Abstract
Decreased CYP2D6 activity is associated with lower levels of active tamoxifen metabolites. We examined the impact of CYP2D6 genotype on tamoxifen pharmacokinetics, biomarker activity, and efficacy in a pooled analysis of low-dose tamoxifen. Four randomized breast cancer prevention trials of very-low-dose (1 mg/day, n = 52 or 10 mg/week, n = 152) or low-dose tamoxifen (5 mg/day, n = 171) were pooled. DNA from 367 subjects was genotyped for CYP2D6 alleles associated with absent (PM allele: *3, *4, *5, *6, *7, *8, *12, and *14), reduced (IM allele: *9, *10, *17, *29, *41), normal (EM allele), or increased (UM: *XN) enzyme activity. Associations of tamoxifen, metabolites, activity biomarkers, and event-free survival with rapid (UM/EM, UM/IM, EM/EM, EM/IM, or EM/PM alleles) versus slow metabolizers (PM/IM or PM/PM) were investigated through random effects models, with ‘study’ as the random factor, and Cox regression models, adjusting for confounders. Rapid metabolizers had higher endoxifen levels than slow metabolizers: 15.3 versus 12.2 ng/mL (P = 0.018) with 5 mg/day, and 3.8 versus 2.8 ng/mL (P = 0.004) with 1 mg/day or 10 mg/week tamoxifen. The IGF-I decrease correlated with endoxifen (P = 0.002) and 4-hydroxytamoxifen levels, demonstrating steeper decreases at higher metabolite levels (P = 0.001). After a median follow-up of 12 years, rapid metabolizers with prior history of breast neoplasms allocated to tamoxifen 5 mg/day had a 60 % reduction of risk of recurrences (HR = 0.40, 95 % CI: 0.16–0.99) compared to slow metabolizers. CYP2D6 genotype may have an impact on tamoxifen efficacy at low doses. Trials investigating tamoxifen dose adjustments based on the woman’s hormonal context and CYP2D6 genotype are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tamoxifen is widely used as adjuvant treatment for women operated for estrogen receptor-positive breast cancer, since it effectively reduces the rate of recurrence and breast cancer mortality [1]. In view of its well-understood side effect profile and efficacy in preventing estrogen receptor-positive breast cancer, tamoxifen has been adopted in the prevention setting too, confirming its benefit also for high-risk women [2]. Tamoxifen-specific adverse events are venous thromboembolic events and endometrial cancers which appear to be dose related [3, 4].
Our group has conducted several prevention trials focusing on the assessment of the optimal dose, able to maintain efficacy, while minimizing tamoxifen side effects. Indeed, our studies indicate that tamoxifen may be reduced to 5 or even 1 mg/day without a significant loss of its antiproliferative activity on breast cancer, as assessed by Ki-67 expression [5], whereas modulation of circulating biomarkers linked to breast cancer risk followed a dose–response relationship [5, 6]. Importantly, a dose of 5 mg/day showed no increased endometrial proliferation compared with placebo [6].
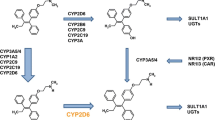
The clinical effectiveness of tamoxifen varies among individuals. Tamoxifen itself has weak affinity for the estrogen receptor and undergoes considerable first-pass oxidative metabolism to more potent metabolites, such as 4-hydroxytamoxifen and 4-hydroxy-N-desmethyltamoxifen (endoxifen), by a multitude of cytochrome P450 (CYP) enzymes. The rate-limiting step in tamoxifen metabolism is mediated primarily by the highly polymorphic CYP2D6 [7].
Numerous studies have shown that CYP2D6 genetic variants that lead to the absence of functional enzyme are associated with lower concentrations of 4-hydroxytamoxifen and endoxifen [8]. Nevertheless, there is still considerable controversy on the clinical relevance of CYP2D6 genotype as a predictor of tamoxifen efficacy at the standard dose of 20 mg/day. Nonfunctional alleles of CYP2D6 were reported to be associated with higher risk of breast cancer recurrence [8–20] in breast cancer patients and increased breast cancer incidence in unaffected women [21], although other studies did not support such an association [22–25]. Evidence has emerged that may clarify some of the causes of these conflicting results [26, 27], including the criteria adopted for subject selection, source of DNA, coverage of genetic variations, and circulating concentrations of tamoxifen metabolites.
Besides CYP2D6 genotyping, given that tamoxifen metabolism also depends on a subjects’ clinical conditions, body mass index (BMI), and menopausal status, an approach would be to directly measure tamoxifen metabolites [27] followed by dose adjustments, if needed. The first study to report on an association between circulating endoxifen levels and breast cancer outcomes suggested a minimal threshold to obtain therapeutic effect [9]. The predictors of this high-risk group were CYP2D6 genotype, higher BMI, and lower tamoxifen concentrations. It is reasonable to assume that CYP2D6 genotype could have an even greater impact at low-dose tamoxifen regimens. Here, we present the results from a pooled analysis of four chemoprevention trials assessing very low doses (1 mg/day, n = 52 or 10 mg/week, n = 152) or low dose (5 mg/day, n = 171) of tamoxifen to investigate the role of CYP2D6 pharmacogenetics on tamoxifen activity through analysis of pharmacokinetics, modulation of breast cancer risk biomarkers (IGF-I and SHBG), and efficacy on breast neoplastic events.
