Abstract
First-generation drug eluting stents (DES) reduced the incidence of restenosis and need for repeated target lesion revascularization but, in autoptic studies, frequently resulted in incomplete endothelial coverage, which is an important predictor of late adverse events and increased mortality after stent implantation. More recently, not only uncovered, but also malapposed or protruding struts have been considered vulnerable structures, as they are deemed to perturb blood flow, whereas only struts well embedded into the vessel wall are considered stable. We compared the number of uncovered and of other vulnerable (protruding or malapposed) struts among three different second-generation drug-eluting stents (DES) (Cre8, Biomatrix, Xience), using optical coherence tomography (OCT) 6 months after implantation. Moreover, we analyzed the relationship between the percentage of vulnerable struts and the clinical characteristics of patients. 60 patients with stable angina or non-ST-Elevation acute coronary syndrome and indication to percutaneous angioplasty were randomly assigned to receive one of the three DES. After 6 months, OCT images were obtained. After 6 months, OCT images were obtained (1289 cross sections; 10,728 struts). None of the three DES showed non-coated struts or areas of stent thrombosis. Significant differences in the average number of protruding struts (Cre8: 33.9 ± 12.6; Biomatrix: 26.2 ± 18.1; Xience: 13.2 ± 8.5; p < 0.001) and in the proportion of malapposed struts (Cre8: 0.7%; Biomatrix: 0.9%; Xience: 0.0%; p = 0.040) and of incomplete stent apposition area (Cre8: 10.4%; Biomatrix: 4.7%; Xience: 0.7%; p < 0.001) were observed. No significant difference was found in neointimal hyperplasia area with a not significant tendency toward greater minimal and maximal struts thickness for Biomatrix. In comparison with Cre8 and Biomatrix, Xience showed a significantly lower proportion of vulnerable struts in all clinical sub-groups considered. In the group of 60 patients a significant relation was found between age and number of vulnerable struts (p = 0.014). The three second-generation DES were similarly effective in permitting neo-intimal formation and complete struts coating 6 months after implantation, but Cre8 and Biomatrix showed a greater proportion of protruding and malapposed struts.
Trail Registry: Clinical Trials.gov Identifier: NCT02850497.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
First-generation drug eluting stents (DES) reduced the incidence of restenosis and need for repeated target lesion revascularization [1, 2] but, in autoptic studies [3], frequently resulted in incomplete endothelial coverage, which is an important predictor of late adverse events and increased mortality after stent implantation [4,5,6,7,8]. Such incomplete endothelial coverage was mainly ascribed to the inflammatory effect of the “drug-polymer binomial” used in these stents [9, 10]. This hypothesis stimulated the search for new technologies which led to the development of second-generation DES, with reduced inflammatory effect on the vessel wall and unchanged power in preventing restenosis [9,10,11]. More recently, not only uncovered, but also malapposed or protruding struts have been considered vulnerable structures, as they are deemed to perturb blood flow, whereas only struts well embedded into the vessel wall are considered stable [12,13,14,15].
Second-generation DES are largely heterogeneous, differing in the type and location of anti-proliferative drugs and polymers on their platform surface. Therefore, interventional cardiologists may find it difficult to select the stent providing the best immediate and mid-term clinical results. We therefore conducted a randomized, prospective trial using optical coherence tomography (OCT), aimed at comparing, the number of uncovered struts and that of other vulnerable (malapposed or protruding) struts among three second-generation DES, 6 months after their implantation during percutaneous coronary intervention (PCI). Moreover, we analyzed the relationship between the proportion of vulnerable struts and clinical and demographic characteristics of patients.
Methods
The CREBX-OCT study (Comparison of uncovered, malapposed, or protruding struts with three types of second-generation DES: CRE8, Biomatrix and Xience with OCT at 6-month follow-up of patients submitted to PCI) is a non-profit, single center, 3-arm, prospective randomized study (Clinical Trials.gov Identifier: NCT02850497; Fig. 1).
Endpoints
The primary endpoint of the trial was to compare, at 6-month follow-up, the number of uncovered struts as assessed by OCT among the three groups. The co-primary endpoint also included other characteristics (malapposition or protrusion) recently reported in the literature [12,13,14,15] as indicating struts’ vulnerability and their relationship to clinical variables.
