Abstract
New compounds to fight cancer are needed due to cancer high incidence and lack of curative treatments for several classes of this disease. Metal-based coordination compounds offer a variety of molecules that can turn into drugs. Among them, coordination copper complexes are emerging as an attractive class of compounds for cancer treatment. A series of [Cu(l-dipeptide)(tmp)] (tmp = 3,4,7,8-tetramethyl-1,10-phenanthroline) complexes were synthesized and characterized in the solid state, including the determination of the crystalline structure of [Cu(Gly-Gly)(tmp)]·3.5 H2O and [Cu2Cl4(tmp)2]. The complexes were studied in solution, where the major species are also ternary ones. The lipophilicity of the complexes was determined and the binding to the DNA was evaluated, suggesting that it occurs in the DNA’s major groove. The cytotoxicity of the complexes was evaluated on different cancer cell lines: human metastatic breast adenocarcinoma MDA-MB-231 (triple negative, ATCC: HTB-26), MCF-7 (ATCC: HTB-22), SK-BR-3 (ATCC: HTB-30), human lung epithelial carcinoma A549 (ATCC: CCL-185), cisplatin resistant-human ovarian carcinoma A2780cis (SIGMA) and nontumoral cell lines: MRC-5 (lung; ATCC: CCL-171) and MCF-10A (breast, ATCC: CRL-10317). [Cu(l-dipeptide)(tmp)] complexes are highly cytotoxic as compared to [Cu(l-dipeptide)(phenanthroline)] and cisplatin. Therefore, [Cu(l-dipeptide)(tmp)] complexes are promising candidates to have their in vivo activity further studied toward new treatments for triple negative breast cancer and other aggressive tumors for which there is no curative pharmacological treatment to the date.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer high incidence makes the search of new compounds to treat it of paramount importance [1]. Metal-based drugs play a key role in this field, with platinum complexes being used in about 50% of cancer treatments. These complexes have been successfully used against a wide range of classes of cancer, being curative in several cases [2]. In spite of that, to date, we are far from having a curative treatment for all classes of cancer. Metal-based coordination compounds offer a variety of molecules that can turn into drugs. Among those, different copper complexes with antitumor activity have been synthesized and characterized [3,4,5,6,7], many with encouraging results. Some of them even present anti-metastatic and anti-angiogenic activities (in vitro assays) [8, 9]. Others are cytotoxic to cancer stem cells [10, 11]. Many Cu(II) complexes are active despite of their ligands bearing no appreciable cytotoxic activity, evidencing the central role of the metal itself in the antitumor activity.
The mechanism of action of copper compounds possibly includes different molecular events, which have not been completely characterized [3, 12, 13]. The lack of specificity against a single molecular target strengthens copper complexes ability to fight a diverse cell population such as those found in a tumor. Deoxyribonucleic acid (DNA) binding and the production of reactive oxygen species (ROS) inducing redox stress, are commonly proposed as molecular events for most anticancer Cu compounds [12, 14]. There are several studies relating the cytotoxic activity with an intracellular copper overload induced by the complexes [8, 15,16,17,18]. A comparative analysis between the activity of several copper complexes and cisplatin on the NIH’s N60 panel of cancer cell lines suggests different mechanisms of action, thus promising also a different spectra of activity in vivo [2].
Trying to find new complexes with antitumor activity, a series of Cu-l-dipeptide, Cu(II)-l-dipeptide–diimine (diimine = 1,10-phenanthroline, phen, 5-NO2-1,10-phenanthroline and neocuproine, neo) complexes have been previously studied by our research group [19,20,21,22,23,24,25,26,27,28,29,30]. Heteroleptic complexes Cu(II)-l-dipeptide–phen are more cytotoxic than both Cu-phen and Cu-l-dipeptide complexes [27, 28, 31,32,33,34,35]. Their mechanism of action possibly includes DNA binding mediated by the phen moiety. Similar results were obtained with Cu(II)-l-dipeptide–5-NO2-phen. For Cu(II)-l-dipeptide–neo complexes, the most active series, the dipeptides only slightly modulated the Cu-neo complex cytotoxicity [36].
Looking to improve the cytotoxicity of these compounds, we used 3,4,7,8-tetramethyl-1,10-phenanthroline (tmp), a practically planar molecule that might be able to intercalate the DNA. The tmp molecule is highly cytotoxic to cancer cells, even to cancer stem cells [37]. The tmp containing complexes are more selective than those of phen, as previously studied, in vitro [37]. As anionic co-ligands we chose a set of l-dipeptides (l-dipeptides: Gly-Gly, Gly-Phe, Ala-Gly, Ala-Phe, Val-Phe, Phe-Ala and Phe-Phe) to cover a range of different side chains and lipophilicity. The complexes were characterized both in the solid state and in aqueous solution and their binding to the DNA molecule was studied. Finally, the cytotoxicity of the complexes were evaluated against MDA-MB-231, MCF-7, SKBR-3 (human metastatic breast adenocarcinomas, the first triple negative), MCF-10A (human nontumoral breast cells), A2780cis (cisplatin resistant-human ovarian carcinoma), A549 (human lung epithelial carcinoma) and MRC-5 (human nontumoral lung epithelial cells).
Experimental
All reagents were used as commercially available: copper(II) carbonate and copper(II) chloride (Fluka), l-dipeptides (SIGMA), 3,4,7,8-tetramethyl-1,10-phenanthroline (tmp, SIGMA) and Calf thymus DNA (CT-DNA, SIGMA).
Synthesis and analytical characterization
[Cu2Cl4(tmp)2]·H2O complex
An ethanolic solution of tmp (4 mM, 5 mL) was added under constant stirring at room temperature to an aqueous solution of CuCl2 (4 mM, 5 mL). The solution turned green instantly. It was allowed to slowly evaporate giving rise to green prismatic single crystals adequate for X-ray diffraction studies. Yield: 20%. [Cu2Cl2(tmp)2]·H2O C0 Calc. for C16H18CuN2OCl2/Found: %C: 49.43/49.09, %N: 7.21/7.08, %H: 4.67/4.61.
