Abstract
Purpose
While intact circulating tumor cells (iCTC) have independent negative prognostic impact on patients with metastatic breast cancer (MBC), the prognostic relevance of apoptotic CTC (aCTC) has not been validated in larger patient cohorts. This study assessed aCTC and iCTC statuses at baseline (CTCBL) and CTC kinetics (CTCKIN) as changes from CTCBL to one completed treatment cycle for their utility in predicting response, progression-free survival (PFS), and overall survival (OS) in MBC.
Methods
Status of iCTC and aCTC was prospectively assessed in 442 patients using the CellSearch™ system. Different cutoffs were analyzed both for iCTC and aCTC (≥5, ≥10, ≥25 and ≥50 CTC/7.5 ml). CTCKIN were characterized by ≥25 % changes in CTC counts.
Results
Numbers of iCTC and aCTC at baseline correlated strongly (r = 0.7). For iCTCBL positive patients, additional detection of aCTCBL had a significant prognostic impact on OS (aCTCBL positive 10.3 vs. aCTCBL negative 16.4 months, p = 0.012). Worst prognosis for OS was observed in patients with ≥50 iCTC/7.5 ml and simultaneously detected aCTC. Determination of aCTCKIN showed stronger discriminating power than iCTCKIN, with higher PFS and OS for the group with decreasing CTCs (PFS 7.7 vs. 6.1; OS 22.2 vs. 16.4).
Conclusions
Intact and aCTC are predictive of outcome in MBC. Apoptotic CTC counts ≥ 5/7.5 ml in conjunction with iCTC at baseline have an independent unfavorable prognostic impact on OS. Decreasing aCTCKIN at ≥ 5/7.5 ml in serial enumeration is associated with favorable outcome. Therefore, separate enumeration of iCTC and aCTC is useful in tailoring systemic treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The greatest challenge in treatment management of metastatic breast cancer (MBC) is to identify early indicators of response to systemic treatment to avoid unnecessary exposure to ineffective but toxic treatment and to enable early prognostication of progression-free (PFS) and overall survival (OS). The presence of ≥5 CTC in 7.5 ml of peripheral blood prior to a new line of treatment is hence an independent factor for poor prognosis in MBC patients [1–6]. The malignant nature and metastasis-initiating potential of CTC is extensively reported in the literature [7, 8]. Previous studies reported that CTC provide more prognostic information than conventional imaging [9], could help identify patients who benefit from a more aggressive treatment regimen, and could be used to monitor treatment response [10–12]. Nevertheless, CTC represent a heterogeneous cell population with phenotypic changes compared to the primary tumor [13, 14] and bear great potential for diagnostic and therapeutic utilization as a liquid biopsy.
Apoptotic CTC (aCTC), which have been reported in 52–79 % of CTC-positive MBC patients as a CTC subtype [15–17], are characterized by altered morphological parameters such as speckled pattern of keratin staining and/or fragmented or disintegrated nuclei. Apoptotic CTC seem to derive from therapy-induced apoptosis and apparently from spontaneous tumor apoptosis as they also appear in patients with progressive disease and no response to systemic therapy [18, 19]. In addition, patients with MBC presented with significantly lower numbers of aCTC compared to patients with early breast cancer [17]. It is suggested that the viability of CTC is related to the stage of disease and aCTC might provide additional prognostic information [20]. Enumeration of disseminated tumor cells (DTC) from bone marrow showed that the appearance of apoptotic DTC (aDTC) is predictive of positive response under neoadjuvant systemic therapy [21, 22]. This underlines the hypothesis that aCTC might serve as a surrogate endpoint for successful systemic therapy [17].
The prognostic impact of both baseline CTC enumeration and kinetics (CTCKIN; change in CTC count from baseline (CTCBL) to first completed cycle of a new line of systemic therapy (CTC1C)) has been demonstrated in recent studies [3]. The present study aimed to prospectively assess the CTC status separately for the apoptotic CTC (aCTC) and intact CTC (iCTC) subtypes in a large group of patients. To this end, we analyzed CTCBL, CTC1C, and CTCKIN for their impact on treatment response, PFS, and OS.
Methods
Patients and study design
This was a prospective, single-center, non-randomized, partially blinded, treatment-based study. Both patients and treating physicians were blinded to CTC status, and hence treatment regimens did not depend on CTC status. All investigators and technical staff who performed or reviewed the CTC data were blinded to patient history and treatment. Independent reviewers confirmed CTC enumeration and characterization. All radiologists performing imaging studies were blinded to the patients’ treatment regimens. The study was conducted at the National Center for Tumor Diseases (NCT), Heidelberg, Germany, and the Department of Obstetrics and Gynecology, University of Heidelberg, Heidelberg, Germany.