Patients
Patients > 18-year-olds planned to be treated with PCI for stable angina (SA) or non-ST segment elevation acute coronary syndrome (N-STE ACS) by a single operator at the catheterization laboratory of the University of Florence from September, 2015 to July, 2017, were considered eligible for the study, provided they did not meet any of the following exclusion criteria: (1) ST segment elevation myocardial infarction; (2) contraindication to dual antiplatelet therapy; (3) surgical intervention planned < 6 months; (4) indication to anticoagulant therapy; (5) comorbidities with expected survival < 6 months; (6) cardiogenic shock; (7) unwillingness to sign informed consent or to undergo 6-month coronary angiography. Informed consent was obtained from each patient. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution’s human research committee.
Randomization to Cre8, Biomatrix, or Xience was performed in computer-generated sequences after coronary angiography resulting in indication to DES implantation. In case of multivessel coronary artery disease, only one vessel was chosen for the study, at operator’s discretion. Further angiographic exclusion criteria were: (1) visually estimated diameter of target vessel ≤ 2.5 mm; (2) target vessel previously treated with stent implantation. Over the study period, 60 patients without exclusion criteria consented to participate in the trial and were hence enrolled, 20 in each randomization arm (Fig. 1).
Stents implantation and OCT imaging
The polymer-free Cre8TM stent (CID s.p.a., Saluggia, Italy) has a cobalt-chromium platform, coated with “carbofilm”, eluting amphilimusTM, which is composed of sirolimus and a mixture of long-chain fatty acids, contained in grooves on stent’s outer surface (Strut’s thickness 80 µm). The BioMatrix FlexTM stent (Biosensors EUROPE SA, Morges, Switzerland) has a steel platform, eluting biolimus A9TM, which is a semi-synthetic sirolimus derivative with improved pharmacokinetic properties. Its poly-lactic acid polymer is biodegradable: the drug and the polymer are present only on stent’s abluminal surface (Strut’s thickness 112 µm; polymer’s thickness 11 µm). The XienceTM V stent (Abbott Vascular, Santa Clara, California, USA) has a cobalt-chromium platform, eluting everolimus and with a non-biodegradable polymer: the drug and the polymer are present on the intraluminal as well as the abluminal stent’s surface (Strut’s thickness 81 µm; polymer’s thickness 3.9 µm × 2).
In all three arms, DES were deployed at a pressure selected at operator’s discretion according to the characteristics of the lesion, and eventual optimization with non-compliant balloon was based on qualitative assessment of post-deployment angiographic images.
OCT was performed with ILUMIENTM PCI Optimization System-St. Jude Medical (resolution power 20 µm) after completion of index PCI and at 6-month follow-up coronary angiography. Briefly, the imaging catheter (DragonflyTM Duo Imaging Catheter, LightLab Imaging, Inc. Westford, USA) was advanced on a standard 0.014 inch PCI guide wire, with the light source distal to the stent. The guide catheter was well engaged into the coronary ostium so as to minimize the reflow into the aorta of contrast medium injected during image acquisition. The OCT catheter was then pulled-back at 20 mm/s by an automated device, while intracoronary injecting contrast medium (20 ml at 4 ml/s or 3 ml/s for left or right coronary artery, respectively). A first, OCT qualitative analysis was performed in the catheterization laboratory, immediately after the index PCI, in order to determine whether stent implantation had to be further optimized with non-compliant balloons. The off-line analysis of OCT data was performed by ILUMENTM OPTISTM St. Jude Medical workstation by two different investigators (C.F. and E.C.), who were blinded to each other’s assessment and to the type of implanted stent. From baseline OCT, only the presence of calcified plaques in the treated segments and the number of malapposed struts in the three groups of patients were recorded. For the 6-month OCT analysis that represented the primary study end-point, each OCT final measure represented the average of measures acquired by the two independent investigators. To this purpose, stents were analyzed by OCT at each millimeter of length, using one frame every 5 acquired frames, the number of measures for each patient thereby depending on the length of implanted stent. Patients with two or more overlapping stents had a number of analyzed frames that was smaller than the sum of individual stent length, because of the overlapping zone. After machine calibration, vessel luminal area, stent area, number of struts, neointimal thickness at each strut level (defined as the distance between luminal surface of strut and vessel lumen, with exclusion of strut thickness), were measured from each analyzed frame. In particular, neointimal area was calculated as: stent area–lumen area. Struts were classified as covered when at the 6-month OCT analysis they had lost the brightness observed at the OCT performed immediately after the stent implantation or if they appeared partially or completely covered by a layer of tissue. Struts were classified as uncovered when they showed the same brightness observed at the OCT analysis performed immediately after stent implantation or if they appeared in any part uncovered by a layer of tissue. Moreover, struts were classified as apposed or malapposed in relation to their adherence to the vessel wall [16, 17].
