Abstract
Background
Breast-conserving surgery (BCS) has been reported to have better survival rates when compared with total mastectomy (TM) in early breast cancer. We evaluated the long-term outcomes of Korean women with early breast cancer who underwent either BCS plus radiotherapy (RT) or TM.
Methods
In this population-based study, we evaluated 45,770 patients from the Korean Breast Cancer Registry (KBCR) who were diagnosed with early breast cancer, and divided them into the BCS + RT and TM groups. To minimize bias caused by factors other than the surgical method, we used exact match pairing of prognostic factors. We compared the 10-year overall survival (OS) and breast cancer-specific survival (BCSS) before and after exact matching. As the KBCR is a multicenter, online-based registry program, we used the Asan Medical Center (AMC) database, a single-center database, to validate the results from the KBCR database.
Results
In both the KBCR and AMC cohorts, the BCS + RT group showed better OS and BCSS than the TM group, before and after exact matching. For the KBCR cohort after exact matching, the hazard ratios for OS and BCSS were 1.541 (95% confidence interval [CI] 1.392–1.707, p < 0.001) and 1.405 (95% CI 1.183–1.668, p < 0.001), respectively, favoring the BCS + RT group. For the AMC cohort after exact matching, the hazard ratios for OS and BCSS were 1.854 (95% CI 1.476–2.328, p < 0.001) and 1.807 (95% CI 1.186–2.752, p = 0.006), respectively.
Conclusions
Our results suggest that BCS + RT is at least equivalent to TM in terms of OS and may affect treatment decisions in early breast cancer patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The randomized controlled trials conducted in the 1980s demonstrated the non-inferiority of breast-conserving surgery (BCS) followed by radiotherapy (RT) compared with mastectomy in patients with early breast cancer. These trials showed that even though patients who had BCS showed increased local recurrence, their overall survival (OS) was equal to that of patients who underwent total mastectomy (TM).1,2 As BCS clearly improved the quality of life of early breast cancer patients, the rate of BCS increased, and BCS combined with RT has become the standard treatment for early breast cancer.
In recent years, many observational studies have been published that reported that early breast cancer patients treated with BCS + RT have superior OS than those treated with TM. van Maaren et al. reported better OS, relative survival, and distant metastasis-free survival for BCS with RT compared with TM in T1N0 stage breast cancer patients in The Netherlands.3 Lagendijk et al. analyzed a Dutch cohort diagnosed between 1999 and 2012 and showed that BCS + RT led to higher OS and relative survival than TM in the T1–2, N0–1 group.4 Although these studies included more prognostic risk factors in the multivariate analysis than other observational studies that showed similar results, these studies lacked information on breast cancer subtype, breast cancer-specific deaths, and patients’ comorbidity.
This study aimed to compare the long-term survival outcomes of Korean breast cancer patients who underwent BCS + RT with those underwent TM, in a large population-based cohort. To overcome bias caused by death not related to breast cancer, we evaluated OS and breast cancer-specific survival (BCSS). Moreover, as we wanted to evaluate the survival difference associated with BCS + RT or TM, we performed 1:1 matching of factors that might affect prognosis, such as patient’s age, T stage, N stage, histological grade, and subtypes.
Methods
In this population-based study, we used the data from the Korean Breast Cancer Registry (KBCR) and Asan Medical Center (AMC) databases.
Korean Breast Cancer Registry
The KBCR is a prospectively maintained, multi-institutional registry of the Korean Breast Cancer Society. Nationwide, breast surgeons in 102 teaching hospitals participate in this program. As of 2004, this registry was estimated to include >50% of all newly diagnosed breast cancer patients in Korea. Essential data include patient’s identification number, sex, age, surgical method, and cancer stage based on the American Joint Committee on Cancer classification. Patients’ age at diagnosis, family history, menopausal status, and tumor characteristics such as subtype and histological grades can also be recorded.
For follow-up, patients were divided into four categories: no evidence of disease (NED), with recurrence, alive with disease, and dead. Type of first recurrence (locoregional or distant metastasis) and causes of death have been further categorized.
Asan Medical Center Database
The AMC database is a prospectively maintained, web-based system that includes information on all patients who have undergone operations for breast diseases at the AMC. This database provides more detailed information on the type of breast tumor and the date and location of breast cancer recurrence.
Patients and Study Design
We included female patients with primary invasive, pathologically staged T1–2, N0–1, M0 breast cancer from the KBCR who were diagnosed between 1998 and 2012. All patients underwent either BCS or TM irrespective of axillary staging or dissection or use of adjuvant systemic therapy. Patients (1) with primary carcinoma in situ, (2) who received neoadjuvant systemic therapy, (3) with unknown information or tumor characteristic needed for 1:1 matching, (4) who received postmastectomy RT, and (5) who did not receive RT following BCS were excluded. Selected patients were divided into the BCS + RT group or TM group, and their 10-year OS and BCSS were compared.