[Cu(dipeptide)(tmp)] complexes
A solution of [Cu(dipeptide)] precursor with general formula [Cu(dipeptide)] was firstly obtained by dissolving the corresponding dipeptide in the minimum volume of H2O. To this solution, a 50% excess of CuCO3 was added and stirred at 60–80 °C for 1 h. The CuCO3 that was not solubilized was filtered off. The resulting blue solution was evaporated at 60–80 °C until an adequate amount of solid is obtained which was then filtered off, washed with cold H2O and air dried, as described by Facchin et al. [23]. The adequate amount of solid (0.1 mmol) was dissolved in hot H2O to make 50 mL of a 2 mM, solution (0.1 mmol). It was mixed under stirring at 60 °C with 10 mL of a 0.01 M ethanolic solution of tmp (0.1 mmol). The dipeptides used were: Gly-Gly, l-Gly-Phe, l-Ala-Gly, l-Ala-Phe, l-Val-Phe, l-Phe-Ala, and l-Phe-Phe. In all cases a dark blue-green solid was obtained after evaporation at 25 °C. Yield: 50–70%. Figure 1 presents a scheme of the synthetic procedure and of the proposed coordination of the complexes. Blue prismatic crystals were obtained for [Cu(Gly-Gly)(tmp)]·6H2O by recrystallization in 95% ethanol. Recrystallization tests for the remaining ternary complexes were unsuccessful.
The obtained solids correspond to the general formula [Cu(l-dipeptide)(tmp)]·xCH3CH2OH⋅xH2O, where the dipeptide acts as a − 2 anion, making neutral complexes of the following formulas and elemental compositions. [Cu(Gly-Gly)(tmp)]·6H2O C1 Calc. for C20H34CuN4O9/Found: %C: 44.64/44.68, %N: 10.41/10.25, %H: 6.36/6.20; [Cu(l-Gly-Phe)(tmp)]·CH3CH2OH·2H2O C2 Calc./Found (C29H38CuN4O6) %C: 57.84/57.39, %N: 9.30/8.96, %H: 6.36/5.90; [Cu(l-Ala-Gly)(tmp)]·3.5H2O C3 Calc./Found (C21H31CuN4O6.5) %C: 49.75/49.75, %N: 11.05/11.12, %H: 6.16/5.99; [Cu(l-Ala-Phe)(tmp)]·3H2O C4 Calc./Found (C28H36CuN4O6) %C: 57.18/57.52, %N: 9.52/9.40, %H: 6.17/6.07; [Cu(l-Val-Phe)(tmp)]·CH3CH2OH C5 Calc./Found (C32H40CuN4O4) %C: 63.19/63.58, %N: 9.21/8.79, %H: 6.62/6.15; [Cu(l-Phe-Ala)(tmp)]·3.5H2O C6 Calc./Found (C28H37CuN4O6.5) %C: 56.31/56.15, %N: 9.38/9.29, %H: 6.24/6.34; [Cu(l-Phe-Phe)(tmp)]·2H2O C7 Calc./Found (C34H38CuN4O5) %C: 63.19/63.60, %N: 8.67/8.59, %H: 5.93/5.74. Only complexes C1, C3 and C4 are soluble in water to mM concentrations, all the complexes are soluble in DMSO and DMSO/water mixtures.
Physical methods
Elemental analyses (C, N and H) of the samples were carried out on a Thermo Flash 2000 analyzer. Infrared spectra were measured on a Shimadzu IR Prestige 21 (4000–400 cm−1) as 1% KBr disks with a 4 cm−1 resolution. UV–visible spectra of 2.5 mM solutions in H2O and/or DMSO of the complexes were recorded on a Thermo Scientific Evolution 60 spectrophotometer in 1 cm path length quartz cells.
Electronic paramagnetic resonance (EPR) measurements (X-band, 9.5 GHz) of frozen DMSO solution (with about 10% of water) samples at 77 K temperature were carried out on a JEOL JES-FA200 spectrometer. The Spin Hamiltonian parameters g and A were determined using spectral simulations with the Easyspin software [38] running in Matlab R2014a.
Crystal structure determination
Diffraction data for suitable crystals of C0 and C1 were obtained with Mo-Kα radiation (λ = 0.71073 Å) at 296(2) K on a Bruker D8 Venture diffractometer equipped with a Photon 100 CMOS detector. Data collection, reduction and multi-scan absorption correction were done on Bruker APEX 3 software [39]. Intrinsic methods were used to solve the structures with SHELXT [40]. Refinement by the full-matrix least squares on F2 method was performed using SHELXL [41] within SHELXLE [42]. Non-hydrogen atoms were refined anisotropically, whereas, hydrogen atoms were geometrically positioned and refined with the riding model using 1.2 Ueq for carbon bonded H atoms and 1.5 for water H atoms. MERCURY was used for structure visualization and image preparation [43]. CIF files were prepared on EnCIFer [44].
In the refinement of C1 structure, a solvent mask procedure [45] within Olex2 [46] was calculated and 65 electrons were found in a volume of 224 Å3 in 1 void per asymmetric unit. This is consistent with the presence of 6.5 water molecules. A summary of crystallographic data, experimental details and refinement results is presented in Table 1.
Lipophilicity assessment
The lipophilicity of the complexes was studied by Thin Layer Reverse Chromatography using ALUGRAM® RP-18W/UV254 plates. Solutions of the complexes in DMSO were applied and the plates were dried for 12 h at 50 °C. Afterwards plates were eluted with a mixture of methanol:water 9:1 mixture in presence of Tris/HCl pH = 7.4 5 mM buffer. The reported RM values were obtained from the determined Rf using the expression RM = log10[(1/Rf) − 1] [47]. Reproducibility was controlled with caffeine, obtaining variations of less than 10%.