The thickness of struts was different in the three type of stents in relation to the different structural characteristics and to the polymer presence: and malapposition was determined by adding the actual strut thickness and polymer thickness to the OCT resolution limit as mentioned above. On the basis of this definition the cutoff to consider a strut malapposed is different in the three types of stents. For malapposed struts, the maximum distance between the external strut surface and vessel wall was calculated. Well-apposed struts were further classified as embedded in the vessel wall or as protruding into vessel lumen. Struts were considered embedded when buried into the vessel wall, with a coverage thickness not reducing the vessel lumen, and protruding when, though covered by tissue, the strut boundary was located above the level of the luminal surface [17].
Pharmacological treatment
Before PCI, all patients received ASA 325 mg orally or 250 mg intravenously and intravenous unfractionated heparin as an initial bolus of 70 UI/kg. Patients treated for SA also received a 600 mg clopidogrel loading dose, those treated for N-STE ACS a 180 mg ticagrelor or a 60 mg prasugrel loading dose. After PCI, all patients were prescribed ASA 100 mg indefinitely and clopidogrel 75 mg daily for 6 months if treated for SA, or ticagrelor 180 mg or prasugrel 10 mg daily for 12 months if treated for N-STE ACS. Other drugs such as statins, beta-blockers, angiotensin-converting enzyme inhibitors, were prescribed in accordance with international guidelines [18, 19].
Response to the Dual antiplatelet therapy was evaluated by light transmittance aggregometry (LTA) using 10 µM/L adenosine-diphosphate (ADP) and 1 mM arachidonic acid (AA) as agonists. Patients with high on-treatment platelet reactivity (HPR) by ADP (≥ 70%) were switched to another P2Y12 antagonist; in patients with high on-treatment platelet reactivity HPR by AA (≥ 20%) acetylsalicylic acid dose was increased if not contraindicated. Platelet function analysis was repeated 48 h after therapy variation [20].
A clinical follow-up at 6 months was performed to evaluate Major Cardiovascular events (MACE) a composite of cardiovascular death, myocardial infarction, stroke, recurring angina or need for target lesion revascularization [21].
Statistical analysis
Data were stored in a dedicated database and analyzed with SPSS 20 for Windows statistical software (SPSS Inc, Chicago, IL, USA). Categorical and continuous variables were reported respectively as percentages, or as mean ± SD, or median and interquartile range (IQR) for non-normal distributions, and compared with Chi square test or with ANOVA or Kruskall-Wallis test. A two-tailed p < 0.05 was considered statistically significant. Association of the continuous variables with the percentage of vulnerable struts was analyzed with a linear or not linear regression on the basis of the best fitting.
Sample size calculation
The sample size needed to achieve a good statistical power was difficult to be obtained a priori because it required the knowledge of how many struts we would have analyzed in our series, that in turn depended on the number and length of stents implanted in our study population. We could only hypothesize, on the basis of previous studies, that a percentage as high as 85% of struts would result embedded at 6 month follow-up, with a difference of ± 10% (with a SD 10) between a group with respect to each other. On this basis, it was estimated that 16 patients per group would be enough to reach a power of 80% and a two sided level of significance of 0.05. We choose to enrol four more patients per group (+ 25%) in order to prevent drop-outs at follow-up.
Results
The main baseline demographic, clinical, and angiographic characteristics (Table 1) were all similar across the three randomization groups. The main laboratory tests at baseline and at 6-month follow-up, including the prevalence of responders to ASA and to P2Y12, were also similar (data not reported).