To minimize the confounding bias caused by variables other than surgical method, we used exact match pairing of variables that affect prognosis. The variables we matched were age at diagnosis, tumor T stage, tumor N stage, histological grade, lymphovascular invasion (LVI) status, tumor subtypes, and year of operation. We compared the OS and BCSS of the two groups.
As the KBCR is a multicenter, online-based registry program, we used single-center data (AMC database) to validate the results of the KBCR. We selected patients from the AMC who were diagnosed with breast cancer between 1998 and 2012. The inclusion and exclusion criteria were the same as those for the KBCR patients.
The follow-up data in the AMC database were more detailed; hence, we decided to compare not only the survival rate but also the recurrence rate of the two groups. Local recurrence and distant recurrence were all referred to as recurrence. For patients from the AMC database, the primary outcome was 10-year OS, BCSS, and disease-free survival (DFS).
In the matched groups, we performed a subgroup analysis of OS, BCSS, and DFS by cancer subtype: luminal A {hormone receptor (HR)-positive [HR+]/human epidermal growth factor receptor (HER2)-negative [HER2−]}, luminal B (HR+/HER2-positive [HER2+]), HER2 type (HR-negative [HR−]/HER2+), and triple negative (HR−/HER2−).
Statistical Analysis
The characteristics of the BCS and TM groups in the KBCR and AMC cohorts were compared using the Chi-square test. To adjust for confounding bias, we performed a 1:1 exact matching of all variables in each cohort, except for adjuvant RT. The Kaplan–Meier method was used to estimate the 10-year OS and BCSS of the KBCR and AMC cohorts, and the 10-year DFS of the AMC cohort, while the log-rank test was used to compare the BCS + RT group and the TM group. To calculate the hazard ratio with 95% confidence interval (CI) for 10-year OS, BCSS, and DFS, we used a multivariate Cox proportional hazard analysis.
All analyses were performed using the SPSS statistical software version 21.0 (IBM Corporation, Armonk, NY, USA) and R (R Core Team, 2019), and the figures were produced using the ggplot2 package (Wickham, 2009).
Results
Korean Breast Cancer Registry
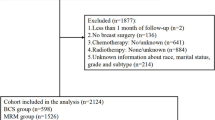
A total of 45,770 patients from the KBCR diagnosed with primary invasive cancer between 1998 and 2012 met the inclusion criteria. Of these patients, 17,147 underwent TM, while 28,623 underwent BCS with RT (Fig. 1a). The TM group was generally older and had a higher percentage of patients with T stage 2, N stage 1, and HER2+ subtype. A total of 14,791 patients in each group were suitable for 1:1 matching. Patient demographics and tumor characteristics are shown in Table 1.
In the multivariate Cox proportional hazard analysis, both before and after 1:1 exact matching, the BCS + RT cohort showed better OS and BCSS compared with the TM group. Before exact matching, the hazard ratios for OS and BCSS in the BCS + RT group were 1.802 (95% CI 1.664–1.950, p < 0.001) and 2.153 (95% CI 1.834–2.528, p < 0.001), respectively. After exact matching, the hazard ratios for OS and BCSS were 1.541 (95% CI 1.392–1.707, p < 0.001) and 1.405 (1.183–1.668, p < 0.001), respectively. The results are shown in Table 2.
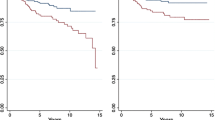
In the Kaplan–Meier survival curve, OS of the BCS + RT group was significantly better both before and after matching. The 10-year OS rates for the BCS + RT group and the TM group before matching were 93.2% and 87.9%, respectively, while the 10-year OS rates for the BCS + RT group and the TM group after matching were 94.3% and 90.6%, respectively (Fig. 2). Furthermore, the BCS + RT group had better BCSS before matching, but no significant difference was observed after matching (electronic supplementary Fig. 1). The median follow-up periods were 68.3 months before matching and 73.4 months after matching.
