Abstract
Purpose
Recent cohort studies demonstrated better overall survival (OS) or breast cancer-specific survival (BCS) for breast-conserving therapy (BCT) followed by radiation (RT) compared to mastectomy alone (MT). This is the first observational study in which adjustments for a comprehensive set of prognostic factors, adjuvant therapies, mode of detection, and comorbidities were possible to investigate OS, BCS, as well as recurrence risk of patients undergoing BCT + RT, MT + RT, or MT.
Methods
Women aged 50–74 years at diagnosis of early-stage invasive breast cancer (I–IIIa) between 2001 and 2005 at the German population-based case–control study (MARIE study) were recruited and followed prospectively as a case cohort until 2015. Kaplan–Meier estimates and stepwise adjusted multivariable Cox models were used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CI).
Results
The 2762 patients included were followed up for a median of 11.9 years (95% CI 11.8–12.0). 74.2% of patients underwent BCT + RT; 10.3% MT + RT and 15.6% MT alone. Compared to patients treated with MT alone, patients treated with BCT + RT showed non-statistically significant improved OS (HR 0.79, 95% CI 0.61–1.02), BCS (HR 0.79, 95% CI 0.55–1.12), and no difference in recurrence risks (HR 1.01, 95% CI 0.74–1.37). For patients treated with MT + RT, there were no differences in OS (HR 1.06, 95% CI 0.75–1.50), BCS (HR 1.17, 95% CI 0.75–1.82), or recurrence risk (HR 1.33, 95% CI 0.89–1.97).
Conclusions
Among patients with early-stage breast cancer, clinical outcomes more than 10 years after diagnosis did not differ between the primary treatment options BCT + RT, MT + RT versus MT alone after full adjustment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the 1980s, several randomized clinical trials (RCT) demonstrated equivalent overall [1,2,3,4,5,6], recurrence-free [2, 4, 5], or disease-free [1, 4] survival after breast-conserving therapy (BCT) followed by radiation (RT) and after mastectomy (MT) for early-stage breast cancer [1,2,3,4,5,6,7]. Consequently, BCT followed by radiation was recommended [8] as an equivalent therapy to MT. Contrary to European data [9,10,11], recent US studies [12, 13] reported increasing rates of MT as treatment option. Reasons for this increasing trend favoring MT as surgery option are still unknown. One might suspect that better reconstruction techniques [12] or concerns regarding radiation therapy (RT) or recurrence after BCT are factors influencing the therapy decision process even though MT seems to be associated with greater morbidity [14, 15]. There are no recent clinical trials investigating prognostic effects of BCT and MT although strategies in breast cancer surveillance, including screening programs and (adjuvant) therapy, have been changed extensively during the last decades.
Recently, population-based cohort studies conducted in the US [16,17,18,19] and Norway [20, 21] demonstrated better overall survival (OS) or breast cancer-specific survival (BCS) for BCT + RT compared to MT in patients 50 years or older. Differences in tumor biology and non-compliance to adjuvant therapy have been suggested to have eventually biased the estimated effects, since at least three studies [17, 19, 20] were conducted in databases with insufficient information to adjust for these factors.
Against this background, the present population-based study, which has collected comprehensive information on covariates, compares BCT + RT and MT + RT against MT alone with regard to OS and BCS for women aged 50–75 years with early-stage breast cancer in Germany. This study can thus account for the influence of strong interdependencies between prognostic factors, adjuvant therapies, mode of detection, as well as comorbidities, which can potentially affect exposure and/or outcome. Furthermore, this is the first observational study investigating recurrence risk for BCT + RT, MT + RT, and MT.
