Abstract
Soil organic matter (SOM) decomposition and organic phosphorus (P) cycling may help sustain plant productivity under elevated CO2 (eCO2) and low-P conditions. Arbuscular mycorrhizal (AM) fungi and their role in P-acquisition and SOM decomposition may become more relevant in these conditions. Yet, experimental evidence of AM fungi and P availability interactive effects on soil carbon (C) cycling under eCO2 is scarce with the potential mechanisms of this control being poorly understood. We performed a pot experiment with soil and a grass from a low-P ecosystem where plant biomass and soil C cycling have been mostly unresponsive to eCO2. We manipulated AM fungi, P, and CO2 levels and assessed their impacts on soil C cycling and plant growth using continuous 13C plant labelling to isolate and measure short-term changes in total and SOM-derived fractions of respired CO2, dissolved organic C (DOC) and microbial biomass (MBC), as relevant components of the soil C cycle. Increases in SOM decomposition and microbial C use were hypothesised to support plant growth under eCO2 and low-P with AM fungi intensifying this effect. However, we did not detect simultaneous significant impacts of the three experimental factors. We observed instead increased root biomass and nutrient uptake with eCO2 and AM presence and lower SOM-derived DOC and MBC with low-P, decreasing further with AM inoculation. Taken together, our findings in this model plant-soil system suggest that, AM fungi can support root biomass growth and nutrient uptake under eCO2 and protect the SOM pool against decomposition even in low-P conditions. Contrary to reports from N-limited ecosystems, our results allow us to conclude that C and P biogeochemical cycles may not become coupled to sustain an eCO2 fertilisation effect and that the role of AM fungi protecting the SOM pool is likely driven by competitive interactions with saprotrophic communities over nutrients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The effect of increased atmospheric CO2 concentrations on plant productivity and its potential impacts on soil carbon (C) are regulated by nutrient availability (Ainsworth and Long 2004; Finzi et al. 2011; Reich et al. 2014; Terrer et al. 2019). Higher plant productivity and increases in soil C with elevated CO2 (eCO2) have been observed when combined with nutrient addition (Maroco et al. 2002; van Groenigen et al. 2006; De Graaff et al. 2006; Hungate et al. 2009; Dieleman et al. 2010; Piñeiro et al. 2017). In the absence of external nutrient supply, higher soil organic matter (SOM) decomposition under eCO2 contributes to meet plant nutrient demands allowing for increases in plant biomass (Hoosbeek et al. 2004; Finzi et al. 2006; Reich et al. 2006; Drake et al. 2011; Phillips et al. 2012; Carrillo et al. 2014). In this case, positive responses of plant biomass with eCO2 are strongly related with decreases in soil C (Terrer et al. 2021). However, it has also been observed that in ecosystems with soil nutrient limitations, eCO2-induced increases in plant productivity are constrained (Reich et al. 2006; Reed et al. 2015; Ellsworth et al. 2017) resulting in no effects on soil C. The current understanding about nutrient availability regulating eCO2 impacts on C cycling has been obtained mainly from northern hemisphere systems, where nitrogen (N) often constrains ecosystem productivity and soil microbial activity. But the impacts of eCO2 on soil C cycling may be dependent on what the most limiting nutrient is (Dijkstra et al. 2013). Phosphorus-limited tropical and subtropical ecosystems are relevant C sinks that cover a vast area (Soepadmo 1993; Pan et al. 2011; Keenan et al. 2015) and although the role of P availability regulating the impact of eCO2 on soil C storage is broadly recognised (Vitousek et al. 2010; Goll et al. 2012; Reed et al. 2015; Sun et al. 2017), the mechanisms of this control are poorly understood (Norby et al. 2015; Ellsworth et al. 2017; Terrer et al. 2019).
In contrast to N, soil P cycling is less coupled to soil C dynamics because P mobilisation is not necessarily linked with SOM decomposition. The P cycle can be divided into inorganic and organic sub-cycles (Mullen 2005; Liu and Chen 2008). Mobilisation of inorganic P occurs mainly via physicochemical mechanisms without SOM mineralisation nor CO2 production, thus C and the inorganic P cycle are not coupled. On the other hand, organic P is made available via P-hydrolytic enzymes that release phosphates without CO2 production (Nannipieri et al. 2011) or as a consequence of substrate degradation (Sharma et al. 2013) driven by the nutrient and energy needs of soil organisms (Tate 1984) and involving CO2 release as a consequence of SOM decomposition. Most P is made available via inorganic P mobilisation and via P-hydrolytic enzymatic activity, particularly with P sufficiency. But in low-P systems, mineral P sources are depleted and P derived from rock weathering is minimal (Reed et al. 2011; Goll et al. 2012). Therefore, the majority of P acquisition occurs via internal recycling of organic P sources (Johnson et al. 2003; Fox et al. 2011; Reed et al. 2011). Most of the research on the effects of eCO2 on soil P availability are focused on its impacts on inorganic P and organic P cycling that do not involve SOM decomposition. For example, higher microbial P enzymatic activity is reported with eCO2 (Moorhead and Linkins 1997; Kelley et al. 2011; Hasegawa et al. 2016; Souza et al. 2017) as well as higher production of organic acids and siderophores involved in P mobilisation (Watt and Evans 1999; Tarnawski and Aragno 2006; Fransson and Johansson 2010; Högy et al. 2010). Moreover, increases in fine root production and changes in pH and soil moisture with eCO2 can indirectly enhance inorganic P cycling in soils (Dijkstra et al. 2012; Jin et al. 2015; Hasegawa et al. 2016). It is likely that organic P cycling involving SOM decomposition is also affected by eCO2 due to the influence of eCO2 on ecosystem stoichiometry (Gifford et al. 2000; Loladze 2002, 2014) but experimental evidence for this is scarce. A better understanding about how eCO2 and P availability influence soil C cycling is important to better predict changes in global soil C due to eCO2 (Finzi et al. 2011; Goll et al. 2012; Yang et al. 2016).
In low-P conditions, organic P cycling may help sustain plant productivity under eCO2 at the expense of higher SOM decomposition. As C allocation belowground increases with eCO2 (Canadell et al. 1995; Cotrufo and Gorissen 1997; Pausch and Kuzyakov 2018), higher labile C availability can lead to increased microbial biomass and activity and higher SOM decomposition may allow for a sustained nutrient release for plant uptake and growth in low-P soils. However, evidence from field experiments exposed to eCO2 suggests that under P-limiting conditions, changes in soil C cycling are minor or undetected (Dijkstra et al. 2013; Drake et al. 2016; Castañeda-Gómez et al. 2020) and that the CO2 fertilization effect is reduced or not observed (Conroy et al. 1992; Peñuelas et al. 2012; Goll et al. 2012; Zhang et al. 2014; Deng et al. 2015; Ellsworth et al. 2017). Thus, if P availability becomes limiting, ecosystem productivity and microbial-mediated SOM decomposition may be hampered, preventing further changes in the soil C pool. Soil microbial communities play a key role in SOM decomposition and both organic and inorganic P cycling. Moreover, the responses of SOM decomposition and C stabilisation to P availability have been found to be largely mediated by changes in saprotrophic microbial community composition and activity (Liu et al. 2018; Soong et al. 2018; Fang et al. 2019; Feng and Zhu 2019; Yuan et al. 2021) and by symbiotic fungi such as arbuscular mycorrhizal (AM) fungi (Xu et al. 2018). Yet the role of saprotrophic and mycorrhizal fungi altering SOM decomposition under eCO2 conditions and low-P availability is poorly understood.
