Abstract
Purpose
Tamoxifen is widely used to reduce the risk of breast cancer (BC) recurrence and extend disease-free survival among women with estrogen-sensitive breast cancers. Tamoxifen efficacy is thought to be attributable to its active metabolite, which is formed through a reaction catalyzed by the P450 enzyme, CYP2D6. Inhibition of tamoxifen metabolism as a result of germline genetic variation and/or use of CYP2D6-inhibiting medications (“inhibitors”) is hypothesized to increase the risk of adverse BC outcomes among women taking tamoxifen.
Methods
The present cohort study of 960 women diagnosed with early-stage BC between 1993 and 1999 examined the association between concomitant use of CYP2D6 inhibitors and adjuvant tamoxifen and the risk of adverse BC outcomes (recurrence, second primary BC, BC mortality), both overall and according to CYP2D6 metabolic phenotype.
Results
Six or more months of CYP2D6 inhibitor use concomitant with tamoxifen was not associated with any appreciable increase in risk of recurrence or second primary BC or BC mortality, and there was no clear evidence of variation by CYP2D6 metabolic phenotype.
Conclusions
These results are consistent with the relatively few other large, population-based studies conducted to date that have not observed an increased risk of adverse BC outcomes associated with CYP2D6 inhibition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tamoxifen (TAM), a selective estrogen receptor modulator, has been widely used to reduce the risk of breast cancer recurrence and prolong disease-free survival among women with non-metastatic hormone receptor-positive cancers. Adjuvant TAM use significantly lowers the risk of breast cancer recurrence and cancer-specific mortality [1, 2], and TAM use exceeding 5 years has been shown to afford further improvement in breast cancer outcomes [3]. Current American Society of Clinical Oncology guidelines recommend up to 10 years of adjuvant endocrine therapy for women with hormone receptor-positive cancer, composed of TAM, aromatase inhibitors, or a combination depending on menopausal status [4]. However, despite the established efficacy of TAM, among women who receive 5 years of adjuvant TAM therapy, approximately a third will experience a breast cancer recurrence and almost a quarter will die from their cancer within 15 years of diagnosis [1, 2]. Significant gaps persist in understanding the factors that modulate TAM response and the subsequent risk of adverse breast cancer outcomes.
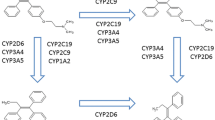
TAM is a prodrug that exhibits relatively weak binding affinity for the estrogen receptor and must undergo biotransformation to achieve its pharmacologic effect [5]. Two metabolites exhibit much greater binding affinity for the estrogen receptor than TAM [6, 7] and one of these, 4-OH-N-desmethyltamoxifen (endoxifen), is found at especially high plasma concentrations and is thought to be TAM’s primary active metabolite [8].
The formation of endoxifen is catalyzed by the product of the CYP2D6 gene, CYP2D6, an enzyme central to the metabolism of many drugs [9]. Co-administration of TAM with medications metabolized through the same pathway has been shown to result in lower plasma concentrations of endoxifen [5, 8]. Concomitant use of TAM and CYP2D6-inhibiting medications has therefore been hypothesized to reduce TAM efficacy. CYP2D6-inhibiting medications span several major drug classes, and some of the strongest inhibitors are antidepressants, which are commonly used by women after breast cancer diagnosis for the treatment of depression, anxiety, and TAM- or chemotherapy-related hormonal side effects [10, 11].
Inherited genetic variation in CYP2D6 has also been shown to correlate with metabolic efficiency and to impact the concentration of TAM and its active metabolites in blood [5, 8, 12,13,14,15], normal breast tissue, and tumor tissue [16]. Up to 10% of the Caucasian population carries variant alleles implicated in near complete loss of enzymatic function (“poor metabolizers”) [17]. Though other enzymes are involved in TAM metabolism [18], between 27 and 58% of the variability in plasma Z-endoxifen concentration has been attributed to CYP2D6, depending on population and phenotype classification [13,14,15]; however, these studies consistently found that at least 90% of those classified as poor metabolizers had endoxifen concentrations at or below the level [19] recommended for inhibition of the estrogen receptor. The combination of CYP2D6-inhibiting medication use in women with impaired CYP2D6 metabolizer status has been shown to result in lower endoxifen levels than seen with either inhibiting factor alone [5, 8, 20] and may therefore be associated with an even greater risk for adverse breast cancer outcomes. Indeed, lower plasma endoxifen levels themselves have been shown to be negatively associated with survival in several studies [19, 21, 22], while others [23, 24] have found no association.