DNA interaction study
A solution of 5 mg of Calf Thymus-DNA (CT-DNA) in 5 mL of water was prepared by at least 12 h of gentle stirring. The concentration of the stock solution was determined spectroscopically using the reported molar absorptivity coefficient at 260 nm (\({\varepsilon }_{260}\) = 6,600 M−1 cm−1/base pair). Protein content was controlled by the A260/A280 ratio which varied in the 1.8–1.9 range [48]. Solutions were stored at 4 °C and were used within 3 days.
DNA intrinsic binding constant (Kb) was determined through UV absorption titration measurements using the Benesi-Hildebrand model. The complexes were dissolved in buffer Tris/HCl pH = 7.5 5 and 50 mM in NaCl. The concentration of the complexes was kept constant at 10–15 μM while adding CT-DNA to obtain concentrations in the 0–250 μM in base pairs range [49,50,51,52,53].
The mode of binding was studied by circular dichroism (CD). The CD spectra of a solution of DNA with fixed concentration (10 μM in a solution containing buffer Tris/HCl pH = 7.2 5 mM buffer) in the presence of increasing complex concentration were recorded. A JASCO J-815 equipment was used and spectra collected in the range of 220–320 nm with a 100 nm/min rate and 6 accumulations.
Cytotoxicity studies
The cytotoxicity of the complexes was evaluated against different human cancer cell lines: human metastatic breast adenocarcinoma MDA-MB-231 (triple negative, ATCC: HTB-26), MCF-7 (ATCC: HTB-22), SK-BR-3 (ATCC: HTB-30), human lung epithelial carcinoma A549 (ATCC: CCL-185), cisplatin resistant-human ovarian carcinoma A2780cis (SIGMA) and nontumoral cell lines: MRC-5 (lung; ATCC: CCL-171) and MCF-10A (breast, ATCC: CRL-10317), using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. The cells were cultured in Dulbecco's Modified Eagle's Medium (DMEM) for MDA-MB-231, A549 and MRC-5, supplemented with 10% fetal bovine serum (FBS), Roswell Park Memorial Institute (RPMI) 1640 Medium for MCF-7, SK-BR-3 and A2780cis, supplemented with 10% FBS or Dulbecco's Modified Eagle Medium Nutrient Mixture F-12 (DMEM F-12) for MCF-10A, containing 5% horse serum, Epidermal growth factor (EGF, 20 ng mL−1), hydrocortisone (0.5 μg mL−1), insulin (0.01 mg mL−1), 1% penicillin and 1% streptomycin, at 310 K in humidified 5% CO2 atmosphere. To retain the resistance of the A2780cis cell line, cisplatin (10 µM) was added every 3 passages during cell culture. To conduct the assay, 1.5 × 104 cells/well were seeded in 150 μL of medium in 96-well plates and incubated at 310 K in 5% CO2 for 24 h to allow cell adhesion. Then the cells were treated with copper complexes for 48 h. Cu-complexes were dissolved in DMSO, and 0.75 μL of solution was added to each well with 150 μL of medium (final concentration of 0.5% DMSO/well). Cisplatin, used as a reference drug, was solubilized in DMF. After the treatment, MTT (50 μL, 1 mg mL−1 in PBS) was added to each well, and the plate was incubated for 4 h. Cell viability was detected by the reduction of MTT to purple formazan by living cells. The formazan crystals were solubilized by isopropanol (150 μL/well), and the optical density of each well was measured using a microplate spectrophotometer at a wavelength of 540 nm. The concentration to 50% (IC50) of cell viability (Table 2) was obtained from the analysis of absorbance data of three independent experiments.
Results and discussion
Crystal structures
Table 1 summarizes crystallographic data as well as refinement details.
[Cu2Cl4(tmp)2]·H2O (C0)
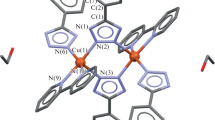
Single crystal X-ray diffraction of suitable crystals of C0 showed a dinuclear compound, with one water molecule per asymmetric unit. The copper center presents a square-based pyramidal coordination sphere with two N atoms from the tmp ligand and three Cl atoms, where two act as bridges between the copper centers. The structure is similar to that of the Cu-phen complex [54]. The contents of the asymmetric unit and the complex generated by symmetry are presented in Fig. 2. Table S1 shows selected distances and angles around the metal centre, Fig. S2 presents the crystal packing.
Ortep representation with 50% probability of C0: a asymmetric unit and b molecule generated by symmetry with atom numbering scheme. The water molecule is not showed in the structure, for the sake of clarity. Selected bond lengths and angles: N1–Cu1A 1.975(9) Å, N2–Cu1A 2.061(9) Å, N1–Cu1A–N2 81.1(3) °
[Cu(Gly-Gly)(tmp)]·3.75 H2O (C1)
The crystal structure of C1 shows pentacoordinated copper centers, with two metal complexes per asymmetric unit. A structural water molecule bridges both molecules by strong O···H–O–H···O intermolecular interactions.
The coordination polyhedron is a square-based pyramid slightly distorted toward a trigonal bipyramid. Figure 3 depicts the contents of the asymmetric unit of C1 (and Fig. S1, Sup. Inf. the Molecule overlay for non-symmetry equivalent molecules). The coordination scheme is similar to that observed in related [Cu(dipeptide)(phen)] complexes [27, 28, 36]. The dipeptide coordinates through the carboxylate oxygen atom and the amidic and aminic nitrogen atoms. The tmp is perpendicular to the dipeptide ligand with a nitrogen atom coordinating on the equatorial plane and the other in the apical position, whit a bite angle slightly lower than that of C0. Table S1 shows the distances and angles of the coordination sphere, Fig. S3 presents the crystal packing.
Infrared spectra
All the studied ternary complexes present similar infrared spectra, which evidences the coordination of both ligands to the metal center. The common characteristic bands in the spectra include a broad, very strong peak around 1600 cm−1, corresponding to ν(C=O) + ν(C–N) + νas(COO), which is characteristic of the coordinated dipeptide moiety [19, 20, 55]. Absorption peaks corresponding to other ring stretching frequencies of the tmp are modified in relation to the free ligand and very close to those of the [Cu2Cl4(tmp)2]·H2O appearing around 1530 and 1400 cm−1, in agreement with the coordination of tmp [56]. The complexes and ligand spectra and their tentative assignment are included in Table S2 and S3 (Sup. Inf.).
