Abstract
In this study, the N,N,O metal chelator 2-pyridinecarboxaldehydeisonicotinoyl hydrazone (HPCIH, 1) and its derivatives 2-acetylpyridine-(HAPIH 2), 2-pyridineformamide-(HPAmIH, 3) and pyrazineformamide-(HPzAmIH, 4) were employed in the synthesis of four copper(II) complexes, [Cu(HPCIH)Cl2]·0.4H2O (5), [Cu(HAPIH)Cl2]·1.25H2O (6), [Cu(HPAmIH)Cl2]·H2O (7) and [Cu(HPzAmIH)Cl2]·1.25H2O (8). The compounds were assayed for their action toward Mycobacterium tuberculosis H37Rv ATCC 27294 strain and the human tumor cell lines OVCAR-8 (ovarian cancer), SF-295 (glioblastoma multiforme) and HCT-116 (colon adenocarcinoma). All copper(II) complexes were more effective in reducing growth of HCT-116 and SF-295 cells than the respective free hydrazones at 5 µg/mL, whereas only complex 7 was more cytotoxic toward OVCAR-8 lines than its ligand HPAmIH. 6 proved to be cytotoxic at submicromolar doses, whose IC50 values (0.39–0.86 µM) are similar to those ones found for doxorubicin (0.23–0.43 µM). Complexes 5 and 6 displayed high activity against M. tuberculosis (MIC = 0.85 and 1.58 µM, respectively), as compared with isoniazid (MIC = 2.27 µM), which suggests the compounds are attractive candidates as antitubercular drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Isoniazid (INH) was first introduced in tuberculosis therapy in the 1950s (Judge et al. 2012). Since then, it is regarded as one of the most commonly used and efficient drugs in treatment of human tuberculosis (Bernardes-Génisson et al. 2013).
In spite of efforts to eradicate tuberculosis, its burden remains substantial (Dheda et al. 2016). Over one-third of the global population is infected by tuberculosis, which causes death in approximately two to three million people annually (WHO 2014). The increased incidence of multidrug-resistant (Prozorov et al. 2012), and more recently, extensively drug-resistant (Sotgiu et al. 2009) strains of Mycobacterium tuberculosis compromise the recurrent effective treatment and evidences the urgency for novel antituberculosis agents (Hoagland et al. 2016).
Modification of existing drugs and the development of novel active compounds have been some of the strategies to improve tuberculosis drug therapy (Wong et al. 2013; Ellis et al. 2014; Chaves et al. 2015). In this sense, INH has become the most researched antitubercular agent (Hearn et al. 2009). A range of isoniazid analogues has been studied for its antitubercular potential and a number of promising candidates has been described (Oludina et al. 2014; Parumasivam et al. 2013; Matei et al. 2013; Ramani et al. 2012; Kumar et al. 2014).
Recently, Ellis and co-workers (2014) noticed that the INH analogue 2-pyridinecarboxaldehyde isonicotinoyl hydrazone (HPCIH), an effective iron chelator, displays potent inhibition of mycobacterial growth, probably acting as a lipophilic vehicle for the transport of the intact INH moiety into the mycobacterium. In spite of its marked antimycobacterial action, it has been shown HPCIH presents limited antiproliferative activity against malignant SK-N-MC (neuroepithelioma) (Becker and Richardson 1999). In our previous work, we demonstrated the analogues 2-acetylpyridine-(HAPIH), 2-benzoylpyridine-(HBPIH), 2-pyridineformamide-(HPAmIH) and 2-pyrazineformamide-(HPzAmIH) isonicotinoyl hydrazones in general exhibit better growth-inhibiting properties towards MCF-7, OVCAR-8 and SF-295 cells than HPCIH (Amim et al. 2016).
Metal complexes of substituted hydrazones have been reported to hold therapeutic activity and have shown pharmacological applications (Lessa et al. 2012, 2013; Raman et al. 2008; Chang et al. 2015). It has been evidenced that the presence of an α(N)heterocyclic ring at azomethine scaffold of hydrazones plays a major role in extending their chelating and/or pharmacological properties (Beraldo and Gambino 2004).
Due to the effectiveness of HPCIH (Armstrong et al. 2003) and its analogues (Ababei et al. 2010; Chang et al. 2015) as tridentate chelating agents for transition metal ions, in this work we report the synthesis of four copper(II) complexes (5–8) with HPCIH (1), HAPIH (2), HPAmIH (3) and HPzAmIH (4) (Fig. 1). As part of our interest in the development of copper(II) complexes as antimycobacterial and anticancer agents, compounds 5–8 were assayed for their action toward Mycobacterium tuberculosis H37Rv ATCC 27294 strain and the human tumor cell lines OVCAR-8 (ovarian cancer), SF-295 (glioblastoma multiforme) and HCT-116 (colon adenocarcinoma).