Results from studies of the association between CYP2D6 inhibition and adverse breast cancer outcomes have been heterogeneous, with several finding an increased risk [10, 25,26,27] (potentially only in relevant subgroups [28, 29]) and others finding a null association [11, 30,31,32,33,34,35,36,37,38,39]. However, the majority of studies have focused exclusively on either CYP2D6-inhibiting medication use or CYP2D6 genotype and have not considered their joint actions. In addition, most studies have employed limited genotyping strategies covering a small number of CYP2D6 alleles and few have assessed the full scope of CYP2D6-inhibiting medications, focusing instead on particular classes of drugs. Lastly, few have attempted to evaluate duration of concomitant use or the intensity of hypothesized CYP2D6 inhibition.
This study examined the associations of concomitant use of TAM and prescription CYP2D6-inhibiting medications (“inhibitors”) and CYP2D6 metabolic phenotype with adverse breast cancer outcomes in a well-characterized, population-based cohort of women with invasive breast cancer. Inhibitor use was assessed for all known prescription CYP2D6 inhibitors and further classified with respect to inhibitor strength and duration of concomitant use. In addition, this study applied a comprehensive CYP2D6 genotyping approach and assessed potential interactions between concomitant inhibitor use and metabolic phenotype on the risk of adverse breast cancer outcomes.
Methods
Study population
The study was conducted within the Quilt Study, a prognostic cohort of 2,337 women diagnosed with invasive breast cancer at ages 45–79 in the Seattle tri-county area between 1993 and 1999 that has been described previously [40]. Cases in the Quilt Study were originally ascertained through the Cancer Surveillance System (CSS), the Surveillance, Epidemiology and End Results (SEER) cancer registry serving the Seattle-Puget Sound region, as part of three prior population-based case–control studies: the Women’s Contraceptive and Reproductive Experiences Study (CARE, Seattle site only) [41]; the Puget Sound Area Breast Cancer Evaluation Study (PACE) [42]; and the Electric Power and the Risk of Breast Cancer (EMF) Study [43] (Fig. 1). The three case study populations were mutually exclusive of one another, and while population-based, the CARE and EMF studies sampled women with respect to race and county, and CARE additionally by age. As part of these three studies, information on demographics, tumor characteristics, and known risk factors for breast cancer incidence was obtained from the CSS and structured in-person interviews. As part of the subsequent Quilt Study, additional information on cancer treatment, recurrences, co-morbidities, prescription medications, and other exposures after diagnosis was collected through follow-up interviews and medical record reviews. Mean and median time from diagnosis to last chart review was 9.2 and 9.7 years, respectively.
Identification of study cohorts, including inclusion and exclusion criteria for each analysis. AA African American, CARE Women’s Contraceptive and Reproductive Experiences, EMF Electric Power and the Risk of Breast Cancer, ER estrogen receptor, Hx history, PACE Puget Sound Area Breast Cancer Evaluation, TAM tamoxifen
The study population for this analysis was restricted to women in the Quilt Study diagnosed with local or regional stage estrogen receptor-positive (ER+) cancers, with no prior history of cancer at breast cancer diagnosis, with medical records available for review, and who used adjuvant TAM for at least 6 months after primary diagnosis and prior to any recurrence or second primary breast cancer (Fig. 1). Those women excluded due to lack of consent or unavailability of medical records were slightly younger, more likely to be non-White and peri-menopausal, to have local disease, and to be alive at end of follow-up, and slightly less likely to receive chemotherapy and radiation than women who met all study criteria (data not shown). The only statistically significant difference in demographic, tumor, or clinical/treatment characteristics between eligible women included and excluded in the study was in the proportion that received radiation therapy (61.6 vs. 68.9%, χ2 p = 0.012). Secondary analyses were restricted to the subset of women from the CARE and PACE studies with genotyping data on CYP2D6.
Data collection
Prescription medication use in targeted categories (including antidepressant/antianxiety medications, antihypertensives, and nonsteroidal anti-inflammatory drugs) and TAM use was abstracted from medical records. For each month following diagnosis and through the date of the last medical record review, women were classified as either having started, stopped, or after starting, continued using a medication in the absence of any evidence of discontinuation. CYP2D6 inhibitors were identified from the classifiers maintained by the U.S. Food and Drug Administration and Indiana University’s Clinical Pharmacology Research Institute, and further categorized with regard to inhibitor strength [44, 45]. Both entities classify inhibitor strength based on the change in in vivo plasma substrate concentration over time with and without co-administration of the inhibitor. Of the medications observed in our data, these two classifiers agreed on four out of five drugs classified as strong or moderate inhibitors, and on all drugs classified as strong inhibitors. For purposes of this study, medications were classified according to the highest inhibition level assigned by either classifier (Table 1).
