Abstract
Purpose
Results of previous studies on the associations between Forkhead box A1 (FOXA1) expression in breast cancer tissues and the prognosis varied depending on the follow-up durations. The present study would investigate whether there is a time-varying effect of FOXA1 in breast cancer tissues on the prognosis.
Methods
FOXA1 expressions were evaluated in 1041 primary invasive breast tumors with tissue microarrays by immunohistochemistry. Cox models with restricted cubic splines and Kaplan–Meier survival analysis were used to examine the associations between FOXA1 and the prognosis. Flexible parametric models were applied to explore the time-varying effect of FOXA1.
Results
Overall, the association between FOXA1 expression and the prognosis was not significant but varied on the time of follow-up. Compared to FOXA1 ≤ 270 of H-score, the hazard ratios (HRs) of death for those with 271–285 of FOXA1 expression increased from 0.35 (95% CI 0.14–0.86) at 6 months after diagnosis to 2.88 (95% CI 1.35–6.15) at 120 months with a crossover at around 36 months. Similar patterns were also observed for FOXA1 > 285 of H-score and for progression free survival (PFS). Moreover, when allowed both FOXA1 and estrogen receptor (ER) to change over time in the model (considering that ER had a similar time-varying effect), these time-varying effects remained for FOXA1 on both overall survival (OS) (P < 0.01) and PFS (P = 0.01) but were attenuated for ER (P = 0.13 for OS).
Conclusions
This study revealed an independent time-varying effect of FOXA1 on breast cancer prognosis, which would provide an insight into the roles of FOXA1 as a marker of breast cancer prognosis and may help optimize the medication strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Forkhead box A1 (FOXA1), as a member of FOX family proteins, is a master regulator in hormone-sensitive tissues [1]. In breast cancer, FOXA1 plays multi-functional roles. It is involved in the interactions of estrogen receptor (ER) with chromatin and promotes the development of breast cancer [1, 2] while it positively regulates the expression of tumor suppressor gene such as E-cadherin and p27 [3,4,5] and prevents tumor invasion and metastasis. In addition, FOXA1 is also a major determinant of endocrine therapy effects by mediating different ER binding profiles which were associated with endocrine therapy response [6,7,8].
The associations between FOXA1 expression in breast cancer tissues and the prognosis have been explored in a large amount of previous studies, but the results were quite different with positive (high expression of FOXA1 related to a better prognosis) [9,10,11,12], negative [13, 14], and null associations [15], particularly for ER-positive breast cancer. Intriguingly, we noticed that the positive associations mostly occurred in the studies with a shorter follow-up time (5–12 years) [9,10,11,12], whereas the null associations occurred mainly in the studies with a longer follow-up time (up to 20 years) [15, 16]. Particularly, one study reported a null association between FOXA1 expression level and distant-metastasis-free-survival during the overall follow-up period (12.5 years) while there was an increased distant metastasis risk among patients with high expression level in the later period of follow-up [17]. These previous findings strongly suggested that the prognostic effects of FOXA1 on breast cancer may change with time.
In the present study, therefore, we investigated whether FOXA1 in breast cancer tissues have an independent time-varying effect on the prognosis, so as to provide an insight into the role of FOXA1 in breast cancer and help us further understand the timing and duration of endocrine therapy.
Materials and methods
Study population
A total of 1063 females with pathologically diagnosed primary invasive breast cancer and > 1 cm of tumor size in diameter were recruited between January 2008 and December 2015 from the Cancer Center of Sun Yat‐sen University in Guangzhou, China. Patients who lacked information of FOXA1 (N = 13) were excluded from the study and 98.6% (N = 1041) of the included patients were successfully followed up until Dec 31, 2019.
Collections of demographic and clinicopathologic information
Demographic characteristics including age and menopausal status were collected in face-to-face interview by trained investigators using structured questionnaires. BMI and clinicopathologic characteristics including clinical stage, histological grade, ER, progesterone receptor (PR), Human epidermal growth factor receptor 2 (HER-2) status and proliferation index factor Ki-67 (Ki-67) etc. were collected from medical records. Detailed definitions of ER, PR, and HER2 status were previously described in detail [18].
