Abstract
Few studies have directly compared vascular responses to second-generation drug-eluting stents (DESs). We performed optical coherence tomography examinations in 56 consecutive patients with implanted single stent [19 cobalt-chromium everolimus-eluting stents (CoCr-EES), 22 platinum-chromium EES (PtCr-EES), and 15 resolute zotarolimus-eluting stents (R-ZES)] for de novo lesions, and who did not have restenosis at their 9-month follow-up. Neointimal thickness (NIT), stent apposition, and neointimal coverage were assessed in every strut. A neointimal unevenness score [(NUS), maximum NIT/average NIT in the same cross-section] was determined for every 1-mm cross-section (CS). A total of 8350 struts and 1159 CSs were analyzed. The CoCr- and PtCr-EES had significantly fewer malapposed struts compared to the R-ZES (CoCr-EES: 0.19 % vs. PtCr-EES: 0.19 % vs. R-ZES: 0.61 %, p = 0.007). Furthermore, the PtCr-EES had a lower frequency of uncovered struts compared to the others (CoCr-EES: 2.0 % vs. PtCr-EES: 1.4 % vs. R-ZES: 2.3 %, p = 0.047). The NUS correlated with the frequency of uncovered struts (p < 0.001, r = 0.54). The EESs demonstrated more homogenous neointimal growth, as shown in the NUS, compared to the R-ZES [CoCr-EES: 1.66 (1.38–1.97) vs. PtCr-EES: 1.67 (1.41–2.00) vs. R-ZES: 1.94 (1.56–2.28), p < 0.001]. Our results demonstrate that unevenness neointimal growth may relate with strut coverage after second-generation DES implantation. The PtCr-EES had a high frequency of strut coverage with a homogeneous neointima, suggesting fewer risks for stent thrombosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
First-generation drug-eluting stents (DESs) have reduced restenosis rates and improved clinical outcomes compared to bare metal stents [1]. However, the occurrence of late or very late stent thrombosis due to incomplete endothelialization and inflammatory reactions remains a clinical concern [2]. Previous pathologic studies have reported that delayed strut endothelialization and late stent malapposition are important factors for stent thrombosis [2].
Second-generation DESs have thin struts with a biocompatible polymer, and have shown more favorable clinical outcomes and a lower stent thrombosis rate compared to first-generation DESs in clinical studies [3]. A past human autopsy analysis also demonstrated that second-generation DESs showed greater strut coverage with less inflammation and fewer stent thromboses compared to first-generation DESs [4].
In recent clinical trials comparing the different types of second-generation DESs, these stents had similar efficacious and safety outcomes in terms of target vessel failure and long-term stent thrombotic events [5, 6]. Although a head-to-head comparison of vascular responses to second-generation DESs was done using an animal model, human in vivo studies about them are limited [7].
Optical coherence tomography (OCT) is a high-resolution imaging modality that can provide a very detailed assessment of stent apposition and tissue strut coverage, and valuable information about the risk of stent thrombosis [8]. Several OCT studies have shown that an absence of neointimal strut coverage and the presence of malapposed struts relate to late stent thrombosis, and second-generation DESs had higher rates of strut coverage compared to first-generation DESs [8, 9]. However, few studies have directly compared OCT findings of neointimal growth and strut apposition among them [10–12].
We aimed to directly compare the in vivo vascular responses and the status of the struts after second-generation DESs implantation, and to assess the differences in these findings by types of stents using OCT.
Methods
Study population
Patients at Yokosuka Kyosai Hospital underwent elective percutaneous coronary intervention (PCI) with single stent implantation of a cobalt-chromium-based everolimus-eluting stent (CoCr-EES), platinum-chromium-based EES (PtCr-EES), or cobalt-chromium-based resolute zotarolimus-eluting stent (R-ZES) for de novo lesions. Of the patients who had received 9-month follow-up coronary angiography and an OCT examination between May 2012 and March 2015, 70 consecutive patients without in-stent restenosis (ISR, >50 % diameter stenosis), overlapping stents, left main trunk disease and major bifurcation lesions were enrolled according to the study protocol. We excluded 14 cases that had inadequate OCT image quality. Thus, ultimately, 56 lesions in 56 patients [51 with stable angina pectoris (SAP) and 5 with unstable angina pectoris (UAP)] were investigated retrospectively.