AM fungi are known to greatly contribute to P mobilisation under eCO2 conditions, especially in ecosystems with low soil P availability (Delucia et al. 1997; Matamala and Schlesinger 2000; Terrer et al. 2016) where AM fungi tend to be more abundant (Treseder and Cross 2006). AM fungi also alleviate P limitation conditions for plants (Willis et al. 2013; Soka and Ritchie 2014) possibly via excretion of P hydrolytic enzymes (Joner et al. 2000; Sato et al. 2015) and certainly via uptake of inorganic nutrients from the soil solution (Javot et al. 2007; Smith et al. 2011). They have also been linked with increased SOM decomposition with climate change (Talbot et al. 2008; Cheng et al. 2012; Carrillo et al. 2015; Wei et al. 2019). Higher C transfer to AM fungi under eCO2 enhances AM fungi growth and activity (Mohan et al. 2014) and so their contribution to plant P acquisition in P-limited ecosystems may be enhanced with eCO2 (Cavagnaro et al. 2011). On the other hand, AM fungi can also suppress eCO2-mediated increases in plant growth due to increased P immobilisation in AM fungal biomass and increased competition over P with saprotrophic communities (Jin et al. 2015) that can simultaneously decrease AM fungi-mediated SOM decomposition (Talbot et al. 2008). If P availability is low, the role of AM fungi mobilising P and promoting plant growth may be constrained due to P-limitation (Treseder and Allen 2002), which might further regulate impacts of eCO2 on ecosystem productivity in P-limited ecosystems. Due to the significant role of AM fungi on P and SOM dynamics, predictions of altered soil C stocks under eCO2 require an understanding how eCO2 can change AM fungal activity, their impact on saprotrophic communities and plant responses to eCO2, particularly under P limitation.
In this study, we explored the role of P availability and AM fungi mediating responses of soil C cycling and plant productivity to eCO2 in a model plant-soil system. Our aim was to answer two questions: How does P availability controls soil C cycling responses to eCO2 and how does AM fungi mediate these responses. We used plant and soil material from the Eucalyptus Free Air CO2 enrichment (EucFACE site) as a model system to answer these questions. At this site, both plant growth and soil C responses to eCO2 have been limited and it has been hypothesised that P limitation is preventing stronger ecosystem responses to eCO2. We manipulated P availability, AM fungal presence and atmospheric CO2 levels. An Australian native grass species (Microlaena stipoides) with the ability to grow in a wide range of P availability conditions and forming arbuscular mycorrhizal associations (Hill et al. 2010; Clark et al. 2014) was grown in controlled environment chambers. This grass species is also a dominant understory species present at the EucFACE site (Hasegawa et al. 2018). The growth chambers used allowed the continuous isotopic labelling of plant tissues so we could directly quantify short-term changes in soil C cycling by measuring relevant total and SOM-derived fractions of relevant soil C components: respired CO2 (R; the product of decomposition), dissolved organic C (DOC; considered an active pool of C) and microbial biomass (MBC; the agent of SOM decomposition. This approach allowed for a comprehensive assessment of the impact of eCO2, P availability and AM fungal presence on short-term soil C cycling and plant growth in a low-P model system. Changes in saprotrophic communities were also measured (using phospholipid fatty acids) in relation to eCO2 conditions, P treatment and AM fungal presence. These responses, along with nutrient contents in plant tissues and available soil nutrients, were collected to test our mechanistic hypotheses (Fig. 1).
Conceptual framework and expected responses of measured variables. (a) Under eCO2 and low P conditions, increases in plant growth and nutrient acquisition will be contingent to increases in soil C cycling facilitated by AM fungi presence. However, (b) if P conditions are too low, soil microbial activity will be limited, including that of AM fungi, and thus, changes to soil C cycling, plant biomass or plant nutrient contents will be constrained. Increased plant biomass under eCO2 and P-sufficiency (c) will not be necessarily accompanied by changes in soil C cycling, with most of P acquisition occurring from the replenished mineral P pool without the involvement of AM fungi. Soil C cycling is measured in this study as soil organic matter-derived (SOMd) C components: R, respired CO2; MB, microbial biomass; DOC, dissolved organic C. Green arrows: increase; red arrows: decrease; equal sign (=): no effect. Box sizes represent the relative size of each pool. The thickness of the arrows from SOM and mineral bound P pools represent the relative flux of P from these pools to plants. The size of the circles on top of each arrow represent the relative role of AM fungi on P acquisition and SOM decomposition from these pools
We hypothesised that under eCO2 and low P conditions, increases in plant growth will be contingent to increases in soil C cycling, evidenced as higher SOM-derived respired CO2, MBC and altered SOM-derived DOC (Fig. 1a). In this scenario, both low-P availability and eCO2 promote AM fungal colonisation rates and activity and thus, AM fungi presence will promote soil C cycling and enhance organic P cycling and nutrient acquisition detected as higher plant P, and lower C:N and C:P in plant tissues. P uptake by AM fungi from the depleted mineral P pool will be therefore reduced in these conditions (AM role reflected on the circles on top of each flux in Fig. 1a). Alternatively, we hypothesised that no fertilization effect would be observed if P conditions were too low due to a limitation of soil microbial activity, including that of AM fungi, without changes to soil C cycling, plant biomass or plant nutrient contents (Fig. 1b). Contrarily, increased plant biomass under eCO2 and P-sufficiency will not be necessarily accompanied by changes in soil C cycling, with most of P acquisition occurring from the replenished mineral P pool (Fig. 1c). In this scenario, AM fungi will not play a relevant role in SOM decomposition nor nutrient acquisition as high P availability decreases AM fungi abundance and activity.
Materials and methods
We set up a factorial experiment with three factors: phosphorus (P) treatment: P addition (+ P)/control; AM fungi: Active AM inoculum (AM)/Non-mycorrhizal (Sterile AM inoculum, −AM) and CO2: ambient (400 ppm—aCO2) and elevated (640 ppm—eCO2) for a total of 8 treatment combinations with 4 replicates each (+ P/+AM/aCO2; +P/+AM/eCO2; +P/−AM/aCO2; +P/−AM/eCO2; control/+AM/aCO2; control/+AM/eCO2; control/−AM/aCO2; control/−AM/eCO2; n = 32, Fig. SI 1a). Plants of Microlaena stipoides (Labill.) R.Br., a native Australian C3 grass with a broad P range, were grown from seed in 125 mm pots containing around 500 g of dry soil. Plants were grown in growth chambers for 12 weeks and soil water content was kept between 15 and 20% gravimetric content by addition of MilliQ water as needed every 2–3 days.
Soil characteristics and treatment preparation
Soil from 0 to 15 cm depth was collected from a Cumberland plain natural woodland in Western Sydney (33° 37′ 01″ S, 150° 44′ 26″ E, 20 m.a.s.l) from the EucFACE site. Soil at this site is an aeric podsol, slightly acidic (pH 5.38 ± 0.02 at 0–10 cm) (Ross et al. 2020), with a N content of 677 mg Kg− 1, low total C (1.8%, 0–15 cm), and P content (76.28 mg Kg− 1) (Hasegawa et al. 2016). Previous studies have demonstrated that both the vegetation (Crous et al. 2015) and soil fauna (Nielsen et al. 2015) are limited by P within this ecosystem. Soil was sieved (to 2 mm) and sterilised (gamma irradiated, 50 kGy) to remove viable AM fungal propagules. To reintroduce a homogenous microbial community to all pots, excluding AM fungi, we prepared a microbial inoculum. For this, approx. 3 Kg of freshly collected soil from a grassland nearby the EucFACE site was mixed with water in a 1:3 proportion by volume and the suspension passed through a 20 μm mesh sieve to remove AM fungal spores (Brundett and Australian Centre for International Agricultural Research 1995). Prior to potting, the filtrate was added to the sterile soil at a rate of 50 mL filtrate per Kg soil. The soil was incubated for a week at room temperature, mixing it daily.
The soil was then divided into four subsets based on whether they were to receive additional P and AM fungal inoculum. For the former, triple super phosphate (Richgro, super phosphate fertiliser supplement 9.1% P w/w) was added in a rate of 0.4 g/kg dry soil. The triple super phosphate powder was weighed, diluted and sprayed into the soil while low-P treatments received MilliQ water only. The soil was then thoroughly mixed and left to settle for a day. Next, AM fungi treatments were created by applying AM fungal inoculum in a 1:10 AM fungi inoculum:soil with non-mycorrhizal controls produced by autoclaving the live AM inoculum (121 °C, 2 h) and applying it to the soil in the same manner (See Supplementary Information for AM inoculum production). Once the four types of soils were prepared, soil was added to pots and five surface sterilised M. stipoides seeds (30% H2O2 for 10 min followed washing) were sown per pot. Pots were randomly split and placed in aCO2 and eCO2 chambers. After 2 weeks of growth, pots were thinned to one plant per pot. Unplanted pots (n = 16) with the different P and CO2 treatments (+ P/−AM/aCO2; +P/−AM/eCO2; control/−AM/aCO2; control/−AM/eCO2 n = 4 each), were included to estimate effects on SOM decomposition in the absence of plants and AM fungi (Fig. SI 1a). Unplanted pots were all prepared using soil with sterile AM inoculum and were kept under the same conditions as the planted pots for the duration of the experiment. The dried soil δ13C from these unplanted pots was measured and an averaged value of all unplanted pots was used for the isotopic partitioning. See “Respired, dissolved organic C (DOC) and microbial biomass isotopic composition and partitioning” section.