Materials and methods
Chemicals
Isoniazid, 2-pyridinecarboxaldehyde, 2-acetylpyridine, 2-pyridinecarbonitrile, pyrazinecarbonitrile and copper(II) chloride dihydrate were purchased from Aldrich and used without further purification.
Physical measurements
Partial elemental analyses were performed on a Perkin Elmer CHN 2400 analyzer. Melting points were determined on Gehaka-PF1500 Farma, a Capillary Melting Point Apparatus. A CG 1800 Gehaka conductivity bridge (conductimetric cell constant 1 cm−1) was employed for molar conductivity measurements of compound solutions (1 × 10−3 mol L−1) in dimethylsulfoxide (DMSO). Infrared spectra were recorded on an attenuated total reflectance/Fourier transform infrared spectrometer (Varian FT-IR 660) in the 4000–600 cm−1 range. Electronic spectra were recorded on an Agilent Technologies 8453 spectrophotometer at room temperature, using a 10 mm beam path quartz cuvette and DMSO as solvent. Magnetic susceptibility measurements were carried out at 298.5 °C on a Johnson Matthey MSB/AUTO balance.
Synthesis of the hydrazones (1–4) and their copper(II) complexes (5–8)
Synthesis of HPCIH (1) (Armstrong et al. 2003), HAPIH (2) (Ababei et al. 2010), HPAmIH (3) (Amim et al. 2016), and HPzAmIH (4) (Glushkov et al. 2004) are described in the literature. Copper(II) complexes 5–8 were obtained by mixing, under reflux and stirring for 4 h, a methanol solution (15 mL) of the desired hydrazone (1 mmol) with CuCl2·2H2O in 1:1 ligand-to-metal molar ratio. The resulting solids were filtered off, then washed with methanol followed by diethylether and dried at 50 °C for 24 h. Single crystals of [Cu(HAPIH)Cl]Cl·H2O (6a) and [Cu(HPzAmIH)Cl2]·H2O (8a) were obtained from mother solutions of 6 and 8, respectively.
Dichloro(2-pyridinecarboxaldehyde-isonicotinoyl hydrazone)copper(II)] hydrate, [Cu(HPCIH)Cl2]· 0.4H2O (5)
Green solid. Anal. Calc. for C12H10.8Cl2CuN4O1.4 (FW = 367.89 g mol−1): C, 39.18; H, 2.96; N, 15.23. Found: C, 39.50; H, 2.79; N, 14.76 %. IR (ATR, cm−1): ν (N + py −H) 2555, ν (C = N) 1523, ρ(py) 644. Molar conductivity (1 × 10−3 mol L−1, DMSO) 46.0 Ω−1 cm2 mol−1. Effective magnetic moment: 1.91 MB. UV–vis (DMSO, λ in nm/ε in mol−1dm2): 386/3.02 × 105, 752/521. Yield 42 %.
Dichloro(2-acetylpyridine-isonicotinoyl hydrazone)copper(II) hidrate, [Cu(HAPIH)Cl2]·1.25 H2O (6)
Green solid. Anal. Calc. for C13H14.5Cl2N4O2.25Cu (FW = 397.23 g mol−1): C, 39.31; H, 3.68; N, 14.10 %. Found: C, 39.47; H, 3.43; N, 13.82 %. IR (ATR, cm−1): ν (N + py −H) 2569, ν (C = N) 1531, ρ(py) 645. Molar conductivity (1 × 10−3 mol L−1, DMSO): 33.4 Ω−1 cm2 mol−1. Effective magnetic moment: 1.85 MB. UV–vis (DMSO, λ in nm/ε in mol−1 dm2): 383/1.34 × 105; 743/136. Yield 77 %.
Dichloro(2-pyridineformamide-isonicotinoyl hydrazone)copper(II) hidrate, [Cu(HPAmIH)Cl2]·H2O (7)
Brown solid. Anal. Calc. for C12H13Cl2CuN5O2 (FW = 393.71 g mol−1): C, 36.61; H, 3.33; N, 17.79 %. Found: C, 36.65; H, 2.85; N, 17.33 %. IR (ATR, cm−1): ν (N–H) 3064, δ(NH2) 1658, ν (C = O), 1622 ν(C = N) 1531, ρ(py) 646. Effective magnetic moment: 1.89 MB. UV–vis (DMSO, λ in nm/ε in mol−1dm2): 398/2.59 × 105; 754/229. Yield 87 %
Dichloro(pyrazineformamide-isonicotinoyl hydrazone)copper(II), hidrate [Cu(HPzAmIH)Cl2]·1.25 H2O (8)
Brown solid. Anal. Calc. for C11H12.25Cl2CuN6O2.25 (FW = 394.70 g mol−1): C, 33.09 %; H, 3.16 %; N, 21.05 %. Found: C, 33.27 %; H, 2.90 %; N, 20.66 %. IR (ATR, cm−1): ν(N–H) 3055, δ(NH2) 1666, ν(C = O) 1616, ν(C = N) 1525, ρ(py) 654. Molar conductivity (1 × 10−3 mol L−1 DMSO): 33.1 Ω−1 cm2 mol−1. Effective magnetic moment: 1.91 MB. UV–vis (DMSO, λ in nm/ε in mol−1dm2): 332/2.60 × 104, 425/6.36 × 104, 764/229. Yield 91 %.