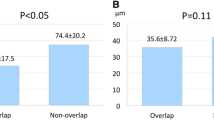
OCT imaging after the index PCI showed 12, 5 and 12 calcified plaques in the Cre8, the Biomatrix and the Xience group, respectively; moreover, two, one and two patients in the Cre8, Biomatrix and Xience group had some malapposed struts, with an overall number of 8, 6 and 7 malapposed struts, respectively : P = NS for all comparisons (Table 2). The 6-month follow-up OCT data deriving from the analysis of 1289 cross sections and 10,728 struts, with similar numbers of frames and struts analyzed across the three groups, are reported in Table 2. The proportion of cross sections with incomplete stent apposition (ISA) was lower in the Xience-group, compared to the other two groups. No uncovered struts, or struts with evidence of thrombus formation, were observed in any of the three groups, whereas the proportion of malapposed struts was smaller, and the maximum distance of malapposition shorter, in the Xience than in the Cre8 or the Biomatrix group. Overall, some malapposed struts were present in 13/60 patients (21.6%), but in five of them (38.4%) some struts had resulted already malapposed in OCT at baseline. The proportion of protruding struts was greater in the Cre8 than in the other two groups. The Xience group also had a higher proportion of well-embedded struts, compared to the other two groups. No significant difference was observed, across the three groups, in neointimal area or in struts’ coverage thickness. Correlations of percentage of vulnerable struts with continuous variables in all patients and in the three groups of treatment are reported in Table 3 A direct linear relation was found between the age of all sixty patients and the percentage of vulnerable struts (Fig. 2)
Comparisons between and within groups of percentage of vulnerable struts regarding clinical characteristics are reported in Table 4. Patients treated with Xience showed a significantly lower proportion of vulnerable struts in all clinical subgroups analyzed.
Over the 6-month follow-up, no patient died or had a myocardial infarction or stroke. However, MACEs occurred in 9/60 patients as recurring angina (n = 6: 1 in the Cre8, 3 in the Biomatrix and 2 in the Xience group, respectively) or as a need for repeated PCI in the target vessel (n = 3; 1 in each group) (P = NS for all comparisons). Overall, 11/60 patients had a second PCI of a coronary branch different from the target one (3 in Cre8, 3 in the Biomatrix, and 5 in the Xience group, respectively).
Discussion
The main findings of this prospective, randomized trial, aimed at comparing OCT images across three second-generation DES (Cre8, Biomatrix and Xience) 6 months after their implantation, are the following: (1) no uncovered struts were observed with any DES, with no difference in neointimal thickness; (2) the proportion of vulnerable (malapposed and/or protruding) struts was lower in patients randomized to receive the Xience than the other two DES, with the Cre8 showing the highest proportion; moreover, the Xience was also associated with a higher proportion of stable, well-embedded struts; (3) age and the number of vulnerable struts were in direct linear relationship; (4) these differences did not translate into different risk of MACEs over the 6-month follow-up (Fig. 3).
In our study population, no uncovered struts were found at 6-month follow-up OCT, reinforcing the concept that all three different second-generation DES ensured a complete neointimal coverage of struts over a relatively short follow-up, irrespective of whether they were implanted in patients with SA or N-STE ACS. Although late-stent thrombosis is a multi-factorial phenomenon in which the uncovered struts, ascribed to delayed and incomplete endothelization, represent only one element [3, 6], this result indirectly reinforces the claim of greater anti-thrombogenic effectiveness of second-generation DES. This finding also confirms previously published data about earlier re-endothelization of second-generation DES that, at 2-week and at 3-month OCT analysis, showed a proportion of uncovered struts similar to that observed at 1-month with bare metal stents [15, 22,23,24,25,26].
Although the true ratio of uncovered struts may be underestimated with OCT because of its partial ability to distinguish a neointimal layer from fibrin, several studies analyzing the healing of second generation DES with OCT, found a ratio of covered struts between 95 and 100% similar to our results [15, 24,25,26,27] (Fig. 4).
Example of optical coherence tomography cross section obtained from our study population. Arrows indicate each type of vulnerable struts. a Vessel cross section; b Optimal stent apposition and embedded struts; c Drug Elutin Stent malappostion with Incomplete Stent Apposition area; d Vessel cross section with protruding struts; e In stent restenosis
Moreover, according to imaging technologies and autoptic studies, the concept of “vulnerable” struts has been extended to include not only the uncovered, but also the malapposed or protruding struts [12,13,14,15, 28, 29]. Although recent analyses of several stent thrombosis registries found that extensively malapposed struts were frequently identified in patients who experienced stent thrombosis, the clinical effects of stent malapposition remain controversial [30,31,32,33,34].