Growth chambers set up
The growth chambers (six in total, three per CO2 treatment) were modified using the approach of Cheng and Dijkstra (2007) to achieve a continuous 13C-labelling of plant tissues in both aCO2 and eCO2 treatments. The chambers were adapted to take an influx of naturally 13C-depleted CO2 (δ13C= − 31.7 ‰ ±1.2) delivered during the photoperiod, combined with a scrubbing system made of a soda lime-filled PVC tube (72 L) that allowed continuous supply of CO2-free air (Fig. SI 1b). Chambers were adjusted to a 16 h/8 h photoperiod with 25 °C/18 °C, 60% relative humidity, and light intensity of 900 µmol/m2s1. The chamber atmosphere was sampled frequently to confirm depletion in 13C. Air samples from the chambers were extracted via a pump system into a gas tight tedlar bag (Tedlar® SCV Gas Sampling Bag) and analysed for δ13C in a PICARRO G2201i isotopic CO2/CH4 analyser (Picarro Inc., Santa Clara, CA, USA). Pots were randomly moved among growth chambers with the same CO2 condition to prevent “chamber effects”. Moreover, to avoid plant-uptake of 13C from outside the chambers, watering and all other manipulations were performed during the night period aided by green light.
Harvest and sample processing
Gas sampling of the plant-soil system
After 12 weeks of growth, we quantified rates of total respired CO2 (R) and its C isotopic composition as described by Carrillo et al. (2014, 2015). Briefly, pots were placed on an elevated platform inside a water filled tray and covered with a PVC chamber (45 cm H x 15 cm D) adapted with an air-tight rubber stopper for air sampling. Free-CO2 air was circulated for 2 h using an aquarium pump connected to a CO2 scrubber (50 cm H x 4 cm D PVC tubing filled with soda lime). After 2 h of scrubbing, an air sample was taken to determine baseline CO2 concentrations using a 7890 A gas chromatograph with a G1888 network headspace sampler (Agilent Technologies, USA). Later, the CO2 scrubbers were removed and the pump reconnected to the PVC chamber to allow for air circulation while pots were incubated. After 2 to 3 h of incubation, a gas sample was collected in an airtight gas collection bag (Tedlar® PVDF, 1 L) using an aquarium pump system and analysed for its C isotopic composition.
Plant and soil harvest
One day after gas sampling, pots were destructively harvested. Aboveground biomass was cut and roots separated from the soil and washed. Plant aboveground biomass, roots and a subsample of fresh soil were oven dried at 60 °C for measurements of dry plant biomass, soil gravimetric water content and total nutrients. A fresh soil subsample was stored at − 20 °C for microbial community analyses and the remaining fresh soil was used for assessments of dissolved nutrients and microbial biomass.
Plant, soil nutrients and microbial biomass C and N
Two sub-samples of soil were weighed. Dissolved nutrients in soil were extracted from the first subsample with a 0.05 M K2SO4 solution in a 4:1 solution to soil ratio, shaking at 180 rpm for an hour. Samples were filtered through a Whatman # 42 filter paper and frozen (− 20 °C) until analyses. The second subsample was fumigated for 5 days with chloroform and then nutrients were extracted as for unfumigated samples. The fumigated and unfumigated extracts were analysed for total dissolved organic C and N (Shimadzu® TN, TOC-L, Japan) and microbial biomass C and N calculated by subtracting fumigated and unfumigated samples(Vance et al. 1987). The volume left of these K2SO4 extractions was used to obtain the isotopic composition of DOC and microbial biomass (see section below). Phosphates were extracted following the Bray 1-P method for acidic soils, in a 1:7 solution:soil ratio using a 0.03 M NH4F solution in 0.025 M hydrochloride (HCl) adjusted pH to 2.6 ± 0.05 with HCl (Rayment et al. 2010). Samples were manually shaken for 60 s and immediately poured over a Whatman # 42 filter paper. Collected extracts were analysed by colorimetry (AQ2 Discrete Analyser, SEAL Analytical, Mequon, WI, USA).
Total P and N were determined from ground oven-dried soil and root samples. Total P concentration was analysed by an X-ray fluorescence spectrometer (PANalytical, εpsilon 3. 10Kv, 0.9 mA. Lelyweg, Almelo, Netherlands) while total C and N from soil, roots and soil extracts were analysed along their isotopic composition.
Respired, dissolved organic C (DOC) and microbial biomass isotopic composition and partitioning
Gas samples from plant-soil system incubations were analysed in a PICARRO analyser (G2201i; Picarro, Santa Clara, CA, USA. Precision values below 0.16‰) for the δ13C and CO2 concentration of the total respired CO2 (R). For the isotopic composition of the DOC and microbial biomass C (MBC), fumigated (f) and unfumigated (uf) soil K2SO4 extracts were oven dried at 60oC. The dried extracts were scraped and weighed for analysis on a Thermo GC-C-IRMS system (Trace GC Ultra gas chromatograph, Thermo Electron Corp., Milan, Italy; coupled to a Delta V Advantage isotope ratio mass spectrometer through a GC/C-III); University of California, Davis Campus, USA). The δ13C of unfumigated soil extracts was used as the isotopic composition of the DOC while δ13C of MBC (δ13CMBC) were calculated from δ13C values of both fumigated (f) and unfumigated (uf) extractions (See Supplementary Information for calculations of the isotopic composition of the microbial biomass). Samples of dried soil were also analysed on the Thermo GC-C-IRMS system for C, N and δ13C.
To calculate the fractions of SOM-derived C in the total respired CO2, DOC and MBC, we used isotopic partitioning applying the formula: SOM.CR,DOC,MBC = (δ13CR,DOC,MBC − δ13Cp)/(δ13Ccontrol − δ13Cp). Where δ13CR,DOC,MBC were the δ13C of either the CO2 measured in the total respired CO2 (R), dissolved organic C (DOC) or microbial biomass C (MBC)) from each pot; δ13Cp is the isotopic ratio of the plant source averaged across P treatments per CO2 condition (root biomass at aCO2 δ13C= − 40.01 ± 0.08 and eCO2 δ13C= − 43.20 ± 0.04) and δ13Ccontrol is the average δ13C of the native SOM source obtained from the dried bulk soil from unplanted pots across CO2 and P conditions (δ13C= − 25.34 ± 0.02). The fractions of plant-derived C were obtained by subtracting SOM-derived fractions from the unit. Measured total respired CO2, DOC and MBC were partitioned into SOM-derived and plant-derived, using these fractions to obtain the mass of SOM derived R, MBC and DOC. In this study, we focus only on the SOM-derived C components.
Microbial community analysis: Phospholipid-derived fatty acids (PLFA) and neutral lipid-derived fatty acids (NLFA)
Soil PLFAs were extracted to assess the overall microbial communities while the 16:1ω5c NLFA was used as an indicator of arbuscular mycorrhizal (AM) fungi presence and abundance (Olsson 1999). Freeze-dried soil from planted pots (n = 32) were extracted following the protocol by Buyer and Sasser (2012) with modifications by Castañeda-Gómez et al. (2020) (See Supplementary Information for PLFA and NLFA analyses). Functional groups were defined as shown in Table SI 1. Fungal to bacterial ratio (F:B) was calculated by dividing fungal PLFAs (not including AM fungi) by the sum of bacterial PLFAs. The sum of individual lipids was used as an indicator of the size of the community (µg PLFA g− 1 dry soil).