DNA extracted from blood from women in the CARE and PACE studies was genotyped for eight common allelic variants in the CYP2D6 gene, as well as the number of copies of the gene, in the Fred Hutch Public Health Sciences’ Molecular Epidemiology Laboratory using validated TaqMan™ Drug Metabolism Genotyping Assay sets (Applied Biosystems, Foster City, CA), with the exception of the *9 allele. This allele was determined by fragment analysis on a 3730xl genetic analyzer, using a fluorescently labeled PCR product covering rs5030656 (primer set: 5′-GACCTGACTGAGCCCTTCCT forward, 5′ FAM-ATTCCTCCTGGGACGCTCAA reverse). Each single nucleotide polymorphism (SNP) corresponds to a known phenotype of tamoxifen metabolism, specifically extensive (EM), intermediate (IM), or poor (PM) metabolic efficiency (Table 2) [46, 47]. Because the SNP defining *2 occurs in multiple other alleles, we considered the *2 allele present only in the absence of those other allele-defining SNPs. Phenotypic diplotypes were determined as being extensive (EM/EM), poor (PM/PM), or intermediate (all others) and further collapsed into EM and IM/PM for analysis.
Breast cancer-specific mortality was identified from CSS, death certificates, or proxy report. Second breast cancer events (SBCEs) were defined as the first local, regional, or distant breast cancer recurrence or second primary breast cancer occurring at least 6 months after the initial cancer diagnosis. Since TAM broadly suppresses the proliferation of estrogen-sensitive tumor, CYP2D6 inhibition of TAM was hypothesized to have similar associations with risk of recurrence and risk of second primary breast cancer, although they were also explored separately. Data on recurrence were obtained from medical record review and interview, and data on second primary cancer were collected primarily from CSS. In addition, for women who died as a result of their breast cancer but for whom there was no record of distant recurrence, distant recurrence dates were imputed by subtracting from death dates the median period between first distant recurrence and death date in cohort members with available dates for both events. If the imputed date preceded the last recorded disease-free date, the later date was used instead.
Analysis
The relative risks of first SBCE and breast cancer-specific mortality associated with concomitant use of TAM and CYP2D6 inhibitors were estimated using Cox proportional hazards models. Women were followed from diagnosis for these events until their death, diagnosis with a non-breast cancer, date of last available medical record, or the end of follow-up on March 31, 2015, at which point they were censored. Because all women had to survive until their original case–control study interview, women entered the analysis (i.e., analyses were left-truncated) on their interview date (mean and median durations between diagnosis and interview were 280 and 242 days, respectively) or after 6 months of TAM use, whichever occurred later. SBCE analyses were also repeated without imputed distant recurrences.
Women who used TAM and a CYP2D6 inhibitor concurrently were considered exposed after 6 months of continuous or cumulative concomitant use and for the remainder of their follow-up time; until this point, they were classified in a time-varying manner as either having no concomitant use or as concomitant users of fewer than 6 months. Exposure assessment was conducted during the period between diagnosis and the first adverse breast cancer outcome; in the mortality analysis, exposure assessment was restricted to the period prior to the first SBCE in order to avoid ambiguous temporality. TAM users who had not used inhibitors concurrently with TAM served as the reference group for all analyses, although it was possible that these medications were used non-concurrently among these women. All analyses further restricted the exposed group to those whose concomitant use was of (i) strong or moderate CYP2D6 inhibitors or (ii) strong inhibitors alone.
Concomitant use of adjuvant TAM and an inhibitor for 6 or more months (main exposure) and duration of adjuvant TAM use were modeled as time-varying covariates. Analyses also adjusted for age at diagnosis (< 55, 55–69, 70+ years), BMI (prior to diagnosis; < 25, 25–29.99, 30+ kg/m2), tumor stage (local or regional), tumor grade (good, moderate, or poor differentiation, or undifferentiated), receipt of radiation, and receipt of chemotherapy. Adjusted Cox models were stratified by diagnosis year (1993–1995, 1996–1997, 1998–1999). All categorical covariates were modeled as dummy variables, and duration of prior adjuvant TAM use was modeled as a time-varying, continuous variable in years.
A subgroup analysis was conducted among women who underwent genotyping for the major CYP2D6 variant alleles from the CARE and PACE studies. Extensive metabolizers served as the reference group to which intermediate and poor metabolizers were compared. In the analysis of the interaction of concomitant inhibitor use and metabolic phenotype, phenotype (EM or IM/PM) and concomitant inhibitor use (none, < 6 months, 6+ months) were included in the model as dummy variable main effect terms along with terms for the interactions of concomitant inhibitor use category and phenotype. Risk estimates were derived using linear combinations of coefficients from the full model including all interaction terms.