Construction of tissue microarray (TMA)
Formalin-fixed and paraffin-embedded tissues of included patients were retrieved. Hematoxylin and eosin (HE)-stained sections of tissue specimens were reviewed by two experienced pathologists, followed by re-slicing and re-staining with HE. Representative tumor tissue regions and adjacent normal tissue regions (If available) were marked on the re-stained HE sections. From the marked regions, two tumor tissue cylinders and one adjacent normal tissue cylinder (If unavailable, it would be replaced with the tumor tissue) with a diameter of 1 mm were punched out of the corresponding paraffin block as donor block and placed into the TMA paraffin block using an automatic tissue arrayer (MiniCore®, Mitogen, UK). The layout of the cores was determined in advanced by TMA Designer 2 Software. Sections of 4-μm cut from TMA blocks were pasted on the coded glass slides and then placed in the oven at 65 °C for 30 min and finally sealed the tissue surface with paraffin.
Immunohistochemistry (IHC)
The TMAs were baked at 60 °C for 2 h and then dewaxed with xylene and ethanol. Next, antigen retrieval was performed in super-pressure kettle using EDTA (PH 9.0) and then endogenous peroxide was blocked using 3% H2O2. After the preparations, slides were incubated in rabbit monoclonal to FOXA1 (EPR10881)-ChIP Grade (ab170933, diluted 1:100, Abcam) overnight at 4 °C and labeled with the EnVision Detection System (Peroxidase/DAB, Rabbit/Mouse) (Dako K5007). Then slides were developed by diaminobenzidine (DAB) and were counterstained by hematoxylin. Finally, slides were dehydrated and mounted.
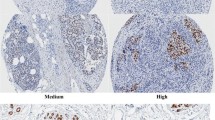
IHC stained sections were digitally imaged using Pannoramic Scanner and CaseViewer software. IHC staining was analyzed by an experienced pathologist and scored for staining intensity (0-no staining, 1-weak, 2-moderate and 3-strong) and percentage of tumor cell nuclear staining (0–100). Multiplying staining intensity with percentages yields an H score ranging from 0 to 300. Mean value of H-score from duplicate cores was taken.
Follow up
Patients were followed up by phone calls or out-patient visits every 3 months in the first year, every 6 months in the second and third year after diagnosis and annually thereafter. The endpoints of this study were overall survival (OS) and progression free survival (PFS), which were defined as the time from diagnosis to death and the time from diagnosis to disease progression including recurrence, metastasis, and death, respectively. Survival status was censored at the latest follow-up date or Dec 31, 2019.
Statistical analysis
Kruskal–Wallis test and Mann–Whitney U test were used to test the associations of FOXA1 H-scores (defined as a continuous variable) with age, BMI, clinical stage, menopausal status, histological grade and expressions of HER-2, ER and PR. FOXA1 H-score was modeled as continuous variable and fitted in a Cox proportional hazard model using restricted cubic splines with knots at the 5th, 35th, 65th, and 95th percentiles to estimate the hazard ratios (HRs) and 95% confidence bands assuming proportional hazard (PH). Then FOXA1 H-score was categorized according to the results of restricted cubic splines and the distribution of FOXA1 H-score. Univariate survival analyses of FOXA1 (defined as categorical variable) were perform using Kaplan–Meier method. Log-rank test was used to estimate the differences in survival curves of FOXA1 and to estimate the associations between demographic and clinicopathologic characteristics and breast cancer prognosis to control the potential confounders.
Flexible parametric models were used to perform time-varying effect analysis. The logarithm of the baseline hazard function was modeled as a natural cubic spline function of log time using a 2 degrees-of-freedom according to Akaike information criterion, where FOXA1 and ER were separately treated as variables with time-dependent effect. We also adjusted age at diagnosis, clinical stage, histological grade, and ER status in the models to estimate HRs and 95% confidence intervals (CI) over time. To confirm the independent time-varying effect of FOXA1, we further treated both FOXA1 and ER as covariates with time-dependent effect in the model, in which HRs and 95% CIs of FOXA1 were calculated separately over time for patients with ER-positive or ER-negative tumors. All analyses were conducted using R 3.6.2 and a two-sided P-value below 0.05 was considered as statistical significance.