Statistical analyses
The effect of CO2 condition, AM fungi treatment and P addition and their interactions on the response variables was analysed with a linear model fitted with the function “lm” from the stats package in R version 3.3.2 (R Core Team 2019). This approach was used instead of a mixed effects modelling approach since pots were moved among chambers with the same CO2 treatment so it was not possible to estimate a random effect associated with each chamber. The normality of the residuals of each model was inspected to check the appropriateness of the fit and transformations were performed when needed. Statistical significance was determined performing an ANOVA (Analyses of variance) with the “Anova” function (“car” package, Fox et al. 2021). Multiple mean comparisons were performed using the Tukey test using the “glht” function (“multcomp” package, Hothorn et al. 2021). As soil moisture can affect soil respiration measurements, we tested for correlations between soil moisture and the total respired CO2, SOM-derived respired CO2 fraction and total mass of SOM-derived respired CO2. For response variables with a significant correlation with moisture, a 3-way ANCOVA was performed with soil moisture as covariate, to account for the variability brought by the slightly different moisture contents at the time of CO2 sampling. The homogeneity of the regression slopes, normality of residuals and homogeneity of variances was tested when performing the ANCOVA. Significance levels were: ≤ 0.1 (.), ≤ 0.05 (*), ≤0.01 (**), ≤ 0.001 (***).
Microbial communities were analysed as the PLFA-based total microbial biomass (sum of all individual lipids, in µg PLFA g− 1 dry soil), as absolute microbial abundance (reflecting the size or biomass of the community, in µg PLFA g− 1 dry soil) and as relative abundance (in percentage, reflecting microbial community composition) of the different microbial groups and as individual lipids. To visualise significant three-way interactions of experimental factors in the abundance of microbial groups from the ANOVA, the “emmip” function from the “emmeans” package (Lenth et al. 2020) was used to show the estimated marginal means from the fitted linear model.
Results
Influence of elevated CO2, P availability and AM fungi on SOM decomposition and soil C cycle components
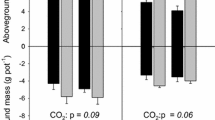
Higher SOM-C losses under eCO2 were expected for control P conditions (low-P), particularly when AM fungi were inoculated (Fig. 1a). However, we found that in general, there was not a significant interactive impact of the three experimental factors on the soil C cycle, represented in the measured SOM-derived fractions of respired CO2, MBC and DOC, as initially expected. Instead, we found that the interaction of AM and P treatments determined the fate of the soil C cycle components, regardless of eCO2. The total respired CO2 (Fig. 2) was higher for control P conditions but only for uninoculated AM pots (−AM. P < 0.05, Tukey’s multiple comparison). However, we did not find any significant effects of CO2 conditions, AM or P treatments on the SOM-derived R (Fig. 2). On the other hand, most of the MBC was derived from SOM (above 80%, Table SI 2) and while neither the total MBC nor the SOM-derived MBC (Fig. 2) were affected by the experimental factors, the C sources used by the MBC were affected by the interaction between AM and P treatments (Table SI 2). The fraction of MBC derived from SOM was marginally lower by 6% for control P and − AM pots (P = 0.1, Tukey’s multiple comparison. Table SI 2), further decreasing for + AM pots. Similar to MBC, most of the DOC was derived from SOM (above 80%, Table SI 2) and both the total DOC and SOM-derived DOC were affected by the interaction between AM and P treatments (Fig. 2) with significantly lower SOM-derived DOC for control P and − AM pots (P = 0.001, Tukey’s multiple comparison) and further decreasing for + AM pots. Finally, although not evidenced in the total or SOM-derived R and DOC, the fractions of SOM-derived R and DOC significantly increased with eCO2 (Table SI 2).
Mean values (± standard error, n = 4) of the total (white) and SOM-derived (grey) respired CO2, microbial biomass C (MBC) and dissolved organic C (DOC) per CO2 condition, AM fungi (with (+ AM) and without (−AM) mycorrhizal fungi) and P addition treatment (C: control and + P: P addition). CO2 conditions are shown on light grey (elevated) and white (ambient) backgrounds. F(1,24) values of ANCOVAs (for respired CO2) and ANOVAs from linear model for response variables displayed on the right. Significance levels: ≤ 0.1 (.), ≤ 0.05 (*), ≤0.01 (**), ≤ 0.001 (***). Only significant interactions of the treatments displayed
Soil nutrient responses to elevated CO2, P availability and AM fungi
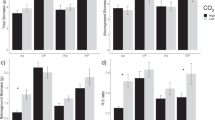
We expected to see decreases in soil dissolved P and N under eCO2, control P and AM fungi presence due to higher nutrient acquisition and immobilisation by the fungal biomass. As hypothesised, dissolved P significantly decreased with eCO2, particularly under control P and + AM conditions (Interactive effects of CO2 by P and CO2 by AM. Table 1). Congruently, we observed significantly higher dissolved C:P in eCO2 conditions for control P and for + AM pots (Fig. 3). Similarly, Dissolved N decreased with AM fungi presence and marginally decreased under eCO2 conditions in + AM pots (Table 1). Thus, higher dissolved C:N observed for control P conditions for AM pots while higher dissolved C:N was detected for AM pots under eCO2 (Fig. 3). Finally, AM fungi significantly decreased total P (−AM: 175.2 ± 11.1 ppm; +AM: 206.8 ± 15.1 ppm. AM_F(1,24) = 5.41, AM_P = 0.03) while higher total soil P was observed as a result of P addition (control: 155.1 ± 8.8 ppm; +P: 226.9 ± 11.7 ppm. P_F(1,24) = 28.01, P_P < 0.00) although soil dissolved P did not increase with P addition (Table 1).
Mean values (± standard error, n = 4) of mass nutrient ratios (columns) in plant and soil solution (dissolved) per treatment. Elevated (eCO2) conditions shown as grey areas and P treatment as white (control) or black (+ P: P addition) circles. +AM (with) and − AM (without) mycorrhizal fungi. F(1,24) values of ANOVAs from linear model for response variables displayed. Significance levels: ≤ 0.1 (.), ≤ 0.05 (*), ≤0.01 (**), ≤ 0.001 (***); only significant interactions of the treatments displayed. Treatments with different letters are significantly different (p ≤ 0.05, Tukey multiple comparison test)
Plant responses to elevated CO2, P availability and AM fungi
We expected that plant biomass would increase with eCO2 and low P conditions in AM fungi presence, thanks to the role of this mycorrhizal fungi in SOM decomposition and nutrient acquisition (Fig. 1). We detected significant increases in root biomass under eCO2 and AM fungi presence, but this effect was independent of P conditions (Table 2). In low P and eCO2 conditions, higher nutrient resorption and immobilization would lead to decreases in plant C-to-nutrient ratios but we did not find a significant impacts of eCO2 on C-to-nutrient ratios and thus no clear evidence for increased P immobilisation under eCO2 was found. However, we detected significant decreases in plant N with eCO2 for AM treatments (Table 2. P = 0.05, Tukey’s multiple comparison). Increases in plant biomass with P addition were not observed either, but we detected increases in plant P concentration as well as the plant P pool in both shoot and roots with P addition (Table 2). As a consequence, plant C:P and N:P significantly decreased with P addition (Fig. 3).
Microbial communities including AM
AM fungi presence and abundance was assessed with the 16:1ω5c neutral lipid. As expected, we detected higher AM fungi presence in + AM pots under control P conditions and a significant decrease in AM fungal neutral lipid with P addition to a level approaching the uninoculated pots (AMxP: F(1,24) = 6.28, P < 0.001 Table SI 2). We assessed the biomass and composition of the microbial community as we expected that AM fungi would alter soil saprotrophic communities as a mechanisms to enhance SOM decomposition under low-P conditions. However, we only observed a significant impact of CO2 conditions on the absolute and relative microbial community abundance (Table SI 3) driven by marginally significant increases in Gram negative bacteria and significant increases in Fungi and Protozoa (Table SI 4).
