longer, and maximal malapposition area and malapposition volume were larger in first generation DES, which were consistent with previous study findings. To avoid VLST due to the delayed arterial healing after DES implantation, improvement of DES including polymer is needed.
Second generation DES
Some resolution about the polymer have been proposed. The first is a biocompatible durable polymer to facilitate vascular healing such as fluoropolymer or anti-CD34 antibody–coatedpolymer capturing endothelial progenitor cells, second is a bioresorbable polymer, and third is polymer-free DES that enables drug elution without a polymer. Second-generation EES has been developed to improve the safety and efficacy of coronary stents by modifying the eluted drug, drug carrying system, and stent design [13, 14, 21]. EES is a cobalt chromium alloy stent with thin (81 μm) strut thickness, which is coated with a thin (7.8 μm), non-adhesive, durable, biocompatible fluorinated co-polymer releasing a reduced dose of everolimus compared with the dose used in first-generation DES. Furthermore, the EES uses a fluorinated copolymer, which is composed of vinylidene fluoride and hexafluoropropylene monomers that might confer a certain degree of thromboresistance and haemocompatibility [13, 14]. These features of EES may provide not only better vascular healing but also low thrombogenicity after stent implantation.
Vascular response following EES at very late phase
Previous OCT studies demonstrated that the delayed arterial healing such as uncovered struts, malapposed struts, and/or evagination after first generation DES implantation were observed not only at late phase (< 1 year) but also at very late phase (> 3 years) [16, 21]. Meanwhile, OCT sub-analysis from the RESET trial disclosed that uncovered strut, malapposed strut, and intra-stent thrombi were significantly less frequently observed in second generation EES compared with first generation SES at 9 months after stent implantation [21]. Furthermore, OCT sub-analysis from the NEXT trial demonstrated that second generation EES has a favorable vascular response at 2 years after stent implantation [22]. However, vascular response after EES implantation at very late phase (> 3 years) was unknown. In the present study, the frequency of maximal malapposition area and malapposition volume were significantly smaller in EES compared to first generation DES at very late phase (> 3 years) after stent implantation. This favorable vascular response at very late phase was thought to be owing to biocompatibility of polymers and the fluorinated copolymer.
Study limitations
The present study has several limitations. First, it is a single center, non-randomized study with relatively small number of patients, which may cause selection bias. However, there were no differences in the clinical characteristics between the two groups. Second, OCT data before and immediately after stent implantation were not available for comparison. Therefore, it was unclear whether there are significant differences in the pre-PCI lesion morphologies including calcification degree or not, and the stent malapposition and intra-stent thrombus were persistent or late acquired. Third, this study includes many patients who underwent PCI at previous version of Japanese guideline era, which did not recommend the short duration of dual antiplatelet therapy after DES implantation and routine use of statin after PCI. Patient population with dual antiplatelet therapy use was about 50%, which is relatively higher, and statin use was about 64–71%, which is relatively low. These may affect to vascular response after DES implantation. Fourth, three types of OCT system were used. The differences in the frame rate and pullback speed among three OCT systems could influence maximum length of segment with either uncovered struts or malapposed struts which were defined as the number of consecutive frames. However, in most of patients, ILUMIEN OPTISTM was used [18 of 22 (82%) patients in EES and 14 of 17 (82%) patients in 1st generation SES], and there was no difference in the rate of it between EES and 1st generation SES (p = 0.999). Fifth, there was a statistical trend (p = 0.079) for shorter OCT follow-up duration since stent implantation in EES compared to the first-generation SES. This shorter duration could have an impact on the maximal neointimal area, neointimal volume, maximal malapposition areas and malapposition volume which were smaller in EES. Sixth, VLST rate itself was definitely lower than rate of malapposed strut and evagination, and the relation between frequency of malapposed strut/evagination and VLST is not one to one relationship. Since the prognostic impact of malapposed strut/evagination has not been fully investigated in a large-scale prospective study, our results considering malapposed strut/evagination as one of the surrogate markers of future VLST are hypothesis-generating and should be carefully interpreted. However, some previous OCT registries of VLST after DES implantation demonstrated that malapposed strut and evagination were identified as the putative causes of VLST. Therefore, patients with malapposed strut and/or evagination need a close follow-up due to high risk for VLST. Finally, clinical and laboratory courses of the patients such as hypertension, diabetes mellitus, and dyslipidemia control between stent implantation and date of follow up were not analyzed. This may have contributed to the vascular response including strut condition and neoatherosclerosis development.