Results
Demographic and clinicopathological characteristics and the associations with FOXA1 expression
The median age at diagnosis was 48 years (interquartile range: 41‐56 years). More than half of the women had a BMI under 23.0 (51.3%) and 58.7% of them were premenopausal. A great part of the women were diagnosed with low histological grade (grade I/II: 73.3%), early stage (stage I/II: 69.6%), ER-positive (73.5%), PR-positive (72.8%), or HER-2 negative (61.3%) (Table 1).
FOXA1 expression was evaluated in the nucleus of breast cancer cells. The H-score of FOXA1 ranged from 0 to 300 with a median (interquartile range) of 280 (270–285). The median (P25, P75) of FOXA1 H-score for ER-positive patients [285.0 (270.0, 285.0)] was significantly higher than that for ER-negative patients [270.0 (0.0, 285.0)] (P < 0.01). In addition, FOXA1 expression was also lower in tumors with higher grade, higher Ki-67, PR negative, or HER-2 negative (all P < 0.05). No marked differences in FOXA1 expression were observed between different age, BMI, menopausal status, clinical stage, tumor size, nodal status and metastasis (Table 1).
Prognostic effects of FOXA1 on breast cancer
Of the 1041 women, 125 died and 217 experienced disease progression with a median follow-up time of 69.5 months. For OS, the risk of death was relatively flat until around 270 of FOXA1 H-score and then slightly increased from 270 to 285 of FOXA1 H-score, but for FOXA1 H-scores > 285, the risk of death was decreased (Fig. 1A), although the association between FOXA1 and OS was not significant (Fig. 1A, P = 0.59, Pnonlinear = 0.49). For PFS, similar pattern was observed (Fig. 1B, P = 0.075, Pnonlinear = 0.59). Based on the results of restricted cubic splines and the distribution of FOXA1 H-score, we categorized FOXA1 H-score into three levels using tertiles as the cut-off points: ≤ 270, 271–285 and > 285.
In Kaplan–Meier analysis, no statistically significant differences were observed both for OS and PFS (log rank P = 0.19 and 0.88, respectively). Whereas, the survival probability was the highest for those with FOXA1 > 285 in the early period of follow-up while it changed to the lowest and the curves crossed over in the later period (Fig. 2).
Time-varying effect of FOXA1 on breast cancer prognosis
Significant time-varying effects of FOXA1 on breast cancer prognosis (both OS and PFS) were observed (all P < 0.01). Compared to FOXA1 ≤ 270, the HRs of death for the patients with H-score = 271–285 of FOXA1 expression increased from 0.35 (95% CI 0.14–0.86) at 6 months after diagnosis to 2.88 (95% CI 1.35–6.15) at 120 months with a crossover at around 36 months, and for those with the highest FOXA1 expression (H-score > 285), the HRs increased from 0.18 (95% CI 0.06–0.53) to 1.25 (95% CI 0.66–2.81) with a crossover at around 84 months in the adjusted model. Similar patterns were also observed for PFS. (Fig. 3; Table 2).
We further found that there was also a time-varying effect for ER on the survival (P = 0.02 for OS and P < 0.01 for PFS, Additional Table 1) and ER was associated with FOXA1 level, which suggested a potential confounding effect of ER on FOXA1 for the time-varying effect. Therefore, we allowed for the effects of both FOXA1 and ER to change over time in the model and let them adjust each other. It turned out that the time-varying effects of FOXA1 on both OS (P < 0.01) and PFS (P = 0.01) remained but the effect of ER disappeared on OS (P = 0.13). In both ER strata, the time-varying patterns of FOXA1 were similar to that in the whole population (Figs. 4, 5; Additional Tables 2, 3).
Discussion
In this study, we found that a higher FOXA1 expression level was associated with less aggressive characteristics of breast cancer, such as lower histological grade, lower Ki-67 expression, positive ER or PR. Overall, FOXA1 expression was not significantly associated with the prognosis of breast cancer patients, while there was a marked time-varying effect of FOXA1 on the prognosis. Compared with the low level of FOXA1, a high level of FOXA1 was associated with a protective effect on the survival of breast cancer patients in the early years after diagnosis, but this protective effect gradually diminished with time and an adverse effect occurred in the later years. These time-varying effects of FOXA1 were independent of ER status.