Discussion
Our experiment investigated whether under low-P conditions, increased SOM decomposition and organic P cycling aided by arbuscular mycorrhizal fungi would allow for a sustained plant fertilisation effect with eCO2. We expected to observe increases in plant biomass under eCO2 conditions and low-P availability paired with higher soil C cycling, represented in the measured SOM-derived fractions of relevant C cycle components (R, respired CO2; MBC, microbial biomass C and DOC, dissolved organic C). Further enhancements in soil C cycling were expected with AM fungi inoculation due to the role of this symbiotic fungi on P acquisition and SOM decomposition, expected to increase further under eCO2 and low-P conditions. We did not find strong evidence suggesting that increases in soil C cycling with low-P availability and AM fungi presence supported plant growth under eCO2. Instead, we found that the impacts of the experimental factors were generally independent of each other. Contrary to our hypotheses (Fig. 1), the detected increases in root biomass with eCO2 conditions and AM presence along with significant decreases in soil dissolved nutrients occurred without negative impacts on soil C cycling (Fig. 4 A). This suggests that AM fungi presence was supporting the eCO2 fertilization effect and facilitating soil nutrient uptake without enhancing SOM decomposition. On the other hand, we observed significantly lower SOM-derived MBC and DOC in low-P and − AM fungi conditions, further decreasing with AM inoculation (Fig. 4). This suggests that low-P conditions were limiting soil C cycling and that AM fungi were protecting SOM against decomposition from saprotrophic communities likely via a more efficient nutrient uptake and immobilization by AM fungi. Finally, soil C cycling (SOM-derived R and DOC) and microbial biomass increased with eCO2 mainly due to the response of Gram negative bacteria, fungi and protozoa (Fig. 4), which points to an increase in microbial-derived SOM decomposition with eCO2. Taken together, our findings in this model plant-soil system demonstrate that C and P biogeochemical cycles may not become strongly coupled to sustain an eCO2 fertilisation effect under low-P conditions and that AM fungi might not support increases in SOM decomposition to sustain positive plant responses to eCO2. Instead, our observations highlight the role of AM fungi protecting the SOM pool against decomposition in eCO2 conditions and facilitating nutrient acquisition without negative impacts on the soil C pool.
Observed responses of relevant measured variables. with the impact of (A) eCO2 alone and when interacting with P and AM fungi treatments. (B) P availability alone on plant variables (green boxes) and (C) AM fungi treatments interacting with P availability on soil organic matter-derived (SOMd) C components: R, respired CO2; MB, microbial biomass; DOC, dissolved organic C. Left side control P, right side + P. Green arrows: increase; red arrows: decrease; equal sign (=): no effect. Box sizes represent the relative size of each pool. The thickness of the arrows from SOM and mineral bound P pools represent the relative flux of P from these pools to plans. The size of the circles on top of each arrow represent the relative role of AM fungi on P acquisition and SOM decomposition from these pools
The CO2 fertilization effect is modulated by the interaction of mycorrhizal associations and nutrient availability (Treseder 2004; Terrer et al. 2016, 2019). Low-P conditions can significantly limit positive responses of root biomass to eCO2 (Jiang et al. 2020), particularly in AM-associated plants (Terrer et al. 2021). In this study however, the observed increase in root biomass under eCO2 conditions occurred when AM fungi were present, regardless of P availability (Table 2; Fig. 4A). A recent study by Frew et al. (2021) found that contrary to our observations, root biomass of Microlaena stipoides did not increase under eCO2 and AM fungi presence. Yet, soils in this experiment were relatively high in P (254.75 mg Kg− 1 P) whereas our soils were low in P (approx. 76.28 mg Kg− 1 P). Despite the lack of significant impacts of P availability in our study, the differences observed on the impact of AM fungi on root growth under eCO2 conditions between this and the study by Frew et al. (2021) suggests that the role of AM fungi on the eCO2 fertilization effect might indeed be dependent on P availability but that the P levels used in this experiment were possibly not high enough to detect this impact. Increases in plant root biomass and root exudation have been linked to enhanced nutrient uptake due to higher plant nutrient demands under eCO2 (Iversen 2010; Dong et al. 2021) and as a response to nutrient deficiency (Shipley and Meziane 2002). In our study, AM fungi were likely facilitating positive root biomass responses to eCO2 as well as enhanced nutrient uptake as supported by the significant decreases in soil dissolved N and P with AM presence under eCO2 conditions (Table 1; Fig. 4A). Lower dissolved nutrients in soil for + AM and eCO2 without any changes on the measured SOM-derived C pools nor altered soil microbial communities, suggests that nutrient uptake by AM fungi in this plant-soil system was not linked with enhanced SOM decomposition. Finally, AM fungi also marginally decreased plant N contents in the aboveground biomass with eCO2 (Table 2; Fig. 4A). This might indicate that the obtained soil dissolved nutrients by this symbiotic fungi are not necessarily being transferred to the aboveground plant biomass. AM fungi can potentially become parasitic and reduce the symbiotic benefits for their plant host if the nutrient exchange efficiency is low, particularly that of P (Cotton 2018) as it might have been the case in our study. Alternatively, greater root biomass growth than the gains in nutrient concentration can be also driving the observed lower plant N concentrations (Dong et al. 2018).
We hypothesised that higher soil C cycling evidenced as increase SOM-derived fractions of soil C components (R, MBC, DOC) would occur with low-P availability as organic P cycling is crucial when mineral P sources are depleted (Reed et al. 2011). We were also expecting that AM fungi would significantly increase SOM decomposition under low-P conditions given their role in both enhanced P acquisition and SOM decomposition (Fig. 1). Studies have shown that AM fungi can increase SOM decomposition under elevated CO2 (Cheng et al. 2012; Kowalchuk 2012; Carrillo et al. 2015) while others show that AM fungi enhance nutrient acquisition from organic substrates, particularly under low-P availability or poor nutrient conditions (Xu et al. 2018; Bunn et al. 2019). But contrary to our expectations, we found that under low-P conditions, SOM decomposition decreased as indicated by significant lower SOM-derived MBC and DOC and that soil C cycling decreased even further with AM fungi presence, regardless of eCO2 (Figs. 2 and 4). This results demonstrate that low-P conditions as well as AM fungi presence were limiting SOM decomposition. Limitation of SOM decomposition in low-P conditions is further supported by the observed increase in soil C cycling with P addition, which might have occurred thanks to the release of saprotrophic communities from P-limiting conditions (Cleveland et al. 2006; Mori et al. 2018). The observed lack of significant increases in SOM decomposition with AM fungi presence might be related to enhanced competition between AM fungi and saprotrophic communities over P and nutrient immobilisation in the fungal biomass with the consequent limitation of saprotrophs and their impact on soil C cycling in this plant-soil system (Zhou et al. 2019). This mechanism for SOM protection against decomposition has been attributed to AM fungi (Frey 2019) and the decreases in soil dissolved nutrients with AM fungi presence under eCO2 further support the idea that these symbiotic fungi might outcompete saprotrophic microbes in nutrient uptake.