Methods
Data source and study population
The cohort consisted of the cases of the population-based case–control MARIE study (Mamma Carcinoma Risk factor Investigation) [22]. Between 2002 and 2005, patients were recruited in two regions of Germany; Hamburg and Rhine-Neckar-Karlsruhe if they were aged 50–74 years at diagnosis and had a histologically confirmed diagnosis of primary invasive (stage I to IV) or in situ breast cancer between January 1, 2001 and September 30, 2005. Information on pre-diagnostic lifestyle factors, socio-economic status, medical history, and information on specific medications, regimen, and duration of use was collected by a standardized face-to-face interview at recruitment. The histological characteristics of the primary breast cancer were extracted from pathology reports. Treatment and clinical course were abstracted from medical records to verify clinical events either self-reported in the interview or reported by treating physicians during a first follow-up in 2009 and a second follow-up in June 2015 (in total > 90% self-reported events verified) resulting in a cohort study with a follow-up time of > 10 years.
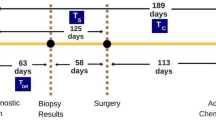
For this study, 3813 patients who were recruited into the baseline case–control study were eligible. Patients were excluded if they had either in situ tumors (N = 232), prior malignant tumors (N = 238), tumors with stage IIIb or higher (N = 386), unknown information on metastases (N = 11), therapy (N = 11), recurrences (N = 45), or radiotherapy (N = 21). Patients treated with BCT but without following radiation therapy (N = 76) or patients with secondary radiotherapy after recurrences or secondary tumors (N = 31) were excluded as well. Thus, 2762 patients were available for analysis (Fig. 1).
All study participants gave written informed consent. The ethics committee of the University of Heidelberg, the Hamburg Medical Council, and the Medical Board of the State of Rhineland-Pfalz gave approval. The study was conducted in accordance with the Declaration of Helsinki.
Outcome assessment
Study participants were prospectively followed until June 30, 2015. Vital status was assessed via information from population registries. Causes of death were derived from death certificates obtained through the local/regional health offices and coded according to the 10th revision of the International Classification of Diseases (ICD-10-GM). The primary endpoints were OS (including death from any cause), BCS (death due to breast cancer; non-breast cancer-related deaths were censored), and recurrences (including ipsilateral, contralateral, local/regional invasive recurrence, and distant recurrence). Participants without an event of interest were censored at the date of last contact or on June 30, 2015, whichever came first.
Exposure
Primary surgery type was assessed through patient’s medical records by defining the exposure status of patients who received either MT and adjuvant RT (MT + RT) or BCT and adjuvant RT (BCT + RT) which is recommended by the guidelines [8] as primary therapy option. MT + RT is recommended only for women who had a higher tumor stage (T3/T4) and other risk factors (i.e., grading G3, invasion of the lymphatic vessels, close resection margin, being premenopausal or younger than 50 years of age) [8].
Covariates
Most factors considered relevant in the German guideline[8] for primary treatment decisions were measured in our study and considered as covariates for analyses: Clinical characteristics: tumor size (T1/T2/T3), grading (G1/G2/G3), number of affected lymph nodes (N0/N1/N2), estrogen/progesterone receptor (ER+PR+/ER+PR− or ER−PR+/ER−PR−), and her2/neu- status (negative/positive); factors related to mode of detection: being diagnosed by routine imaging (mammography or sonography) (yes/no), and benign breast disease (yes/no); comorbidities: diabetes mellitus and cardiovascular diseases (CVDs) (yes/no, respectively); adjuvant therapy after surgery including chemotherapy, tamoxifen use or aromatase use (yes/no, respectively). Furthermore, study center (Hamburg/Rhine-Neckar Karlsruhe) and age at diagnosis (years) were included (for categories see Table 1). Neoadjuvant chemotherapy was not considered as it is not recommended for early-stage breast cancer. Furthermore, due to exclusion criteria of patients with tumor stage IIIb or higher, our study sample did not include patients who received neoadjuvant chemotherapy.
Statistical analysis
The Kaplan–Meier method was used to estimate unadjusted OS, BCS, and recurrence risk by treatment group. The log-rank test was applied to evaluate differences between the groups.