Crystallography
Single-crystal X-ray diffraction methods were used to determine the structures of [Cu(HAPIH)Cl]Cl·H2O (6a) and [Cu(HPzAmIH)Cl2]·H2O (8a). Data were collected at room temperature on a Bruker D8 VENTURE equipped with Mo Kα high-brilliance IµS radiation (λ = 0.71073 Å) and a PHOTON 100 CMOS detector. The instrument was controlled by the APEX2 software package (Bruker 2014). Data were processed using the integrate plug-in in the controlling software package (SAINT) and corrected for absorption by the multiscan semi-empirical method implemented in SADABS (Bruker 2014). Using Olex2 (Dolomanov et al. 2009) the structure was solved with the SHELXS-97 (Sheldrick 2008) structure solution program by means of Direct Methods and refined with the SHELXL-2013 (Sheldrick 2008) refinement package using Least Squares minimization. Positional and anisotropic atomic displacement parameters were refined for all non-hydrogen atoms. Hydrogen atoms were placed geometrically and the positional parameters were refined using a riding model.
In vitro biological activity assays
Cytotoxicity toward human tumor cell lines
The cytotoxic activity of compounds 1–8 was tested against SF-295 (glioblastoma multiforme), HCT-116 (colon adenocarcinoma) and OVCAR-8 (ovarian cancer), from National Cancer Institute (Bethesda, MD, USA). The cells were maintained in RPMI 1640 medium supplemented with 10 % fetal bovine serum, 2 mM glutamine, 100 μg/mL penicillin, and 100 μg/mL streptomycin at 37 °C/5 % CO2. Each compound was previously dissolved in DMSO (stock solution), whose final concentration in the RPMI culture medium was kept below 0.1 % (v/v). For initial cytotoxic activity evaluation, compounds 1–8 (5 μg/mL) were incubated with SF-295, HCT-116 and OVCAR-8 cell lines, for 72 h. Cell viability was determined by dye reduction 3-(4,5-dimethyl-2-thiazole)-2,5-diphenyl-2H-tetrazole bromide (MTT) assay to yield the formazan, which is detected by electronic spectroscopy (Mosman 1983). Compounds that inhibited the proliferation in more than 50 % were selected for determination of the half maximal inhibitory concentration (IC50). To this end, 5–0.009 μg mL−1 range for compound concentration was used. All experiments were performed in least three replicates per compound and results are shown as the average and 95 % confidence interval of three independent experiments.
Antitubercular activity
Antimycobacterial activities of compounds 1–8, as well as isoniazid and copper(II) chloride, were assessed against Mycobacterium tuberculosis H37Rv ATCC 27294 using the Micro plate Alamar Blue Assay (MABA) (Franzblau et al. 1998). This methodology is nontoxic, uses a thermally-stable reagent and shows good correlation with proportional and BACTEC radiometric methods (Tortoli et al. 2002; Kontos et al. 2004). The method is described as follows: 200 ml of sterile deionized water was added to all outer-perimeter wells of 96 sterile well plates (falcon, 3072: Becton–Dickinson, Lincoln Park, NJ) to minimize evaporation of the medium in the test wells during incubation. The 96 plates received 100 mL of the Middlebrook 7H9 broth (Difco laboratories, Detroit, MI, USA) and successive dilution of the compounds was performed directly on the plate. The final drug concentrations tested were 0.01–20.0 mg/mL. Plates were covered and sealed with parafilm and incubated at 37°C for 5 days. Twenty five milliliter of a freshly prepared 1:1 mixture of Alamar Blue (Accumed International, WestlakeOhio) reagent and 10 % tween 80 were then added to the plate and incubated for 24 h. A blue color in the well was interpreted as no bacterial growth, and a pink color was scored as growth. The minimal inhibition concentration (MIC) was defined as the lowest drug concentration, which prevents a color change from blue to pink.