Several mechanisms have been proposed to explain AM fungi contribution to soil C cycling and how this impact will shift with climate change factors such as eCO2 Talbot et al. 2008; Wei et al. 2019; Frey 2019; Parihar et al. 2020). Yet, multifactorial experiments assessing how nutrient availability, specifically P, impact AM fungi-mediated SOM decomposition under eCO2 are scarce. We aimed to capture tripartite interactions between P availability, eCO2 conditions and AM fungi presence, however we could not detect significant simultaneous effects of these experimental factors on the measured variables. First, the observed increases in root biomass under eCO2 and AM fungi presence occurred independently of P availability and without impacts to the SOM pool. Second, decreases in soil C cycling for low-P conditions and AM fungi presence occurred independently of eCO2 conditions (Fig. 4). The lack of significant interactive impacts of P availability on root biomass responses with AM fungi presence and eCO2 might be related with the chosen methodology for P addition. In this study, only one addition of P as triple superphosphate was done at the start of this experiment. Triple superphosphate is highly soluble in water and becomes rapidly available for plant uptake (Mullins et al. 1995) by the initial weeks of application (Ghosal and Chakraborty 2012). Moreover, plant P luxury consumption, where higher P immobilisation is not necessarily related to increases in growth (Brar and Tolleson 1975) has been observed for Microlaena stipoides (Robinson et al. 1993; Nie et al. 2009), which explains the observed higher P concentrations in plants with P addition without increases in biomass under any CO2 or AM fungi treatment (Fig. 4). Furthermore, the lack of impacts of AM fungi on the SOM pool under eCO2 conditions, despite enhanced nutrient uptake and positive impacts on root biomass, could potentially be related with the preferential C allocation of this C3 plant species to increase root biomass rather than to sustain a mutualistic relationship with AM fungi (Frey 2019). Thus, the lack of a more efficient funnelling of labile C to saprotrophic communities by AM fungi likely restricted an impact on the SOM pool in these conditions. Higher DOC is usually used as evidence for increased C allocation belowground and higher rhizodeposition with eCO2 (Lukac et al. 2003; Freeman et al. 2004; Drake et al. 2011; Phillips et al. 2011) but it can also be the result of higher SOM decomposition (Hagedorn et al. 2002, 2008). Supporting the idea that not enough photosynthate was reaching soil microbial communities, our isotopic analyses allowed us to detect that the observed increases in DOC with eCO2 were the result of enhanced SOM decomposition given the observed increases in SOM-derived DOC fraction (Table SI 2), rather than the result of higher C allocation to belowground microbial communities. An inefficient C transfer to AM fungi and saprotophs under low-P conditions might also explain the lack of a significant impact of eCO2 on soil C cycling in low-P + AM fungi pots. Although this well-controlled experiment was able to provide mechanistic insights into potential effects of P availability on plant and microbe responses to eCO2, it was a short-term pot study with a single grass species and relatively low replication number. Longer-term investigations using a variety of species and higher replication at the pot level to enhance statistical power are needed to more fully assess potential future responses of P-limited ecosystems to eCO2. Moreover, impacts of P availability and P manipulations are likely to be more relevant if applied at continuous rates in the form of a nutrient solution rather than as a single application.
Current understanding of the nutrient dependency of the impacts of eCO2 on plant productivity has focused on N-limited ecosystems. Higher SOM decomposition under eCO2 when N availability is low occurs as a mechanism to sustain nutrient supply and plant growth, with mycorrhizal fungi aiding to deliver the mined nutrients to the plants. For P-limited ecosystems however, low-P availability generally constrains ecosystem responses to CO2 enrichment (Reich et al. 2006; Reed et al. 2015; Ellsworth et al. 2017; Jiang et al. 2020) and the role of AM fungi mediating plant and soil C responses to eCO2 with low-P availability is not fully clear. This pot study was carried out with natural soils and a grass species growing in a P-limited ecosystem where the EucFACE site is located, and thus it serves as a model system that allows us to infer possible responses at the ecosystem level. Previous studies at the EucFACE site have shown that higher soil C losses at this site are not driven by the impact of eCO2 on soil microbial communities but are rather reliant on seasonal increases in water and nutrient availability (Castañeda-Gómez et al. 2020, 2021). Our observations from this soil-plant system also suggest that the low-P conditions in this Australian native woodland might enhance competition over nutrients with AM fungi that outcompete saprotrophic communities over these limited resources, leading to an overall decrease in SOM decomposition and limitation the eCO2 fertilization effect unless a more efficient transfer of photosynthate can be allocated to the mycorrhizal symbionts which may potentially allow for enhanced AM fungi mediated soil C cycling.
In this experiment we show that the impacts of CO2 enrichment and P availability on plant growth and soil C cycling were independent of each other and are not likely to become more coupled with eCO2 conditions. We also demonstrate that AM fungi presence is contributing to SOM protection, likely via competition over nutrients with saprotrophic communities or maybe due to a reduced transfer of C from the plant host to AM fungi and therefore, to the saprotrophic communities. Our findings highlight that ecosystem responses to eCO2 with P limitation differ from those reported for N-limited systems in that low-P conditions do not necessarily lead to higher SOM decomposition as a mechanism to sustain plant growth as usually observed for N-limited systems. Thus, inferences of the behaviour of P-limited ecosystems based on current knowledge about N-limited ecosystems are not ideal. In this way, our results also contribute to the current gap in knowledge regarding the impacts of soil C cycling with low-P availability exposed to eCO2 conditions.
Data availability
Data available at Figshare: https://doi.org/10.6084/m9.figshare.19964411.v1.
Code availability
Sample R code is included in the supplementary material.
References
Ainsworth EA, Long SP (2004) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2: Tansley review. New Phytol 165:351–372. https://doi.org/10.1111/j.1469-8137.2004.01224.x
Brar GS, Tolleson EL (1975) The luxury uptake phenomenon for removal of phosphates from municipal wastewater. Water Res 9:71–77. https://doi.org/10.1016/0043-1354(75)90154-2
Brundett M (1995) Mycorrhizas for plantation forestry in Asia : proceedings of an international symposium and workshop, Kaiping, Guangdong Province, P.R. China, 7–11 November, 1994/editors: M. Brundett ... [et al.]. Australian Centre for International Agricultural Research, Canberra
Bunn RA, Simpson DT, Bullington LS et al (2019) Revisiting the ‘direct mineral cycling’ hypothesis: arbuscular mycorrhizal fungi colonize leaf litter, but why? ISME J 13:1891–1898. https://doi.org/10.1038/s41396-019-0403-2
Buyer JS, Sasser M (2012) High throughput phospholipid fatty acid analysis of soils. Appl Soil Ecol 61:127–130. https://doi.org/10.1016/j.apsoil.2012.06.005
Canadell JG, Pitelka LF, Ingram JSI (1995) The effects of elevated [CO2] on plant-soil carbon below-ground: a summary and synthesis. Plant Soil 187:391–400. https://doi.org/10.1007/BF00017102
Carrillo Y, Dijkstra FA, Pendall E et al (2014) Plant rhizosphere influence on microbial C metabolism: the role of elevated CO2, N availability and root stoichiometry. Biogeochemistry 117:229–240. https://doi.org/10.1007/s10533-014-9954-5