Characterization in solution
To have an insight of the major species in aqueous solution, UV–Vis and EPR spectroscopy studies were conducted.
UV–Vis spectra (Table S4, Fig. S4, Sup. Inf.), both in aqueous and DMSO solution, present a maximum absorption wavelength (λmax) in the 620–650 nm range with a shoulder at 850 nm. This is typical of Cu(II) in pentacordinated complexes [31, 33, 34, 57, 58], as observed in the solid state structure. Empiric correlations between the λmax and the donor atoms coordinated to the Cu(II) [59,60,61,62] were used to further analyze it. The λmax of the visible spectra, calculated according to Prenesti et al. [61, 62] for the proposed coordination scheme shown in Fig. 1—corresponding to the crystal structure of C1 is around 610–620 nm. Spectra were recorded in H2O for C1, C3 and C4 only, due to solubility limitations. The λmax is around 650 nm with a shoulder at 850 nm. This λmax is higher than the calculated one, but is not the calculated (and determined) for any of the binary complexes [19, 20, 23]. The spectra in DMSO present a λmax, around 620 nm, similar to the calculated for the pentacoordinated ternary complex, suggesting this is the major species. According to this analysis, the major species in solution are ternary ones. Spectra of the complexes (both in H2O and in DMSO) remain constant for at least 48 h, evidencing complexes are stable.
The EPR spectra of these ternary complexes are in agreement with a distorted square pyramidal geometry (Fig. S5, Sup. Inf.). There is no evidence of the coexistence of multiple species with different coordination environments. The g and A values are in accordance with previous reports of copper coordination centres with N and O donor atoms [31, 33, 34, 57, 58, 63, 64].
The combination of results from UV–Vis and EPR spectroscopies suggest that the coordination sphere of the major species observed in aqueous or DMSO solution is similar to the one observed for C1 in the solid state, depicted in Fig. 1.
Lipophilicity
Lipophilicity was determined as RM for the tmp ligand and the studied ternary complexes. The cationic binary complex C0 runs with the front, as well as C1 and C3. Lipophilicity values expressed as RM are: tmp 0.35, C2 − 0.02, C4 − 0.01, C5 0.20, C6 0.07 and C7 0.70. Lipophilicity follows the trend of the dipeptide complexity, increasing by addition of alkyl and aromatic substituent. Only C7, containing l-Phe-Phe, is more lipophilic than tmp, as expected and already reported for [Cu(dipeptide)(phen)] complexes [27]. The order of the residues on isomeric ligands affects lipophilicity, as observed for C4 and C6, which correlates also with the difference in solubility in water which is higher for C4 than C6.
Complex–DNA interaction studies
Values of intrinsic binding constants to DNA (Kb) of all the heteroleptic complexes are relatively similar, in the order of 1 × 103(Table S5 and Fig. S6, Sup. Inf.), evidencing a similar DNA binding strength. The Kb values are around ten-fold lower than their analogous for Cu-dipeptide-phen complexes [27], suggesting that methyl groups of tmp impair DNA binding as compared to phen. Their order of magnitude is consistent with groove binding [65], and/or partial intercalation, possibly of the tmp moiety.
CD studies show that the complexes induce a DNA conformational change from the B form to the A form (Fig. S6 presents the spectra) [66]. The A form presents a deeper major groove, suggesting that the complexes bind the DNA in the major groove.
Cytotoxicity
The complexes are highly cytotoxic against the studied cell lines, as presented in Table 2, showing a tenfold increase in activity compared to cisplatin. Their activity is especially remarkable in the cisplatin resistant line A2780cis, suggesting a different mechanism of action that overcomes resistance. When comparing the activity on the different breast cancer cells, compounds are highly cytotoxicity to MDA-MB-231 cells, a triple-negative breast cancer cell line, compared with the hormone-dependent MCF-7 cell line.
When compared with other Cu-compounds, the complexes can be classified as potent or remarkable cytotoxic agents according to the classification of Santini et al. [3]. The activity is high as compared with other Cu-complexes such as Casiopeínas [67], [Cu(bimda)(tmp)] [68], [Cu(trien)(tmp)](ClO4)2 [69] other phen containing ternary complexes with tridentate ligands such as [Cu(4-bromo-6-(((5-chloro-2-hydroxyphenyl)amino)methylene)cyclohexa-2,4-ien-1-one)(phen)] [70], [Cu(phen)2Cl]Cl∙p-aminobenzoic acid∙4H2O [71], complexes with highly functionalised phen derivatives such as with a triphenylphosphine group linked by an amide [8], complexes of tridentate N,N,O-chelating salphen-like ligand scaffold [72], complexes with tripodal ligands as [Cu(tris(2-pyridyamine)Cl2] [73], complexes with heteroescorpionate ligands [74] and comparable to the highly cytotoxic Cu-carbene compound WBC4 [75].
To the best of our knowledge, there is no relation between the structural descriptors determined in this work with the cytotoxicity. When comparing with other [Cu(dipeptide)(diimine)] complexes, tmp complexes present are the more active (present lower IC50), in spite of showing the lower Kb, suggesting that DNA binding is not the determinant of the cytotoxic activity [27, 36].
As a first approach to the toxicity of the complexes, their cytotoxicity toward nontumoral cells were determined and a Selectivity Index was calculated (SI, IC50 on non-tumor cells/IC50 on tumor cells of the same origin except for A2780cis cells for which in absence of a nontumoral model MRC-5 cells were used).