Results and discussion
Formation of the copper(II) complexes
Microanalyses suggest the formation of [Cu(HPCIH)Cl2]·0.4H2O (5), [Cu(HAPIH)Cl2]·1.25H2O (6), [Cu(HPAmIH)Cl2]·H2O (7) and [Cu(HPzAmIH)Cl2]·1.25H2O (8). For complexes 5 and 6, the hydrazones coordinate as zwitterionic forms (based on infrared spectroscopy), whereas neutral ligands are attached to the metal center in 7 and 8. Molar conductivities of 5–8 weren’t determined in commonly used solvents (Geary 1971) due to its low solubility. Instead, molar conductivities were measured in DMSO whose values suggest the compounds behave as weak electrolytes in solution (Zianna et al. 2016). This behavior is probably consequence of either the labile nature of chloro ligands or metal coordinating feature of the solvent. At room temperature, powdered samples of complexes display effective magnetic moments (μeff) in the 1.85–1.91 BM range, which is higher than the spin-only value. Such divergence, which is not quite uncommon in mononuclear copper(II) complexes, is due to mixing-in of some orbital angular momentum from the closely lying excited states via spin–orbit coupling (Bhattacharyya et al. 1996).
Infrared spectra
Absorptions observed between 1545 and 1557 cm−1 assigned to the ν(C = N) in the IR spectra of free hydrazones (1–4) shift to 1523–1531 cm−1 in the spectra of the copper(II) complexes (5–8), suggesting coordination through azomethine nitrogen (Parrilha et al. 2014). Absorptions attributed to ρ(py) observed at 613–665 cm−1 for 1–4 exhibit pronounced shift in spectra of complexes 5–8 (644–654 cm−1), suggesting the pyridine nitrogen coordination (Ferraz et al. 2013).
Bands attributed to ν(N–H) of secondary amines are absent in the spectra of 5 and 6. On the other hand, a fairly strong and very broad absorption attributed to the ν(N+–H) stretching vibration of the pyridinium group (Lessa et al. 2011) is observed around 2600 cm−1. Moreover, the ν(C = O) absorptions at 1665–1677 cm−1 in the spectra of the uncomplexed hydrazones disappear in those ones of complexes 5 and 6, in agreement with coordination of an enolate oxygen (Mondal et al. 2013). Thus, for 5 and 6 the hydrazones are attached to copper(II) as zwitterionic species. Nonetheless, for complexes 7 and 8, ν(C = O) vibrations are present at 1616–1622 cm−1, which are shifted in relation to the free hydrazones, in accordance with coordination through a keto oxygen (Mishra and Sharma 2009).
Electronic spectra
The electronic absorption spectra of hydrazones (1–4) and their complexes (5–8) were recorded at room temperature using DMSO as the solvent. The absorption spectra of the ligands are characterized by one band and a sholder or by two bands in the 297–377 nm region, which are assigned to n → π* and π → π* transitions of azomethine and the carbonyl groups (Cohen and Flavian 1967; Gegiou et al. 1996; Sorrell 1989). In the UV–vis spectra of 5–8 these absorptions are shifted. Also, the complexes show a single broad band centred in 743–764 nm, which is typical for d–d transition of Jahn–Teller distorted copper(II) complexes in square pyramidal geometries (Tabbì et al. 2013).
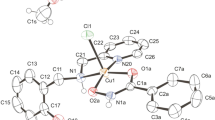
Structural study of [Cu(HAPIH)Cl]Cl·H2O (6a) and [Cu(HPzAmIH)Cl2]·H2O (8a)
Crystal data and structure refinement for 6a and 8a are summarized in supplementary information (online resource). Selected bond distances and angles for 6a and 8a are shown in Table 1. Compounds 6a and 8a crystallized in the triclinic and monoclinic systems, respectively. ORTEP (Farrugia 1997) drawings (Figs. 2, 3) display the hydrazones tridentate to the metal ion through NazoNaromO system for both complexes, giving rise to two five-membered rings. Despite the similarities in ligand structures, metal ions adopt different coordination numbers and geometries, that is, 6a shows a distorted square planar geometry, in which one chloride is attached to the metal center, whereas 8a is a pentacoordinated compound, in which two chloride ions complete its coordination. According to Addison and co-workers (1984), for distorted pentacoordinate structures, the parameter τ (τ = (β−α)/60°, where α and β are the largest angles around the metal center) can be used to rationalize its geometries. Value τ is 0 for perfectly square-pyramidal geometry and 1 for perfectly trigonal–bipyramidal geometry. For 8a, τ is 0.36, which suggests it is likely a distorted square–pyramidal compound.