OS by operation method. (a) OS of KBCR patients before matching. (b) OS of KBCR patients after matching. (c) OS of AMC patients before matching. (d) OS of AMC patients after matching. OS overall survival, KBCR Korean Breast Cancer Registry, AMC Asan Medical Center, TM total mastectomy, BCS breast-conserving surgery, RT radiotherapy, Op method operation method
In the subgroup analysis by breast cancer subtype, the BCS + RT patients with luminal A and triple-negative subtypes showed better OS than TM patients, whereas the OS of those with luminal B and HER2 subtypes did not show a significant difference (Fig. 3). The analysis of BCSS showed different results, reporting that luminal A, B, and triple-negative subtypes did not show a significant difference in terms of survival benefit, while the BCS + RT group with HER2 subtype showed better BCSS compared with the TM group, with a p-value of 0.049 (electronic supplementary Fig. 2).
OS by operation method; subgroup analysis by cancer subtype in KBCR patients after matching. (a) OS of HR+/HER2− subtype. (b) OS of HR+/HER2+ subtype. (c) OS of HR−/HER2+ subtype. (d) OS of HR−/HER2− subtype. OS overall survival, KBCR Korean Breast Cancer Registry, HR hormone receptor, HER2 human epidermal growth factor receptor, TM total mastectomy, BCS breast-conserving surgery, RT radiotherapy, Op method operation method
Asan Medical Center Database
A total of 10,016 patients from the AMC database who were diagnosed with primary invasive cancer between 1998 and 2012 met the inclusion criteria. Of these patients, 3633 underwent TM and 6383 underwent BCS with RT. A total of 2927 patients in each group were suitable for 1:1 matching (Fig. 1b). The TM group was generally older and had a higher percentage of patients with higher T stage and HER2+ subtype. The characteristics of patients and tumors according to the type of surgery are presented in Table 1.
In the multivariate Cox proportional hazard analysis, both before and after 1:1 exact matching, the BCS + RT cohort showed better OS, BCSS, and DFS compared with the TM cohort. Before exact matching, the hazard ratios for OS, BCSS, and DFS in the BCS + RT group were 1.634 (95% CI 1.363–1.960, p < 0.001), 1.536 (95% CI 1.109–2.129, p = 0.010), and 1.371 (95% CI 1.199–1.568, p < 0.001), respectively. After exact matching, the hazard ratios for OS, BCSS, and DFS were 1.854 (95% CI 1.476–2.328, p < 0.001), 1.807 (95% CI 1.186–2.752, p = 0.006), and 1.310 (95% CI 1.090–1.574, p = 0.004), respectively (Table 3).
In the Kaplan–Meier survival curve, the BCS + RT group showed better OS, BCSS, and DFS, both before and after matching. The 10-year OS rates for the BCS + RT group and the TM group after matching were 95.8% and 91.9%, respectively (Fig. 2); the 10-year BCSS rates for the BCS + RT group and TM group after matching were 98.8% and 97.6%, respectively (electronic supplementary Fig. 1); and the 10-year DFS rates for the BCS + RT group and the TM group after matching were 90.2% and 87.6%, respectively (electronic supplementary Fig. 3). The median follow-up periods were 97.0 months before matching and 99.0 months after matching.
In subgroup analysis, the BCS + RT group with luminal A and triple-negative subtypes showed significantly better OS compared with the TM group, whereas those with the HER2+ subtype did not show significant OS difference (Fig. 4). In the AMC matching cohort, unlike the KBCR matching cohort, BCSS showed a pattern similar to OS, where patients with luminal A and triple-negative subtypes treated with BCS + RT showed better outcomes than those with luminal B and HER2 subtypes (electronic supplementary Fig. 4). In terms of DFS, only patients with luminal A subtype treated with BCS + RT showed significantly better survival (electronic supplementary Fig. 5).
OS by operation method; subgroup analysis by cancer subtype in AMC patients after matching. (a) OS of HR+/HER2− subtype. (b) OS of HR+/HER2+ subtype. (c) OS of HR−/HER2+ subtype. (d) OS of HR−/HER2− subtype. OS overall survival, AMC Asan Medical Center, HR hormone receptor, HER2 human epidermal growth factor receptor 2, TM total mastectomy, BCS breast-conserving surgery, RT radiotherapy, Op method operation method
Discussion
Since the publication of randomized trials demonstrating the non-inferiority of BCS followed by RT compared with TM, by Veronesi et al. and Fisher et al., although the incidence of local recurrence was higher in patients treated with BCS, as it was deemed acceptable with similar OS, the rate of BCS for early breast cancer increased;1,2,5 however, these trials were carried out when the clinical, biological, or genetic prognostic factors were unknown, and adjuvant treatment was not tailored to disease biology. In the meantime, treatment of breast cancer has improved dramatically over time, and recent studies showed an impressive decrease in local recurrence in BCS + RT patients with appropriate adjuvant treatment, i.e. 2% in 10 years.6 As the incidence of local recurrence decreased over time, there might have been changes in the survival outcome between early breast cancer patients treated with TM and those treated with BCS + RT.