All patients were taking 100 mg/day aspirin. In addition, 200 mg/day ticlopidine or 75 mg/day clopidogrel were given for at least 6 months after stenting. The study protocol was approved by the ethics committee of our hospital. All of the enrolled study patients gave written informed consent to undergo the follow-up OCT and for enrollment into the study.
OCT imaging and analysis
A frequency-domain OCT (C7/C8 OCT Intravascular Imaging System, St. Jude Medical, St. Paul, MN, USA) was used. An OCT imaging catheter (Dragonfly OCT imaging catheter, St. Jude Medical, St. Paul, MN, USA) was advanced to the distal end of the stented lesion. The entire length of the stent was automatically imaged at 20 m/s (C7) or 36 mm/s (C8) to the proximal end of the stented lesion with continuous-flushing methods by the injection of contrast media or low molecular weight dextran from the guiding catheter. OCT images were analyzed by two investigators using an offline review workstation (LightLab Imaging Inc.).
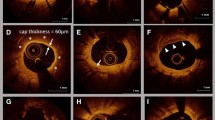
Cross-sectional OCT images were analyzed at 1-mm intervals between both edges of the stent. The stent and lumen area were measured manually and the neointimal hyperplasia (NIH) areas were derived from these two areas. In each stent strut, the neointimal thickness was measured from the endoluminal surface of the neointima to the surface of the strut (Fig. 1a) [12]. Struts were defined as uncovered by their exposure to the lumen and absence of a definite neointima (Fig. 1b) [11]. Strut apposition was assessed in every strut by measuring the distance between the endoluminal leading edge of the strut and the luminal surface of the vessel (Fig. 1c, d) [11]. Strut malapposition at each stent was defined as the distance from the vessel wall by >89 μm for the CoCr-EES and PtCr-EES, and >97 μm for the R-ZES [11]. To assess for stent asymmetric expansion, a stent eccentricity index (SEI) was determined by dividing the minimum stent diameter by the maximum stent diameter in each cross-section [13]. For the assessment of unevenness of neointimal growth, a neointimal unevenness score (NUS) was calculated for each cross-section as the maximum neointimal thickness in one cross-section divided by the average neointimal thickness of the same cross-section [13].
Representative optical coherence tomography images a Strut level (white arrows in a) and cross-sectional OCT measurements; b covered struts (white arrows in b) and uncovered struts (red arrows in b); c malapposed struts (red arrows in c); d assessment of strut apposition by measuring the distance between the strut surface and the lumen wall (red arrows in d). OCT optical coherence tomography
Statistical analysis
Statistical analysis was conducted using JMP 12 (SAS Institute, Cary, North Carolina). Categorical data were expressed as absolute frequencies and percentages, and were compared using the Chi square test or Fisher’s test, as appropriate. Continuous variables were presented as mean ± standard deviation (SD) for normally distributed variables and as median [interquartile range (IQR)] for non-normally distributed variables, and were compared using a one-way ANOVA or Kruskal–Wallis test, as appropriate. If a significant difference was found, a post hoc test for multiple comparisons among the three DESs was performed using a Tukey’s test or Steel–Dwass test for continuous variables, and a Bonferroni correction for categorical variables. Simple correlations between the average SEI, average NUS, and the frequency of uncovered struts were examined with a Spearman correlation [13]. When simple correlations between the frequencies of uncovered struts and malapposed struts were calculated, patients were divided into 2 groups according to the median value of SEI. P values <0.05 were considered statistically significant except for post-hoc analysis with a Bonferroni correction, where <0.017 (0.05 divided by 3) was required for significance.
Results
The median period to the follow-up OCT study was 274 days (range 256–302 days) after stenting. Aspirin was continued until the follow-up OCT for all cases and the rate of dual antiplatelet therapy was similar among the 3 groups. The number of cases with patients taking β-blockers in the R-ZES group was significantly higher than that in the others. Otherwise, there were no significant differences among the 3 groups in the patient and lesion characteristics (Tables 1, 2).