Hydrazone ligands are nearly planar whose rms deviation of atoms from the least-squares plane is 0.0782 Å for 6a and 0.0588 Å for 8a. Metal ions lay close onto this plane (at 0.0481(10) and 0.0715(10) Å for 6a and 8a, respectively), as well as chlorine ligand in 6a (0.638(2) Å).
The N3–C8 bond lengths 1.320(2) and 1.306(3) Å found for hydrazones in 6a and 8a, respectively, is shorter than the similar free hydrazone HBPIH (1.3601 (1) Å) (Ababei et al. 2010). Furthermore, C8–O1 bond is marked longer in 6a (1.280(2) Å) and 8a (1.288(2) Å) in comparison with HBPIH (1.2152 (1) Å). The C8–O1 bond is most likely to change from a double to a predominantly single bond and N3–C8 acquires some double bond character when hydrazones are attached to copper(II) in the enolate form (Despaigne et al. 2012; Mondal et al. 2013).
Besides, the enolate ligands are protonated at the para-substituted pyridine nitrogen in both complexes, indicating it is attached to the metal ion in the zwitterionic form. Complex 8a is also protonated in the powder, whereas zwitterionic form of 6a was obtained only through the crystallization process.
The dihedral angles C2−C7−N2−N3 and N2−N3−C8−O1 are 179.87(17) and 2.5(3), respectively, for 6a as well as −179.59(16) and 1.8(3) respectively for 8, which are in accordance with EZ conformation adopted by the hydrazones when attached to copper(II).
Interactions in crystal packing for 6a and 8a are described in Table 2. The interaction between Cu1 and Cl1(1−x, 2−y, 1−z) (2.6375(6) Å) is the main contact in structure of compound 6a, which results in the formation of a dimeric arrangement. Study of hydrogen bonds reveals a chain along the [1–2 1] direction. For compound 8a, a three-dimensional hydrogen-bonding network is observed connecting water molecules and coordination compound (see supplementary information).
Cytotoxicity against tumor cell lines
Figure 4 reports growth inhibition of the human tumor cell lines OVCAR-8, SF-295 and HCT-116 induced by compounds 1−4 (Amim et al. 2016) and its complexes 5–8. INH and copper chloride dihydrate were also tested for comparison.
According to results, isoniazid proved to be poorly effective against the three cell lines. Copper chloride, in turn, reduced in 75 % the SF-295 cells growth, whereas it presented moderate to low activity against the other cell lines.
All copper(II) complexes were more effective in reducing growth of HCT-116 and SF-295 cells than the respective free hydrazones. Coordination led to significant higher cytotoxity of 7 to OVCAR-8 cells than hydrazone 3. 2 as well as its complex 6 also strongly inhibited OVCAR-8 cells growth. It is noteworthy that complexes 6 and 7 were able to inhibit the growth of all cell lines in more than 90 %.
The most potent compounds HAPIH (2), [Cu(HAPIH)Cl2]·1.25H2O (6) and [Cu(HPAmIH)Cl2]·H2O (7) were selected to determine the concentration which inhibits 50 % of cell growth (IC50) (Table 3 ). Complex 6 was found to be the most active compound against all strains, whose activity is superior to the free hydrazone 2. Besides, 6 is as potent as the anticancer drug doxorubicin. Thus, coordination of 2 to copper(II) was an efficient approach to obtain compound with improved action against tumor SF-295, OVCAR-8 and HCT-116 cell lines.
Antimycobacterial activity
Determined values of minimum inhibitory concentrations (MIC) of hydrazones 1−4, their complexes 5–8, INH and copper(II) chloride salt against Mycobacterium tuberculosis H37Rv (ATCC 27294) are listed in Table 4.
Selected hydrazones display different behavior toward M. tuberculosis. HPCIH (1) shows moderate activity, whereas substitution of hydrogen at imine carbon by a methyl group in HAPIH (2) increases the antimycobacterial potency. HAPIH (MIC = 2.60 µM) is as effective as the reference antitubercular drug INH (MIC = 2.27 µM) in inhibit M. tuberculosis. Nonetheless, the presence of formamide in HPAmIH (3) as well as pyrazine substituent in HPzAmIH (4) leads to moderate and lost of action, respectively. In general, coordination of hydrazones to copper(II) promotes reduction in MIC values. [Cu(HPCIH)Cl2]·0.4H2O (5), for example, exhibited sub-micromolar MIC value (0.85 µM) and was around 15-fold more effective than HPCIH (1) (13.79 µM) in inhibit growth of M. tuberculosis. Complexes 5 and [Cu(HAPIH)Cl2]·1.25H2O (6) displayed high activity against M. tuberculosis, as compared with isoniazid, which suggests the compounds are attractive candidates as antitubercular drugs.