Study design
MBC patients were consecutively enrolled between March 2010 and May 2015 at the beginning of new line of systemic therapy. Blood samples were taken prior to treatment to determine the baseline counts (CTCBL) of iCTC and aCTC. Counts ≥5 CTC/7.5 ml peripheral blood were considered CTCBL-positive [23]. After the first cycle of systemic therapy, a second blood sample was taken (CTC1C).
The first 100 patients in this study had no further blood samples taken after a negative CTCBL result. Every 3 months patients were categorized as showing progressive disease (PD), stable disease (SD), complete remission (CR), or partial response (PR) based on the Response Evaluation Criteria in Solid Tumors [24]. Survival status was recorded until death or loss to follow-up.
Enumeration of CTC
For CTC enumeration, 7.5 ml peripheral whole blood was collected in a CellSave tube (J Janssen Diagnostics, LLC, Raritan, NJ, USA). Blood samples were kept at room temperature for ≤96 h until analysis using the CellSearch™ assay (CellSearch™ Epithelial Cell Kit/CellSpotter™ Analyzer, Janssen Diagnostics, LLC, Raritan, NJ, USA). Sample processing and analysis were done strictly according to the manufacturer’s instructions. The assay uses a ferrofluid coated with antibodies to epithelial cell adhesion molecule (EpCAM) to immunomagnetically separate cells of epithelial origin from blood, and fluorescent staining to differentiate between debris, hematopoietic cells, and epithelial cells [13]. It provides high intra-observer, inter-observer, and inter-instrument agreements [2, 5, 15, 25]. Cells enriched by anti-EpCAM antibodies were labeled with the nuclear dye 4′,6-diamidino-2-phenylindole (DAPI) and immunostained with monoclonal antibodies specific for keratins and for the leukocyte common antigen CD45. CTC detection was performed by trained staff using the CellSpotter™ Analyzer, a semi-automated fluorescence-based microscopy system that enables computer-generated reconstruction of cellular images [16, 21, 26]. Morphologically intact, CD45-negative CTC without obvious alterations of nuclei and non-speckled keratin immunofluorescence were defined as iCTC and enumerated by trained operators. Patients with counts ≥5 intact CTC/7.5 ml blood were considered iCTC-positive.
Characterization of apoptotic CTC (aCTC)
Apoptotic CTC were visually characterized by altered morphological parameters such as speckled keratin staining patterns and/or fragmented or disintegrated nuclei. In select cases, classification as aCTC was proven by addition of the M30 antibody for the detection of caspase-cleaved Keratin-18 (VLV bio, 1:100) in the fourth channel of the CellSearch system. The vast majority of CTC with characteristic morphologic changes also was positive for M30 (Fig. 2). Patients with counts ≥5 aCTC/7.5 ml blood were considered aCTC-positive.
Evaluation of CTC kinetic
To analyze CTC kinetics from baseline (CTCBL) until the end of first cycle of systemic therapy (CTC1C), changes were categorized as CTC negative (<5 CTC for both CTCBL and CTC1C), stable (<25 % change), indicating decrease (≥25 % decrease), or indicating increase (≥25 % increase) [12]. Kinetics were separately evaluated for the following cutsoff: ≥5, ≥10, ≥25, and ≥50 CTC/7.5 ml (Supplementary Figs. 1, 2; Supplementary Table 1, 2).
Data collection and analysis
Demographic data and clinical characteristics were described as frequency and percentage, median and range, or mean and standard deviation. Groups were compared using the Wilcoxon rank test or Fisher’s exact test, as appropriate. Kaplan–Meier plots by aCTC and iCTC statuses were generated for PFS and OS (time from enrollment to disease progression and death from any cause, respectively), with data being censored at last follow-up if progression or death had not occurred. PFS and OS times were estimated as medians with 95 % confidence intervals (CIs). Differences in PFS and OS by iCTC and aCTC statuses were assessed by the log-rank test. Statistical analyses were performed using R (version 3.1.2) [27]. A significance level of 5 % was chosen.
Results
CTC status and enumeration at baseline (CTCBL)
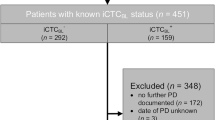
442 consecutive patients were enrolled in the study. Figure 1 shows the flow of patients through the study, which also details the reasons for exclusion from or non-availability for further analysis. Patient characteristics at baseline are summarized in Table 1. Of the 442 available patients with CTCBL counts, 155 (35.1 %) were iCTCBL-positive and 128 (29.1 %) patients were aCTCBL-positive (Fig. 2). 108 (24.4 %) patients were both positive for iCTCBL and aCTCBL (pos pos), and 267 (60.4 %) patients were both negative for iCTCBL and aCTCBL (neg neg), as shown in Table 3. Positivity rates for higher cutoff values (≥10, ≥25 and ≥50) are listed in Table 4.