OCT findings
In the 1159 cross-sections, 8350 struts were identified. The neoitnimal thickness by struts and the percentage of NIH area in the cross-sections were significantly smaller in the R-ZES group (Table 3). Compared to the CoCr-EES, the PtCr-EES had thinner neointimal thickness and a smaller percentage of NIH area. The frequency of uncovered struts in the PtCr-EES was the lowest of all (Fig. 2). While not significant, there was a lower frequency of uncovered struts in the PtCr-EES compared to the CoCr-EES. The CoCr- and PtCr-EES had a lower frequency of malapposed struts and lower NUS compared to the R-ZES. These EESs showed similar levels of NUS and rates of malapposed struts (Fig. 2; Table 3). Stent expansion of the CoCr-EES was more symmetrical compared to the PtCr-EES (Table 3).
The relationships among the SEI, NUS, and frequency of uncovered struts are shown in Fig. 3. The NUS had a significant positive correlation with the frequency of uncovered struts in the whole population (Fig. 3a). Moreover, a significant relationship was found between the SEI and NUS (Fig. 3e). There was no significant relationship between the SEI and the frequency of uncovered struts (Fig. 3c). In lesions without malapposed struts, similar relationships were found (Fig. 3).
The frequency of uncovered struts had a significant positive correlation with the frequency of malapposed struts in the whole population (Fig. 4a). When patients were divided into 2 groups according to their median values of SEI (low SEI group ≤0.89, n = 30; high SEI group >0.89, n = 26), the frequency of uncovered struts significantly correlated with the frequency of malapposed struts in both groups (Fig. 4b, c).
Discussion
The present study demonstrated the following: (1) the incidence of malapposed struts was less frequently observed in the CoCr- and PtCr-EES, and the PtCr-EES had the lowest frequency of uncovered struts; (2) the R-ZES had smaller neointimal thickness and percent NIH area than CoCr- and PtCr-EES; (3) the frequency of uncovered struts correlated not to the SEI but the NUS in the whole stent; and (4) the CoCr- and PtCr-EES had a lower NUS compared to the R-ZES.
Neointimal coverage and strut malapposition
Previous studies showed that a greater percentage of uncovered struts (cut-off value of ≥5.9 % uncovered struts) and the presence of incomplete strut apposition (ISA) at mid-term follow-up were associated with adverse clinical events, such as cardiovascular death, myocardial infarction and stent thrombosis during long-term follow-up [8, 14, 15]. Moreover, a past study reported that the presence of neoatherosclerosis at follow-up OCT, which might be caused by vascular responses to DES, was independently associated with major adverse cardiac events [16]. They suggest that OCT findings at follow-up are important to understand mechanism of late stent failure.
We aimed to use OCT to directly compare the findings of three kinds of DESs: the CoCr-EES, PtCr-EESs and R-ZES, and they had differences in the amount of neointima, the frequency of strut coverage, and apposition. Second-generation DESs with an advanced architecture of stent, biocompatible drugs, and polymers have significantly improved endothelialization of struts and reduced thrombus formation compared to first-generation DESs [4]. We hypothesized that the different antiproliferative drugs, design of the stent platform, and the polymer played a role in determining neointimal suppression and strut coverage, and that these parameters would influence differences seen in OCT findings among the second-generation DESs.
The CoCr- and PtCr-EES had similarly low frequencies of malapposed struts and a lower frequency compared to the R-ZES in our study. These EESs have the same antiproliferative drug, durable biocompatible fluorocopolymer, and thin struts, but differ in stent alloy and design. These differences might not significantly influence the proportion of malapposed struts, which was consistent with a previous study [10]. However, thin struts were more likely to be apposed to the vessel wall compared to thick struts, thicker struts of R-ZES (91 μm) compared to EESs (81 μm) might involve the frequency of malapposed struts [17].