Conclusion
In this work, copper(II) complexes 5–8 were evaluated toward three tumor cell lines (OVCAR-8, SF-295 and HCT-116). In most cases, chelation with metals gave rise to enhancement of the ligands activity against the tested cells. 6 and 7 were appointed as lead cytotoxic complexes. Additionally, 6 has proved to be as effective as the anticancer drug doxorubicin. Further work will be needed to understand the mechanism whereby the complex disturbs cellular proliferation.
Upon coordination to copper(II), activity against Mycobacterium tuberculosis H37Rv growth significantly improved except for 7. Copper(II) chloride is poorly effective, suggesting the action is probably due to the complex per se. Coordination of HPCIH (1) to copper(II) was an efficient strategy to produce a compound (5) with improved antimycobacterial action. Complex 5 was also more active than isoniazid, suggesting it is a promising compound, which should be considered for further studies aiming to confirm its potential as novel antitubercular drug candidate.
References
Ababei LV, Kriza A, Musuc AM, Andronescu C, Rogozea EA (2010) Thermal behavior and spectroscopic studies of complexes of some divalent transitional metals with 2-benzoil pyridilizonicotinoylhydrazone. J Therm Anal Calorim 101:987–996
Addison AW, Rao TN, Reedijk J, Rijin J, Verschoo GC (1984) Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen-sulphur donor ligands; the Crystal and molecular structure of aqua[1,7-bis(N-methylbenzimidazol-2’-yl)-2,6-dithiaheptane]copper(II)perchlorate. J Chem Soc, Dalton Trans 7(7):1349–1356
Amim RS, Firmino GSS, Rego ACPD, Nery AL, Da-Silva SAG, Souza MVN, Pessoa C, Resende JALC, Figueroa-Villar JD, Lessa JA (2016) Cytotoxicity and leishmanicidal activity of isoniazid-derived hydrazones and 2-pyrazineformamide thiosemicarbazones. J Braz Chem Soc 27:769–777
Armstrong CM, Bernhardt PV, Chin P, Richardson DR (2003) Structural variations and formation constants of first-row transition metal complexes of biologically active aroylhydrazones. Eur J Inorg Chem 6:1145–1156
Becker E, Richardson DR (1999) Development of novel aroylhydrazone ligands for iron chelation therapy: 2-Pyridylcarboxaldehyde isonicotinoyl hydrazone analogs. J Lab Clin Med 134:510–521
Beraldo H, Gambino D (2004) The wide pharmacological versatility of semicarbazones, thiosemicarbazones and their metal complexes. Mini-Rev Med Chem 4:31–39
Bernardes-Génisson V, Deraeve C, Chollet A, Bernadou J, Pratviel G (2013) Isoniazid: an update on the multiple mechanisms for a singular action. Curr Med Chem 20(35):4370–4385
Bhattacharyya S, Kumar SB, Dutta SK, Tiekink ERT, Chaudhury M (1996) Zinc(II) and copper(II) complexes of pentacoordinating (N4S) ligands with flexible pyrazolyl arms: syntheses, structure, and redox and spectroscopic properties. Inorg Chem 35:1967–1973
Bruker (2014) APEX2, SAINT and SADABS. Bruker AXS Inc, Madison, Wisconsin
Chang H, Jia L, Xu J, Xu Z, Chen R, Wu W, Bie H, Zhu T, Ma T, Wang Y (2015) Syntheses, characterizations, antitumor activities and cell apoptosis induction of Cu(II), Zn(II) and Cd(II) complexes with hydrazone Schiff base derived from isonicotinohydrazide. Inorg Chem Comm 57:8–10
Chaves JDS, Damasceno JL, Paula MCF, de Oliveira PF, Azevedo GC, Matos RC, Lourenço MCS, Tavares DC, Silva H, Fontes APS, de Almeida MV (2015) Synthesis, characterization, cytotoxic and antitubercular activities of new gold(I) and gold(III) complexes containing ligands derived from carbohydrates. Biometals 28:845–860
Cohen MD, Flavian S (1967) Topochemistry. Part XXVI. The absorption spectra of some thermochromic N-salicylideneanilines and hydroxynaphthylideneanilines in the crystal. J Chem Soc B 317:329–334
Despaigne AAR, Da Costa FB, Piro OE, Castellano EE, Louro SRW, Beraldo H (2012) Complexation of 2-acetylpyridine- and 2-benzoylpyridine-derived hydrazones to copper(II) as an effective strategy for antimicrobial activity improvement. Polyhedron 38:285–290
Dheda K, Barry CE 3rd, Maartens G (2016) Tuberculosis. Lancet 387:1211–1226
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Cryst 42:339–341