As can be seen from Table 2, the complexes are less selective than cisplatin in this model, and are more selective for breast cancer than for lung cell lines. Complexes are very cytotoxic to the MRC-5 cells. Despite that, compound C0 was tested decades ago on mice and was relatively well tolerated at doses at least ten-fold the determined IC50 [76]. Therefore, as the complexes are highly cytotoxic and present similar selectivity to C0 which presented tolerable side effects in animals, we consider that both C0 and [Cu(dipeptide)(tmp)] complexes deserve being studied in animal models to check their activity and toxicity in vivo. In particular complex C3 presents the most adequate profile for in vivo testing on breast and ovarian cancer models, due to its selectivity index and water solubility.
Looking for the active species for this series of complexes two different approaches have been followed. EPR spectra of DMSO solutions of the complexes with 10% culture medium were measured, as complexes in solution with culture medium can experiment speciation equilibria that can extend to coordinate media ligands. Spectra do not evidence major changes upon medium addition, suggesting that although speciation equilibria are possibly happening, the ternary complexes remain a major fraction of the Cu. As an example, Fig. S8 presents the simulated EPR spectra with the parameters (g, A) of compound C4 overlapped to the EPR of the same complex with culture medium. This does not rule out the formation of species where Cu is partially coordinated to, for instance, albumin of the media, as demonstrated for Cu-phen complexes. Moreover, the EPR spectra remain the same after 72 h (Fig. S9), evidencing that the compound maintains its coordination over time. This is only an approach to the equilibrium at culture conditions where Cu levels are much lower (but not adequate for EPR) and expected to favor complex dissociation, specially of the dipeptide. Once inside the cell endogenous copper is present as Cu(I). When the cells are exposed to copper complexes, they induce an increase of copper uptake, this copper is also believed to be Cu(I) [77, 78]. We expect it to be the case for our complexes although this point is outside the reach of this work. If complexes were reduced to Cu(I), as suggested for the Cu-phen complexes [77], that would lead to the dissociation of the dipeptide and possible also the tmp.
As a complementary approach, the activity of all the components of the heteroleptic complexes was determined. The copper salt and free dipeptides present low cytotoxicity (with no detectable activity up to 50 µM). Therefore, if the compound were to dissociate in the culture medium nor the copper or the dipeptide alone are responsible for the observed cytotoxicity. In relation to free tmp, for the breast-derived cells, its activity and selectivity toward cancer cells is lower than C0 and the ternary complexes. On the other cells, the activity is similar and the selectivity is somewhat higher for tmp than C0 and the heteroleptic complexes. The activity of the free diimines as phen and tmp was recently reviewed with authors proposing that their cytotoxicity is due to the formation of complexes with the Cu of the culture medium [79], an effect that cannot be excluded to occur, at least partially, in our studies. However, this is not the cause of the observed differences of relative activity of tmp versus the complexes, as studies for cells with different relative behavior were performed in exactly the same medium being an intrinsic different cytotoxicity of tmp on certain cancer cell lines. According to our results, it cannot be excluded that for the A549, A2780cis and MRC-5 cells the tmp is responsible for the activity, whereas for the breast-derived cells the complexation seems to play a more significant role. This issue is becoming a new focus of research in the literature, and was studied mostly for the related Cu-phen system. One work suggests that the ultimate active species are Cu(I) and phen, separately inside the cell, but as Cu distribution depends on the ligands of the complexes, the ternary complexes still play a role inside the cell [77]. In vivo experiments showed that Cu-phen, but not free phen, present significant antitumor activity in a mouse cancer model [79], evidencing the central role of the complexation. This is a subject that deserves yet more research to understand the active species of Cu-complexes.
Conclusions
Seven new ternary [Cu(l-dipeptide)(tmp)] complexes were synthesized and characterized both in solid state and in solution, by analytical and spectroscopic methods. Two new structures were determined, including [Cu(l-Gly-Gly)(tmp)]⋅6H2O, which presents the typical [Cu(l-dipeptide)(phen)] structure. The coordination environment of the metal observed in the solid state is maintained in the major species in aqueous solution.
Tmp impairs DNA binding as compared to phen, possibly favoring major groove binding. The introduction of tmp as a ligand augmented the cytotoxic activity of the complexes, as compared to the related [Cu(l-dipeptide)(phen)], suggesting that the DNA interaction is not determinant in the cytotoxicity of the compounds. The complexes are highly cytotoxic as compared with other Cu-complexes and cisplatin, and are potential candidates to further study in vivo as new treatments of triple negative breast cancer and other aggressive tumors for which there is no available curative pharmacological treatment yet.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424