Our study demonstrated that EESs had a lower frequency of uncovered struts and relatively thicker neointima growth compared to the R-ZES. In the RESOLUTE All Comers trial, no significant differences were found in strut coverage, apposition, and neointimal thickness, although neointimal thickness tended to be thinner in the R-ZES compared to the CoCr-EES at 13-month follow-up [11]. The R-ZES has the same cobalt-chromium stent alloy with a different durable hydrophilic polymer as the CoCr-EES. The R-ZES and PtCr-EES differ in the antiproliferative drug, polymer, stent platform and alloy. Notably, the polymer of R-ZES was designed with a longer drug elution of up to approximately 6 months compared to EESs. This may have resulted in the greater neointimal suppression and higher frequency of uncovered struts at the 9-month follow-up in our study. Moreover, our study enrolled most of the patients with stable angina pectoris, although the Resolute All Comers trial included nearly 50 % of patients with acute coronary syndrome of the whole. Possible reasons of discrepancies between our study and the RESOLUTE All Comers trial include these differences of study protocol.
Past studies identified that several patient and procedural factors also influenced the neointimal growth or vascular healing after stent implantation, such as diabetes mellitus, clinical presentation and a presence of stent overlap [18–21]. Additionally, complex lesion characteristics including lipid and calcium content related with delayed neointimal coverage of stent struts in a past OCT study [19]. To minimize the influence of clinical status, plaque morphology and procedural factors excepting stent types, only patients without acute myocardial infarction and major bifurcation lesions who implanted a single second-generation DES were enrolled in this retrospective observational study. Moreover, the study population did not show significantly different clinical characteristics related with vascular responses. Therefore, the differences of stent performance might greatly influence on vascular responses in this study population.
Symmetrical stent expansion and homogenous neointimal growth
We demonstrated that uneven neointimal growth had a significant correlation with the frequency of uncovered struts. However, there was no significant relationship between asymmetric stent expansion and the frequency of uncovered struts. The mechanisms of uneven neointimal growth are not fully understood. Our study showed that there was a weak, but significant, relationship between asymmetric stent expansion and uneven neointimal growth. This relationship has been previously reported in CoCr-EES [22]. One possible mechanism is that asymmetric stent expansion may cause heterogeneous strut placement and a local drug concentration, and therefore results in heterogeneous neointimal suppression [23].
The presences of diabetes mellitus and hypertension have been reported to be independent factors related with uneven neointimal growth after CoCr-EES implantation [19]. Ordinarily, a dense tissue matrix of plaque, calcification, and tortuosity of vessel would hamper symmetric stent expansion and strut attachment to the vessel wall. A previous study reported that asymmetric stent expansion could be an important factor in thrombus formation by increasing the number of uncovered struts after implantation of first-generation DESs [13]. First-generation DESs in particular may tend to expand asymmetrically in those complex lesions, because they have thicker struts and a higher rigidity compared to the second-generation DESs. Improvement of asymmetrical expansion in stent implantation may cause homogeneous neointimal growth related with the frequency of the uncovered stent; that is a key factor in late stent thrombosis. This finding may have important clinical implications. Meanwhile, high symmetrical stent expansion did not always show a high frequency of stent coverage. One possible reason may be related to the fact that there was a significant relationship between the frequency of uncovered struts and the frequency of malapposed struts regardless of degree of SEI. This may suggest that strut malapposition is a powerful predictor of strut coverage.
Predominance of PtCr-EES in neointimal growth
In our study, there was a significant difference in the NUS among the three stents types. The CoCr-EES and PtCr-EES were observed to have more homogenous neointimal growth compared to the R-ZES. In addition, the PtCr-EES showed relatively asymmetrical stent expansion but a higher frequency of strut coverage with comparable homogenous and thinner neointimal growth compared to CoCr-EESs. From bench data, the PtCr alloy shows greater radial strength and less post-deployment recoil than CoCr alloy [24, 25]. Further, because of the reduced injury and low thrombogenicity due to the thin strut and the PtCr alloy itself, the PtCr-EES may offer modest vessel healing with a high incidence of strut coverage [26].
Additionally, stent flexibility and vessel conformability might be potential clinical advantages when treating tortuous and calcified lesions. We speculate that the flexibility and short stent segment design of PtCr-EES help to keep natural vessel curvature in tortuous and complex lesions without heterogeneous strut placement, and therefore result in less vascular injury and homogenous neointima formation.