Ellis S, Kalinowski DS, Leotta L, Huang ML, Jelfs P, Sintchenko V, Richardson DR, Triccas JA (2014) Potent antimycobacterial activity of the pyridoxal isonicotinoyl hydrazone analog 2-pyridylcarboxaldehyde isonicotinoyl hydrazone: a lipophilic transport vehicle for isonicotinic acid hydrazide. Mol Pharm 85:269–278
Farrugia LJ (1997) ORTEP-3 for windows—a version of ORTEP-III with a graphical user interface (GUI). J Appl Cryst 30:565
Ferraz KSO, da Silva JG, Costa FM, Mendes BM, Rodrigues BL, dos Santos RG, Beraldo H (2013) N(4)-Tolyl-2-acetylpyridine thiosemicarbazones and their platinum(II, IV) and gold(III) complexes: cytotoxicity against human glioma cells and studies on the mode of action. Biometals 26:677–691
Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH (1998) Rapid, lowtechnology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar blue assay. J Clin Microbiol 36:362–366
Geary WJ (1971) The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord Chem Rev 7:81–122
Gegiou D, Lambi E, Hadjoudis E (1996) Solvatochromism in N-(2-Hydroxybenzylidene)aniline, N-(2-Hydroxybenzylidene)benzylamine, and N-(2-Hydroxybenzylidene)-2-phenylethylamine. J Phys Chem 100(45):17762–17765
Glushkov RG, Modnikova GA, L’vov IA, Krylova LY, Pushkina TV, Gus’kova TA, Solov’eva NP (2004) Synthesis and antituberculosis activity in vitro of amidine and hydrazidine analogs of pyrazinamide and isoniazid. Pharm Chem J 38:16–19
Hearn MJ, Cynamon MH, Chen MF, Coppins R, Davis J, Joo-On Kang H, Noble A, Tu-Sekine B, Terrot MS, Trombino D, Thai M, Webster ER, Wilson R (2009) Preparation and antitubercular activities in vitro and in vivo of novel Schiff bases of isoniazid. Eur J Med Chem 44:4169–4178
Hoagland DT, Liu J, Lee RB, Lee RE (2016) New agents for the treatment of drug-resistant Mycobacterium tuberculosis. Adv Drug Del Rev 102:55–72
Judge V, Narasimhan B, Ahuja M (2012) Isoniazid: the magic molecule. Med Chem Res 21:3940–3957
Kontos F, Maniati M, Costopoulos C, Gitti Z, Nicolaou S, Petinaki E, Anagnostou S, Tselentis I, Maniatis AN (2004) Evaluation of the fully automated Bactec MGIT 960 system for the susceptibility testing of Mycobacterium tuberculosis to first-line drugs: a multicenter study. J Microbiol Methods 56(2):291–294
Kumar HSN, Parumasivam T, Jumaat F, Ibrahim P, Asmawi MZ, Sadikun A (2014) Synthesis and evaluation of isonicotinoyl hydrazone derivatives as antimycobacterial and anticancer agents. Med Chem Res 23(1):269–279
Lessa JA, Guerra JC, Miranda LF, Romeiro CFD, Da Silva JG, Mendes IC, Speziali NL, Souza-Fagundes EM, Beraldo H (2011) Gold(I) complexes with thiosemicarbazones: cytotoxicity against human tumor cell lines and inhibition of thioredoxin reductase activity. J Inorg Biochem 105:1729–1739
Lessa JA, Ferraz KSO, Guerra JC, de Miranda LF, Romeiro CFD, Souza-Fagundes EM, Barbeira PJS, Beraldo H (2012) Spectroscopic and electrochemical characterization of gold(I) and gold(III) complexes with glyoxaldehyde bis(thiosemicarbazones): cytotoxicity against human tumor cell lines and inhibition of thioredoxin reductase activity. Biometals 25:587–598
Lessa JA, Soares MA, dos Santos RG, Mendes IC, Salum LB, Daghestani HN, Andricopulo AD, Day BW, Vogt A, Beraldo H (2013) Gallium(III) complexes with 2-acetylpyridine-derived thiosemicarbazones: antimicrobial and cytotoxic effects and investigation on the interactions with tubulin. Biometals 26:151–165
Matei L, Bleotu C, Baciu I, Draghici C, Ionita P, Paun A, Chifiriuc MC, Sbarcea A, Zarafu I (2013) Synthesis and bioevaluation of some new isoniazid derivatives. Bioorg Med Chem 21:5355–5361
Mishra AP, Sharma N (2009) Synthesis, characterization, X-ray and thermal studies of some schiff base metal complexes. J Ind Council Chem 26(2):125–129
Mondal S, Naskara S, Deya AK, Sinnb E, Eribalb C, Herronc SR, Chattopadhyaya K (2013) Mononuclear and binuclear Cu(II) complexes of some tridentate aroyl hydrazones. X-ray crystal structures of a mononuclear and a binuclear complex. Inorg Chim Acta 398:98–105