Flow of patients through the study. 466 consecutive patients were assessed for eligibility, 24 (5.2 %) were excluded from the study because no CTCBL data were available, or no follow-up was performed. Of the 442 patients included in the study, 190 were excluded from further analysis for the following reasons. During the initial phase of the study, CTC1C status of the first 100 patients was routinely determined only in CTCBL positive patients, resulting in 64 patients without measured CTC1C counts. Of the remaining 116 patients without CTC1C counts, 18 were excluded because blood samples were not obtained within the predefined study timeframe of 2–14 weeks, 30 did not survive to CTC1C assessment, 10 had no survival data, and 68 patients had not yet proceeded to CTC1C
aCTC and iCTC morphologic characteristics. CellSearch image gallery displaying morphologically intact CTC (1–5) and apoptotic CTC (6–10). KER/DAPI represents a composite image of PE-keratin (KER) and DAPI (nuclei). CD45 positivity of leukocytes is demonstrated in the APC channel and applying a FITC-labeled anti-M30 antibody in the fourth channel, M30-FITC-positive CTC present with speckled keratin staining patterns and/or fragmented nuclei
While in most cases iCTCBL positivity corresponded with aCTCBL positivity, 47 (10.6 %) patients were iCTCBL-positive and aCTCBL-negative (pos neg) and 20 (4.5 %) were iCTCBL-negative and aCTCBL positive (neg pos) (p < 0.001). Figure 3 illustrates the correlation of iCTC and aCTC counts per 7.5 ml (r = 0.7).
CTC status and survival at baseline
Follow-up data were available for 442 patients with a median [95 % confidence interval (CI)] follow-up of 34.1 [32.0–36.9] months for OS. Median [95 % CI] OS for iCTCBL-positive versus iCTCBL-negative patients was 13.1 [10.0–15.9] versus 27.0 [24.0–31.7] months (p < 0.001), respectively (Kaplan–Meier plots not shown). Median PFS was 4.9 [4.1–6.1] versus 7.8 [6.9–9.2] months (p < 0.001), respectively (Table 2). Median [95 % CI] OS for aCTCBL-positive versus aCTCBL-negative patients was 10.9 [7.9–15.5] versus 26.9 [22.5–29.9] months (p < 0.001), respectively (Suppl. Material). Median PFS was 4.6 [4.0–6.1] versus 7.7 [6.5–9.2] months (p < 0.001), respectively (Table 2).
Figure 4 shows Kaplan–Meier plots for PFS and OS by iCTC and aCTC status at baseline. Median [95 % CI] OS for iCTCBL-positive/aCTCBL-positive (pos pos), iCTCBL-positive/aCTCBL-negative (pos neg), iCTCBL-negative/aCTCBL-positive (neg pos) and iCTCBL-negative/aCTCBL-negative (neg neg) patients was 10.3 [7.4–15.0], 16.4 [11.1–37.9], 29.9 [10.4–NA], and 27.9 [24.0–32.5] months (p < 0.001) and median PFS was 4.4 [3.5–5.9], 6.1 [4.2–9.8], 6.3 [3.4–19.4] and 7.9 [6.9–9.4] months (p < 0.001) as shown in Table 3.
Kaplan–Meier plots—OS and PFS separated in apoptotic and intact CTC at baseline (aCTCBL and iCTCBL). OS (left) and PFS (right) by CTC status at baseline in 442 patients with MBC; pos pos (iCTCBL-positive and aCTCBL-positive), pos neg (iCTCBL-positive and aCTCBL-negative), neg pos (iCTCBL-negative and aCTCBL-positive), and neg neg (iCTCBL-negative and aCTCBL-negative)
This indicates that iCTCBL-positive patients in conjunction with positive aCTCBL (pos pos) had a significantly lower OS compared to iCTCBL-positive/aCTCBL-negative patients (pos neg) (PFS 4.4 vs. 6.1 months, p = 0.166; OS 10.3 vs. 16.4 months, p = 0.012). In contrast, the iCTCBL-negative/aCTCBL-positive (neg pos) group showed no significant impact on PFS and OS compared to iCTCBL-negative and aCTCBL-negative (neg neg) (PFS 6.3 vs. 7.9 months, p = 0.665; OS 29.9 vs. 27.9 months, p = 0.360 univariate).
The group iCTCBL-positive/aCTCBL-positive (pos pos) also showed a significantly higher iCTCBL count when compared to iCTCBL-positive/aCTCBL-negative (pos neg) patients (103.6 (179.0), respectively 28.9 (75.1), mean (STD); Wilcoxon test, p < 0.001).