In consistent with our study, a lot of previous population studies have also found that FOXA1 expression level was higher in tumors with less aggressive characteristics [16, 19, 20]; Cellular experiments revealed that upregulation of FOXA1 inhibited epithelial to mesenchymal transition (EMT), migration and invasion in breast cancer cells [21, 22]. Moreover, another finding of the present study that the significant association of a high FOXA1 expression level with a better prognosis of breast cancer in the early stage after diagnosis also supported this result.
It was a quite interesting phenomenon that the protective effect on breast cancer survival of a high FOXA1 expression level gradually diminished and shifted to a detrimental effect in later years after diagnosis. One of the possible reasons for this time-varying effect was the altered FOXA1 expression level. It has been found that FOXA1 expression level decreased in long-term tamoxifen-treated MCF7 cells [23] and ER-negative cells exposed to bisphenol A [22]. Another reason was the termination of endocrine therapy, which may result in the diminished protective effect of high FOXA1 expression level because FOXA1 plays its role depending on endocrine therapy [6]; the Chinese Anti-Cancer Association (CSCA) Diagnosis and Treatment Guidelines for breast cancer (version 2008) [24] recommended ER positive patients to receive endocrine therapy for 5 years after surgery and most of the patients complied with the guideline [25], which was also consistent with our results that the protective effect of highest FOXA1 expression on cancer progression shifted to an detrimental effect at 60 months after diagnosis. The third reason was the acquisition of endocrine resistance caused by FOXA1 through transcription reprogramming in breast cancer cells with the extension of endocrine therapy time [8, 14, 26,27,28].
We found that the time-varying effects of FOXA1 on breast cancer were independent on ER status while the same effects of ER were affected by FOXA1 to some extent. This phenomenon may be explained by that FOXA1 was essential for sustained ER expression [29] and was the upstream of ER-chromatin interactions, regulating more than 90% of ER binding events [6, 30]. In addition, this result likely suggested that the time-varying effects of FOXA1 may also be mediated through other pathways, such as androgen receptor [31] and AGR2 [32, 33]. The exact mechanisms remained to be explored.
There were some limitations in this study. First, only patients with tumor > 1 cm were included and that may lead to selective bias. However, FOXA1 expression was independent of tumor size in this study, causing non-differential bias on the associations between FOXA1 and prognosis. Second, we were unable to collect the information on the changes of FOXA1 over time that made us fail to make sure whether the time-varying effect was due to changes in FOXA1 expression. Third, we didn’t collect the information about treatment which was associated with prognosis. However, since the treatment was determined according to the clinicopathological characteristics such as ER status and tumor stage, adjustment of these characteristics in the statistic models largely controlled the confounding effects of the treatment. Finally, a follow-up time up to 10 years may lead a bias estimation in the later stage of follow-up. However, the crossover time-points of time-varying effects occurred before the median follow-up time (72.2 months), indicating that the results of the time-varying effect were reliable.
Conclusion
This study firstly revealed the time-varying effect of FOXA1 on breast cancer prognosis: a higher expression of FOXA1 was associated with a better survival in the early stage after diagnosis while it associated with a poor survival in the late stage. Similar results were observed when further treated ER as covariates with time-dependent effect in the model. These findings provided an insight into the roles of FOXA1 as a marker of breast cancer prognosis and argued in favor of an extended endocrine therapy rather than 5 years.