Multivariable delayed entry cox models were used, adjusted for all therapy-relevant confounders, adjuvant therapies, comorbidities, and diagnostic mode. Hazard ratios (HR) and 95% confidence intervals (95% CI) for OS, BCS, and recurrence risk were estimated for BCT + RT and for MT + RT compared with MT. Variables which did not fulfill the proportional hazard assumption (her2/neu, tamoxifen treatment, chemotherapy) were included in the model as stratification variables. Since we consider the comparisons between BCT + RT, MT + RT, and MT, no main effect for RT is specified. For sensitivity analyses, full adjusted models were stratified either by grade (grade 3 vs. grade 1 and grade 2) and by ER/PR-status (ER−/PR− vs. ER+/PR+ and mixed ER/PR-status) to compare estimates between the different strata.
A reverse Kaplan–Meier method was used to estimate the median follow-up time in the cohort.
Results
Median follow-up was 11.9 years (95% CI 11.8–12.0). BCT + RT was conducted in 2048 patients (74.2%), MT + RT in 283 patients (10.3%), and MT in 431 patients (15.6%). The mean age of patients undergoing BCT + RT or MT + RT was 62.2 (SD: 5.9), respectively, and for MT alone 62.9 (SD: 6.4) (Table 1). In the BCT + RT treatment group, 336 (16.4%) deaths, including 96 (4.7%) breast cancer-specific deaths, and 288 (14.1%) recurrences occurred; portions were similar in patients treated with MT alone but higher in patients treated with MT + RT (Table 1).
No differences between the patients of the three treatment groups were found for age, ER−/PR-status, aromatase and tamoxifen therapy, diabetes, and CVDs (Table 1). A higher percentage of patients with MT + RT had a larger tumor size, more affected lymph nodes, higher tumor grading, more her2/neu-positive carcinoma, and underwent chemotherapy more often than patients treated with BCT + RT or patients treated with MT alone (Table 1). Patients with BCT + RT were more likely to have had their tumor detected by routine imaging than patients who underwent MT + RT or MT alone (Table 1).
Overall survival (OS), breast cancer-specific survival (BCS), and recurrence risk
Kaplan–Meier estimates showed significantly improved OS, BCS, and lower recurrence risk favoring BCT + RT over MT. Compared to MT alone, patients treated with MT + RT had poorer OS, BCS, and higher recurrence risk. Log-rank tests indicated statistically significant differences between the survival curves (p < 0.001, respectively for all outcomes) (Fig. 2a–c).
Univariate Cox models of OS, BCS, and recurrence risk as well as prognostic factors and possible other covariates
Univariate Cox models showed that in comparison to patients who received MT alone, those patients who received BCT + RT had improved OS (HR 0.75, 95% CI 0.59–0.94). Compared to patients treated with MT alone BCS (HR 0.81, 95% CI 0.58–1.31) and recurrence risk (HR 0.94, 95% CI 0.71–1.25) were favorable but CIs included the 1 (Table 2). Patients who received MT + RT had poorer OS (HR 1.44, 95% CI 1.07–1.93), poorer BCS (HR 2.08, 95% CI 1.39–3.09), and higher recurrence risk (HR 1.99, 95% CI 1.42–2.81) compared to patients receiving MT alone.
Age at diagnosis was not associated with the respective outcomes. The established prognostic factors (tumor size, nodal status, tumor grade, negative ER−/PR-status, positive her2/neu-status and chemotherapy) were associated with lower OS and BCS, and with higher recurrence risk (Supplemental Table 1). Benign breast disease, a diagnosis by routine imaging and tamoxifen therapy was associated with improved OS. For both other outcomes (BCS and recurrence risk), similar results were found for the respective covariates (Supplemental Table 1). Comorbidities were associated with poorer OS and BCS but were not statistically significantly associated with risk of recurrences.