Results
Nine-hundred sixty women from the Quilt Study cohort met study inclusion criteria (269 from CARE, 464 from PACE, and 227 from EMF, Fig. 1). Women who were concomitant inhibitor users for 6 or more months were slightly older than women without concomitant inhibitor use, were more likely to have been diagnosed in the years 1996–1997, had higher pre-diagnosis BMI, and were somewhat less likely to be postmenopausal than never concomitant users (Table 3). They were also more likely to be diagnosed at a regional stage, to receive chemotherapy, and had slightly longer durations of TAM use overall [median (IQR): 59 (36–61) vs. 57 (31–61) months].
There were 252 women who experienced a breast cancer recurrence or second primary breast cancer. Of these SBCEs, 19 were local recurrences, 3 were regional recurrences, 134 were distant recurrences (22 imputed), and 75 were second primary cancers; the remaining 21 SBCEs encompassed simultaneous events (e.g., a local and distant recurrence diagnosed at the same time). Two-hundred and twenty-two women were censored in the SBCE analysis at the time of their diagnosis with a non-breast cancer (n = 158) or death from another cause (n = 64). A total of 168 breast cancer deaths occurred during the follow-up period, with a mean cause-specific survival time of 14.2 years and a median of 16.6 years. In the mortality analysis, 214 women were censored at the time of their death due to another cause.
Approximately 70% of the observed follow-up time in the SBCE analysis occurred among never users of any concomitant CYP2D6 inhibitor, with an additional 8% among women who used a CYP2D6 inhibitor concomitant with TAM for fewer than 6 months, and the remaining 22% among women who used an inhibitor concomitant with TAM for 6 months or longer. The distribution of follow-up time was similar in the mortality analysis. Among women who used any CYP2D6 inhibitor concomitant with TAM for at least 6 months, approximately a third first used inhibitors at diagnosis, a third started them within 2 years following diagnosis, and a third initiated use more than 2 years after diagnosis. Approximately 45% of women who used inhibitors had no unopposed TAM use before beginning inhibitor use, and 27% did not begin inhibitor use until completing at least 2 years of unopposed TAM use.
Women who used CYP2D6 inhibitors concurrently with TAM for fewer than 6 months were considered to be at low risk for adverse events attributable to concomitant use, and the hazard ratios (HRs) associated with short-term concomitant use were not significantly different from 1.0 across all inhibitor strength categories (Fig. 2). Overall, 6 or more months of any CYP2D6 inhibitor use concomitant with TAM was not associated with any appreciable change in risk of SBCE or breast cancer mortality relative to non-concomitant users (Table 4; Fig. 2). Hazard ratio estimates were similar for SBCE and recurrences alone (data not shown), in line with the hypothesis that TAM inhibition would affect recurrence and second primary risk similarly; only results for the combined SBCE endpoint are presented. Among women whose first event was a second primary breast cancer, there were no significant differences in ER positivity by concomitant inhibitor use (χ2 p = 0.894), but information on ER status was available only for a small number of women (n = 42). Associations were not observed in any category of inhibitor strength for either SBCE or breast cancer-specific mortality (Table 4), and results did not differ when imputed distant recurrences were excluded. Concomitant inhibitor use appeared inversely related to the risk of adverse breast cancer outcomes, but the bounds of the 95% confidence intervals did not exclude the null.
Crude Kaplan–Meier survival curves showing time to earliest recurrence or second primary breast cancer by time-varying TAM-CYP2D6 inhibitor concomitant use. Because exposure status was assessed in the first 6 months following diagnosis, no events in this analysis could occur during this period. Individuals were classified as nonusers prior to first record of concomitant use, at which point they become exposed for < 6 months; after 6 or more months of cumulative concomitant use, they were considered exposed in the main analysis. Mos. months
Six hundred and sixty-five women had CYP2D6 genotyping results available, of which 111 (17%) were classified as EMs, 512 (77%) as IMs, and 42 (6%) as PMs. All metabolizer phenotype groups had similar distributions of concomitant use categories; overall, 64% were classified as never users and 26% were classified as concomitant users for 6 months or longer. No significant association between metabolizer phenotype and SBCE risk or breast cancer-specific mortality was observed among never users of concomitant inhibitors in adjusted analyses (Table 4). Use of CYP2D6 inhibitors was not associated with risk of SBCE or cancer mortality regardless of a women’s metabolic phenotype.