Abbreviations
- CI:
-
Confidence interval
- DAB:
-
Diaminobenzidine
- ER:
-
Estrogen receptor
- EMT:
-
Epithelial to mesenchymal transition
- FOXA1:
-
Forkhead box A1
- HE:
-
Hematoxylin and eosin
- HER-2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hazard ratio
- IHC:
-
Immunohistochemistry
- Ki-67:
-
Proliferation index factor Ki-67
- OS:
-
Overall survival
- PFS:
-
Progression free survival
- PR:
-
Progesterone receptor
- TMA:
-
Tissue microarray
References
Augello MA, Hickey TE, Knudsen KE (2011) FOXA1: master of steroid receptor function in cancer. Embo J 30:3885–3894. https://doi.org/10.1038/emboj.2011.340
Bernardo GM, Keri RA (2012) FOXA1: a transcription factor with parallel functions in development and cancer. Biosci Rep 32:113–130. https://doi.org/10.1042/bsr20110046
Zheng L, Qian B, Tian D, Tang T, Wan S, Wang L, Zhu L, Geng X (2015) FOXA1 positively regulates gene expression by changing gene methylation status in human breast cancer MCF-7 cells. Int J Clin Exp Pathol 8:96–106
Liu YN, Lee WW, Wang CY, Chao TH, Chen Y, Chen JH (2005) Regulatory mechanisms controlling human E-cadherin gene expression. Oncogene 24:8277–8290. https://doi.org/10.1038/sj.onc.1208991
Williamson EA, Wolf I, O’Kelly J, Bose S, Tanosaki S, Koeffler HP (2006) BRCA1 and FOXA1 proteins coregulate the expression of the cell cycle-dependent kinase inhibitor p27(Kip1). Oncogene 25:1391–1399. https://doi.org/10.1038/sj.onc.1209170
Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS (2011) FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet 43:27–33. https://doi.org/10.1038/ng.730
Robinson JL, Carroll JS (2012) FoxA1 is a key mediator of hormonal response in breast and prostate cancer. Front Endocrinol (Lausanne) 3:68. https://doi.org/10.3389/fendo.2012.00068
Ross-Innes CS, Stark R, Teschendorff AE, Holmes KA, Ali HR, Dunning MJ, Brown GD, Gojis O, Ellis IO, Green AR, Ali S, Chin SF, Palmieri C, Caldas C, Carroll JS (2012) Differential oestrogen receptor binding is associated with clinical outcome in breast cancer. Nature 481:389–393. https://doi.org/10.1038/nature10730
Hisamatsu Y, Tokunaga E, Yamashita N, Akiyoshi S, Okada S, Nakashima Y, Aishima S, Morita M, Kakeji Y, Maehara Y (2012) Impact of FOXA1 expression on the prognosis of patients with hormone receptor-positive breast cancer. Ann Surg Oncol 19:1145–1152. https://doi.org/10.1245/s10434-011-2094-4
Rangel N, Fortunati N, Osella-Abate S, Annaratone L, Isella C, Catalano MG, Rinella L, Metovic J, Boldorini R, Balmativola D, Ferrando P, Marano F, Cassoni P, Sapino A, Castellano I (2018) FOXA1 and AR in invasive breast cancer: new findings on their co-expression and impact on prognosis in ER-positive patients. BMC Cancer 18:703. https://doi.org/10.1186/s12885-018-4624-y
Albergaria A, Paredes J, Sousa B, Milanezi F, Carneiro V, Bastos J, Costa S, Vieira D, Lopes N, Lam EW, Lunet N, Schmitt F (2009) Expression of FOXA1 and GATA-3 in breast cancer: the prognostic significance in hormone receptor-negative tumours. Breast Cancer Res 11:R40. https://doi.org/10.1186/bcr2327
Jiang G, Wang X, Sheng D, Zhou L, Liu Y, Xu C, Liu S, Zhang J (2019) Cooperativity of co-factor NR2F2 with pioneer factors GATA3, FOXA1 in promoting ERα function. Theranostics 9:6501–6516. https://doi.org/10.7150/thno.34874
Zhang YW, Ma J, Shi CT, Han W, Gao XJ, Zhou MH, Ding HZ, Wang HN (2020) Roles and correlation of FOXA1 and ZIC1 in breast cancer. Curr Probl Cancer. https://doi.org/10.1016/j.currproblcancer.2020.100559