Mosman T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Oludina YN, Voloshina AD, Kulik MV, Zobov VV, Bukharov SV, Tagasheva RG, Nugumanova GN, Burilov AR, Kravchenko MA, Skornyakov SN, Rusinov GL (2014) Synthesis, toxicity, and antituberculosis activity of isoniazid derivatives containing sterically hindered phenols. Pharm Chem J 48:5–7
Parrilha GL, Ferraz KSO, Lessa JA, de Oliveira KN, Ramos JP, Souza-Fagundes EM, Ott I, Beraldo H (2014) Metal complexes with 2-acetylpyridine-N(4)-orthochlorophenylthiosemicarbazone: cytotoxicity and effect on the enzymatic activity of thioredoxin reductase and glutathione reductase. Eur J Med Chem 84:537–544
Parumasivam T, Kumar HSN, Ibrahim P, Sadikun A, Mohamad S (2013) Anti-tuberculosis activity of lipophilic isoniazid derivatives and their interactions with first-line anti-tuberculosis drugs. J Pharm Res 7:313–317
Prozorov AA, Zaichikova MV, Danilenko VN (2012) Mycobacterium tuberculosis mutants with multidrug resistance: history of origin, genetic and molecular mechanisms of resistance, and emerging challenges. Rus J Gen 48(1):1–14
Raman N, Raja JD, Sakthivel A (2008) Design, synthesis, spectroscopic characterization, biological screening, and DNA nuclease activity of transition metal complexes derived from a tridentate Schiff base. Russ J Coord Chem 34:400–406
Ramani AV, Monika A, Indira VL, Karyavardhi G, Venkatesh J, Jeankumar VU, Manjashetty TH, Yogeeswari P, Sriram D (2012) Synthesis of highly potent novel anti-tubercular isoniazid analogues with preliminary pharmacokinetic evaluation. Bioorg Med Chem Lett 22:2764–2767
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr 64:112–122
Sorrell TN (1989) Synthetic models for binuclear copper proteins. Tetrahedron 54:10–12
Sotgiu G, Ferrara G, Matteelli A et al (2009) Epidemiology and clinical management of XDRTB: a systematic review by TBNET. Eur Respir J 33(4):871–881
Tabbì G, Giuffrida A, Bonomo RP (2013) Determination of formal redox potentials in aqueous solution of copper(II) complexes with ligands having nitrogen and oxygen donor atoms and comparison with their EPR and UV–Vis spectral features. J Inorg Biochem 128:137–145
Tortoli E, Benedetti M, Fontanelle A, Simonetti MT (2002) Evaluation of automated BACTEC MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to four major antituberculous drugs: comparison with the radiometric bactec 460 tb method and the agar plate method of proportion. J Clin Microbiol 40(2):607–610
WHO (2014) Global tuberculosis Report WHO. Geneva, Switzerland: World Health Organization.http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf. Accessed 01 Aug 2016
Wong EB, Cohen KA, Bishai WR (2013) Rising to the challenge: new therapies for tuberculosis. Trends Microbiol 21(9):493–501
Zianna A, Psomas G, Hatzidimitriou A, Lalia-Kantouri M (2016) Ni(II) complexes with 2,2-dipyridylamine and salicylaldehydes: synthesis, crystal structure and interaction with calf-thymus DNA and albumins. J Inorg Biochem. doi:10.1016/j.jinorgbio
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ). The authors express sincere thanks to the LDRX-UFF for the X-ray facilities and measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10534_2016_9968_MOESM1_ESM.pdf
Supplementary material 1 (PDF 890 kb). Supplementary information (infrared and UV–vis spectra used for the identification of compounds, as well as X-ray crystal data of [Cu(HAPIH)Cl]Cl·H2O (6a) and [Cu(HPzAmIH)Cl2]·H2O (8a)) is available free of charge at http://springerlink.bibliotecabuap.elogim.com as PDF file. CCDC 1497179 and CCDC 1497180 contain supplementary crystallographic data for 6a and 8a, respectively. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html, or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: (+44) 1223- 336-033; or e-mail: deposit@ccdc.cam.ac.uk
Rights and permissions
About this article
Cite this article
Firmino, G.S.S., de Souza, M.V.N., Pessoa, C. et al. Synthesis and evaluation of copper(II) complexes with isoniazid-derived hydrazones as anticancer and antitubercular agents. Biometals 29, 953–963 (2016). https://doi.org/10.1007/s10534-016-9968-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-016-9968-7