Discussion
Overall, this study found no increased risk of adverse breast cancer outcomes associated with concomitant use of CYP2D6 inhibiting medications and adjuvant TAM for 6 months or longer. No evidence of an increased risk of SBCE or cancer mortality was observed at any level of inhibitor strength. Furthermore, no association with metabolizer phenotype was observed among never users of concomitant CYP2D6 inhibitors, nor was there any evidence for an interaction of metabolizer phenotype and concomitant inhibitor use.
The main results of this study are consistent with other observational studies that have observed no association between CYP2D6 inhibiting medications, with or without additional information on CYP2D6 genotype, and risk of adverse breast cancer outcomes [11, 30,31,32,33, 37, 39]. Despite evidence that both use of CYP2D6 inhibitors and metabolizer phenotype are associated with reduced plasma endoxifen levels, only one observational study has observed a significant association between inhibitor usage and adverse clinical breast cancer outcomes [10].
Effects of impaired metabolic efficiency or pharmacologic inhibition of CYP2D6 on breast cancer outcomes have been hypothesized to be minimal, as TAM and its metabolites may still overwhelm estrogens in competition for ER binding sites in estrogen-sensitive tumor cells at standard doses [48]. If anything, deleterious effects of CYP2D6 inhibition are hypothesized to be most pronounced among pre-menopausal women [49], who have higher levels of endogenous estrogens than postmenopausal women and for whom TAM remains the preferred endocrine therapy. In an exploratory analysis, stratification by menopausal status revealed no difference in the association between concomitant inhibitor use and adverse breast cancer outcomes (data not shown).
This study has several notable strengths. As noted earlier, this study employed a population-based design and followed participants for events for up to 22 years. Population-based ascertainment of cases increases the generalizability of our findings, but also must be considered in light of nonparticipation or data unavailability. Overall, approximately 15% of women eligible declined to participate in the original case–control studies, and mortality among these women is likely to be greater, as has been shown in previous case–control studies of breast cancer [50]. In this analysis of the Quilt cohort, approximately 27% of women were excluded due to unavailability of medical charts. If the association between CYP2D6 inhibition and event-free survival differs in those not included in this analysis, this could introduce bias into our study.
This was also one of a few studies to assess CYP2D6 inhibition due to both medication use and genetic variation. The use of a broader genotyping strategy in this study represents a significant advantage over previous work and allowed for more precise classification of CYP2D6 metabolic phenotypes. Our study utilized germline DNA from blood rather than tumor, the specimen source for many past studies, alleviating possible concern regarding genotypic misclassification due to loss of heterozygosity in tumor tissue [51, 52]. In addition, this study included all prescription medications known to inhibit CYP2D6 activity in exposure assessment, rather than restricting to a single class of medications, and concomitant inhibitor use was classified with regard to intensity and duration of concomitant use, rather than relying on ever use of TAM and inhibitors. As prescribing practices for TAM changed over the study period, this approach allowed for variation in TAM usage among the study population when assessing concomitant use. However, it is unknown when during follow-up, after what duration, and for how long concomitant inhibitor use may affect risk of adverse breast cancer outcomes. Although longer durations of TAM have been found to provide greater clinical benefit [3], even 2 years of TAM use confers a long-term survival benefit relative to no use [53]. This study found that the majority of concomitant use began within 2 years of diagnosis, but we lacked sufficient data to explore the robustness of our findings to other risk periods and exposure definitions. Incorrect specification of the relevant exposure and/or risk period could explain our null findings.
This analysis is limited to prescription CYP2D6 inhibitors. While all inhibitors currently classified as having strong or moderate inhibiting activity are prescription medications likely to appear in medical records, several weak inhibitors are not prescription medications and therefore may not be captured (e.g., diphenhydramine). The use of medical records to classify drug exposures may also be less accurate than insurance claims or pharmacy fill data.
The present study is also limited by a small number of events among exposed women, leading to imprecise effect estimates and precluding assessment of associations with longer periods of CYP2D6 inhibition. The timing of inhibition and the duration of previous or subsequent unopposed TAM use may also affect long-term risk of adverse outcomes, but this study was underpowered to assess these factors.
Lastly, the only observed uses of moderate or strong CYP2D6 inhibitors in this cohort were of medications indicated for the treatment of depression or anxiety, conditions that may be independently associated with adverse cancer outcomes [54]. Despite concerns that this may have led to confounding by indication, no increased risk was observed.