All proven higher cutoffs for CTC positivity (≥10, ≥25 and ≥50/7.5 ml) were significantly associated with decreasing overall survival (Fig. 8), thereby reducing the prognostic impact of additionally enumerating apoptotic CTC (Table 4).
CTC status at first cycle (CTC1C)
The results for CTC1C enumeration are shown in Table 5. The percentage of aCTC-positive patients decreased significantly to 41/252 (16.3 %) from CTCBL to CTC1C (p < 0.001, McNemar test). Figure 5 shows Kaplan–Meier plots for PFS and OS by iCTC and aCTC status after the first cycle of a new line of systemic therapy (CTC1C). Median OS [95 % CI] for iCTC1C-positive and aCTC1C-positive (pos pos), pos neg, neg pos and neg neg patients were 6.7 [5.0–13.9], 13.3 [10.9–22.5], 10.4 [4.1–NA], and 31.8 [26.9–37.9] months (p < 0.001), respectively, 3.5 [3.2–5.5], 5.2 [3.8–7.7], 2.9 [2.7–NA] and 8.0 [6.9–10.1] months for PFS (p < 0.001) as shown in Table 5.
Kaplan–Meier plots—OS and PFS separated in apoptotic and intact CTC at first cycle (aCTC1C and iCTC1C). OS (left) and PFS (right) by CTC status after the first cycle of a new line of systemic therapy 252 patients with MBC; pos pos (iCTC1C-positive and aCTC1C-positive), pos neg (iCTC1C-positive and aCTC1C-negative), neg pos (iCTC1C-negative and aCTC1C-positive), and neg neg (iCTC1C-negative and aCTC1C-negative)
The pos pos group showed significantly higher iCTC1C counts than the pos neg group (96.1 (170.1) vs. 24.5 (31.0), mean (STD), p = 0.015).
CTC kinetics from baseline to first cycle (CTCKIN)
Intact CTC and aCTC enumeration for CTCKIN is shown in Table 6. High numbers in the diagonal cells indicate that iCTCKIN and aCTCKIN evolve similarly in most cases (Cramer’s V = 0.50). Most patients were negative for both iCTC and aCTC at CTCBL and CTC1C (50.0 %), followed by decreased iCTC and aCTC levels (17.5 %).
CTCKIN and survival
Figures 5 and 6 show Kaplan–Meier plots for PFS and OS by CTC kinetics. Patients with counts <5 for iCTCKIN, aCTCKIN, and (iCTC + aCTC)KIN combined in both samples had the best outcomes for OS (32.9, 27.9, and 31.8 months, respectively) and PFS (7.9, 7.7, and 7.9 months, respectively). Stable or increased iCTCKIN, aCTCKIN, and (iCTC + aCTC)KIN counts showed the shortest OS (10.7/9.5, 5.1/5.7, and 10.8/7.4 months, respectively) and PFS (4.6/4.0, 4.1/3.2, and 5.5/3.3 months, respectively) as shown in Table 7. Decreased aCTCKIN had most favorable outcomes compared to iCTCKIN and decreased (iCTC + aCTC)KIN for OS (22.2 vs. 16.4 and 21.1 months) and PFS (7.7 vs. 6.1 and 6.1 months). aCTCKIN showed greater discriminatory power for OS and PFS between the favorable groups (<5 CTC at any time or decrease) and the unfavorable groups (stable/increase) than did iCTCKIN. Kaplan–Meier plots are shown in Figs. 5, 6. However, this discriminating power decreases with the increasing CTC cutoff values applied, suggesting that separate counting of aCTC is most meaningful at CTC levels of <10/7.5 ml (Supplementary Figs. 1, 2).
Kaplan–Meier plots—Kinetic of intact CTC between baseline and first cycle (iCTCKIN). OS (left) and PFS (right) by CTC status between baseline and first cycle in 252 patients with MBC: <5 CTC (<5 CTC in cells in both CTCBL and CTC1C), stable (<25 % change), decrease (≥25 % decrease), and increase (≥25 % increase)
CTCKIN and systemic therapy
The regimen of individual treatment for patients depended on the molecular subtype of both primary tumor tissue and metastatic tissue, previous therapeutic response, and clinical situation of the patient, following the German guidelines of the AGO [28]. During the first cycle of systemic therapy, 68 % of patients were treated with chemotherapy (CHT), including mono and poly-CHT after enrollment in the study, 20 % received Bevacizumab in addition to the CHT. 29 % of patients received endocrine therapy (such as Tamoxifen, Fulvestrant, Exemestane, and Letrozole) and 16 % of patients received Anti-HER2 therapy (such as Trastuzumab, Pertuzumab, and Lapatinib).