Multivariable analysis of OS, BCS, and RFS
After full adjustment, non-statistically significant favorable OS (HR 0.79, 95% CI 0.61–1.02) and BCS (HR 0.78, 95% CI 0.55–1.11) for patients treated with BCT + RT compared to patients treated with MT alone were observed. Recurrence risk (HR 1.01, 95% CI 0.74–1.37) after BCT + RT did not differ from MT alone (Table 2). In patients treated with MT + RT compared to MT alone HR indicate no differences in risk of dying from breast cancer (1.17 (95% CI 0.75–1.82)) or in risk of recurrence (1.33 (95% CI 0.89–1.97)) as well as regarding OS (HR 1.05, 95% CI 0.74–1.49) (Table 2).
Adjustment for only therapy-relevant variables revealed similar results but with borderline significant improved OS for BCT + RT compared to MT alone (HR 0.76, 95% CI 0.60–0.98) (Supplemental Table 2).
Estimates for the stepwise inclusion of covariates are displayed in Supplemental Table 2.
Sensitivity analyses stratified by grade and ER/PR-status did not reveal relevant changes of the point estimates for OS, BCS, or recurrence risk (data not shown).
Discussion
This is the first German population-based study of routinely treated breast cancer patients to examine the impact of the different surgery (treatment) options BCT + RT, MT + RT, and MT on long-term outcomes, including OS, BCS and recurrence risk, taking into account comprehensively the interdependencies between primary treatment, prognostic factors, adjuvant therapies, mode of detection, and comorbidities. Overall, we found no statistically significant differences between the primary surgical options in OS, BCS, and recurrence risks after more than 10 years of follow-up.
Due to the observational study design, it was necessary to control for potential group differences between the surgery types not only by adjustment for prognostic factors but also by further covariates like adjuvant therapies, mode of detection, and comorbidities, which are known to have an influence on long-term outcome [23,24,25,26]. Prior published observational studies [16,17,18,19,20] generally adjusted for prognostic factors; however, only one of them adjusted additionally for adjuvant therapies and for mode of detection [21], whereas none of the studies adjusted for comorbidities. We were able to take all these covariates into account and investigated their influence separately on the respective outcomes in a stepwise approach.
In the full adjusted model, we found statistically non-significant favorable BCS (HR 0.79, 95% CI 0.55–1.12) for BCT + RT compared to MT alone. Prior studies reported comparable estimates of BCS ranging from 0.76 (95% CI 0.72–0.80) [17] to 0.61 (95% CI 0.53–0.70) [20] after adjustment for only prognostic factors as well as after additional adjustment for adjuvant therapies and mode of detection (HR 0.59, 95% CI 0.42–0.77) [21].
The results reported by observational studies are contrary to the general consensus that patients undergoing BCT + RT compared to MT have similar long-term OS and BCS as observed in several RCT studies [1, 2, 6, 27, 28] conducted during the previous decade. These RCTs found no differences between treatment groups at 5-year follow-up [29, 30] and also after long-term follow-up (10–20 years) [6, 27]. One reason might be that these RCTs were conducted in the late 1980s or early 1990s. Since then, breast cancer treatment as well as detection techniques, adjuvant systemic therapy, i.e., radiation therapy indications and the quality of treatment procedures have substantially improved. Additionally, the increased use of adjuvant systemic treatment for better local tumor control might have led to the supposed survival advantage of patients undergoing BCT in our study compared to those diagnosed more than decades before.