Casini A, Vessières A, Meier-Menches SM (2019) Metal-based anticancer agents. Royal Society of Chemistry, Cambridge
Santini C, Pellei M, Gandin V, Porchia M, Tisato F, Marzano C (2014) Advances in copper complexes as anticancer agents. Chem Rev 114:815–862
McGivern TJP, Afsharpour S, Marmion CJ (2018) Copper complexes as artificial DNA metallonucleases: from Sigman’s reagent to next generation anti-cancer agent? Inorg Chim Acta 472:12–39
Mahalakshmi R, Raman N (2016) A therapeutic journey of mixed ligand complexes containing 1,10-phenanthroline derivatives: a review. Chemistry 16:18
Mejía C, Ortega-Rosales S, Ruiz-Azuara L (2018) Mechanism of action of anticancer metallodrugs, biomedical applications of metals. Springer, Berlin, pp 213–234
Kellett A, Molphy Z, McKee V, Slator C (2019) Recent advances in anticancer copper compounds. In: Casini A, Vessieres A, Meier-Menches SM (eds) Metal-based anticancer agents. Metallobiology, vol 14. RSC, pp 91–119
Shi X, Chen Z, Wang Y, Guo Z, Wang X (2018) Hypotoxic copper complexes with potent anti-metastatic and anti-angiogenic activities against cancer cells. Dalton Trans 47:5049–5054
Qin X-Y, Wang Y-N, Yang X-P, Liang J-J, Liu J-L, Luo Z-H (2017) Synthesis, characterization, and anticancer activity of two mixed ligand copper(II) complexes by regulating the VEGF/VEGFR2 signaling pathway. Dalton Trans 46:16446–16454
Laws K, Bineva-Todd G, Eskandari A, Lu C, O’Reilly N, Suntharalingam K (2018) A copper(II) phenanthroline metallopeptide that targets and disrupts mitochondrial function in breast cancer stem cells. Angew Chem 130:293–297
Laws K, Suntharalingam K (2018) The next generation of anticancer metallopharmaceuticals: cancer stem cell-active inorganics. ChemBioChem 19:2246–2253
Serment-Guerrero J, Bravo-Gomez ME, Lara-Rivera E, Ruiz-Azuara L (2017) Genotoxic assessment of the copper chelated compounds Casiopeinas: clues about their mechanisms of action. J Inorg Biochem 166:68–75
Marzano C, Tisato F, Porchia M, Pellei M, Gandin V (2019) Phosphine copper(I) complexes as anticancer agents: biological characterization. Part II, copper(I) chemistry of phosphines, functionalized phosphines and phosphorus heterocycles. Elsevier, Amsterdam, pp 83–107
Nunes P, Yildizhan Y, Adiguzel Z, Marques F, Costa Pessoa J, Acilan C, Correia I (2021) Copper(II) and oxidovanadium(IV) complexes of chromone Schiff bases as potential anticancer agents. J Biol Inorg Chem. https://doi.org/10.1007/s00775-021-01913-4
Tardito S, Bassanetti I, Bignardi C, Elviri L, Tegoni M, Mucchino C, Bussolati O, Franchi-Gazzola R, Marchio L (2011) Copper binding agents acting as copper ionophores lead to caspase inhibition and paraptotic cell death in human cancer cells. J Am Chem Soc 133:6235–6242
Gaál A, Mihucz VG, Bősze S, Szabó I, Baranyi M, Horváth P, Streli C, Szoboszlai N (2018) Comparative in vitro investigation of anticancer copper chelating agents. Microchem J 136:227–235
Shi X, Fang H, Guo Y, Yuan H, Guo Z, Wang X (2019) Anticancer copper complex with nucleus, mitochondrion and cyclooxygenase-2 as multiple targets. J Inorg Biochem 190:38–44
Nagababu P, Barui AK, Thulasiram B, Devi CS, Satyanarayana S, Patra CR, Sreedhar B (2015) Antiangiogenic activity of mononuclear copper(II) polypyridyl complexes for the treatment of cancers. J Med Chem 58:5226–5241
Facchin G, Torre MH, Kremer E, Piro OE, Castellano EE, Baran EJ (2000) Structural and spectroscopic characterization of two new Cu(II)-dipeptide complexes. Z Naturforsch B 55:1157–1162
Facchin G, Torre MAH, Kremer E, Piro OE, Castellano EE, Baran EJ (2002) Synthesis and characterization of three new Cu(II)-dipeptide complexes. J Inorg Biochem 89:174–180
Facchin G, Torre M, Kremer E, Baran E, Mombrú A, Pardo H, Araujo M, Batista A, Costa-Filho A (2003) Cu(II) complexation with His-Gly and His-Ala. X-ray structure of [Cu(his–gly)2(H2O)2]· 6H2O. Inorg Chim Acta 355:408–413
Vieira ED, Casado NM, Facchin G, Torre MH, Costa-Filho AJ, Calvo R (2006) Weak exchange interaction supported by a biologically relevant long chemical bridge in a Cu-peptide model compound. Inorg Chem 45:2942–2947
Facchin G, Kremer E, Baran EJ, Castellano EE, Piro OE, Ellena J, Costa-Filho AJ, Torre MH (2006) Structural characterization of a series of new Cu-dipeptide complexes in solid state and in solution. Polyhedron 25:2597–2604
Sanchiz J, Kremer C, Torre M, Facchin G, Kremer E, Castellano EE, Ellena J (2006) Magnetic properties of copper(II) complexes containing peptides. Crystal structure of [Cu(phe-leu)]. J Mol Struct 797:179–183
Facchin G, Kremer E, Barrio DA, Etcheverry SB, Costa-Filho AJ, Torre MH (2009) Interaction of Cu-dipeptide complexes with Calf Thymus DNA and antiproliferative activity of [Cu(ala-phe)] in osteosarcoma-derived cells. Polyhedron 28:2329–2334
Iglesias S, Noble C, González R, Torre MH, Kremer E, Kramer G, Facchin G (2013) Towards the development of new copper compounds for the treatment of cancer: study of the cytotoxic activity of Cu(l-dipeptide)(1,10-phenanthroline) complexes. In: Proceedings—12th international symposium of metal ions in biology and medicine, p 1
Iglesias S, Alvarez N, Torre MH, Kremer E, Ellena J, Ribeiro RR, Barroso RP, Costa-Filho AJ, Kramer MG, Facchin G (2014) Synthesis, structural characterization and cytotoxic activity of ternary copper(II)–dipeptide–phenanthroline complexes. A step towards the development of new copper compounds for the treatment of cancer. J Inorg Biochem 139:117–123