Methods
Study population
This pooled analysis includes patients participating in four different double-blind breast cancer prevention trials. The study protocols were approved by the local institutional review boards, and each participant gave her written informed consent. The pooled analysis included patients randomized to any tamoxifen dose. Three different low-dose tamoxifen schedules were administered: 1 mg/day (n = 52), 10 mg/week (n = 152), and 5 mg/day (n = 171). Two studies included arms of low-dose tamoxifen in combination with anastrozole [28] and fenretinide [29, 30]. The combination of these drugs did not affect tamoxifen metabolite concentrations, nor the biomarker activity [28, 29].
Brief description of the four biomarker trials
S109: Postmenopausal women with breast intraepithelial neoplasia were allocated to 10 mg/week tamoxifen alone (n = 25), 1 mg/day anastrozole (n = 25), or in combination with 1 mg/day anastrozole (n = 25) for 12 months. Main results have previously been published [28].
S52: Healthy postmenopausal women on HRT were randomly assigned to tamoxifen 1 mg/day (n = 52), tamoxifen 10 mg/week (n = 52), tamoxifen 5 mg/day (n = 53), or placebo (n = 53) for 12 months. Main results have previously been published [6].
007: Premenopausal women with intraepithelial neoplasia, microinvasive disease, or 5-year Gail risk >1.3 % were randomly allocated either to tamoxifen 5 mg/day (n = 58), fenretinide 200 mg/day (n = 59), their combination (n = 60), or placebo (n = 58) for two years. Trial registration number CNR-IEO-007 web link: http://www.cancer.gov/clinicaltrials/search/view?cdrid=305999&version=HealthProfessional&protocols. Main results have previously been published [29].
S162: Premenopausal women with early estrogen receptor-positive breast cancer (n = 50) were assigned to tamoxifen 10 mg/week (n = 50), raloxifene 60 mg/day, or placebo (n = 25) for 6 weeks before surgery. Trial registration number ISRCTN86894592; web link: www.isrctn.com/ISRCTN86894592. Main results have previously been published [31].
Sample collection and DNA extraction
Morning fasting blood samples were collected at baseline and at different sampling intervals according to trial design. The shortest interval of tamoxifen exposure was 6 weeks in trial S162, which showed drug levels similar to 12 months. The samples were stored at −80 °C until assayed. Circulating concentrations of tamoxifen, its metabolites, and biomarkers were determined on serum. Genomic DNA was extracted from frozen whole EDTA-treated blood samples with the QIAamp DNA Blood Kit (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions, by the use of the automated Qiacube. DNA concentration was adjusted to 20–70 ng/μl before genotyping.
Genotyping and metabolic activity classification
The CYP2D6 genotyping was performed in a blinded fashion by a fully automated multiplex analyzer, the INFINITI™ (AutoGenomics, Carlsbad, CA, USA), based on a detection primer hybridization and extension method applying fluorescent nucleotides. The analyses were performed according to manufacturer instructions. INFINITI CYP450 2D6T assay (*2, *3, *4, *5, *6, *9, *29, *41) was applied in 246 subjects and CYP450 2DI assay in 121 subjects. The latter assay additionally included CYP2D6*2A, *7, *8, *10, *12, *14, *17, and *XN. According to the predicted enzyme activity, the different alleles are categorized in four groups. Full enzyme activity: *1 (wt), *2 (2850C>T; rs16947), *2A (−1584 C>G; rs1080985); Null activity: *3 (2549delA; rs35742686), *4 (1846G>A; rs3892097), *5 (CYP2D6 deleted), *6 (1707 delT; rs5030655), *7 (2935A>C; rs5030867), *8 (1758G>T; rs5030865), *12 (124G>A; rs5030862), *14 (1758G>A; rs5030865); Reduced activity: *9 (2615–2617delAGG; rs5030656), *10 (100C>T; rs1065852), *17 (1023C>T rs28371706), *29 (1659G>A; rs61736512), and *41 (2988G>A; rs28371725); Duplications: *XN: INFINITI does not establish how many duplicates, nor which specific allele type. If the subject had fully functional alleles in association with a duplication, we assumed the patient was an ultrarapid metabolizer.
Phenotypes, or metabolic activity classification, were assigned to subjects depending on the combination of the two alleles, determining the overall genotype effect. Women were classified into four different metabolic phenotypes according to predictive enzymatic activity, as previously described [9, 32]. Women were defined as extensive metabolizers (EM), if they had two fully functional alleles or if they carried one reduced function alleles or one nonfunctional allele in combination with a fully function allele; intermediate metabolizers (IM), if they carried two reduced function alleles or one reduced and one nonfunctional allele, while poor metabolizers (PM) carried two nonfunctional alleles; ultrarapid metabolizers (UM) had fully functional alleles and a duplication.