Table 8 shows the proportion of favorable aCTCKIN (<5 aCTC and decreased aCTC) from baseline to first cycle in relation to systemic therapy during the first cycle after patient enrollment regarding OS and PFS. The patient subset receiving endocrine therapy exhibited the longest OS compared to monochemotherapy, polychemotherapy, and chemotherapy with Bevacizumab (33.4 months vs. 18.1, 22.1, 20.4 months, respectively) and displayed the highest proportion of negative and decreasing aCTC (92 % vs. 89, 86, 87 %, respectively). Anti-HER2 treatment compared to no Anti-HER2 therapy in the first cycle of systemic therapy was associated with longer OS (27.9 vs. 20.4 months) and PFS (8.1 vs. 6.3 months) and a higher proportion of favorable aCTCKIN (97 vs. 87 %).
Discussion
As reported previously, iCTC at baseline have a high impact on both OS and PFS [3, 10]. Our results from a large cohort of 442 patients confirm that iCTC-positivity has the strongest negative impact on prognosis [3]. However, aCTC might have the potential to tip the scale in undecided situations whether to change, intensify, or stay with the therapy scheme [20].
Prognostic role of aCTC at baseline
The presence of aCTC alone at baseline has a similar favorable outcome compared to the complete absence of CTC (iCTC/aCTC) as evidenced by OS 29.9 versus 27.9 and PFS 6.3 versus 7.9 months. iCTCBL-negative/aCTCBL-positive patients (neg pos) had an even better, though not significantly better OS compared to patients without any CTC (iCTC/aCTC; neg neg). These aCTC in the bloodstream may represent tumor tissue undergoing chemotherapy-induced apoptosis. In line with Fehm et al. [21, 22], aCTCs could be similar to aDTC used as surrogate for systemic therapy response. However, only a small fraction of patients (4.5 %) were iCTCBL-negative/aCTCBL-positive (neg pos) at CTCBL, and the number of events in this subgroup is limited. Therefore, these results should be interpreted with care even though they might support the hypothesis.
Our data suggest that aCTC enumeration in combination with elevated iCTC at baseline compared to negative aCTC with positive iCTC at baseline has a strong impact on OS (10.3 vs. 16.4 months) and PFS (4.4 vs. 6.1 months). Proliferating tumor tissue is associated with necrosis and apoptosis, and therefore aCTC have been considered a sign of tumor proliferation [16, 29]. Following this reasoning, aCTC appear to be the result of therapy-induced apoptosis and tumor cell apoptosis in the context of carcinoma equilibrium and could be regarded as a natural side product of iCTC and tumor tissue [19, 29]. This hypothesis is supported by the fact that iCTCBL-positive/aCTCBL-positive (pos pos) patients had significantly higher amount of iCTC counts and showed the worst prognosis for OS and PFS.
Our results suggest that in the presence of ≥5 iCTC/7.5 ml, aCTC do not predict positive therapy response but outline a heterogeneous picture including tumor cell homeostasis and therapy-induced cell death and thus extend the picture of therapy response status [18, 19]. However, applying increasing cutoff values for iCTC positivity from ≥5 CTC to ≥50 CTC/7.5 ml reduced the additional prognostic impact of aCTC (Fig. 8).
Prognostic role of aCTC at first cycle
To investigate the diagnostic relevance of aCTC, we analyzed the enumeration of aCTC during treatment with the clinical prognosis. The data show a strong relation of iCTC and aCTC kinetics (Cramer’s V = 0.49). Eighty-five percent of patients were either positive for both iCTC and aCTC (pos pos) or negative for both iCTC and aCTC (neg neg). iCTCBL negative patients had the most favorable prognosis for OS (p < 0.001) and PFS (p < 0.001) independent of the aCTC status.
Prognostic role of aCTC kinetics
Differences in OS and PFS were observed for the aCTC kinetics (aCTC decrease (OS 22.2, PFS 7.7) and stable/increased aCTC count (OS 5.1/5.7, PFS 4.1/3.2)). A decrease of ≥25 % in aCTC had a better long-term outcome than stable or increased aCTC count independent of iCTC status.
Apoptotic CTCKIN has more discriminatory power for treatment response than iCTCKIN or the combination of iCTC and aCTC kinetics ((iCTC + aCTC)KIN). The Kaplan–Meier plots for aCTCKIN better reflect the prognostic situation for the single categories than iCTCKIN (as indicated by Figs. 6 and 7). Stronger discriminating results were observed for aCTC compared to iCTC decrease or iCTC + aCTC decrease for OS (22.2 vs. 16.4 and 21.1) and PFS (7.7 vs. 6.1 and 6.1). However, since aCTC levels are significantly associated with iCTC levels, separate enumeration of aCTC is only of prognostic relevance at CTC levels below 10 CTC/7.5 ml Fig. 8. The discriminating power decreases with the increasing CTC cutoff values applied (Supplementary Figs. 1 and 2).