Iglesias S, Alvarez N, Kramer G, Torre MH, Kremer E, Ellena J, Costa-Filho AJ, Facchin G (2015) Structural characterization and cytotoxic activity of heteroleptic copper(II) complexes with l-dipeptides and 5-NO2-phenanthroline. Crystal structure of [Cu(Phe-Ala)(5-NO2-Phen)]. 4H2O. Struct Chem Crystallogr Commun 1:1–7. https://doi.org/10.21767/2470-9905.100007
Facchin G, Veiga N, Kramer MG, Batista AA, Várnagy K, Farkas E, Moreno V, Torre MH (2016) Experimental and theoretical studies of copper complexes with isomeric dipeptides as novel candidates against breast cancer. J Inorg Biochem 162:52–61
Alvarez N, Noble C, Torre MH, Kremer E, Ellena J, de Araujo MP, Costa-Filho AJ, Mendes LF, Kramer MG, Facchin G (2017) Synthesis, structural characterization and cytotoxic activity against tumor cells of heteroleptic copper(I) complexes with aromatic diimines and phosphines. Inorg Chim Acta 466:559–564
Lim M, Sinn E, Martin RB (1976) Crystal structure of a mixed-ligand complex of copper(II), 1,10-phenanthroline, and glycylglycine dianion: glycylglycinato (1,10-phenanthroline) copper(II) trihydrate. Inorg Chem 15:807–811
Sugimori T, Shibakawa K, Masuda H, Odani A, Yamauchi O (1993) Ternary metal(II) complexes with tyrosine-containing dipeptides. Structures of copper(II) and palladium(II) complexes involving l-tyrosylglycine and stabilization of copper(II) complexes due to intramolecular aromatic ring stacking. Inorg Chem 32:4951–4959
Bhirud RG, Srivastava TS (1991) Synthesis, characterization and superoxide dismutase activity of some ternary copper(II) dipeptide-2, 2′-bipyridine, 1,10-phenanthroline and 2,9-dimethyl-1,10-phenanthroline complexes. Inorg Chim Acta 179:125–131
Deshpande S, Srivastava T (1983) Preparation and spectral studies of some ternary 2,2′-bipyridine and 1,10-phenanthroline copper(II) dipeptide complexes. Inorg Chim Acta 78:75–80
García-Raso A, Fiol JJ, Adrover B, Moreno V, Mata I, Espinosa E, Molins E (2003) Synthesis, structure and nuclease properties of several ternary copper(II) peptide complexes with 1,10-phenanthroline. J Inorg Biochem 95:77
Alvarez N, Viña D, Leite CM, Mendes LF, Batista AA, Ellena J, Costa-Filho AJ, Facchin G (2020) Synthesis and structural characterization of a series of ternary copper(II)-l-dipeptide-neocuproine complexes Study of their cytotoxicity against cancer cells including MDA-MB-231, triple negative breast cancer cells. J Inorg Biochem 203:110930
Robin P, Singh K, Suntharalingam K (2020) Gallium(III)-polypyridyl complexes as anti-osteosarcoma stem cell agents. Chem Commun 56:1509–1512. https://doi.org/10.1039/C9CC08962D
Stoll S, Schweiger A (2006) EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J Magn Reson 178:42–55
Bruker (2012) APEX 3. Bruker AXS Inc., Madison
Sheldrick GM (2014) SHELXT: integrating space group determination and structure solution. Acta Crystallogr Sect A Found Adv 70:C1437
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr Sect C Struct Chem 71:3–8
Hübschle CB, Sheldrick GM, Dittrich B (2011) ShelXle: a Qt graphical user interface for SHELXL. J Appl Crystallogr 44:1281–1284
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, Streek J (2006) Mercury: visualization and analysis of crystal structures. J Appl Crystallogr 39:453–457
Allen FH, Johnson O, Shields GP, Smith BR, Towler M (2004) CIF applications. XV. enCIFer: a program for viewing, editing and visualizing CIFs. J Appl Crystallogr 37:335–338
Jiang J-S, Brünger AT (1994) Protein hydration observed by X-ray diffraction: solvation properties of penicillopepsin and neuraminidase crystal structures. J Mol Biol 243:100–115
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JA, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42:339–341
Eadsforth CV, Moser P (1983) Assessment of reverse-phase chromatographic methods for determining partition coefficients. Chemosphere 12:1459–1475
Sirajuddin M, Ali S, Badshah A (2013) DRUG-DNA Interactions and their study by UV-visible, fluorescence spectroscopies and cyclic voltametry. J Photochem Photobiol B Biol 124:1–19
Benesi HA, Hildebrand JH (1949) A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J Am Chem Soc 71:2703–2707
Schmechel DEV, Crothers DM (1971) Kinetic and hydrodynamic studies of the complex of proflavine with poly A·poly U. Biopolymers 10:465–480
Wolfe A, Shimer GH, Meehan T (1987) Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. Biochemistry 26:6392–6396
Jenkins TC (1997) Optical absorbance and fluorescence techniques for measuring DNA–drug interactions. In: Fox KR (ed) Drug–DNA interaction protocols. Humana Press, Totowa, pp 195–218
Sirajuddin M, Ali S, Badshah A (2013) Drug–DNA interactions and their study by UV–visible, fluorescence spectroscopies and cyclic voltametry. J Photochem Photobiol B 124:1–19
Viossat B, Gaucher JF, Mazurier A, Selkti M, Tomas A (1998) Crystal structure of bis(μ-chloro)bis[chloro-(o-phenanthroline-N,N′)-coppeг(II)], Cu2(C12H8N2)2(Cl2)2. Zeitschrift für Kristallographie New Cryst Struct 213:343–344
Nakamoto K (2009) Infrared and Raman spectra of inorganic and coordination compounds, applications in coordination, organometallic, and bioinorganic chemistry, 6th edn. Wiley-Interscience, Hoboken
Yuan C-Q, Peng Z-H, Pan Q-C, Li D-C, Shen Y-F (2006) Spectroscopic and theoretical studies on copper(II) complex of maleonitriledithiolate and 5-nitro-1,10-phenanthroline. J Mol Struct 789:52–58
Cotton FA, Wilkinson G, Murillo CA, Bochmann M, Grimes R (1999) Advanced inorganic chemistry. Wiley, New York