Fu X, Jeselsohn R, Pereira R, Hollingsworth EF, Creighton CJ, Li F, Shea M, Nardone A, De Angelis C, Heiser LM, Anur P, Wang N, Grasso CS, Spellman PT, Griffith OL, Tsimelzon A, Gutierrez C, Huang S, Edwards DP, Trivedi MV, Rimawi MF, Lopez-Terrada D, Hilsenbeck SG, Gray JW, Brown M, Osborne CK, Schiff R (2016) FOXA1 overexpression mediates endocrine resistance by altering the ER transcriptome and IL-8 expression in ER-positive breast cancer. Proc Natl Acad Sci USA 113:E6600–E6609. https://doi.org/10.1073/pnas.1612835113
Habashy HO, Powe DG, Rakha EA, Ball G, Paish C, Gee J, Nicholson RI, Ellis IO (2008) Forkhead-box A1 (FOXA1) expression in breast cancer and its prognostic significance. Eur J Cancer 44:1541–1551. https://doi.org/10.1016/j.ejca.2008.04.020
Mehta RJ, Jain RK, Leung S, Choo J, Nielsen T, Huntsman D, Nakshatri H, Badve S (2012) FOXA1 is an independent prognostic marker for ER-positive breast cancer. Breast Cancer Res Treat 131:881–890. https://doi.org/10.1007/s10549-011-1482-6
Horimoto Y, Sasahara N, Sasaki R, Hlaing MT, Sakaguchi A, Saeki H, Arakawa A, Himuro T, Saito M (2020) High FOXA1 protein expression might predict late recurrence in patients with estrogen-positive and HER2-negative breast cancer. Breast Cancer Res Treat 183:41–48. https://doi.org/10.1007/s10549-020-05751-x
He JR, Tang LY, Yu DD, Su FX, Song EW, Lin Y, Wang SM, Lai GC, Chen WQ, Ren ZF (2011) Epstein-Barr virus and breast cancer: serological study in a high-incidence area of nasopharyngeal carcinoma. Cancer Lett 309:128–136. https://doi.org/10.1016/j.canlet.2011.05.012
He K, Zeng H, Xu X, Li A, Cai Q, Long X (2016) Clinicopathological significance of forkhead box protein A1 in breast cancer: a meta-analysis. Exp Ther Med 11:2525–2530. https://doi.org/10.3892/etm.2016.3229
Cheng TD, Yao S, Omilian AR, Khoury T, Buas MF, Payne-Ondracek R, Sribenja S, Bshara W, Hong CC, Bandera EV, Davis W, Higgins MJ, Ambrosone CB (2020) foxa1 protein expression in ER(+) and ER(-) breast cancer in relation to parity and breastfeeding in black and white women. Cancer Epidemiol Biomark Prev 29:379–385. https://doi.org/10.1158/1055-9965.Epi-19-0787
Xu Y, Qin L, Sun T, Wu H, He T, Yang Z, Mo Q, Liao L, Xu J (2017) Twist1 promotes breast cancer invasion and metastasis by silencing Foxa1 expression. Oncogene 36:1157–1166. https://doi.org/10.1038/onc.2016.286
Zhang XL, Wang HS, Liu N, Ge LC (2015) Bisphenol A stimulates the epithelial mesenchymal transition of estrogen negative breast cancer cells via FOXA1 signals. Arch Biochem Biophys 585:10–16. https://doi.org/10.1016/j.abb.2015.09.006
Yamaguchi N, Nakayama Y, Yamaguchi N (2017) Down-regulation of Forkhead box protein A1 (FOXA1) leads to cancer stem cell-like properties in tamoxifen-resistant breast cancer cells through induction of interleukin-6. J Biol Chem 292:8136–8148. https://doi.org/10.1074/jbc.M116.763276
Chinese Anti-Cancer Association Committee of Breast Cancer Society (2008) Chinese Anti-Cancer Association (CSCA) diagnosis and treatment guidelines for breast cancer (version 2008). Chin Oncol 19:448–474. https://doi.org/10.3969/j.issn.1007-3639.2009.06.012
Xu H, Jin F, Zhang XJ, Wang DQ, Yu SF, Wang AP (2020) Adherence status to adjuvant endocrine therapy in Chinese Women with Early Breast Cancer and its influencing factors: a cross-sectional survey. Cancer Med 9:3703–3713. https://doi.org/10.1002/cam4.3017
Fu X, Pereira R, De Angelis C, Veeraraghavan J, Nanda S, Qin L, Cataldo ML, Sethunath V, Mehravaran S, Gutierrez C, Chamness GC, Feng Q, O’Malley BW, Selenica P, Weigelt B, Reis-Filho JS, Cohen O, Wagle N, Nardone A, Jeselsohn R, Brown M, Rimawi MF, Osborne CK, Schiff R (2019) FOXA1 upregulation promotes enhancer and transcriptional reprogramming in endocrine-resistant breast cancer. Proc Natl Acad Sci USA 116:26823–26834. https://doi.org/10.1073/pnas.1911584116