Overall, this study found no evidence of increased risk of adverse breast outcomes among women who used CYP2D6-inhibiting medications concomitant with TAM, and no interaction of medication use with CYP2D6 metabolic phenotype. Findings were similar across all levels of inhibitor strengths and among pre-/peri- and postmenopausal women. If anything, there was suggestion of a reduced risk of adverse breast cancer outcomes in relation to concomitant inhibitor use, although the confidence limits for the risk estimates did not exclude the null. This could be suggestive of some degree of “healthy user/adherer” bias among long-term users of CYP2D6-inhibiting medications, which in this study were indicated primarily for the treatment of psychiatric and cardiac conditions. The suggested reduced risk associated with concomitant inhibitor use could be partially attributable to the influence of this uncontrolled confounding, as well as by other factors like social support and care access that were also not controlled for in this analysis.
Our findings are consistent with previous large observational studies and suggest that there is little clinical evidence at this time to support avoidance of CYP2D6-inhibiting medications among women using TAM endocrine therapy. It is possible that accounting only for CYP2D6 inherited variation and CYP2D6-inhibiting medication use does not fully capture factors impacting TAM efficacy, since additional enzymes are involved in TAM metabolism. A more comprehensive approach that can account for the entire TAM metabolic pathway may be needed to fully capture the effect of medication use and endogenous metabolic phenotypes on risk of adverse breast cancer outcomes.
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365(9472):1687–1717
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378(9793):771–784
Davies C, Pan H, Godwin J, Gray R, Arriagada R et al (2013) Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 381:805–816
Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE et al (2014) Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: American Society of Clinical Oncology Clinical Practice guideline focused update. J Clin Oncol 32(21):2255–2269
Jin Y, Desta Z, Stearns V, Ward B, Ho H et al (2005) CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J Natl Cancer Inst 97:30–39
Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM et al (2004) Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat 85:151–159
Lim YC, Desta Z, Flockhart DA, Skaar TC (2005) Endoxifen (4-hydroxy-N-desmethyl-tamoxifen) has anti-estrogenic effects in breast cancer cells with potency similar to 4-hydroxy-tamoxifen. Cancer Chemother Pharmacol 55:471–478
Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, et al (2003) Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst 95:1758–1764
Stearns V, Rae JM (2008) Pharmacogenetics and breast cancer endocrine therapy: CYP2D6 as a predictive factor for tamoxifen metabolism and drug response? Expert Rev Mol Med 10:e34
Kelly CM, Juurlink DN, Gomes T, Duong-Hua M, Pritchard KI et al (2010) Selective serotonin reuptake inhibitors and breast cancer mortality in women receiving tamoxifen: a population based cohort study. BMJ 340:c693
Chubak J, Buist DS, Boudreau DM, Rossing MA, Lumley T et al (2008) Breast cancer recurrence risk in relation to antidepressant use after diagnosis. Breast Cancer Res Treat 112(1):123–132
Gjerde J, Hauglid M, Breilid H, Lundgren S, Varhaug JE et al (2008) Effects of CYP2D6 and SULT1A1 genotypes including SULT1A1 gene copy number on tamoxifen metabolism. Ann Oncol 19(1):56–61
Mürdter TE, Schroth W, Bacchus-Gerybadze L, Winter S, Heinkele G et al (2011) Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther 89(5):708–717
Hennig EE, Piatowska M, Karczmarski J, Goryca K, Brewczynska E et al (2015) Limited predictive value of achieving beneficial plasma (Z)-endoxifen threshold level by CYP2D6 genotyping in tamoxifen-treated Polish women with breast cancer. BMC Cancer 15:570
Schroth W, Winter S, Mürdter T et al (2017) Improved prediction of endoxifen metabolism by CYP2D6 genotype in breast cancer patients treated with tamoxifen. Front Pharmacol 8:582
Kisanga ER, Gjerde J, Guerrieri-Gonzaga A, Pigatto F, Pesci-Feltri A et al (2004) Tamoxifen and metabolite concentrations in serum and breast cancer tissue during three dose regimens in a randomized preoperative trial. Clin Cancer Res 10(7):2336–2343