Hathaway B, Billing D (1970) The electronic properties and stereochemistry of mono-nuclear complexes of the copper(II) ion. Coord Chem Rev 5:143–207
Billo E (1974) Copper(II) chromosomes and the rule of average environment. Inorg Nucl Chem Lett 10:613–617
Sigel H, Martin RB (1982) Coordinating properties of the amide bond. Stability and structure of metal ion complexes of peptides and related ligands. Chem Rev 82:385–426
Prenesti E, Daniele P, Prencipe M, Ostacoli G (1999) Spectrum–structure correlation for visible absorption spectra of copper(II) complexes in aqueous solution. Polyhedron 18:3233–3241
Prenesti E, Daniele PG, Berto S, Toso S (2006) Spectrum–structure correlation for visible absorption spectra of copper(II) complexes showing axial co-ordination in aqueous solution. Polyhedron 25:2815–2823
Peisach J, Blumberg WE (1974) Structural implications derived from the analysis of electron paramagnetic resonance spectra of natural and artificial copper proteins. Arch Biochem Biophys 165:691–708
Tabbì G, Giuffrida A, Bonomo RP (2013) Determination of formal redox potentials in aqueous solution of copper(II) complexes with ligands having nitrogen and oxygen donor atoms and comparison with their EPR and UV–Vis spectral features. J Inorg Biochem 128:137–145
Islam MM, Chakraborty M, Pandya P, Al Masum A, Gupta N, Mukhopadhyay S (2013) Binding of DNA with Rhodamine B: spectroscopic and molecular modeling studies. Dyes Pigm 99:412–422
Dickerson RE (1992) [5] DNA structure from A to Z, methods enzymol. Elsevier, Amsterdam, pp 67–111
Bravo-Gómez ME, Dávila-Manzanilla S, Flood-Garibay J, Muciño-Hernández MÁ, Mendoza Á, García-Ramos JC, Moreno-Esparza R, Ruiz-Azuara L (2012) Secondary ligand effects on the cytotoxicity of several Casiopeína’s group II compounds. J Mex Chem Soc 56:85–92
Loganathan R, Ramakrishnan S, Ganeshpandian M, Bhuvanesh NSP, Palaniandavar M, Riyasdeen A, Akbarsha MA (2015) Mixed ligand copper(II) dicarboxylate complexes: the role of co-ligand hydrophobicity in DNA binding, double-strand DNA cleavage, protein binding and cytotoxicity. Dalton Trans 44:10210–10227
Sharma M, Ganeshpandian M, Majumder M, Tamilarasan A, Sharma M, Mukhopadhyay R, Islam NS, Palaniandavar M (2020) Octahedral copper(II)-diimine complexes of triethylenetetramine: effect of stereochemical fluxionality and ligand hydrophobicity on Cu II/Cu I redox, DNA binding and cleavage, cytotoxicity and apoptosis-inducing ability. Dalton Trans 49:8282–8297
Mohammadizadeh F, Falahati-Pour SK, Rezaei A, Mohamadi M, Hajizadeh MR, Mirzaei MR, Khoshdel A, Fahmidehkar MA, Mahmoodi M (2018) The cytotoxicity effects of a novel Cu complex on MCF-7 human breast cancerous cells. Biometals 31:233–242
Hammud HH, McManus GJ, Zaworotko MJ, Tabesh RN, Ibrahim HIM, Ayub K, Ludwig R (2021) The co-crystal of copper(II) phenanthroline chloride complex hydrate with p-aminobenzoic acid: structure, cytotoxicity, thermal analysis, and DFT calculation. Monatshefte für Chemie Chem Mon 152:323–336
Peña Q, Sciortino G, Maréchal J-D, Bertaina S, Simaan AJ, Lorenzo J, Capdevila M, Bayón P, Iranzo O, Palacios Ò (2021) Copper(II) N,N,O-chelating complexes as potential anticancer agents. Inorg Chem 60:2939–2952
Jopp M, Becker J, Becker S, Miska A, Gandin V, Marzano C, Schindler S (2017) Anticancer activity of a series of copper(II) complexes with tripodal ligands. Eur J Med Chem 132:274–281
Pellei M, Gandin V, Marchiò L, Marzano C, Bagnarelli L, Santini C (2019) Syntheses and biological studies of Cu(II) complexes bearing bis(pyrazol-1-yl)-and bis(triazol-1-yl)-acetato heteroscorpionate ligands. Molecules 24:1761
Walther W, Fichtner I, Hackenberg F, Streciwilk W, Tacke M (2014) In vitro and in vivo investigations into the carbene copper bromide anticancer drug candidate WBC4. Lett Drug Des Discov 11:825–832
Dwyer F, Mayhew E, Roe E, Shulman A (1965) Inhibition of landschuetz ascites tumour growth by metal chelates derived from 3,4,7,8-tetramethyl-1,10-phenanthroline. Br J Cancer 19:195
Nunes P, Correia I, Marques F, Matos AP, Dos Santos MM, Azevedo CG, Capelo J-L, Santos HM, Gama S, Pinheiro T (2020) Copper complexes with 1,10-phenanthroline derivatives: underlying factors affecting their cytotoxicity. Inorg Chem 59:9116–9134
Zheng P, Eskandari A, Lu C, Laws K, Aldous L, Suntharalingam K (2019) Biophysical analysis of cancer stem cell-potent copper(II) coordination complexes. Dalton Trans 48:5892–5896
Pinho JO, Amaral JD, Castro RE, Rodrigues CM, Casini A, Soveral G, Gaspar MM (2019) Copper complex nanoformulations featuring highly promising therapeutic potential in murine melanoma models. Nanomedicine 14:835–850
Han B-J, Jiang G-B, Wang J, Li W, Huang H-L, Liu Y-J (2014) The studies on bioactivity in vitro of ruthenium(II) polypyridyl complexes towards human lung carcinoma A549 cells. RSC Adv 4:40899–40906
Acknowledgements
The authors thank Comisión Sectorial de Investigación Científica (CSIC Uruguay, I+D Grant to GF), Programa de Desarrollo de las Ciencias Básicas (PEDECIBA Química), Agencia Nacional de Investigación e Innovación (ANII) (Uruguay), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Brazil) for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alvarez, N., Leite, C.M., Napoleone, A. et al. Tetramethyl-phenanthroline copper complexes in the development of drugs to treat cancer: synthesis, characterization and cytotoxicity studies of a series of copper(II)-l-dipeptide-3,4,7,8-tetramethyl-phenanthroline complexes. J Biol Inorg Chem 27, 431–441 (2022). https://doi.org/10.1007/s00775-022-01938-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-022-01938-3