Kaplan–Meier plots—Kinetic of apoptotic CTC between baseline and first cycle (aCTCKIN). OS (left) and PFS (right) by CTC status between baseline and first cycle in 252 patients with MBC: <5 CTC (<5 CTC in cells in both CTCBL and CTC1C), stable (<25 % change), decrease (≥25 % decrease), increase (≥25 % increase)
Different types of systemic treatment had no independent influence on aCTC. Treatment groups with high proportions of favorable aCTCKIN (<5 and decreased aCTC) showed longer OS and lower numbers of aCTC at baseline as shown in Tables 1 and 8. Thus, aCTCKIN seems to be mostly influenced by treatment response but by treatment regimen.
Alongside the response of the tumor tissue to treatment, the aCTC levels also appear to subside in the long term. These results underline the hypothesis that aCTC are a side product of metastatic disease [29]. Apoptotic CTC enumeration could thus be used as an extended parameter to monitor systemic therapy in MBC patients.
Limitations
When interpreting CTC kinetics, it must be borne in mind that only patients who survived the first cycle of systemic therapy were included in this statistic, which inherently leads to an overestimation of OS and PFS. This possibly affects CTCBL-positive patients more than CTCBL-negative patients. The first 100 patients in this study had no further blood samples taken after receiving a negative CTCBL result at baseline. This change may have introduced a potential source of bias in the CTC1C results. Also, the sensitivity of CTC detection has limits since certain EpCAM/keratin-negative CTC are not detected via CellSearch©, representing another limitation of the study. Especially systems using EpCAM might miss cells undergoing epithelial–mesenchymal transition (EMT) [2]. This potentially leads to underestimation of CTC levels. Nevertheless, EpCAM-positive CTC measurement is a very reliable and reproducible procedure offering potentially relevant clinical information with little effort and little discomfort to the patient [26]. As an additional prognostic factor, the identification of aCTC as a subgroup of CTC provides further clinically relevant information. Whether morphologic criteria or automated analysis platforms such as M30 epitopes [16] as methods for identification of aCTC are chosen, needs to be evaluated in further trials.
Impact of aCTC in MBC
In summary, our study demonstrates that serial CTC monitoring of iCTC and aCTC is a versatile tool for predicting treatment outcome in MBC and a useful adjunct to standard diagnostic tests for tailoring therapy. The data presented here support the hypothesis that monitoring aCTC is a promising source of biological information toward predicting the course of disease and its responsiveness to targeted agents, thus paving the way for individualized therapy [8, 10, 30].
Conclusions
Separate classification and enumeration of aCTC (≥5 aCTC/7.5 ml) at baseline can tip the scale as a predictive marker for OS. Similarly, increasing cutoff values for unfavorable iCTC counts go along with an increasing discriminating power for OS. Positive aCTCBL in conjunction with positive iCTCBL have an unfavorable prognostic impact on OS. In addition, the decrease in aCTCKIN over time is associated with improved outcomes in terms of both OS and PFS and has more discriminatory power compared to iCTCKIN or (iCTC + aCTCKIN) enumeration.
Abbreviations
- 1C:
-
One cycle of systemic chemotherapy
- aCTC:
-
Apoptotic circulating tumor cell(s)
- BL:
-
Baseline
- CHT:
-
Chemotherapy
- CI:
-
Confidence interval
- CTC:
-
Circulating tumor cell(s)
- DTC:
-
Disseminated tumor cell(s)
- EMT:
-
Epithelial–mesenchymal transition
- EpCAM:
-
Epithelial cell adhesion molecule
- iCTC:
-
Intact circulating tumor cell(s)
- KIN:
-
Kinetics
- MBC:
-
Metastatic breast cancer
- NA:
-
Not available/not applicable
- NCT:
-
National Center for Tumor Diseases, Heidelberg, Germany
- NST:
-
Neoadjuvant systemic therapy
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- STD:
-
Standard deviation
References
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW et al (2004) Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 351(8):781–791
Giordano A, Gao H, Anfossi S, Cohen E, Mego M, Lee BN, Tin S, De Laurentiis M, Parker CA, Alvarez RH et al (2012) Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Mol Cancer Ther 11(11):2526–2534
Wallwiener M, Riethdorf S, Hartkopf AD, Modugno C, Nees J, Madhavan D, Sprick MR, Schott S, Domschke C, Baccelli I et al (2014) Serial enumeration of circulating tumor cells predicts treatment response and prognosis in metastatic breast cancer: a prospective study in 393 patients. BMC Cancer 14:512