Circulating biomarkers and tamoxifen metabolites
Serum insulin-like growth factor-I (IGF-I) and sex hormone-binding globulin (SHBG) were determined, as previously described [29, 30].
A high-pressure liquid chromatography–tandem mass spectrometry system was used to determine tamoxifen and its metabolites in serum [33, 34]. The assay was modified to improve the sensitivity by changing the API 2000/Qtrap mass spectrometry system from Applied Biosystems (AB MDS Sciex, Concord, Canada) to the API 4000, equipped with TurboIonSpray. In this method, N-desmethyl-α-hydroxytamoxifen, N-desmethyl-3-hydroxytamoxifen, α-hydroxytamoxifen, 3-hydroxytamoxifen, and the isoforms E and Z of endoxifen and 4-hydroxytamoxifen were not determined. This may result in some overestimation of endoxifen and 4-hydroxytamoxifen [35]. Tamoxifen citrate and 4-hydroxytamoxifen were purchased from Sigma-Aldrich (Steinheim, Germany); the internal standard deuterated 5-tamoxifen (D5Tam) and tamoxifen-N-oxide (TamNox) from Beta Chem Inc. (Kansas, USA), and endoxifen from Sintef Materials and Chemistry (Oslo, Norway). N-desmethyltamoxifen (NDTam) and N-desdimethyltamoxifen (NDDTam) were gifts from the Pharmaceuticals Division of Imperial Chemical Industries PLC (Macclesfield, UK).
Statistical analysis
This paper describes a pooled analysis of individual patients data from four randomized clinical trials. Median values, interquartile ranges, and results from nonparametric Wilcoxon tests were presented to investigate differences in biomarker levels and metabolites according to dose arms (1 mg/day or 10 mg/week vs 5 mg/day) and CYP2D6 metabolic status (‘Impaired or poor’ vs ‘normal or ultrarapid’ metabolizers). Between-study heterogeneity of frequencies of CYP2D6 metabolic status was checked with Chi-square tests. Circulating biomarkers were investigated as continuous variables, evaluating changes in time. Changes from baseline of biomarkers and differences of tamoxifen metabolites by CYP2D6 metabolic status were analyzed through random effects models, for each treatment groups, with ‘study’ as random factor, and adjusting for baseline values (for biomarkers), age, BMI, and disease status when significant (PROC MIXED, SAS). The validity of the assumptions of the analysis was checked using residual plots from saturated models.
We evaluated the association of CYP2D6 metabolic status with breast cancer events during follow-up among subjects at high risk, i.e., history of prior intraepithelial neoplasia or minimally invasive disease, pT1a. Time to death and time to breast recurrence were defined as the time from study entry until the event of interest. All patients alive or free of disease at last follow-up date were considered right censored. Kaplan–Meier curves of Disease-Free Survival and Cox proportional hazards models adjusted for ‘study’ and relevant confounders were presented to assess the association of CYP2D6 metabolic status, adjusted for confounder. All statistical tests were two-sided, and P < 0.05 was considered statistically significant.
The statistical analyses were performed with the Statistical Analysis System Version 9.2 (SAS Institute, Cary, NC).
Results and discussion
Overall study Flow
A flow diagram of participants included in the pooled analysis is presented in Fig. 1. Blood draw was performed at baseline and at different time intervals according to study design. A total of 375 women were randomized to tamoxifen at three different low-dose regimens. Because we already know that the addition of 1 mg/day anastrozole to 10 mg/week tamoxifen does not affect tamoxifen pharmacokinetics [28], likewise the combination of 200 mg/day fenretinide to 5 mg/day tamoxifen [29], we also included these arms into the pooled analysis. Consent for genotyping was obtained from 368 participants, and 367 were successfully genotyped. Of these subjects, 322 had detectable serum concentrations of tamoxifen at follow-up blood draw.
Patient characteristics
Baseline characteristics of subjects are illustrated in Table 1. We grouped women randomized to either 10 mg/week or 1 mg/day into the very-low-dose group since drug levels were similar, whereas women allocated to tamoxifen 5 mg/day were defined as the low-dose group. The latter were younger (median age 49 years) than women in the former group (median age 54 years).
Genotype profiles
The allele frequencies are presented in appendix 1. All subjects were Caucasians with European descent. All subjects consenting for DNA analysis were successfully genotyped, except for one woman in whom DNA was re-extracted from another blood aliquot, but neither the INFINITI assay nor the Taq Man assay detected the genotype, pointing to a genetic alteration that hampered primer ligation. The allele frequencies of CYP2D6*2, *2A, *4, and *41 were in Hardy–Weinberg Equilibrium (HWE). The frequencies of the other variants were too rare in relation to sample size to estimate HWE. CYP2D6*7, *12, *14, *17, *29 were not represented in our population (Supplementary table S1). According to the combination of alleles, the predictive phenotype distribution was as follows: 268 extensive metabolizers, 70 intermediate metabolizers, 23 poor metabolizers, and 6 ultrarapid metabolizers.
Serum concentrations of tamoxifen and its metabolites by CYP2D6
To analyze the effect of CYP2D6 genotypes on serum concentrations of tamoxifen and metabolites according to tamoxifen dose, we grouped women with impaired or poor metabolic status into “slow metabolizers” and compared them with extensive or ultrarapid (“rapid”) metabolizers.