Indeed, many recent large-scale population studies have shown better outcome in BCS + RT compared with TM. Agarwal et al. examined 132,000 patients with early-stage breast cancer from the Surveillance, Epidemiology, and End Results (SEER) database. In this study, patients with tumor size from 0 to 4 cm and 0–3 positive nodes were included and were divided according to the type of local treatment (BCS + RT, mastectomy alone, and mastectomy + RT). Disease-specific survival was better in the BCS + RT cohort, which was confirmed in the subgroup analyses based on tumor size and lymph node involvement.7 This study had some major limitations. Systemic therapy was not analyzed and tumor characteristics were limited to HR status, grade, and lymph node status, with no data on LVI or HER2 status. van Maaren et al. analyzed patients from the Netherlands Cancer Registry and reported a similar conclusion that BCS + RT showed improvement in 10-year OS and relative survival, when compared with TM, in early breast cancer. However, in a subgroup analysis of 10-year distant metastasis-free survival, only the T1N0 patients in the BCS + RT group showed significantly better survival, indicating possible confounding by severity.3 More than 75% of patients in the T1N0 subgroup did not receive adjuvant systemic therapy, and HR and HER2 status were not included as prognostic factors.
In the current study, we tried to avoid the above limitations and only included patients with enough information on tumor biology and subtype. To adjust for various prognostic risk factors between the two groups, a 1:1 exact matching for age at diagnosis, T stage, N stage, histological grade, lymphovascular invasiveness, subtype, and period of operation was performed. We added period of operation as a factor, as adjuvant systemic therapy has improved dramatically over time. We used a nationwide, multicentered database in order to include a large number of patients, with the single-center database used to validate the results. We performed a long-term follow-up, with a median follow-up of 68.33 months before matching and 73.43 months after matching for KBCR, and 97.0 months before matching and 99.0 months after matching for the AMC database. For the primary endpoint, we evaluated OS and BCSS. BCSS is more reliable in estimating the treatment effects than OS by eliminating the large influence of age and comorbidities.
Our study reported that Korean early breast cancer patients who underwent BCS + RT showed better OS and BCSS than those who underwent TM. In terms of BCSS, the BCS + RT group showed better survival based on the results of the multivariate Cox proportional hazard analysis of all groups. In the Kaplan–Meier survival analysis, the KBCR matching group showed no significant difference. As the KBCR is a nationwide online-based database, the cause of death might not have been specifically registered, and therefore the number of events could be underestimated (electronic supplementary Fig. 1b).
As the AMC database had more detailed information and patients were more thoroughly followed, we had enough information to evaluate DFS. In our raw data, we classified patients with recurrence into two categories; locoregional recurrence and distant recurrence, which were all referred to, as recurrence in our study and the BCS + RT group showed better DFS than the TM group. Although not shown in our results, we performed subgroup analysis by type of recurrence. In the AMC matching data, of 5854 patients, 601 patients had recurrence and 322 patients had locoregional recurrence, while 279 patients had distant recurrence. In the Kaplan–Meier survival curve of patients with distant recurrence, the BCS + RT group showed better DFS than the TM group. The 10-year DFS rate for the BCS + RT group was 95.3%, and 94.1% for the TM group. Patients with locoregional recurrence did not show significant difference in DFS between two groups. One of the reasons for this result could be that as BCS + RT has a higher chance of ipsilateral breast cancer tumor recurrence, this might have affected the result.