Carrillo Y, Dijkstra FA, LeCain D, Pendall E (2015) Mediation of soil C decomposition by arbuscular mycorrizhal fungi in grass rhizospheres under elevated CO2. Biogeochemistry. https://doi.org/10.1007/s10533-015-0159-3
Castañeda-Gómez L, Walker JKM, Powell JR et al (2020) Impacts of elevated carbon dioxide on carbon gains and losses from soil and associated microbes in a Eucalyptus woodland. Soil Biol Biochem 143:107734. https://doi.org/10.1016/j.soilbio.2020.107734
Castañeda-Gómez L, Powell JR, Ellsworth DS et al (2021) The influence of roots on mycorrhizal fungi, saprotrophic microbes and carbon dynamics in a low-phosphorus Eucalyptus forest under elevated CO2. Funct Ecol. https://doi.org/10.1111/1365-2435.13832
Cavagnaro TR, Gleadow RM, Miller RE (2011) Plant nutrient acquisition and utilisation in a high carbon dioxide world. Funct Plant Biol 38:87–96. https://doi.org/10.1071/FP10124
Cheng W, Dijkstra FA (2007) Theoretical proof and empirical confirmation of a continuous labeling method using naturally 13 C-depleted carbon dioxide. J Integr Plant Biol 49:401–407
Cheng L, Booker FL, Tu C et al (2012) Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337:1084–1087. https://doi.org/10.1126/science.1224304
Clark CEF, Mitchell ML, Islam MR, Jacobs B (2014) Phosphorus content of the soil influences the growth and productivity of Themeda triandra Forssk. and Microlaena stipoides (Labill.) R.Br. Rangel J 36:233–237. https://doi.org/10.1071/RJ13108
Cleveland CC, Reed SC, Townsend AR (2006) Nutrient regulation of organic matter decomposition in a tropical rain forest. Ecology 87:492–503. https://doi.org/10.1890/05-0525
Conroy JP, Milham PJ, Barlow EWR (1992) Effect of nitrogen and phosphorus availability on the growth response of Eucalyptus grandis to high CO2. Plant Cell Environ 15:843–847. https://doi.org/10.1111/j.1365-3040.1992.tb02152.x
Cotrufo MF, Gorissen A (1997) Elevated CO2enhances below-ground C allocation in three perennial grass species at different levels of N availability. New Phytol 137:421–431. https://doi.org/10.1046/j.1469-8137.1997.00839.x
Cotton TA (2018) Arbuscular mycorrhizal fungal communities and global change: an uncertain future. FEMS Microbiol Ecol 94:fiy179. https://doi.org/10.1093/femsec/fiy179
Crous KY, Ósvaldsson A, Ellsworth DS (2015) Is phosphorus limiting in a mature Eucalyptus woodland? Phosphorus fertilisation stimulates stem growth. Plant Soil 391:293–305. https://doi.org/10.1007/s11104-015-2426-4
De Graaff M-A, Groenigen K-JV, Six J et al (2006) Interactions between plant growth and soil nutrient cycling under elevated CO2: a meta-analysis. Glob Change Biol 12:2077–2091. https://doi.org/10.1111/j.1365-2486.2006.01240.x
Delucia EH, Callaway RM, Thomas EM, Schlesinger WH (1997) Mechanisms of phosphorus acquisition for ponderosa pine seedlings under high CO2 and temperature. Ann Bot 79:111–120. https://doi.org/10.1006/anbo.1996.0320
Deng Q, Hui D, Luo Y et al (2015) Down-regulation of tissue N:P ratios in terrestrial plants by elevated CO2. Ecology 96:3354–3362. https://doi.org/10.1890/15-0217.1
Dieleman WIJ, Luyssaert S, Rey A et al (2010) Soil [N] modulates soil C cycling in CO2-fumigated tree stands: a meta-analysis. Plant Cell Environ 33:2001–2011. https://doi.org/10.1111/j.1365-3040.2010.02201.x
Dijkstra FA, Pendall E, Morgan JA et al (2012) Climate change alters stoichiometry of phosphorus and nitrogen in a semiarid grassland. New Phytol 196:807–815. https://doi.org/10.1111/j.1469-8137.2012.04349.x
Dijkstra FA, Carrillo Y, Pendall E, Morgan JA (2013) Rhizosphere priming: a nutrient perspective. Front Microbiol. https://doi.org/10.3389/fmicb.2013.00216
Dong Y, Wang Z, Sun H et al (2018) The response patterns of arbuscular mycorrhizal and ectomycorrhizal symbionts under elevated CO2: a meta-analysis. Front Microbiol 9:1248. https://doi.org/10.3389/fmicb.2018.01248
Dong J, Hunt J, Delhaize E et al (2021) Impacts of elevated CO2 on plant resistance to nutrient deficiency and toxic ions via root exudates: a review. Sci Total Environ 754:142434. https://doi.org/10.1016/j.scitotenv.2020.142434
Drake JE, Gallet-Budynek A, Hofmockel KS et al (2011) Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol Lett 14:349–357. https://doi.org/10.1111/j.1461-0248.2011.01593.x
Drake JE, Macdonald CA, Tjoelker MG et al (2016) Short-term carbon cycling responses of a mature eucalypt woodland to gradual stepwise enrichment of atmospheric CO2 concentration. Glob Change Biol 22:380–390. https://doi.org/10.1111/gcb.13109
Ellsworth DS, Anderson IC, Crous KY et al (2017) Elevated CO2does not increase eucalypt forest productivity on a low-phosphorus soil. Nat Clim Change 7:279–282. https://doi.org/10.1038/nclimate3235
Fang X-M, Zhang X-L, Chen F-S et al (2019) Phosphorus addition alters the response of soil organic carbon decomposition to nitrogen deposition in a subtropical forest. Soil Biol Biochem 133:119–128. https://doi.org/10.1016/j.soilbio.2019.03.005
Feng J, Zhu B (2019) A global meta-analysis of soil respiration and its components in response to phosphorus addition. Soil Biol Biochem 135:38–47. https://doi.org/10.1016/j.soilbio.2019.04.008
Finzi AC, Sinsabaugh RL, Long TM, Osgood MP (2006) Microbial community responses to atmospheric carbon dioxide enrichment in a warm-temperate forest. Ecosystems 9:215–226. https://doi.org/10.1007/s10021-005-0078-6
Finzi AC, Austin AT, Cleland EE et al (2011) Responses and feedbacks of coupled biogeochemical cycles to climate change: examples from terrestrial ecosystems. Front Ecol Environ 9:61–67. https://doi.org/10.1890/100001
Fox TR, Miller BW, Rubilar R et al (2011) Phosphorus nutrition of forest plantations: the role of inorganic and organic phosphorus. In: Bünemann E, Oberson A, Frossard E et al (eds) Phosphorus in action: biological processes in soil phosphorus cycling. Springer, Berlin, Heidelberg, pp 317–338
Fox J, Weisberg S, Price B et al (2021) Car: companion to applied regression. Version 3.0-11. https://CRAN.R-project.org/package=car
Fransson PMA, Johansson EM (2010) Elevated CO2 and nitrogen influence exudation of soluble organic compounds by ectomycorrhizal root systems. FEMS Microbiol Ecol 71:186–196. https://doi.org/10.1111/j.1574-6941.2009.00795.x
Freeman C, Fenner N, Ostle NJ et al (2004) Export of dissolved organic carbon from peatlands under elevated carbon dioxide levels. Nature 430:195–198. https://doi.org/10.1038/nature02707
Frew A, Price JN, Oja J et al (2021) Impacts of elevated atmospheric CO2 on arbuscular mycorrhizal fungi and their role in moderating plant allometric partitioning. Mycorrhiza 31:423–430. https://doi.org/10.1007/s00572-021-01025-6
Frey SD (2019) Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu Rev Ecol Evol Syst 50:237–259. https://doi.org/10.1146/annurev-ecolsys-110617-062331
Ghosal P, Chakraborty T (2012) Comparative solubility study of four phosphatic fertilizers in different solvents and the effect of soil. Resour Environ 2:175–179. https://doi.org/10.5923/j.re.20120204.07
Gifford RM, Barrett DJ, Lutze JL (2000) The effects of elevated [CO2] on the C:N and C:P mass ratios of plant tissues. Plant Soil 224:1–14. https://doi.org/10.1023/A:1004790612630
Goll DS, Brovkin V, Parida BR et al (2012) Nutrient limitation reduces land carbon uptake in simulations with a model of combined carbon, nitrogen and phosphorus cycling. Biogeosciences 9:3547–3569. https://doi.org/10.5194/bg-9-3547-2012
Hagedorn F, Blaser P, Siegwolf R (2002) Elevated atmospheric CO2 and increased N deposition effects on dissolved organic carbon—clues from δ13C signature. Soil Biol Biochem 34:355–366. https://doi.org/10.1016/S0038-0717(01)00191-2
Hagedorn F, van Hees PAW, Handa IT, Hättenschwiler S (2008) Elevated atmospheric CO2 fuels leaching of old dissolved organic matter at the alpine treeline. Glob Biogeochem Cycles. https://doi.org/10.1029/2007GB003026
Hasegawa S, Macdonald CA, Power SA (2016) Elevated carbon dioxide increases soil nitrogen and phosphorus availability in a phosphorus-limited Eucalyptus woodland. Glob Change Biol 22:1628–1643