Struts’ malapposition may be acquired or may result from incomplete stent expansion during the index procedure, especially in the presence of calcified plaques. Late, or acquired malapposition may be due either to the presence of thrombus or dissection between stent and plaque at stent implantation, which eventually disappeared at follow-up, or to positive vessel remodeling [35]. In our study population, the number of malapposed struts observed after the index procedure was similar across the three groups. Therefore, the differences observed at OCT follow-up cannot be attributed to potential differences in stent deployment during index PCI. In particular, the lower percentage of malapposed struts found in our study in the Xience group is in agreement with that found by Katayama et al. [36], that comparing healing between everolimus and biolimus stent. These authors observed that a late-acquired malapposition, due to a positive vessel-remodeling, was less frequent in the permanent fluoropolymer-coated everolimus stents than in the biodegradable polymer-coated biolimus stents suggesting that the Xience polymer may have a higher biocompatibility.
In our trial, protrusion was defined as struts projecting into the lumen in the absence of an obvious separation from vessel wall. Although the concept of struts protrusion with potentially altered flow pattern is not widely recognized, it is likely that a foreign body protruding into the coronary lumen may disturb the flow at the blood-intima interface, with complex and turbulent flow patterns potentially associated with delayed endothelization and/or thrombogenic effects [37,38,39,40,41]. For this reason, Moore et al. [12] suggested that stent protrusion ratio is an important parameter to be taken into account when evaluating stent’s safety. Moreover, protruding struts, even when properly covered by neointima, may impair coronary flow by reducing vessel lumen because of the greater thickness of tissue needed to cover them. Protruding struts may represent a stage of healed, formerly malapposed struts related to incomplete stent apposition at the time of DES implantation or may be the result of an outward remodeling of vessel wall giving the appearance of coronary evagination between the struts. Hypothetically, coronary evaginations may represent an early stage of positive remodeling [13, 36].
In contrast, embedded struts are considered “stable” because they are buried into the vessel wall and well covered by a neointima layer without significant lumen loss. In our study, the Xience group showed the lowest proportion of malapposed or protruding, and the highest proportion of well-embedded struts in all clinical sub-groups considered, whereas the Cre8 group showed the highest proportion of protruding or malapposed struts and the highest proportion of cross sections with incomplete stent apposition. Despite these differences, the neointimal thickness measured by OCT was similar across the three groups, supporting a similar efficacy of all three DES types in preventing re-stenosis. However, the Biomatrix stent was associated with a minimal and maximal coverage thickness that were largely greater, although not significantly (so, because of large SD and variability coefficients, togliere?) than those observed in the other two groups. In particular, the mean value of the minimal thickness observed in the Biomatrix group was almost twice that observed in the Xience and Cre8 groups, suggesting a lower efficacy of this stent in limiting neointimal hyperplasia.
It could be hypothesized that the different percentage of vulnerable struts observed at 6-month OCT analysis in the three groups of patients was related to differences in the flexibility, elasticity and geometric structure of the metallic platform which may have affected the stent apposition to the vessel wall in addition to the different type and location of anti-proliferative drugs and polymers [35,36,37,38].
Moreover, a direct relation was found between the age of the overall group and the percentage of vulnerable struts probably reflecting the increased stiffness due to a more extensive atherosclerotic disease of the vessel wall, with the increase in age.
Clinical implications
The results of the present study may also have implications in the current interventional practice; they suggest that all three types of stent used can be safely implanted but Xience showed a lower percentage of protruding and malapposed struts at follow-up. These results can be translated in the daily use of Xience as the best option of drug eluting stent with respect to the other two ones analyzed. Moreover, our study suggests that struts vulnerability may be mainly determined by structural characteristics of the stent independently of implantation technique.
Limitations
Some limitations of our study have to be acknowledged. First, the study enrolled only 60 patients and was therefore not powered for detecting differences in the clinical outcomes and MACE.
Secondly, OCT analysis performed at the index procedure was not analyzed, with the exception of data, available for 51/60 patients (85%) and relating to malapposed struts only.
Future directions
Studies performed in a wider population of patients will provide further informations on the relation among stents structure, their healing and adverse events at follow-up.
Conclusion
The results of this study demonstrate that all the three DES used are safe since they allow a complete strut coverage at 6-month OCT follow-up. However, the use of Cre8 and Biomatrix with respect to Xience, have been associated with a higher proportion of malapposed and protruding struts at follow-up in all clinical subgroups considered showing a differential healing among the three stents analyzed.