OCT findings and clinical implications.
We demonstrated that favorable neointimal coverage and stent apposition in each second-generation DES as past studies described, so expect low rates of major adverse cardiac events in the future even if we discontinue dual-anti platelet therapy [11, 12, 14]. In our study that directly compared second-generation DESs, the CoCr- and PtCr-EES especially showed low incidences of uncovered and malapposed struts, and it suggests that EESs are more likely to maintain long-term safety outcomes.
Moreover, we showed the predominance of the PtCr-EES in homogenous neointimal growth. It is an advantage in the treatment of complex lesions when we consider the underlying mechanisms of unevenness neointimal growth.
Limitations
First, this was a single-center, retrospective, and observational study, and had a limited sample size, which raises the concern of selection bias. Because of this study design, no power calculation was performed. Second, pre- and post- interventional OCT data were not analyzed. Third, our study demonstrated that second-generation DESs had lower frequencies of uncovered and malapposed struts with thinner NIH compared to past OCT studies [10, 11]. One possible reason why our study showed these findings was that there were many patients with stable angina pectoris and relatively simple lesions treated using a single stent. Finally, we evaluated patients without in-stent restenosis or other cardiac events until their 9-month follow-up and could not show correlations between the differences of OCT findings and clinical outcomes such as stent thrombosis. This follow-up time is relatively short, however it was similar to past OCT studies which assessing vascular responses after DESs implantation [12, 15, 22]. There were limited evidences that how much difference of these OCT findings among the three kind of DESs influence on long-term clinical outcomes [14, 15, 22]. Second-generation DESs showed a favorable vascular healing than first-generation DESs, however, the presence of durable polymer was still concerned as a potential trigger of vascular inflammation and late stent failure [27]. Further studies and longer follow-up periods are required to show the clinical relevance of these OCT findings.
Conclusion
In this OCT study at 9-month follow-up, the quality of neointima coverage differed in the three kinds of second-generation DESs. Our study showed that the PtCr-EES provided homogenous neointimal growth and a high rate of strut coverage, suggesting that it poses fewer risks for stent thrombosis in the future.
References
Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, Jaeger JL, Kuntz RE; SIRIUS Investigators (2003) Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med 349:1315–1323
Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R (2006) Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol 48:193–202
Tada T, Byrne RA, Simunovic I, King LA, Cassese S, Joner M, Fusaro M, Schneider S, Schulz S, Ibrahim T, Ott I, Massberg S, Laugwitz KL, Kastrati A (2013) Risk of stent thrombosis among bare-metal stents, first-generation drug-eluting stents, and second-generation drug-eluting stents: results from a registry of 18,334 patients. JACC Cardiovasc Interv 6:1267–1274
Otsuka F, Vorpahl M, Nakano M, Foerst J, Newell JB, Sakakura K, Kutys R, Ladich E, Finn AV, Kolodgie FD, Virmani R (2014) Pathology of second-generation everolimus-eluting stents versus first-generation sirolimus- and paclitaxel-eluting stents in humans. Circulation 129:211–223