Between-study heterogeneity of frequencies of CYP2D6 metabolic status was not significant (Chi Square P = 0.44), nor was between-dose heterogeneity (P = 0.13).
Serum concentrations of tamoxifen and its metabolites are presented according to tamoxifen dose (1 mg/day or 10 mg/week vs 5 mg/day) in Table 2. Subjects being compliant at blood draw were included (tamoxifen concentration > 0 ng/mL, n = 322). An important point is timing between the last pill intake and date of blood draw: For the 1 mg/daily and 5 mg/daily doses, the last pill was taken the night before or early in the morning, the same day of blood draw, whereas concerns the 10 mg/weekly dose, the time elapse was longer, usually between 5–7 days. Considering that the steady-state concentrations for tamoxifen are achieved in about 4 weeks and that the most abundant metabolite, N-desmethyltamoxifen, main CYP2D6 substrate, has a half-life of approximately 2 weeks, the time elapse of 5–7 days appear less critical. At the very low doses (1 mg/day or 10 mg/week), the metabolite levels appear to be proportionally similar. In the S52 dose-ranging trial [6], after 12 months tamoxifen at 1 mg/day (n = 40), unadjusted mean serum concentration of tamoxifen was 6.1 and 2.9 ng/ml of endoxifen, while with 10 mg/week (n = 41), mean levels were 8.4 and 3.7 ng/ml, respectively (unpublished results). Furthermore, we did not find any relevant difference in treatment duration in this pooled analysis. Comparing serum concentrations of endoxifen and other metabolites in different settings with 10 mg tamoxifen/week, 6-week [31] and 12-month [28] treatment showed similar results. Specifically, considering the 6-week tamoxifen administration in the presurgical trial S162 (n = 45 premenopausal breast cancer patients), the mean serum concentrations of tamoxifen were 9.5 ng/ml and endoxifen 3.5 ng/ml, thus very similar to the S52 trial.
We detected higher concentrations of N-desmethyltamoxifen in slow versus rapid metabolizers, pointing to an accumulation of the metabolite in these subjects. The difference reached statistical significance at the very-low-dose tamoxifen, where the median level was 20 ng/ml in slow metabolizers versus 16 ng/ml in rapid metabolizers (P = 0.007). Rapid metabolizers had higher endoxifen levels than slow metabolizers: 15.3 versus 12.2 ng/mL (P = 0.018) with 5 mg/day and 3.8 versus 2.8 ng/mL (P = 0.004) with 1 mg/day or 10 mg/week tamoxifen (Fig. 2).
Serum concentrations of insulin-like growth factor-I and sex hormone-binding globulin according to tamoxifen dose and composite genotype
Tamoxifen 5 mg/day has a favorable effect on IGF-I [29] and SHBG [30], with a decrease in IGF-I and an increase in SHBG, while the very low doses did not significantly modulate these biomarkers in this pooled analysis (data not shown). The change in IGF-I levels correlated strongly and inversely with the circulating concentrations of endoxifen (Fig. 3, panel A, 4OHNdesTam; P = 0.002), and 4-hydroxytamoxifen (Fig. 3, panel B, 4OHTam; P = 0.001), demonstrating steeper decreases in IGF-I at higher levels of active metabolites. Five women had falls in IGF-I levels of over 100 ng/ml. These presented with quite high levels at baseline. Our experience is that the fall in IGF-I by tamoxifen tends to be steeper in subjects having higher baseline levels. The IGF-I levels tend to rise to baseline values after treatment cessation (Supplementary table S2). As opposed to IGF-I, the SHBG increase was independent of circulating endoxifen levels (not shown). The CYP2D6 phenotype alone did not predict tamoxifen ability to lower circulating levels of IGF-I (Supplementary table S3) nor SHBG.
Role of CYP2D6 composite genotype on low-dose tamoxifen efficacy on recurrence
In two studies, S109 and 007, patients had been followed up for a median time of 11 years, so that we were able to investigate the predictive value of CYP2D6 genotype on low-dose tamoxifen prevention efficacy in women who had an intraepithelial neoplasia and were allocated either to 10 mg/week tamoxifen (n = 50) or tamoxifen 5 mg/day (n = 118). Rapid metabolizers with prior history of breast neoplasms (n = 118) had 32 (27 %) second breast events, whereas slow metabolizers (n = 19) had 8 (42 %) second breast events, accounting for a 52 % borderline significant reduction (HR = 0.48, 95 % CI, 0.22–1.05; P = 0.06 adjusted for study). Since there was an indication for a between-study heterogeneity (P value for study = 0.07), we investigated also the 007 trial alone, and with 5 mg/day, the risk reduction was similar but even stronger, with a 60 % risk reduction (HR = 0.40; 95 % CI, 0.16–0.99; P = 0.046 adjusted for age, Fig. 4).
There was no significant association between endoxifen levels and breast neoplastic events in the trial 007. Out of 24 women with endoxifen levels below the lower quartile (<12.3 ng/mL), 33 % developed a second breast neoplasm, compared to 30 % among 73 women having endoxifen levels above the lower quartile (>12.3 ng/mL, Chi square P = 0.5).