References
Sarno G, Lagerqvist B, Frobert O, Nilsson J, Olivecrona G, Omerovic E, Saleh N, Venetzanos D, James S (2012) Lower risk of stent thrombosis and restenosis with unrestricted use of “new-generation” drug-eluting stents: a report from the nationwide Swedish Coronary Angiography and Angioplasty Registry (SCAAR). Eur Heart J 33:606–613
Van Dyck CJ, Hoymans VY, Haine S, Vrints CJ (2013) New-generation drug-eluting stents: focus on Xience V® Everolimus-eluting stent and Resolute® Zotarolimus-Eluting Stent. J Interv Cardiol 3:278–286
Nakano M, Virmani R (2015) Histopathology of vascular response to drug-eluting stents: an insight from human autopsy into daily practice. Cardiovasc Interv Ther 30:1–11
Ong AT, McFadden EP, Regar E, De Jaegere PP, Van Domburg RT, Serruys PW (2005) Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J Am Coll Cardiol 45:2088–2092
Pfisterer M, Brunner-LaRocca HP, Buser PT, Rickenbacher P, Hunziker P, Mueller C, Jeger R, Bader F, Osswald S, Kaiser C, BASKET-LATE Investigators (2006) Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am Coll Cardiol 48:2584–2591
Farb A, Burke AP, Kolodgie FD, Virmani R (2003) Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation 108:1701–1706
Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R (2006) Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 48:193–202
Cutlip DE, Baim DS, Ho KK, Popma JJ, Lansky AJ, Cohen DJ, Carrozza JP Jr, Chauhan MS, Rodriguez O, Kuntz RE (2001) Stent thrombosis in the modern era: a pooled analysis of multicenter coronary stent clinical trials. Circulation 103:1967–1971
Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, Airoldi F, Chieffo A, Montorfano M, Carlino M, Michev I, Corvaja N, Briguori C, Gerckens U, Grube E, Colombo A (2005) Incidence, predictors and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA 293:2126–2130
Lagerqvist B, James SK, Stenestrand U, Lindbäck J, Nilsson T, Wallentin L, SCAAR Study Group (2007) Long-term outcomes with drug-eluting stents versus bare metal stents in Sweden. N Engl J Med 356:1009–1019
Kim BK, Shin DH, Kim JS, Ko YG, Choi D, Jang Y, Hong MK (2012) Optical coherence tomography-based evaluation of malapposed strut coverage after drug-eluting stent implantation. Int J Cardiovasc Imaging 28:1887–1894
Moore P, Barlis P, Spiro J, Ghimire G, Roughton M, Di Mario C, Wallis W, Ilsley C, Mitchell A, Mason M, Kharbanda R, Vincent P, Sherwin S, Dalby M (2009) A randomized optical coherence tomography study of coronary stent strut coverage and luminal protrusion with rapamycin-eluting stents. JACC Cardiovasc Interv 2:437–444
Radu M, Jørgensen E, Kelbæk H, Helqvist S, Skovgaard L, Saunamäki K (2011) Optical coherence tomography at follow-up after percutaneous coronary intervention: relationship between procedural dissections, stent strut malapposition and stent healing. EuroIntervention 7:353–361
Radu M, Jørgensen E, Kelbaek H, Helqvist S, Skovgaard L, Saunamäki K (2010) Strut apposition after coronary stent implantation visualised with optical coherence tomography. EuroIntervention 6:86–93
Räber L, Zanchin T, Baumgartner S, Taniwaki M, Kalesan B, Moschovitis A, Garcia-Garcia HM, Justiz J, Pilgrim T, Wenaweser P, Meier B, Jüni P, Windecker S (2014) Differential healing response attributed to culprit lesions of patients with acute coronary syndromes and stable coronary after implantation of drug-eluting stents: an optical coherence tomography study. Int J Cardiol 173:259–267
Prati F, Regar E, Mintz G, Arbustini E, Di Mario C, Jang IK, Akasaka T, Costa M, Guagliumi G, Grube E, Ozaki Y, Pinto F, Serruys PW, Expert’s OCT Review Document (2010) Expert review document on methodology, terminology and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 31:401–415