Hayes DF, Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Miller MC, Matera J, Allard WJ, Doyle GV, Terstappen LW (2006) Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 12(14 Pt 1):4218–4224
Giuliano M, Giordano A, Jackson S, Hess KR, De Giorgi U, Mego M, Handy BC, Ueno NT, Alvarez RH, De Laurentiis M et al (2011) Circulating tumor cells as prognostic and predictive markers in metastatic breast cancer patients receiving first-line systemic treatment. Breast Cancer Res 13(3):R67
Dawood S, Broglio K, Valero V, Reuben J, Handy B, Islam R, Jackson S, Hortobagyi GN, Fritsche H, Cristofanilli M (2008) Circulating tumor cells in metastatic breast cancer: from prognostic stratification to modification of the staging system? Cancer 113(9):2422–2430
Fehm T, Sagalowsky A, Clifford E, Beitsch P, Saboorian H, Euhus D, Meng SD, Morrison L, Tucker T, Lane N et al (2002) Cytogenetic evidence that circulating epithelial cells in patients with carcinoma are malignant. Clin Cancer Res 8(7):2073–2084
Baccelli I, Schneeweiss A, Riethdorf S, Stenzinger A, Schillert A, Vogel V, Klein C, Saini M, Bauerle T, Wallwiener M et al (2013) Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol 31(6):539–544
Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, Matera J, Repollet M, Doyle GV, Terstappen LW et al (2006) Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin Cancer Res 12(21):6403–6409
Wallwiener M, Hartkopf AD, Baccelli I, Riethdorf S, Schott S, Pantel K, Marme F, Sohn C, Trumpp A, Rack B et al (2013) The prognostic impact of circulating tumor cells in subtypes of metastatic breast cancer. Breast Cancer Res Treat 137(2):503–510
Smerage JB, Barlow WE, Hortobagyi GN, Winer EP, Leyland-Jones B, Srkalovic G, Tejwani S, Schott AF, O’Rourke MA, Lew DL et al (2014) Circulating tumor cells and response to chemotherapy in metastatic breast cancer: sWOG S0500. J Clin Oncol 32(31):3483–3489
Hartkopf AD, Wagner P, Wallwiener D, Fehm T, Rothmund R (2011) Changing levels of circulating tumor cells in monitoring chemotherapy response in patients with metastatic breast cancer. Anticancer Res 31(3):979–984
Fehm T, Hoffmann O, Aktas B, Becker S, Solomayer EF, Wallwiener D, Kimmig R, Kasimir-Bauer S (2009) Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res 11(4):R59
Wallwiener M, Hartkopf AD, Riethdorf S, Nees J, Sprick MR, Schonfisch B, Taran FA, Heil J, Sohn C, Pantel K et al (2015) The impact of HER2 phenotype of circulating tumor cells in metastatic breast cancer: a retrospective study in 107 patients. BMC Cancer 15:403
Mehes G, Witt A, Kubista E, Ambros PF (2001) Circulating breast cancer cells are frequently apoptotic. Am J Pathol 159(1):17–20
Rossi E, Basso U, Celadin R, Zilio F, Pucciarelli S, Aieta M, Barile C, Sava T, Bonciarelli G, Tumolo S et al (2010) M30 neoepitope expression in epithelial cancer: quantification of apoptosis in circulating tumor cells by Cell Search analysis. Clin Cancer Res 16(21):5233–5243
Kallergi G, Konstantinidis G, Markomanolaki H, Papadaki MA, Mavroudis D, Stournaras C, Georgoulias V, Agelaki S (2013) Apoptotic circulating tumor cells in early and metastatic breast cancer patients. Mol Cancer Ther 12(9):1886–1895
Krawczyk N, Hartkopf A, Banys M, Meier-Stiegen F, Staebler A, Wallwiener M, Rohm C, Hoffmann J, Hahn M, Fehm T (2014) Prognostic relevance of induced and spontaneous apoptosis of disseminated tumor cells in primary breast cancer patients. BMC Cancer 14:394
Russo A, Terrasi M, Agnese V, Santini D, Bazan V (2006) Apoptosis: a relevant tool for anticancer therapy. Ann Oncol 17(Suppl 7 vii):115–123
Smerage JB, Budd GT, Doyle GV, Brown M, Paoletti C, Muniz M, Miller MC, Repollet MI, Chianese DA, Connelly MC et al (2013) Monitoring apoptosis and Bcl-2 on circulating tumor cells in patients with metastatic breast cancer. Mol Oncol 7(3):680–692
Fehm T, Becker S, Becker-Pergola G, Sotlar K, Gebauer G, Durr-Storzer S, Neubauer H, Wallwiener D, Solomayer EF (2006) Presence of apoptotic and nonapoptotic disseminated tumor cells reflects the response to neoadjuvant systemic therapy in breast cancer. Breast Cancer Res 8(5):R60
Hartkopf AD, Taran FA, Wallwiener M, Hagenbeck C, Melcher C, Krawczyk N, Hahn M, Wallwiener D, Fehm T (2013) The presence and prognostic impact of apoptotic and nonapoptotic disseminated tumor cells in the bone marrow of primary breast cancer patients after neoadjuvant chemotherapy. Breast Cancer Res 15(5):R94