The relative risks for MT + RT compared to MT alone revealed no statistically significant differences after full adjustment. This is in line with several RCT studies [2, 6, 7, 31] that reported no differences in local recurrences between the two treatment groups (BCT and MT), whereas more recent RCTs [29, 30, 32, 33] found higher local recurrence rates after BCT alone in younger women (≤ 40 years of age) but no differences in distant recurrence-free survival after about 10 years of follow-up [34, 35]. Unfortunately, we could not distinguish between local recurrence- and distant recurrence-free survival in our analysis, due to insufficient power. Furthermore, analysis regarding MT + RT versus MT alone may be underpowered. This could also partly explain the findings regarding different point estimates between the primary surgical options BCT + RT and MT + RT, as our results were statistically not significant throughout treatment groups and outcomes. In addition, this study has been conducted in the pre-her2-targeted era, meaning only a small proportion of the women included in this study received Trastuzumab, Pertuzumab etc., a therapy, which is nowadays hold as standard for her2/neu-positive tumors. As almost 70% of all tumors in the MT + RT patient group were her2/neu positive, outcomes for this patient group might be different in more contemporary settings. However, in our study, only 58 patients have been treated with Trastuzumab, of whom 39 were treated primary with BCT + RT, while only 12 patients received MT + RT and, 7 were treated with MT alone as primary therapy option. MT with subsequent RT is recommended for women with higher tumor stage (T3/T4) or other risk factors [8], indicating that these patient groups have in general a poorer survival prognosis. Our finding of a statistically non-significant increased risk for this group could still be due to residual confounding although we were able to employ a large set of adjusting covariates. Due to exclusion criteria, our study population comprises early-stage breast cancer cases and only 283 women received MT + RT in our study, a choice of treatment procedure, which may well depend on individual preferences, reasonable indications for RT after MT (e.g., higher grade tumors), and/or clinic-specific decision processes. However, sensitivity analyses by stratification of grade or ER/PR status did not reveal relevant changes of OS and BCS between the different patient groups (data not shown).
We found no difference regarding recurrence risk for women treated with BCT + RT compared to MT alone.
As unadjusted estimates for MT + RT were more than twice as high for BCS and almost 50% higher for OS and recurrence risk, a substantial amount of confounding is controlled for in our multivariable analyses. In order to compare our results with that of prior studies which adjusted only for prognostic factors, we also limited our analyses to the adjustment of similar risk factors than used in the prior studies. However, beside adjustment for therapy-related covariates, further adjustment does not suggest a strong influence of other covariates in our study, which indicates that results of other observational studies may not be different even if all possible confounders would have been accounted for. Nevertheless, we cannot exclude bias due to residual confounding.
Limitations of our study are mostly due to the retrospective nature of collected information and the possibility for recall bias due to self-reported baseline characteristics. However, self-reported information on MT or BCT as well as adjuvant therapies was validated through patient records. Histopathological characteristics were also extracted from patient records. There are still 45 patients with unknown information on their recurrence status which were excluded from analyses to reduce potential outcome misclassification bias. Additionally, in a patient subsample, a part of the questionnaire was tested for reliability; the MARIE data have being rated as being of good quality with only a low potential of misclassification [36].
Unfortunately, we could not stratify our analysis for patients receiving BCT plus radiation (N = 2031) and patients receiving BCT alone (N = 74) as numbers were too low. We therefore excluded patients without subsequent radiation as this is not a standard treatment according to guidelines [8].
The main strength of the study is the detailed information on prognostic factors, therapy data, and the completeness of follow-up information of recurrence, vital status, and causes of death. Furthermore, we have detailed information about compliance of the respective treatments as the patients have been asked for the specific treatment they had been taken.
Several single-institution reports [12, 37, 38] as well as nationwide studies [13, 39] have shown an increasing trend of mastectomy rates in the US during the last decades while some other studies [40] found discrepant results. However, studies based on European [10, 11, 41] or German [9] data reported no increasing trend of mastectomy rates even though these rates differ also in comparable health care systems.
Our data suggest no statistical significant survival advantage or reduced recurrence between the primary therapy options of BCT + RT or MT + RT compared to MT alone in patients with early-stage invasive breast carcinoma. In order to lead to advantageous long-term survival, patients need to be informed in an understandable way on advantages and disadvantages of eligible treatments based on the respective clinical indications to compile the best integrated treatment plan.