Discussion
The activity of tamoxifen is considered to reside in its conversion to the more active metabolites having greater binding affinity for the estrogen receptor along with their potent inhibitory effects on breast cancer cell proliferation [36]. The broad interindividual variability in serum concentrations of tamoxifen and its metabolites is well documented [37] and provides an opportunity for a personalized treatment dose of tamoxifen based upon clinical context, metabolic state, and genetic features, including CYP2D6 profiling. Lower doses of tamoxifen have been used in an attempt to reduce risks while retaining benefits in the prevention setting [5].
Pooling four different low- (5 mg/day) or very-low-dose (1 mg/day or 10 mg/week) tamoxifen breast cancer prevention trials enabled us to gain insight into the associations of CYP2D6 genotype at different low-dose tamoxifen regimens, demonstrating that CYP2D6 composite genotype impacts on circulating tamoxifen metabolites even at low-dose tamoxifen.
The minimal active dose of tamoxifen in breast cancer prevention is still a matter of debate. Observational studies indicate that low-dose tamoxifen is effective in preventing recurrence of highly endocrine ductal neoplasia [38]. Findings from our window-of-opportunity presurgical trial point to 5 mg/day or even 1 mg/day as the minimal dose required to retain a sufficient inhibitory activity on breast cancer proliferation, while limiting its adverse side effects [5]. Conversely, several circulating breast cancer risk biomarkers follow a dose–response relationship with tamoxifen and its metabolite levels [37], including IGF-I and SHBG [5]. We observed a direct association between serum concentrations of endoxifen and IGF-I decrease. Still, the CYP2D6 phenotype did not independently predict tamoxifen effect in decreasing circulating levels of IGF-I. There were differences between trials that may have contributed to variability in IGF-I levels. Specifically, women taking the 5 mg/day dose of tamoxifen were younger and more often premenopausal and thus naïve from hormone replacement therapy (68 %). They had higher baseline levels of IGF-I and SHBG compared to the very-low-dose group. Sex steroids, including exogenous hormone replacement therapy, are known to enhance the IGF system and increase SHBG, which could in part mask the effect of tamoxifen on IGF-I and SHBG [39, 40].
Importantly, the CYP2D6 composite genotype predicted efficacy of low-dose tamoxifen in preventing second breast neoplasms. With the 5 mg/day dose, tamoxifen attained a 60 % reduction of recurrence in rapid metabolizers compared to slow metabolizers. At randomization, the majority of these unaffected premenopausal women had high endogenous estradiol concentrations, characteristic of an active ovarian cycle [31]. Since tamoxifen is a selective estrogen receptor modulator with antagonistic activity in breast tissue, circulating estrogens are obvious competitors of tamoxifen metabolites for the estrogen receptor. Additionally, Lien et al. recently reported that circulating levels of tamoxifen and metabolites are lower in premenopausal women than in postmenopausal women [27]. These aspects may contribute to a potentially greater impact of the CYP2D6 genotype on estrogen-dependent cancer growth in premenopause women, as higher levels of active metabolites are expected to be needed to effectively decrease estrogen receptor-dependent proliferative activity of the breast. In prevention of postmenopausal breast cancer in women of normal weight and low serum estrogens, even a very-low-dose tamoxifen might be considered for CYP2D6 rapid metabolizers. This hypothesis is strengthened by the evidence coming from case–control studies nested in prevention trials [22] and adjuvant trials [23, 24], reporting a lack of a clinical significance of CYP2D6 genotype in predicting tamoxifen response in postmenopausal women. Furthermore, a Swedish study [10] reported an effect on recurrence mainly in premenopausal patients. In a case–control study nested within the Italian chemoprevention trial of tamoxifen 20 mg daily, we previously reported an association between the CYP2D6 *4/*4 genotype and breast cancer risk [21]. Furthermore, the efficacy of tamoxifen in preventing breast cancer in that trial was greater in hormone replacement users [41] and high-risk women [42].
It is of note that although CYP2D6 genotype predicted efficacy of low-dose tamoxifen, we did not observe any significant association between endoxifen levels and breast neoplastic events. Several hypotheses can be advanced. Tamoxifen accumulates in adipose and breast tissue, and CYP2D6 is metabolically active not only in the liver, but also in breast tissue. It is possible that at low doses of tamoxifen, methods for detecting tamoxifen metabolites may not have sufficient sensitivity to detect differences at low concentrations of tamoxifen. Thus, any potential differences due to individual metabolic characteristics tend to flatten at these low ranges of endoxifen, and the statistical power to detect them is insufficient.