Prati F, Guagliumi G, Mintz GS, Costa M, Regar E, Akasaka T, Barlis P, Tearney GJ, Jang IK, Arbustini E, Bezerra HG, Ozaki Y, Bruining N, Dudek D, Radu M, Erglis A, Motreff P, Alfonso F, Toutouzas K, Gonzalo N, Tamburino C, Adriaenssens T, Pinto F, Serruys PW, Di Mario C, Expert’s OCT Review Document (2012) Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J. 33:2513–2520
Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol Ç, Fitzsimons D, Halle M, Hamm C, Hildick-Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J, Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (2016) 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 37:267–315
Authors/Task Force members, Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Jüni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W (2014) Witkowski A (2014) ESC/EACTS Guidelines on myocardial revascularization. The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 35:2541–2619
Cecchi E, Marcucci R, Chiostri M, MecarocciV Spini V, Innocenti L, Calabretta R, Cordisco A, Romano SM, Abbate R, Gensini GF, Giglioli C (2015) Dual antiplatelet therapy tailored on platelet function test after coronary stent implantation: a real-world experience. Int and Emerg Med 10:805–814
Windecker S, Remondino A, Eberli FR, Jüni P, Räber L, Wenaweser P, Togni M, Billinger M, Tüller D, Seiler C, Roffi M, Corti R, Sütsch G, Maier W, Lüscher T, Hess OM, Egger M, Meier B (2005) Sirolimus-eluting and paclitaxel-eluting stents for coronary revascularization. N Engl J Med 353:653–662
Oka N, Fujii K, Kadohira T, Kitahara H, Fujimoto Y, Takahara M, Himi T, Ishikawa K, Sano K, Kobayashi Y (2019) Assessment of strut coverage of everolimus-eluting platinum chromium stent 2 weeks after implantation by optical coherence tomography. Heart Vessels 34(8):1258–1265
Prati F, Romagnoli E, Valgimigli M, Burzotta F, De Benedictis M, Ramondo A, Mehran R, Stella PR (2014) Randomized comparison between 3-month Cre8 DES vs 1-month Vision/Multilink8 BMS neointimal coverage assessed by OCT evaluation: the demostrate study. Int J Cardiol. 176:904–909
Pruski M Jr, Blachut A, Kachel M, Michalak M, Janas A, Fernandez C, Buszman P, Milewski K, Buszman P (2017) Determinants of stent healing in optical coherence tomography Validation study with histpathlogy in the porcine coronary restenosis model. JACC 70(Suppl. B):202
Qian J, Zhang YJ, Xu B, Yang YJ, Yan HB, Sun ZW, Zhao YL, Tang YD, Gao Z, Chen J, Cui JG, Mintz GS, Gao RL (2014) Optical coherence tomography assessment of a PLGA-polymer with electro-grafting base layer versus a PLA-polymer sirolimus-eluting stent at three-month follow-up: the BuMA-OCT randomised trial. EuroIntervention. 10(7):806–814
Suwannasom P, Onuma Y, Benit E, Gach O, von Birgelen C, Hofma SH, Sotomi Y, Bo X, Zhang YJ, Gao R, García-García HM, Wykrzykowska JJ, de Winter RJ, Serruys PW (2016) Evaluation of vascular healing of polymer-free sirolimus-eluting stents in native coronary artery stenosis: a serial follow-up at three and six months with optical coherence tomography imaging. EuroIntervention. 12(5):e574–e583
Kim BK, Ha J, Mintz GS, Kim JS, Shin DH, Ko YG, Choi D, Jang Y, Hong MK (2014) Randomised comparison of strut coverage between Nobori biolimus-eluting and sirolimus-eluting stents: an optical coherence tomography analysis. EuroIntervention. 9(12):1389–1397
Barlis P, Dimopoulos K, Tanigawa J, Dzielicka E, Ferrante G, Del Furia F, Di Mario C (2010) Quantitative analysis of intracoronary optical coherence tomography measurements of stent strut apposition and tissue coverage. Int J Cardiol 141:151–156
Tanigawa J, Barlis P, Di Mario C (2007) Intravascular optical coherence tomography: optimization of image acquisition and quantitative assessment of stent strut apposition. EuroIntervention. 3:128–136
Hong SJ, Park KH, Ahn CM, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Hong MK (2019) Severe acute stent malapposition follow-up: 3-month and 12-month serial quantitative analyses by optical coherence tomography. Int J Cardiol. https://doi.org/10.1016/j.ijcard.2019.06.075