Crews KR, Gaedigk A, Dunnenberger HM, Leeder JS, Klein TE et al (2014) Clinical pharmacogenetics implementation consortium guidelines for cytochrome P450 2D6 genotype and codeine therapy: 2014 update. Clin Pharmacol Ther 95(4):376–382
Binkhorst L, Mathijssen RH, Jager A, van Gelder T (2015) Individualization of tamoxifen therapy: much more than just CYP2D6 genotyping. Cancer Treat Rev 41(3):289–299
Madlensky L, Natarajan L, Tchu S, Pu M, Mortimer J et al (2011) Tamoxifen metabolite concentrations, CYP2D6 genotype and breast cancer outcomes. Clin Pharmacol Ther 89(5):718–725
Borges S, Desta Z, Li L, Skaar TC, Ward BA et al (2006) Quantitative effect of CYP2D6 genotype and inhibitors on tamoxifen metabolism: implication for optimization of breast cancer treatment. Clin Pharmacol Ther 80(1):61–74
Saladores P, Mürdter T, Eccles D, Chowbay B, Zgheib NK et al (2015) Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenomics J 15(1):84–94
Helland T, Henne N, Bifulco E, Naume B, Borgen E et al (2017) Serum concentrations of active tamoxifen metabolites predict long-term survival in adjuvantly treated breast cancer patients. Breast Cancer Res 19:125
Johansson H, Gandini S, Serrano D, Gjerde J, Lattanzi M et al (2016) A pooled analysis of CYP2D6 genotype in breast cancer prevention trials of low-dose tamoxifen. Breast Cancer Res Treat 159(1):97–108
Neven P, Jongen L, Lintermans A, Van Asten K, Blomme C et al (2018) Tamoxifen metabolism and efficacy in breast cancer: a prospective multicenter trial. Clin Cancer Res 24(10):2312–2318
Schroth W, Antoniadou L, Fritz P, Schwab M, Mürdter T et al (2007) Breast cancer treatment outcome with adjuvant tamoxifen relative to patient CYP2D6 and CYP2C19 genotypes. J Clin Oncol 25(33):5187–5193
Schroth W, Goetz MP, Hamann U, Fasching PA, Schmidt M et al (2009) Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA 302(13):1429–1436
Goetz MP, Suman VJ, Hoskin TL, Gnant M, Filipits M et al (2013) CYP2D6 metabolism and patient outcome in the Austrian Breast and Colorectal Cancer Study Group trial (ABCSG) 8. Clin Cancer Res 19(2):500–507
Thompson AM, Johnson A, Quinlan P, Hillman G, Fontecha M et al (2011) Comprehensive CYP2D6 genotype and adherence affect outcome in breast cancer patients treated with tamoxifen monotherapy. Breast Cancer Res Treat 125(1):279–287
Chubak J, Bowles EJA, Yu O, Buist DSM, Fujii M et al. Boudreau DM. (2016). Breast cancer recurrence in relation to antidepressant use. Cancer Causes Control. 27(1):125–136
Dezentjé VO, van Blijderveen NJ, Gelderblom H, Putter H, van Herk-Sukel MP et al (2010) Effect of concomitant CYP2D6 inhibitor use and tamoxifen adherence on breast cancer recurrence in early-stage breast cancer. J Clin Oncol 28(14):2423–2429
Lash TL, Cronin-Fenton D, Ahern TP, Rosenberg CL, Lunetta KL et al (2010) Breast cancer recurrence risk related to concurrent use of SSRI antidepressants and tamoxifen. Acta Oncol 49(3):305–312
Lash TL, Cronin-Fenton D, Ahern TP, Rosenberg CL, Lunetta KL et al (2011) CYP2D6 inhibition and breast cancer recurrence in a population-based study in Denmark. J Natl Cancer Inst 103(6):489–500
Azoulay L, Dell’Aniello S, Huiart L, du Fort GG, Suissa S (2011) Concurrent use of tamoxifen with CYP2D6 inhibitors and the risk of breast cancer recurrence. Breast Cancer Res Treat 126(3):695–703
Regan MM, Leyland-Jones B, Bouzyk M, Pagani O, Tang W et al Breast International Group (BIG) 1–98 Collaborative Group. (2012). CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1–98 trial. J Natl Cancer Inst. 104(6):441–451
Rae JM, Drury S, Hayes DF, Stearns V, Thibert JN et al (2012) CYP2D6 and UGT2B7 genotype and risk of recurrence in tamoxifen-treated breast cancer patients. J Natl Cancer Inst 104(6):452–460
Markkula A, Hjertberg M, Rose C, Ingvar C, Jernström H (2014) No association found between CYP2D6 genotype and early breast cancer events in tamoxifen-treated patients. Acta Oncol 53(2):195–200
Haque R, Shi J, Schottinger JE, Ahmed SA, Cheetham TC et al (2016) Tamoxifen and antidepressant drug interaction among a cohort of 16,887 breast cancer survivors. J Natl Cancer Inst 108(3):djv337
Hertz DL, Kidwell KM, Hilsenbeck SG et al (2017) CYP2D6 genotype is not associated with survival in breast cancer patients treated with tamoxifen: results from a population-based study. Breast Cancer Res Treat 166(1):277–287