Riethdorf S, Muller V, Zhang L, Rau T, Loibl S, Komor M, Roller M, Huober J, Fehm T, Schrader I et al (2010) Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res 16(9):2634–2645
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Riethdorf S, Pantel K (2008) Disseminated tumor cells in bone marrow and circulating tumor cells in blood of breast cancer patients: current state of detection and characterization. Pathobiology 75(2):140–148
Cristofanilli M (2009) The biological information obtainable from circulating tumor cells. Breast 18(Suppl 3):S38–S40
R: A Language and environment for statistical computing. http://www.r-project.org
Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, Beitsch PD, Leitch M, Hoover S, Euhus D et al (2004) Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res 10(24):8152–8162
Fehm T, Muller V, Aktas B, Janni W, Schneeweiss A, Stickeler E, Lattrich C, Lohberg CR, Solomayer E, Rack B et al (2010) HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat 124(2):403–412
Acknowledgments
The authors gratefully acknowledge all patients whose data were used in this study. We also thank the medical and nursing staff, especially Martina Scharpff, at the National Center for Tumor Diseases (NCT; Heidelberg) for excellent management and care of our patients; the NCT laboratory staff; and Antje Andreas; Cornelia Coith; and Oliver Mauermann (Hamburg), who provided excellent technical assistance with the CTC determinations. This study was supported by NCT in-house funds, made available to AS and AT, and by grants to AT from the BioRN Leading Edge Cluster “Molecular and Cell Based Medicine” (BRN 02GS1893), supported by the German Federal Ministry of Education and Research (BMBF), Berlin, Germany (BMBF N02/74829), and the Dietmar Hopp Foundation. Moreover, this study was supported by the ERC-2010-AdG_20100317 DISSECT to KP. We acknowledge the financial support from the German Research Foundation (DFG) and Ruprecht-Karls-Universität Heidelberg through the funding program for Open Access Publishing.
Authors’ contributions
MW, ADH, SR, KP, AT, and AS jointly conceived the study and developed its design. MW and AS supervised the study. SR and KP developed the methodology. TMD, SR, JN, ADH, MRS, BS, CS, KP, AT, AS, and MW participated in patient recruitment, patient management, clinical data collection, sample collection, and sample analysis. BS and TMD organized and reported the data, constructed the databases, and conducted data management. BS performed the statistical analysis. TMD, JN, ADH, SS, SYB, SR, FS, CD, MRS, BS, CS, AT, AS, and MW participated in data analysis and interpretation. TMD, JN, ADH, BS, AS, and MW drafted the manuscript. MW, SR, MRS, CS, KP, SR, SS, TMD, ADH, BS, SYB, AS, and AT revised the draft manuscript for important intellectual input. MW prepared the final manuscript. All the authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Thomas M. Deutsch and Sabine Riethdorf are joint first authors. Andreas Schneeweiss and Markus Wallwiener are joint senior authors.
Thomas M. Deutsch, Sabine Riethdorf, Andreas Schneeweiss, and Markus Wallwiener have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (EPS 404 kb)
Supplementary Fig. 1 Kaplan–Meier plots for OS with different cutoff values for iCTCKIN and aCTCKIN. OS by CTC status between baseline and first cycle in 252 patients with MBC: <5 CTC (<5 CTC in cells in both CTCBL and CTC1C), stable (<25 % change), decrease (≥25 % decrease), and increase (≥25 % increase)
Supplementary material 2 (EPS 342 kb)
Supplementary Fig. 2 Kaplan–Meier plots for PFS with different cutoff values for iCTCKIN and aCTCKIN. PFS by CTC status between baseline and first cycle in 252 patients with MBC: <5 CTC (<5 CTC in cells in both CTCBL and CTC1C), stable (<25 % change), decrease (≥25 % decrease), and increase (≥25 % increase)
Supplementary material 3 (EPS 106 kb)
Supplementary Fig. 3. Kaplan–Meier plots—Kinetic of apoptotic CTC between first cycle and progression disease. OS by CTC status between first cycle and progression disease in 189 patients with MBC: < 5 CTC (<5 CTC in cells in both CTCBL and CTC1C), stable (<25 % change), decrease (≥25 % decrease), increase (≥25 % increase)
Rights and permissions
About this article
Cite this article
Deutsch, T.M., Riethdorf, S., Nees, J. et al. Impact of apoptotic circulating tumor cells (aCTC) in metastatic breast cancer. Breast Cancer Res Treat 160, 277–290 (2016). https://doi.org/10.1007/s10549-016-3997-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3997-3