Lee S-Y, Im E, Hong SJ, Ahn C-M, Kim J-S, Kim B-K, Ko Y-G, Choi D, Jang Y, Hong M-K (2019) Severe acute stent malapposition after drug-eluting stent implantation: effects on long-term clinical outcomes. J Am Heart Assoc 8:e012800
Souteyrand G, Amabile N, Mangin L, Chabin X, Meneveau N, Cayla G, Vanzetto G, Barnay P, Trouillet C, Rioufol G, Range G, Teiger E, Delaunay R, Dubreuil O, Lhermusier T, Mulliez A, Levesque S, Belle L, Caussin C, Motreff P (2016) Mechanisms of stent thrombosis analysed by optical coherence tomography: insights from the national PESTO French registry. Eur Heart J 37:1208–1216
Taniwaki M, Radu MD, Zaugg S, Amabile N, Garcia-Garcia HM, Yamaji K, Jorgensen E, Kelbaek H, Pilgrim T, Caussin C, Zanchin T, Veugeois A, Abildgaard U, Juni P, Cook S, Koskinas KC, Windecker S, Raber L (2016) Mechanisms of very late drug-eluting stent thrombosis assessed by optical coherence tomography. Circulation 133:650–660
Adriaenssens T, Joner M, Godschalk TC, Malik N, Alfonso F, Xhepa E, De Cock D, Komukai K, Tada T, Cuesta J, Sirbu V, Feldman LJ, Neumann FJ, Goodall AH, Heestermans T, Buysschaert I, Hlinomaz O, Belmans A, Desmet W, Ten Berg JM, Gershlick AH, Massberg S, Kastrati A, Guagliumi G, Byrne RA (2017) Optical coherence tomography findings in patients with coronary stent thrombosis: a report of the PRESTIGE Consortium. Circulation 136:1007–1021
Yamamura S, Fujisue K, Tsujita K, Sakamoto K, Miyazaki Y, Kaikita K, Hokimoto S, Ogawa H (2016) Optical coherence tomography visualization of stent deformation with subsequent thrombus adhesion at very early phase after everolimus-eluting stent implantation: a case report. BMC Cardiovasc Disord. 16:116
Katayama Y, Kubo T, Akasaka T, Ino Y, Kimura K, Okura H, Shinke T, Igarashi K, Kadota K, Kozuma K, Tanabe K, Nakagawa Y, Muramatsu T, Morino Y, Kimura T, NEXT investigators (2017) Two-year vascular responses to drug-eluting stents with biodegradable polymer versus durable polymer: An optical coherence tomography sub-study of the NEXT. J Cardiol 70(6):530–536
Räber L, Baumgartner S, Garcia-Garcia HM, Kalesan B, Justiz J, Pilgrim T, Moschovitis A, Khattab AA, Buellesfeld L, Wenaweser P, Meier B, Serruys PW, Jüni P, Windecker S (2012) Long-term vascular healing in response to sirolimus- and paclitaxel-eluting stents: an optical coherence tomography study. JACC Cardiovasc Interv. 5:946–957
Radu MD, Pfenniger A, Räber L, de Marchi SF, Obrist D, Kelbæk H, Windecker S, Serruys PW, Vogel R (2014) Flow disturbances in stent-related coronary evaginations: a computational fluid-dynamic simulation study. EuroIntervention. 10:113–123
Foin N, Gutiérrez-Chico JL, Nakatani S, Torii R, Bourantas CV, Sen S, Nijjer S, Petraco R, Kousera C, Ghione M, Onuma Y, Garcia-Garcia HM, Francis DP, Wong P, Di Mario C, Davies JE, Serruys PW (2014) Incomplete stent apposition causes high shear flow disturbances and delay in neointimal coverage as a function of strut to wall detachment distance: implications for the management of incomplete stent apposition. Circ Cardiovasc Interv. 7:180–189
Cook S, Wenaweser P, Togni M, Billinger M, Morger C, Seiler C, Vogel R, Hess O, Meier B, Windecker S (2007) Incomplete stent apposition and very late stent thrombosis after drug-eluting stent implantation. Circulation 115:2426–2434
Cook S, Eshtehardi P, Kalesan B, Räber L, Wenaweser P, Togni M, Moschovitis A, Vogel R, Seiler C, Eberli FR, Lüscher T, Meier B, Jüni P, Windecker S (2012) Impact of incomplete stent apposition on long-term clinical outcome after drug-eluting stent implantation. Eur Heart J 33:1334–1343
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Giglioli, C., Formentini, C., Romano, S.M. et al. Vulnerable struts with CRE8, Biomatrix and Xience stents assessed with OCT and their correlation with clinical variables at 6-month follow-up: the CREBX-OCT study. Int J Cardiovasc Imaging 36, 217–230 (2020). https://doi.org/10.1007/s10554-019-01719-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-019-01719-1