Tokunaga E, Hisamatsu Y, Tanaka K, Yamashita N, Saeki H, Oki E, Kitao H, Maehara Y (2014) Molecular mechanisms regulating the hormone sensitivity of breast cancer. Cancer Sci 105:1377–1383. https://doi.org/10.1111/cas.12521
Piggin CL, Roden DL, Law AMK, Molloy MP, Krisp C, Swarbrick A, Naylor MJ, Kalyuga M, Kaplan W, Oakes SR, Gallego-Ortega D, Clark SJ, Carroll JS, Bartonicek N, Ormandy CJ (2020) ELF5 modulates the estrogen receptor cistrome in breast cancer. PLoS Genet 16:e1008531. https://doi.org/10.1371/journal.pgen.1008531
Bernardo GM, Lozada KL, Miedler JD, Harburg G, Hewitt SC, Mosley JD, Godwin AK, Korach KS, Visvader JE, Kaestner KH, Abdul-Karim FW, Montano MM, Keri RA (2010) FOXA1 is an essential determinant of ERalpha expression and mammary ductal morphogenesis. Development 137:2045–2054. https://doi.org/10.1242/dev.043299
Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, Fox EA, Silver PA, Brown M (2005) Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell 122:33–43. https://doi.org/10.1016/j.cell.2005.05.008
Robinson JL, Macarthur S, Ross-Innes CS, Tilley WD, Neal DE, Mills IG, Carroll JS (2011) Androgen receptor driven transcription in molecular apocrine breast cancer is mediated by FoxA1. Embo J 30:3019–3027. https://doi.org/10.1038/emboj.2011.216
Cocce KJ, Jasper JS, Desautels TK, Everett L, Wardell S, Westerling T, Baldi R, Wright TM, Tavares K, Yllanes A, Bae Y, Blitzer JT, Logsdon C, Rakiec DP, Ruddy DA, Jiang T, Broadwater G, Hyslop T, Hall A, Laine M, Phung L, Greene GL, Martin LA, Pancholi S, Dowsett M, Detre S, Marks JR, Crawford GE, Brown M, Norris JD, Chang CY, McDonnell DP (2019) The lineage determining factor GRHL2 collaborates with FOXA1 to establish a targetable pathway in endocrine therapy-resistant breast cancer. Cell Rep 29:889-903.e810. https://doi.org/10.1016/j.celrep.2019.09.032
Wright TM, Wardell SE, Jasper JS, Stice JP, Safi R, Nelson ER, McDonnell DP (2014) Delineation of a FOXA1/ERα/AGR2 regulatory loop that is dysregulated in endocrine therapy-resistant breast cancer. Mol Cancer Res 12:1829–1839. https://doi.org/10.1158/1541-7786.Mcr-14-0195
Acknowledgements
We sincerely thank the patients who participated in this study, the staff who conducted the baseline and the follow‐up data collection, and the medical staff in the breast departments of the First Affiliated Hospital, and the Cancer Center of Sun Yat‐Sen University.
Funding
This research was funded by National Natural Science Foundation of China (81773515 and 81973115) and Science and Technology Planning Project of Guangdong Province, China (2019B030316002). The founders have no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
QC, ZR, and JY designed and directed the study, wrote and/or revise the manuscript. YY and YL constructed the TMAs. YY contributed to the IHC. ZW, XZ, JG, and LT contributed to digital imaging of IHC-stained sections and the assessment of immunohistochemical expression. QC, ZL, ZH, JC and YL contributed to clinical data collection and curation. QC, ZL, and ZH participated in the statistical analysis plan and interpretation of results. ZR, and JY provided administrative support and supervision for the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The study was approved by the ethics committee of School of Public Health, Sun Yat-sen University.
Consent to participate
All participants provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Qx., Yang, Yz., Liang, Zz. et al. Time-varying effects of FOXA1 on breast cancer prognosis. Breast Cancer Res Treat 187, 867–875 (2021). https://doi.org/10.1007/s10549-021-06125-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-021-06125-7