Abbreviations
- BCS:
-
Breast cancer-specific survival
- BCT:
-
Breast-conserving therapy
- CVD:
-
Cardiovascular disease
- ER/PR receptor:
-
Estrogen/progesterone receptor
- HR:
-
Hazard ratio
- ICD-10-GM:
-
International classification of diseases, 10th revision
- MT:
-
Mastectomy
- OS:
-
Overall survival
- RT:
-
Radiation
- RCT:
-
Randomized clinical trials
References
Fisher B, Redmond C, Poisson R, Margolese R, Wolmark N, Wickerham L, Fisher E, Deutsch M, Caplan R, Pilch Y (1989) Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med 320:822–828
Blichert-Toft M, Nielsen M, Düring M, Møller S, Rank F, Overgaard M, Mouridsen HT (2008) Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol 47:672–681
Litiere S, Werutsky G, Fentiman IS, Rutgers E, Christiaens MR, Van LE, Baaijens MH, Bogaerts J, Bartelink H (2012) Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial 324 324 35244. Lancet Oncol 13:412–419
Simone NL, Dan T, Shih J, Smith SL, Sciuto L, Lita E, Lippman ME, Glatstein E, Swain SM, Danforth DN, Camphausen K (2012) Twenty-five year results of the national cancer institute randomized breast conservation trial. Breast Cancer Res Treat 132:197–203
Wilkinson JB, Vicini FA, Shah C, Shaitelman S, Jawad MS, Ye H, Kestin LL, Goldstein NS, Martinez AA, Benitez P, Chen PY (2012) Twenty-year outcomes after breast-conserving surgery and definitive radiotherapy for mammographically detected ductal carcinoma in situ. Ann Surg Oncol 19:3785–3791
Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, Aguilar M, Marubini E (2002) Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med 347:1227–1232
van Dongen JA, Bartelink H, Fentiman IS, Lerut T, Mignolet F, Olthuis G, van der Schueren E, Sylvester R, Winter J, van Zijl K (1992) Randomized clinical trial to assess the value of breast-conserving therapy in stage I and II breast cancer, EORTC 10801 trial. J Natl Cancer Inst Monogr 11:15–18
AWMF-Register-Nummer: 032-045OL K 3 (2012) Interdisziplinäre S3-Leitlinie für die Diagnostik, Therapie und Nachsorge des Mammakarzinoms. Cited 2016 Mar 24; http://www.awmf.org/uploads/tx_szleitlinien/032-045OL_k_S3__Brustkrebs_Mammakarzinom_Diagnostik_Therapie_Nachsorge_2012-07.pdf
Heil J, Rauch G, Szabo AZ, Garcia-Etienne CA, Golatta M, Domschke C, Badiian M, Kern P, Schuetz F, Wallwiener M, Sohn C, Fries H et al (2013) Breast cancer mastectomy trends between 2006 and 2010: association with magnetic resonance imaging, immediate breast reconstruction, and hospital volume. Ann Surg Oncol 20:3839–3846
Güth U, Myrick ME, Viehl CT, Weber WP, Lardi AM, Schmid SM (2012) Increasing rates of contralateral prophylactic mastectomy—a trend made in USA? Eur J Surg Oncol 38:296–301
Garcia-Etienne CA, Tomatis M, Heil J, Friedrichs K, Kreienberg R, Denk A, Kiechle M, Lorenz-Salehi F, Kimmig R, Emons G, Danaei M, Heyl V et al (2012) Mastectomy trends for early-stage breast cancer: a report from the EUSOMA multi-institutional European database. Eur J Cancer 48:1947–1956
Katipamula R, Degnim AC, Hoskin T, Boughey JC, Loprinzi C, Grant CS, Brandt KR, Pruthi S, Chute CG, Olson JE, Couch FJ, Ingle JN et al (2009) Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol 27:4082–4088
Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA, Investigation O, Kummerow KL, Du L, Penson DF, Shyr Y, Hooks MA, Investigation O (2014) Nationwide trends in mastectomy for early-stage breast cancer. JAMA Surg 37232:1–8