Several previous studies have shown similar results and have attempted to explain the reason for better survival in the BCS + RT group compared with the TM group, with the most obvious reason being the impact of RT. Onitilo and colleagues investigated the OS of the BCS group with and without RT and compared it with that of the TM group. The results showed that OS of BCS patients without RT was equal to that of TM patients, but the OS was longer in BCS patients with RT than in TM patients. The better OS of the BCS + RT group is likely to be related to the addition of RT than to the surgery itself.8 In the Netherlands study, more than 75% of patients in the T1N0 subgroup did not receive adjuvant systemic therapy; therefore, adjuvant RT partly attributed to the improvement of distant metastasis-free survival in the BCS + RT group compared with the TM group.3 The concept of RT as a local treatment having a systemic effect is now easier to understand after the overview on the impact of postoperative RT.9 In our study, even though adjuvant systemic therapy was not included in the matching factors, we assumed that as age, stage, subtype, and time of diagnosis were matched, similar adjuvant systemic treatments have been used; hence, adjuvant RT possibly attributed to the improvement in patients’ survival. Another reason is the possibility of depressed immune response after undergoing a more extensive surgery; however, the complex relationship between surgical trauma, RT, and immune response remained unknown.5
In the subgroup analysis by cancer subtype, results showed interesting patterns. Excluding the BCSS of the KBCR matching group, patients with luminal A and triple-negative subtypes in the BCS + RT group showed better survival compared with the TM group, whereas HER2+ subtypes showed no significant difference in OS and BCSS. This finding could be due to the fact that as HER2+ tumor outcome has dramatically improved after the introduction of targeted therapy, systemic therapy has more influence on the nature of HER2+ tumor regardless of the provision of local treatment such as surgery and RT.10
This study has some limitations. First, although we evaluated BCSS to differentiate overall mortality from disease-specific mortality, several causes of death were not specified in the database, indicating that the disease-specific mortality could be underestimated. Second, when exact matching was performed, tumor size was matched by T stage, and the TM group showed larger tumor size compared with the BCS group. In future studies, a more precise stratification of tumor size could lead to more accurate results. Third, we did not include the status of adjuvant systemic therapy when we performed the exact matching or in the multivariate analysis. We just made an assumption that in the same period of time, with the same tumor stage and subtype, most patients will probably receive similar adjuvant therapy. Fourth, one of the key characteristics of Korean breast cancer is the higher incidence of young-age breast cancer than in Western countries.11,12 About half of the patients diagnosed with breast cancer were under the age of 50 years (Table 1). Because of the difference in age distribution, our study results may not represent the global population of breast cancer patients. Finally, because of the retrospective and voluntary nature of the KBCR database, for many patients the data are incomplete, which may have affected the results of our study. To minimize the bias, we only included patients with sufficient data, and, to validate the KBCR database, we used the more detailed AMC database, which showed a similar result.
Conclusion
Even after adjusting for confounding factors by exact matching, the BCS + RT group showed better OS and BCSS in both the KBCR and AMC databases. As the AMC database had more precise and accurate information regarding the date and site of recurrence, we analyzed DFS and found the BCS + RT group showed better DFS compared with the TM group. These results suggest that BCS + RT is at least equivalent to TM in terms of recurrence-free survival and OS, and may affect the treatment decisions in early breast cancer patients. Hence, randomized controlled trials should be conducted and should include adjuvant treatment to confirm whether early breast cancer patients treated with BCS + RT have a better survival rate than those treated with TM.
References
Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–32.
Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41.
van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. 2016;17(8):1158–70.
Lagendijk M, van Maaren MC, Saadatmand S, et al. Breast conserving therapy and mastectomy revisited: breast cancer-specific survival and the influence of prognostic factors in 129,692 patients. Int J Cancer. 2018;142(1):165–75.
Gentilini OD, Cardoso MJ, Poortmans P. Less is more. Breast conservation might be even better than mastectomy in early breast cancer patients. Breast. 2017;35:32–3.
Poortmans PMP, Arenas M, Livi L. Over-irradiation. Breast. 2017;31:295–302.
Agarwal S, Pappas L, Neumayer L, et al. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014;149(3):267–74.
Onitilo AA, Engel JM, Stankowski RV, et al. Survival comparisons for breast conserving surgery and mastectomy revisited: community experience and the role of radiation therapy. Clin Med Res. 2015;13(2):65–73.
Early Breast Cancer Trialists’ Collaborative Group, McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–35.
Arteaga CL, Sliwkowski MX, Osborne CK, et al. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat Rev Clin Oncol. 2012;9(1):16–32.
Kang SY, Kim YS, Kim Z, et al. Breast cancer statistics in Korea in 2017: data from a breast cancer registry. J Breast Cancer. 2020;23(2):115–28.
Emily H, Andrew H, Noah E, et al. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. 2020;8(8):e1027–37.
Acknowledgement
This study was supported by a Grant (Elimination of Cancer Project Fund) from the Asan Cancer Institute of the AMC, Seoul (2017-1341), and this article was supported by the Korean Breast Cancer Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Hakyoung Kim, Sae Byul Lee, Seok-Jin Nam, Eun Sook Lee, Byeong-Woo Park, Ho Yong Park, Hyouk Jin Lee, Jisun Kim, Il Yong Chung, Hee Jeong Kim, Beom Seok Ko, Jong Won Lee, Byung Ho Son, Sei Hyun Ahn declare they have no conflicts of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, H., Lee, S.B., Nam, SJ. et al. Survival of Breast-Conserving Surgery Plus Radiotherapy versus Total Mastectomy in Early Breast Cancer. Ann Surg Oncol 28, 5039–5047 (2021). https://doi.org/10.1245/s10434-021-09591-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-09591-x