Conclusion
Compared with first generation SES, second generation EES showed significantly lower incidence of very late malapposition and evagination, and smaller neointimal and malapposition areas. Second generation EES might have more favourable vascular responses at the very late phase (> 3 years) after stent implantation.
Impact on daily practice
Second generation everolimus-eluting stent (EES) might have more favourable vascular responses at the very late phase (> 3 years) after stent implantation, which suggests the superior efficacy and safety of EES beyond 3 years after stent implantation compared with first generation SES.
References
Morice MC, Serruys PW, Sousa JE, Fajadet J, Ban Hayashi E, Perin M, Colombo A, Schuler G, Barragan P, Guagliumi G, RAVEL Study Group et al (2002) A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med 346:1773–1780
Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, SIRIUS Investigators et al (2003) Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 349:1315–1323
Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Jüni P, Sianos G, Hellige G et al (2007) Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 369:667–678
Kuriyama N, Kobayashi Y, Nakama T, Mine D, Nishihira K, Shimomura M, Nomura K, Ashikaga K, Matsuyama A, Shibata Y (2011) Late restenosis following sirolimus-eluting stent implantation. JACC Cardiovasc Interv 4:123–128
Nakazawa G, Finn AV, Joner M, Ladich E, Kutys R, Mont EK, Gold HK, Burke AP, Kolodgie FD, Virmani R (2008) Delayed arterial healing and increased late stent thrombosis at culprit sites after drug-eluting stent placement for acute myocardial infarction patients: an autopsy study. Circulation 118:1138–1145
Nakazawa G, Finn AV, Vorpahl M, Ladich ER, Kolodgie FD, Virmani R (2011) Coronary responses and differential mechanisms of late stent thrombosis attributed to first-generation sirolimus- and paclitaxel-eluting stents. J Am Coll Cardiol 57:390–398
Taniwaki M, Radu MD, Zaugg S, Amabile N, Garcia-Garcia HM, Yamaji K, Jørgensen E, Kelbæk H, Pilgrim T, Caussin C et al (2016) Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation 133:650–660
Guagliumi G, Sirbu V, Musumeci G, Gerber R, Biondi-Zoccai G, Ikejima H, Ladich E, Lortkipanidze N, Matiashvili A, Valsecchi O et al (2012) Examination of the in vivo mechanisms of late drug-eluting stent thrombosis: findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc Interv 5:12–20
Otsuka F, Byrne RA, Yahagi K, Mori H, Ladich E, Fowler DR, Kutys R, Xhepa E, Kastrati A, Virmani R et al (2015) Neoatherosclerosis: overview of histopathologic findings and implications for intravascular imaging assessment. Eur Heart J36:2147–2159
Park SJ, Kang SJ, Virmani R, Nakano M, Ueda Y (2012) In-stent neoatherosclerosis: a final common pathway of late stent failure. J Am Coll Cardiol 59:2051–2057
Ino Y, Kubo T, Kameyama T, Shimamura K, Terada K, Matsuo Y, Kitabata H, Shiono Y, Kashiwagi M, Kuroi A et al (2018) Clinical utility of combined optical coherence tomography and near-infrared spectroscopy for assessing the mechanism of very late stent thrombosis. JACC Cardiovasc Imaging 11:772–775
Giglioli C, Formentini C, Romano SM, Cecchi E, Baldereschi GJ, Landi D, Chiostri M, Prati F, Marchionni N (2019) Vulnerable struts with CRE8, Biomatrix and Xience stents assessed with OCT and their correlation with clinical variables at 6-month follow-up: the CREBX-OCT study. Int J Cardiovasc Imaging. https://doi.org/10.1007/s10554-019-01719-1
Sabate M, Cequier A, Iñiguez A, Serra A, Hernandez-Antolin R, Mainar V, Valgimigli M, Tespili M, den Heijer P, Bethencourt A et al (2012) Everolimus-eluting stent versus bare-metal stent in ST-segment elevation myocardial infarction (EXAMINATION): 1 year results of a randomized controlled trial. Lancet 380:1482–1490