Gomez SL, Lichtensztajn D, Kurian AW, Telli ML, Chang ET, Keegan THM, Glaser SL, Clarke CA (2010) Increasing mastectomy rates for early-stage breast cancer? Population-based trends from California. J Clin Oncol 28:e155–e157; author reply e158
El-Tamer MB, Ward BM, Schifftner T, Neumayer L, Khuri S, Henderson W (2007) Morbidity and mortality following breast cancer surgery in women: national benchmarks for standards of care. Ann Surg 245:665–671
Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA (2013) Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer 119:1402–1411
Agarwal S, Pappas L, Neumayer L, Kokeny K, Agarwal J (2014) Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg 149:267–274
Onitilo AA, Engel JM, Stankowski RV, Doi SAR (2015) Survival comparisons for breast conserving surgery and mastectomy revisited: Community experience and the role of radiation therapy. Clin Med Res 13:65–73
Grover S, Nurkic S, Diener-West M, Showalter SL (2016) Survival after breast-conserving surgery with whole breast or partial breast irradiation in women with early stage breast cancer: a SEER data-base analysis. Breast J. http://doi.wiley.com/10.1111/tbj.12729
Hartmann-Johnsen OJ, Kåresen R, Schlichting E, Nygård JF (2015) Survival is better after breast conserving therapy than mastectomy for early stage breast cancer: a registry-based follow-up study of Norwegian women primary operated between 1998 and 2008. Ann Surg Oncol 22:3836–3845
Hofvind S, Holen Å, Aas T, Roman M, Sebuødegård S, Akslen LA (2015) Women treated with breast conserving surgery do better than those with mastectomy independent of detection mode, prognostic and predictive tumor characteristics. Eur J Surg Oncol 41:1417–1422
Flesch-Janys D, Slanger T, Mutschelknauss E, Kropp S, Obi N, Vettorazzi E, Braendle W, Bastert G, Hentschel S, Berger J, Chang-Claude J (2008) Risk of different histological types of postmenopausal breast cancer by type and regimen of menopausal hormone therapy. Int J Cancer 123:933–941
Soerjomataram I, Louwman MWJ, Ribot JG, Roukema JA, Coebergh JWW (2008) An overview of prognostic factors for long-term survivors of breast cancer. Breast Cancer Res Treat 107:309–330
Søgaard M, Thomsen RW, Bossen KS, Sørensen HT, Nørgaard M (2013) The impact of comorbidity on cancer survival: A review. Clin Epidemiol 5:3–29
Anampa J, Makower D, Sparano JA (2015) Progress in adjuvant chemotherapy for breast cancer: an overview. BMC Med 13:195
Shen Y, Yang Y, Inoue LYT, Munsell MF, Miller AB, Berry DA (2005) Role of detection method in predicting breast cancer survival: Analysis of randomized screening trials. J Natl Cancer Inst 97:1195–1203
Fisher B, Jeong J-H, Anderson S, Bryant J, Fisher ER, Wolmark N (2002) Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med 347:567–575
Sarrazin D, Lê MG, Arriagada R, Contesso G, Fontaine F, Spielmann M, Rochard F, Le Chevalier T, Lacour J (1989) Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother Oncol 14:177–184
van der Sangen MJC, van Wiel FMM, Poortmans PMP, Tjan-Heijnen VCG, Nieuwenhuijzen GAP, Roumen RMH, Ernst MF, Tutein Nolthenius-Puylaert MCBJE, Voogd AC (2011) Are breast conservation and mastectomy equally effective in the treatment of young women with early breast cancer? Long-term results of a population-based cohort of 1,451 patients aged ≤ 40 years. Breast Cancer Res Treat 127:207–215
Elkhuizen PH, van de Vijver MJ, Hermans J, Zonderland HM, van de Velde CJ, Leer JW (1998) Local recurrence after breast-conserving therapy for invasive breast cancer: high incidence in young patients and association with poor survival. Int J Radiat Oncol Biol Phys 40:859–867