Our study indicates that the CYP2D6 genotype do influence metabolite levels and may affect the preventive efficacy of low doses of tamoxifen. Low-dose tamoxifen in breast cancer prevention is safe and active among CYP2D6 rapid metabolizers and further supports the use of 5 mg/day in the prevention setting. This dose might be better tolerated with regards to menopausal symptoms like hot flashes, endometrial polyps, and other menopausal symptoms than the standard dose of 20 mg/day [43]. Whereas the weekly dose of tamoxifen slightly increased endometrial thickness, the 5 mg/day dose was not associated with an increase in endometrial proliferation as assessed by Ki-67 compared to placebo [6]. To validate our findings, CYP2D6 genotype is being analyzed within two phase III placebo-controlled trials of tamoxifen at 5 mg/day [44, 45]. Theoretically, a personalized preventive treatment would entail a different dose according to the CYP2D6 composite genotype, such as 5 mg/day tamoxifen to rapid metabolizers and a higher tamoxifen dose, or a different compound (e.g., an aromatase inhibitor) in slow metabolizers.
Although our study includes a relatively small sample size, a strength is that all four trials were designed and conducted at our Institution, and participant adherence to study protocol was very high. The pooled analysis enabled us to gain statistical power that could not be obtained in the single trials because poor metabolic status is not frequent enough. Furthermore, three different tamoxifen doses were adopted in one of the trials.
In conclusion, our analysis of four randomized low-dose tamoxifen breast cancer prevention trials yields the first evidence of an association between CYP2D6 composite genotype and circulating levels of 4-hydroxylated tamoxifen metabolites at 5 mg/day, or even 1 mg/day of tamoxifen. A clinically relevant finding was that CYP2D6 composite genotype may increase the preventive efficacy of low doses of tamoxifen. Trials investigating tamoxifen dose adjustments based on the woman’s hormonal context and CYP2D6 genotype are warranted.
Abbreviations
- 4OHTam:
-
4-hydroxytamoxifen
- 4OHNdesTam:
-
4-hydroxy-N-desmethyltamoxifen, or endoxifen
- BMI:
-
Body mass index
- EM:
-
Extensive metabolizers
- HRT:
-
Hormone replacement therapy
- IGF:
-
Insulin-like growth factor
- IM:
-
Intermediate metabolizers
- IQR:
-
Interquartile ranges
- NDDTam:
-
N-desdimethyltamoxifen
- NDTam:
-
N-desmethyltamoxifen
- PM:
-
Poor metabolizers
- SHBG:
-
Sex hormone-binding globulin
- UM:
-
Ultrarapid metabolizers
References
Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S et al (2011) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 378:771–784
Cuzick J, Sestak I, Bonanni B, Costantino JP, Cummings S, Decensi A et al (2013) Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet 381:1827–1834
Rutqvist LE, Johansson H, Signomklao T, Johansson U, Fornander T, Wilking N (1995) Adjuvant tamoxifen therapy for early stage breast cancer and second primary malignancies. Stockholm Breast Cancer Study Group. J Natl Cancer Inst 87:645–651
Early Breast Cancer Trialists’ Collaborative Group (1998) Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 351:1451–1467
Decensi A, Robertson C, Viale G, Pigatto F, Johansson H, Kisanga ER et al (2003) A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst 95:779–790
Decensi A, Gandini S, Serrano D, Cazzaniga M, Pizzamiglio M, Maffini F et al (2007) Randomized dose-ranging trial of tamoxifen at low doses in hormone replacement therapy users. J Clin Oncol 25:4201–4209
Klein DJ, Thorn CF, Desta Z, Flockhart DA, Altman RB, Klein TE (2013) PharmGKB summary: tamoxifen pathway, pharmacokinetics. Pharmacogenet Genomics 23:643–647
Kiyotani K, Mushiroda T, Imamura CK, Hosono N, Tsunoda T, Kubo M et al (2010) Significant effect of polymorphisms in CYP2D6 and ABCC2 on clinical outcomes of adjuvant tamoxifen therapy for breast cancer patients. J Clin Oncol 28:1287–1293
Madlensky L, Natarajan L, Tchu S, Pu M, Mortimer J, Flatt SW et al (2011) Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther 89:718–725
Margolin S, Lindh JD, Thoren L, Xie H, Koukel L, Dahl ML et al (2013) CYP2D6 and adjuvant tamoxifen: possible differences of outcome in pre- and post-menopausal patients. Pharmacogenomics 14:613–622
Schroth W, Goetz MP, Hamann U, Fasching PA, Schmidt M, Winter S et al (2009) Association between CYP2D6 polymorphisms and outcomes among women with early stage breast cancer treated with tamoxifen. JAMA 302:1429–1436
Newman WG, Hadfield KD, Latif A, Roberts SA, Shenton A, McHague C et al (2008) Impaired tamoxifen metabolism reduces survival in familial breast cancer patients. Clin Cancer Res 14:5913–5918