von Birgelen C, Basalus MW, Tandjung K, van Houwelingen KG, Stoel MG, Louwerenburg JH, Linssen GC, Saïd SA, Kleijne MA, Sen H, Löwik MM, van der Palen J, Verhorst PM, de Man FH (2012) A randomized controlled trial in second-generation zotarolimus-eluting Resolute stents versus everolimus-eluting Xience V stents in real-world patients: the TWENTE trial. J Am Coll Cardiol 59:1350–1361
Stone GW, Teirstein PS, Meredith IT, Farah B, Dubois CL, Feldman RL, Dens J, Hagiwara N, Allocco DJ, Dawkins KD; PLATINUM Trial Investigators (2011) A prospective, randomized evaluation of a novel everolimus-eluting coronary stent: the PLATINUM (a Prospective, Randomized, Multicenter Trial to Assess an Everolimus-Eluting Coronary Stent System [PROMUS Element] for the Treatment of Up to Two de Novo Coronary Artery Lesions) trial. J Am Coll Cardiol 57:1700–1708
Yazdani SK, Sheehy A, Nakano M, Nakazawa G, Vorpahl M, Otsuka F, Donn RS, Perkins LE, Simonton CA, Kolodgie FD, Virmani R (2013) Preclinical evaluation of second-generation everolimus- and zotarolimus-eluting coronary stents. J Invasive Cardiol 25:383–930
Guagliumi G, Sirbu V, Musumeci G, Gerber R, Biondi-Zoccai G, Ikejima H, Ladich E, Lortkipanidze N, Matiashvili A, Valsecchi O, Virmani R, Stone GW (2012) Examination of the in vivo mechanisms of late drug-eluting stent thrombosis findings from optical coherence tomography and intravascular ultrasound imaging. JACC Cardiovasc Interv 5:12–20
Choi HH, Kim JS, Yoon DH, Hong KS, Kim TH, Kim BK, Ko YG, Choi D, Jang Y, Hong MK (2012) Favorable neointimal coverage in everolimus-eluting stent at 9 months after stent implantation: comparison with sirolimus-eluting stent using optical coherence tomography. Int J Cardiovasc Imaging 28:491–497
Guagliumi G, Capodanno D, Ikejima H, Bezerra HG, Sirbu V, Musumeci G, Fiocca L, Lortkipanidze N, Vassileva A, Tahara S, Valsecchi O, Costa MA (2013) Impact of different stent alloys on human vascular response to everolimus-eluting stent: an optical coherence tomography study: the OCTEVEREST. Catheter Cardiovasc Interv 81:510–518
Gutiérrez-Chico JL, van Geuns RJ, Regar E, van der Giessen WJ, Kelbæk H, Saunamäki K, Escaned J, Gonzalo N, di Mario C, Borgia F, Nüesch E, García-García HM, Silber S, Windecker S, Serruys PW (2011) Tissue coverage of a hydrophilic polymer-coated zotarolimus-eluting stent vs. a fluoropolymer-coated everolimus-eluting stent at 13-month follow-up: an optical coherence tomography substudy from the RESOLUTE All Comers trial. Eur Heart J 32:2454–2463
Kim JS, Kim BK, Jang IK, Shin DH, Ko YG, Choi D, Hong MK, Cho YK, Nam CW, Hur SH, Choi JH, Song YB, Hahn JY, Choi SH, Gwon HC, Jang Y (2012) Comparison of neointimal coverage between zotarolimus-eluting stent and everolimus-eluting stent using Optical Coherence Tomography (COVER OCT). Am Heart J 163:601–607
Otake H, Shite J, Ako J, Shinke T, Tanino Y, Ogasawara D, Sawada T, Miyoshi N, Kato H, Koo BK, Honda Y, Fitzgerald PJ, Hirata K (2009) Local determinants of thrombus formation following sirolimus-eluting stent implantation assessed by optical coherence tomography. JACC Cardiovasc Interv 2:459–466
Won H, Shin DH, Kim BK, Mintz GS, Kim JS, Ko YG, Choi D, Jang Y, Hong MK (2013) Optical coherence tomography derived cut-off value of uncovered stent struts to predict adverse clinical outcomes after drug-eluting stent implantation. Int J Cardiovasc Imaging 29:1255–1263
Cook S, Eshtehardi P, Kalesan B, Räber L, Wenaweser P, Togni M, Moschovitis A, Vogel R, Seiler C, Eberli FR, Lüscher T, Meier B, Jüni P, Windecker S (2012) Impact of incomplete stent apposition on long-term clinical outcome after drug-eluting stent implantation. Eur Heart J 33:1334–1343
Kuroda M, Otake H, Shinke T, Takaya T, Nakagawa M, Osue T, Taniguchi Y, Iwasaki M, Nishio R, Kinutani H, Konishi A, Hirata KI (2015) The impact of in-stent neoatherosclerosis on long-term clinical outcomes: an observational study from the Kobe University Hospital optical coherence tomography registry. EuroIntervention 11:doi:10.4244/EIJY15M12_05