Ino Y, Kubo T, Tanaka A, Liu Y, Tanimoto T, Kitabata H, Shiono Y, Shimamura K, Orii M, Komukai K et al (2015) Comparison of vascular response between everolimus-eluting stent and bare metal stent implantation in ST-segment elevation myocardial infarction assessed by optical coherence tomography. Eur Heart J Cardiovasc Imaging 16:513–520
Dangas GD, Serruys PW, Kereiakes DJ, Hermiller J, Rizvi A, Newman W, Sudhir K, Smith RS Jr, Cao S, Theodoropoulos K et al (2013) Meta-analysis of everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease: final 3-year results of the SPIRIT clinical trials program (clinical evaluation of the Xience V everolimus-eluting coronary stent system in the treatment of patients with de novo native coronary artery lesions). JACC Cardiovasc Interv 6:914–922
Usui E, Yonetsu T, Kanaji Y, Hoshino M, Yamaguchi M, Hada M, Hamaya R, Kanno Y, Murai T, Lee T et al (2018) Prevalence of neoatherosclerosis in sirolimus-eluting stents in a very late phase after implantation. EuroIntervention. https://doi.org/10.4244/EIJ-D-18-00486
Ino Y, Kubo T, Matsuo Y, Yamaguchi T, Shiono Y, Shimamura K, Katayama Y, Nakamura T, Aoki H, Taruya A et al (2016) Optical coherence tomography predictors for edge restenosis after everolimus-eluting stent implantation. Circ Cardiovasc Interv 9:e004231
Uchimura Y, Itoh T, Oda H, Taguchi Y, Sasaki W, Kaneko K, Sakamoto T, Goto I, Sakuma M, Ishida M et al (2019) Cut-off value of mal-apposition volume and depth for resolution at early phase of acute incomplete stent apposition after CoCr-EES implantation. Int J Cardiovasc Imaging 35:1979–1987
Galløe AM, Kelbæk H, Thuesen L, Hansen HS, Ravkilde J, Hansen PR, Christiansen EH, Abildgaard U, Stephansen G, Lassen JF, SORT OUT II Investigators et al (2017) 10 year clinical outcome after randomization to treatment by sirolimus- or paclitaxel-eluting coronary stents. J Am Coll Cardiol 69:616–624
Takano M, Yamamoto M, Mizuno M, Murakami D, Inami T, Kimata N, Murai K, Kobayashi N, Okamatsu K, Ohba T (2010) Late vascular responses from 2 to 4 years after implantation of sirolimus-eluting stents: serial observations by intracoronary optical coherence tomography. Circ Cardiovasc Interv 3:476–483
Kubo T, Akasaka T, Kozuma K, Kimura K, Kawamura M, Sumiyoshi T, Ino Y, Morino Y, Tanabe K, Kadota K, RESET Investigators et al (2015) Comparison of neointimal coverage between everolimus-eluting stents and sirolimus-eluting stents: an optical coherence tomography substudy of the RESET (randomized evaluation of sirolimus-eluting versus everolimus-eluting stent trial). EuroIntervention 11:564–571
Katayama Y, Kubo T, Akasaka T, Ino Y, Kimura K, Okura H, Shinke T, Igarashi K, Kadota K, Kozuma K, NEXT investigators et al (2017) Two-year vascular responses to drug-eluting stents with biodegradable polymer versus durable polymer: an optical coherence tomography sub-study of the NEXT. J Cardiol 70:530–536
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Kubo has received lecture fees from Abbott Vascular and Terumo. Dr. Akasaka has received lecture fees from Abbott Vascular and Terumo, and research grants from Abbott Vascular and Terumo. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khalifa, A.K.M., Ino, Y., Kubo, T. et al. Very late-phase vascular response after everolimus-eluting stent implantation assessed by optical coherence tomography. Int J Cardiovasc Imaging 36, 1627–1635 (2020). https://doi.org/10.1007/s10554-020-01877-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-020-01877-7