Xu Y, Sun Y, Yao L, Shi L, Wu Y, Ouyang T et al (2008) Association between CYP2D6 *10 genotype and survival of breast cancer patients receiving tamoxifen treatment. Ann Oncol 19:1423–1429
Bijl MJ, van Schaik RH, Lammers LA, Hofman A, Vulto AG, van GT et al (2009) The CYP2D6*4 polymorphism affects breast cancer survival in tamoxifen users. Breast Cancer Res Treat 118:125–130
Cajal T, Altes A, Pare L, del RE, Alonso C, Barnadas A et al (2010) Impact of CYP2D6 polymorphisms in tamoxifen adjuvant breast cancer treatment. Breast Cancer Res Treat 119:33–38
Park HS, Choi JY, Lee MJ, Park S, Yeo CW, Lee SS et al (2011) Association between genetic polymorphisms of CYP2D6 and outcomes in breast cancer patients with tamoxifen treatment. J Korean Med Sci 26:1007–1013
Teh LK, Mohamed NI, Salleh MZ, Rohaizak M, Shahrun NS, Saladina JJ et al (2012) The risk of recurrence in breast cancer patients treated with tamoxifen: polymorphisms of CYP2D6 and ABCB1. AAPS J 14:52–59
Sukasem C, Sirachainan E, Chamnanphon M, Pechatanan K, Sirisinha T, Ativitavas T et al (2012) Impact of CYP2D6 polymorphisms on tamoxifen responses of women with breast cancer: a microarray-based study in Thailand. Asian Pac J Cancer Prev 13:4549–4553
Damodaran SE, Pradhan SC, Umamaheswaran G, Kadambari D, Reddy KS, Adithan C (2012) Genetic polymorphisms of CYP2D6 increase the risk for recurrence of breast cancer in patients receiving tamoxifen as an adjuvant therapy. Cancer Chemother Pharmacol 70:75–81
Goetz MP, Suman VJ, Hoskin TL, Gnant M, Filipits M, Safgren SL et al (2013) CYP2D6 metabolism and patient outcome in the Austrian Breast and Colorectal Cancer Study Group trial (ABCSG) 8. Clin Cancer Res 19:500–507
Bonanni B, Macis D, Maisonneuve P, Johansson H, Gucciardo G, Oliviero P et al (2006) Polymorphism in the CYP2D6 Tamoxifen-Metabolizing Gene Influences Clinical Effect but Not Hot Flashes: data From the Italian Tamoxifen Trial. J Clin Oncol 24:3708–3709
Goetz MP, Schaid DJ, Wickerham DL, Safgren S, Mushiroda T, Kubo M et al (2011) Evaluation of CYP2D6 and Efficacy of Tamoxifen and Raloxifene in Women Treated for Breast Cancer Chemoprevention: results from the NSABP P1 and P2 Clinical Trials. Clin Cancer Res 17:6944–6951
Rae JM, Drury S, Hayes DF, Stearns V, Thibert JN, Haynes BP et al (2012) CYP2D6 and UGT2B7 Genotype and Risk of Recurrence in Tamoxifen-Treated Breast Cancer Patients. J Natl Cancer Inst 104:452–460
Regan MM, Leyland-Jones B, Bouzyk M, Pagani O, Tang W, Kammler R et al (2012) CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1-98 trial. J Natl Cancer Inst 104:441–451
Sestak I, Kealy R, Nikoloff M, Fontecha M, Forbes JF, Howell A et al (2012) Relationships between CYP2D6 phenotype, breast cancer and hot flushes in women at high risk of breast cancer receiving prophylactic tamoxifen: results from the IBIS-I trial. Br J Cancer 107:230–233
Kiyotani K, Mushiroda T, Zembutsu H, Nakamura Y (2013) Important and critical scientific aspects in pharmacogenomics analysis: lessons from controversial results of tamoxifen and CYP2D6 studies. J Hum Genet 58:327–333
Lien EA, Soiland H, Lundgren S, Aas T, Steen VM, Mellgren G et al (2013) Serum concentrations of tamoxifen and its metabolites increase with age during steady-state treatment. Breast Cancer Res Treat 141:243–248
Bonanni B, Serrano D, Gandini S, Guerrieri-Gonzaga A, Johansson H, Macis D et al (2009) Randomized biomarker trial of anastrozole or low-dose tamoxifen or their combination in subjects with breast intraepithelial neoplasia. Clin Cancer Res 15:7053–7060
Decensi A, Robertson C, Guerrieri-Gonzaga A, Serrano D, Cazzaniga M, Mora S et al (2009) Randomized double-blind 2 x 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol 27:3749–3756
Johansson H, Bonanni B, Gandini S, Guerrieri-Gonzaga A, Cazzaniga M, Serrano D et al (2013) Circulating hormones and breast cancer risk in premenopausal women: a randomized trial of low-dose tamoxifen and fenretinide. Breast Cancer Res Treat 142:569–578
Serrano D, Lazzeroni M, Gandini S, Macis D, Johansson H, Gjerde J et al (2013) A randomized phase II pre-surgical trial of weekly low-dose tamoxifen versus raloxifene versus placebo in premenopausal women with estrogen receptor positive breast cancer. Breast Cancer Res 15:R47
Serrano D, Lazzeroni M, Zambon CF, Macis D, Maisonneuve P, Johansson H et al (2011) Efficacy of tamoxifen based on cytochrome P450 2D6, CYP2C19 and SULT1A1 genotype in the Italian Tamoxifen Prevention Trial. Pharmacogenomics J 11:100–107
Gjerde J, Kisanga ER, Hauglid M, Holm PI, Mellgren G, Lien EA (2005) Identification and quantification of tamoxifen and four metabolites in serum by liquid chromatography-tandem mass spectrometry. J Chromatogr A 1082:6–14