Tanigawa J, Barlis P, Dimopoulos K, Dalby M, Moore P, Di Mario C (2009) The influence of strut thickness and cell design on immediate apposition of drug-eluting stents assessed by optical coherence tomography. Int J Cardiol 134:180–188
Kim C, Kim BK, Lee SY, Shin DH, Kim JS, Ko YG, Choi D, Jang Y, Hong MK (2015) Incidence, clinical presentation, and predictors of early neoatherosclerosis after drug-eluting stent implantation. Am Heart J 170:591–597
Ishigami K, Uemura S, Morikawa Y, Soeda T, Okayama S, Nishida T, Takemoto Y, Onoue K, Somekawa S, Takeda Y, Kawata H, Horii M, Saito Y (2009) Long-term follow-up of neointimal coverage of sirolimus-eluting stents-evaluation with optical coherence tomography. Circ J 73:2300–2307
Gutiérrez-Chico JL, Räber L, Regar E, Okamura T, di Mario C, van Es GA, Windecker S, Serruys PW (2013) Tissue coverage and neointimal hyperplasia in overlap versus nonoverlap segments of drug-eluting stents 9 to 13 months after implantation: in vivo assessment with optical coherence tomography. Am Heart J 166:83–94
Kim JS, Fan C, Choi D, Jang IK, Lee JM, Kim TH, Park SM, Paik SI, Ko YG, Hong MK, Jang Y, Chung N (2011) Different patterns of neointimal coverage between acute coronary syndrome and stable angina after various types of drug-eluting stents implantation; 9-month follow-up optical coherence tomography study. Int J Cardiol 146:341–346
Iwasaki M, Otake H, Shinke T, Nakagawa M, Hariki H, Osue T, Inoue T, Taniguchi Y, Nishio R, Kinutani H, Konishi A, Hiranuma N, Kuroda M, Shite J, Hirata K (2014) Vascular responses in patients with and without diabetes mellitus after everolimus-eluting stent implantation. Circ J 78:2188–2196
Hwang CW, Wu D, Edelman ER (2001) Physiological transport forces govern drug distribution for stent-based delivery. Circulation 104:600–605
Menown IB, Noad R, Garcia EJ, Meredith I (2010) The platinum chromium element stent platform: from alloy, to design, to clinical practice. Adv Ther 27:129–411
Kereiakes DJ, Cannon LA, Feldman RL, Popma JJ, Magorien R, Whitbourn R, Dauber IM, Rabinowitz AC, Ball MW, Bertolet B, Kabour A, Foster MC, Wang JC, Underwood P, Dawkins KD (2010) Clinical and angiographic outcomes after treatment of de novo coronary stenoses with a novel platinum chromium thin-strut stent: primary results of the PERSEUS (Prospective Evaluation in a Randomized Trial of the Safety and Efficacy of the Use of the TAXUS Element Paclitaxel-Eluting Coronary Stent System) trial. J Am Coll Cardiol 56:264–271
Eppihimer MJ, Sushkova N, Grimsby JL, Efimova N, Kai W, Larson S, Forsyth B, Huibregtse BA, Dawkins KD, Wilson GJ, Granada JF (2013) Impact of stent surface on thrombogenicity and vascular healing: a comparative analysis of metallic and polymeric surfaces. Circ Cardiovasc Interv 6:370–377
Chamié D, Abizaid A, Costa JR Jr, Feres F, Almiro da Silva JF, Mattos LA, Staico R, Costa RA, Abizaid A, Tanajura LF, Sousa AG, Sousa JE (2011) Serial angiographic and intravascular ultrasound evaluation to interrogate the presence of late “catch-up” phenomenon after Cypher® sirolimus-eluting stent implantation. Int J Cardiovasc Imaging 27:867–874
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Rights and permissions
About this article
Cite this article
Ohtani, H., Kimura, S., Sugiyama, T. et al. Comparison of vascular responses after different types of second-generation drug-eluting stents implantation detected by optical coherence tomography. Int J Cardiovasc Imaging 33, 177–186 (2017). https://doi.org/10.1007/s10554-016-1001-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-016-1001-2