Donneyong MM, Bykov K, Bosco-Levy P, Dong YH, Levin R, Gagne JJ (2016) Risk of mortality with concomitant use of tamoxifen and selective serotonin reuptake inhibitors: multi-database cohort study. BMJ 354:i5014
Reding KW, Doody DR, McTiernan A, Hsu L, Davis S et al (2011) Age-related variation in the relationship between menopausal hormone therapy and the risk of dying from breast cancer. Breast Cancer Res Treat 126(3):749–761
Marchbanks PA, McDonald JA, Wilson HG, Burnett NM, Daling JR et al (2002) The NICHD Women’s Contraceptive and Reproductive Experiences Study: methods and operational results. Ann Epidemiol 12(4):213–221
Li CI, Malone KE, Porter PL, Weiss NS, Tang MT et al (2003) Relationship between long durations and different regimens of hormone therapy and risk of breast cancer. JAMA 289(24):3254–3263
Davis S, Mirick DK, Stevens RG (2002) Residential magnetic fields and the risk of breast cancer. Am J Epidemiol 155(5):446–454
Food US, Administration D (2016) Table 3–2: Examples of clinical inhibitors for P450-mediated metabolisms (for concomitant use clinical DDI studies and/or drug labeling). https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm093664.htm#table3-2. Accessed 8 Jan 2018
Indiana University Clinical Pharmacology Research Institute (2016) Flockhart™ P450 Drug interaction table–inhibitors. http://medicine.iupui.edu/clinpharm/ddis/main-table. Accessed 8 Jan 2018
Lyon E, Gastier Foster JM, Palomaki GE, Pratt VM, Reynolds K et al, working group of the Molecular Genetics Subcommittee on behalf of the American College of Medical Genetics and Genomics (ACMG) Laboratory Quality Assurance Committee (2012) Laboratory testing of CYP2D6 alleles in relation to tamoxifen therapy. Genet Med 14(12):990–1000
Hertz DL, Snavely AC, McLeod HL, Walko CM, Ibrahim JG et al (2015) In vivo assessment of the metabolic activity of CYP2D6 diplotypes and alleles. Br J Clin Pharmacol 80(5):1122–1130
Cronin-Fenton DP, Lash TL (2011) Clinical epidemiology and pharmacology of CYP2D6 inhibition related to breast cancer outcomes. Expert Rev Clin Pharmacol 4(3):363–377
Lash TL, Lien EA, Sørensen HT, Hamilton-Dutoit S (2009) Genotype-guided tamoxifen therapy: time to pause for reflection? Lancet Oncol 10(8):825–833
Malone KE, Daling JR, Doody DR, O’Brien C, Resler A et al (2011) Family history of breast cancer in relation to tumor characteristics and mortality in a population–based study of young women with invasive breast cancer. Cancer Epidemiol Biomarkers Prev 20(12):2560–2571
Goetz MP, Sun JX, Suman VJ, Silva GO, Perou CM et al (2014) Loss of heterozygosity at the CYP2D6 locus in breast cancer: implications for germline pharmacogenetic studies. J Natl Cancer Inst 107(2):dju401
Ahern TP, Hertz DL, Damkier P, Ejlertsen B, Hamilton-Dutoit SJ et al (2017) Cytochrome P-450 2D6 (CYP2D6) genotype and breast cancer recurrence in tamoxifen-treated patients: evaluating the importance of loss of heterozygosity. Am J Epidemiol 185(2):75–85
Ekholm M, Bendahl PO, Fernö M, Nordenskjöld B, Stål O, Rydén L (2016) Two years of adjuvant tamoxifen provides a survival benefit compared with no systemic treatment in premenopausal patients with primary breast cancer: long-term follow-up (> 25 years) of the phase III SBII:2pre trial. J Clin Oncol 34(19):2232–2238
Satin JR, Linden W, Phillips MJ (2009) Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer 115(22):5349–5361
Acknowledgments
This research was supported by the following National Cancer Institute grants issued to the Fred Hutch/University of Washington: CA173795, CA098858, CA015704, and CA009168. We are very grateful for the generous contributions of our study participants and research team.
Funding
This research was supported by NIH Grants R01 CA098858, R03 CA173795, and T32 CA009168. This research was also supported in part through the NIH/NCI Cancer Center Support Grant P30 CA015704.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Mayer, S.E., Weiss, N.S., Chubak, J. et al. CYP2D6-inhibiting medication use and inherited CYP2D6 variation in relation to adverse breast cancer outcomes after tamoxifen therapy. Cancer Causes Control 30, 103–112 (2019). https://doi.org/10.1007/s10552-018-1117-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10552-018-1117-x