Gjerde J, Hauglid M, Breilid H, Lundgren S, Varhaug JE, Kisanga ER et al (2008) Effects of CYP2D6 and SULT1A1 genotypes including SULT1A1 gene copy number on tamoxifen metabolism. Ann Oncol 19:56–61
Jager NGL, Rosing H, Linn SC, Schellens JHM, Beijnen JH (2012) Importance of highly selective LC-MS/MS analysis for the accurate quantification of tamoxifen and its metabolites: focus on endoxifen and 4-hydroxytamoxifen. Breast Cancer Res Treat 133:793–798
Murdter TE, Schroth W, Bacchus-Gerybadze L, Winter S, Heinkele G, Simon W et al (2011) Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther 89:708–717
Kisanga ER, Gjerde J, Guerrieri-Gonzaga A, Pigatto F, Pesci-Feltri A, Robertson C et al (2004) Tamoxifen and metabolite concentrations in serum and breast cancer tissue during three dose regimens in a randomized preoperative trial. Clin Cancer Res 10:2336–2343
Guerrieri-Gonzaga A, Botteri E, Lazzeroni M, Rotmensz N, Goldhirsch A, Varricchio C et al (2010) Low-dose tamoxifen in the treatment of breast ductal intraepithelial neoplasia: results of a large observational study. Ann Oncol 21:949–954
Johansson H, Baglietto L, Guerrieri-Gonzaga A, Bonanni B, Mariette F, Macis D et al (2004) Factors associated with circulating levels of insulin-like growth factor-I and insulin-like growth factor binding protein-3 in 740 women at risk for breast cancer. Breast Cancer Res Treat 88:63–73
Johansson H, Gandini S, Bonanni B, Mariette F, Guerrieri-Gonzaga A, Serrano D et al (2008) Relationships between circulating hormone levels, mammographic percent density and breast cancer risk factors in postmenopausal women. Breast Cancer Res Treat 108:57–67
Veronesi U, Maisonneuve P, Costa A, Sacchini V, Maltoni C, Robertson C et al (1998) Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women. Italian Tamoxifen Prevention Study. Lancet 352:93–97
Veronesi U, Maisonneuve P, Rotmensz N, Bonanni B, Boyle P, Viale G et al (2007) Tamoxifen for the Prevention of Breast Cancer: late Results of the Italian Randomized Tamoxifen Prevention Trial Among Women With Hysterectomy. J Natl Cancer Inst 99:727–737
Decensi A, Bonanni B, Maisonneuve P, Serrano D, Omodei U, Varricchio C et al (2013) A phase-III prevention trial of low-dose tamoxifen in postmenopausal hormone replacement therapy users: the HOT study. Ann Oncol 24:2753–2760
Iqbal J, Ginsburg OM, Wijeratne TD, Howell A, Evans G, Sestak I et al (2012) Endometrial cancer and venous thromboembolism in women under age 50 who take tamoxifen for prevention of breast cancer: a systematic review. Cancer Treat Rev 38:318–328
Zanardi S, Branchi D, Ponti A, Cruciani G, D’Amico C, Cortesi L et al (2011) Abstract A56: randomized, placebo-controlled, phase III trial of low-dose tamoxifen in women with intraepithelial neoplasia. Cancer Prev Res 4:A56
Acknowledgments
We thank Medical Systems S.p.A. (Genoa, Italy) for providing instruments for CYP analysis and Tiziana Chiesa for her technical assistance.
Funding
Lega Italiana per la Lotta contro i Tumori, Italian Foundation for Cancer Research, National Cancer Institute (Grant No CA-77188), A regional Grant (1068/2005) on second tumors from the Italian Association for Cancer Research, Susan Komen Breast Cancer Foundation (Grant No. BCTR01-00537), Italian Health Ministry (Ricerca Finalizzata 2004/86), Gruppo Bancario Credito Valtellinese (Research Fellowship), European Institute of Oncology Foundation, Western Norway Regional Health Authority.
Author contribution
HJ, SG, and DS contributed to the conception and design of the study. For the clinical interpretation of the data, HJ was assisted by DS and ADC. SG performed the statistical analysis. AGG assisted with her expertise in data management and coordination of clinical trial reporting. JG performed tamoxifen metabolite measurements. DM and VA performed DNA extraction and genotyping of CYP2D6, assisted by ML at Medical System S.p.A. (Genoa, Italy). HJ gave technical support for laboratory measurements and quality check of lab analysis. GM and EAL participated in the trial design as experts in the field of tamoxifen pharmacokinetics studies. BB and ADC are PIs of the four chemoprevention trials and Senior coauthors of this manuscript. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no competing interests.
Additional information
Andrea DeCensi and Bernardo Bonanni are Senior co-authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Johansson, H., Gandini, S., Serrano, D. et al. A pooled analysis of CYP2D6 genotype in breast cancer prevention trials of low-dose tamoxifen. Breast Cancer Res Treat 159, 97–108 (2016). https://doi.org/10.1007/s10549-016-3932-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3932-7