Jacobson JA, Danforth DN, Cowan KH, D’Angelo T, Steinberg SM, Pierce L, Lippman ME, Lichter AS, Glatstein E, Okunieff P (1995) Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. New Engl J Med 332:907–911
de Bock GH, van der Hage JA, Putter H, Bonnema J, Bartelink H, van de Velde CJ (2006) Isolated loco-regional recurrence of breast cancer is more common in young patients and following breast conserving therapy: long-term results of European Organisation for Research and Treatment of Cancer studies. Eur J Cancer 42:351–356
Jones HA, Antonini N, Hart AAM, Peterse JL, Horiot JC, Collin F, Poortmans PM, Oei SB, Collette L, Struikmans H, Van Den Bogaert WF, Fourquet A et al (2009) Impact of pathological characteristics on local relapse after breast-conserving therapy: a subgroup analysis of the EORTC boost versus no boost trial. J Clin Oncol 27:4939–4947
Coulombe G, Tyldesley S, Speers C, Paltiel C, Aquino-Parsons C, Bernstein V, Truong PT, Keyes M, Olivotto IA (2007) Is mastectomy superior to breast-conserving treatment for young women? Int J Radiat Oncol Biol Phys 67:1282–1290
Kroman N, Holtveg H, Wohlfahrt J, Jensen M-B, Mouridsen HT, Blichert-Toft M, Melbye M (2004) Effect of breast-conserving therapy versus radical mastectomy on prognosis for young women with breast carcinoma. Cancer 100:688–693
Slanger T, Mutschelknauss E, Kropp S, Braendle W, Flesch-Janys D, Chang-Claude J (2007) Test-retest reliability of self-reported reproductive and lifestyle data in the context of a German case-control study on breast cancer and postmenopausal hormone therapy. Ann Epidemiol 17:993–998
Tuttle TM, Habermann EB (2011) Are mastectomies on the rise? A 13-year trend analysis of the selection of mastectomy versus breast conservation therapy in 5865 patients. Breast Dis 22:289–290
Sorbero MES, Dick AW, Beckjord EB, Ahrendt G (2009) Diagnostic breast magnetic resonance imaging and contralateral prophylactic mastectomy. Ann Surg Oncol 16:1597–1605
Tuttle TM, Habermann EB, Grund EH, Morris TJ, Virnig BA (2007) Increasing use of contralateral prophylactic mastectomy for breast cancer patients: a trend toward more aggressive surgical treatment. J Clin Oncol 25:5203–5209
Habermann EB, Abbott A, Parsons HM, Virnig BA, Al-Refaie WB, Tuttle TM (2010) Are mastectomy rates really increasing in the United States? J Clin Oncol 28:3437–3441
van Nes JGH, Seynaeve C, Jones S, Markopoulos C, Putter H, van de Velde CJH, Hasenburg A, Rea DW, Vannetzel J-M, Dirix L, Hozumi Y, Kerin MJ et al (2010) Variations in locoregional therapy in postmenopausal patients with early breast cancer treated in different countries. Br J Surg 97:671–679
Acknowledgements
We are grateful to all MARIE study participants. We also thank U. Eilber, S. Behrens, J. Mertin, J. Roczen, and T. Olchers for most valuable technical assistance and data management. The MARIE, MARIEplus, and MARIEplus2 study were funded by the Deutsche Krebshilfe e.V. (#70-2892-BR I, #108253 and #108419, #70110826 and #70112562).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thöne, K., Rudolph, A., Obi, N. et al. Prognostic impact of surgery for early-stage invasive breast cancer on breast cancer-specific survival, overall survival, and recurrence risk: a population-based analysis. Breast Cancer Res Treat 170, 381–390 (2018). https://doi.org/10.1007/s10549-018-4754-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-018-4754-6