Hasegawa S, Piñeiro J, Ochoa-Hueso R et al (2018) Elevated CO2 concentrations reduce C4 cover and decrease diversity of understorey plant community in a Eucalyptus woodland. J Ecol. https://doi.org/10.1111/1365-2745.12943
Hill JO, Simpson RJ, Ryan MH, Chapman DF (2010) Root hair morphology and mycorrhizal colonisation of pasture species in response to phosphorus and nitrogen nutrition. Crop Pasture Sci 61:122. https://doi.org/10.1071/CP09217
Högy P, Keck M, Niehaus K et al (2010) Effects of atmospheric CO2 enrichment on biomass, yield and low molecular weight metabolites in wheat grain. J Cereal Sci 52:215–220. https://doi.org/10.1016/j.jcs.2010.05.009
Hoosbeek MR, Lukac M, van Dam D et al (2004) More new carbon in the mineral soil of a poplar plantation under Free Air Carbon Enrichment (POPFACE): cause of increased priming effect? Glob Biogeochem Cycles. https://doi.org/10.1029/2003GB002127
Hothorn T, Bretz F, Westfall P et al (2021) Multcomp: simultaneous inference in general parametric models. Version 1.4-17. https://CRAN.R-project.org/package=multcomp
Hungate BA, Groenigen K-JV, Six J et al (2009) Assessing the effect of elevated carbon dioxide on soil carbon: a comparison of four meta-analyses. Glob Change Biol 15:2020–2034. https://doi.org/10.1111/j.1365-2486.2009.01866.x
Iversen CM (2010) Digging deeper: fine-root responses to rising atmospheric CO2 concentration in forested ecosystems. New Phytol 186:346–357. https://doi.org/10.1111/j.1469-8137.2009.03122.x
Javot H, Pumplin N, Harrison MJ (2007) Phosphate in the arbuscular mycorrhizal symbiosis: transport properties and regulatory roles: phosphate transport in the AM symbiosis. Plant Cell Environ 30:310–322. https://doi.org/10.1111/j.1365-3040.2006.01617.x
Jiang M, Caldararu S, Zhang H et al (2020) Low phosphorus supply constrains plant responses to elevated CO2: a meta-analysis. Glob Change Biol 26:5856–5873. https://doi.org/10.1111/gcb.15277
Jin J, Tang C, Sale P (2015) The impact of elevated carbon dioxide on the phosphorus nutrition of plants: a review. Ann Botany 116:987–999. https://doi.org/10.1093/aob/mcv088
Johnson AH, Frizano J, Vann DR (2003) Biogeochemical implications of labile phosphorus in forest soils determined by the Hedley fractionation procedure. Oecologia 135:487–499. https://doi.org/10.1007/s00442-002-1164-5
Joner EJ, van Aarle IM, Vosatka M (2000) Phosphatase activity of extra-radical arbuscular mycorrhizal hyphae: a review. Plant Soil 226:12
Keenan RJ, Reams GA, Achard F et al (2015) Dynamics of global forest area: results from the FAO Global Forest Resources Assessment 2015. For Ecol Manag 352:9–20. https://doi.org/10.1016/j.foreco.2015.06.014
Kelley AM, Fay PA, Polley HW et al (2011) Atmospheric CO2 and soil extracellular enzyme activity: a meta-analysis and CO2 gradient experiment. Ecosphere 2:art96. https://doi.org/10.1890/ES11-00117.1
Kowalchuk GA (2012) Bad news for soil carbon sequestration? Science 337:1049–1050. https://doi.org/10.1126/science.1227303
Lenth R, Singmann H, Love J et al (2020) Emmeans: estimated marginal means, aka least-squares means. Version 1.4.6. Available at https://CRAN.R-project.org/package=emmeans
Liu Y, Chen J (2008) Phosphorus Cycle. In: Jørgensen SE, Fath BD (eds) Encyclopedia of Ecology. Academic Press, Oxford, pp 2715–2724
Liu Y, Zang H, Ge T et al (2018) Intensive fertilization (N, P, K, Ca, and S) decreases organic matter decomposition in paddy soil. Appl Soil Ecol 127:51–57. https://doi.org/10.1016/j.apsoil.2018.02.012
Loladze I (2002) Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry? Trends Ecol Evol 17:457–461. https://doi.org/10.1016/S0169-5347(02)02587-9
Loladze I (2014) Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife. https://doi.org/10.7554/eLife.02245
Lukac M, Calfapietra C, Godbold DL (2003) Production, turnover and mycorrhizal colonization of root systems of three Populus species grown under elevated CO2 (POPFACE). Glob Change Biol 9:838–848. https://doi.org/10.1046/j.1365-2486.2003.00582.x
Maroco JP, Breia E, Faria T et al (2002) Effects of long-term exposure to elevated CO2 and N fertilization on the development of photosynthetic capacity and biomass accumulation in Quercus suber L. Plant Cell Environ 25:105–113. https://doi.org/10.1046/j.0016-8025.2001.00800.x
Matamala R, Schlesinger WH (2000) Effects of elevated atmospheric CO2 on fine root production and activity in an intact temperate forest ecosystem. Glob Change Biol 6:967–979. https://doi.org/10.1046/j.1365-2486.2000.00374.x
Mohan JE, Cowden CC, Baas P et al (2014) Mycorrhizal fungi mediation of terrestrial ecosystem responses to global change: mini-review. Fungal Ecol 10:3–19. https://doi.org/10.1016/j.funeco.2014.01.005
Moorhead DL, Linkins AE (1997) Elevated CO2 alters belowground exoenzyme activities in tussock tundra. Plant Soil 189:321–329. https://doi.org/10.1023/A:1004246720186
Mori T, Lu X, Aoyagi R, Mo J (2018) Reconsidering the phosphorus limitation of soil microbial activity in tropical forests. Funct Ecol 32:1145–1154. https://doi.org/10.1111/1365-2435.13043
Mullen MD (2005) Phosphorus in soils | biological interactions. In: Hillel D (ed) Encyclopedia of Soils in the Environment. Elsevier, Oxford, pp 210–216
Mullins GL, Sikora FJ, Williams JC (1995) Effect of water-soluble phosphorus on the effectiveness of triple superphosphate fertilizers. Soil Sci Soc Am J 59:256–260. https://doi.org/10.2136/sssaj1995.03615995005900010040x
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In: Bünemann E, Oberson A, Frossard E (eds) Phosphorus in action. Springer, Berlin, Heidelberg, pp 215–243
Nie ZN, Zollinger RP, Jacobs JL (2009) Performance of 7 Australian native grasses from the temperate zone under a range of cutting and fertiliser regimes. Crop Pasture Sci 60:943. https://doi.org/10.1071/CP09067
Nielsen UN, Prior S, Delroy B et al (2015) Response of belowground communities to short-term phosphorus addition in a phosphorus-limited woodland. Plant Soil 391:321–331. https://doi.org/10.1007/s11104-015-2432-6
Norby RJ, Kauwe MGD, Domingues TF et al (2015) Model–data synthesis for the next generation of forest free-air CO2 enrichment (FACE) experiments. New Phytol 209:17–28. https://doi.org/10.1111/nph.13593
Olsson PA (1999) Signature fatty acids provide tools for determination of the distribution and interactions of mycorrhizal fungi in soil. FEMS Microbiol Ecol 29:303–310
Pan Y, Birdsey RA, Fang J et al (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–993. https://doi.org/10.1126/science.1201609
Parihar M, Rakshit A, Meena VS et al (2020) The potential of arbuscular mycorrhizal fungi in C cycling: a review. Arch Microbiol 202:1581–1596. https://doi.org/10.1007/s00203-020-01915-x
Pausch J, Kuzyakov Y (2018) Carbon input by roots into the soil: quantification of rhizodeposition from root to ecosystem scale. Glob Change Biol 24:1–12. https://doi.org/10.1111/gcb.13850
Peñuelas J, Sardans J, Rivas-ubach A, Janssens IA (2012) The human-induced imbalance between C, N and P in Earth’s life system. Glob Change Biol 18:3–6. https://doi.org/10.1111/j.1365-2486.2011.02568.x
Phillips RP, Finzi AC, Bernhardt ES (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett 14:187–194. https://doi.org/10.1111/j.1461-0248.2010.01570.x
Phillips RP, Meier IC, Bernhardt ES et al (2012) Roots and fungi accelerate carbon and nitrogen cycling in forests exposed to elevated CO 2. Ecol Lett 15:1042–1049. https://doi.org/10.1111/j.1461-0248.2012.01827.x
Piñeiro J, Ochoa-Hueso R, Delgado-Baquerizo M et al (2017) Effects of elevated CO2 on fine root biomass are reduced by aridity but enhanced by soil nitrogen: a global assessment. Sci Rep. https://doi.org/10.1038/s41598-017-15728-4
Rayment GE, Lyons DJ, Shelley B (2010) Soil chemical methods: Australasia. CSIRO Publishing, Victoria
R Core Team (2019) R: the R project for statistical computing. https://www.r-project.org/. Accessed 24 Mar 2018
Reed SC, Townsend AR, Taylor PG, Cleveland CC (2011) Phosphorus cycling in tropical forests growing on highly weathered soils. In: Bünemann E, Oberson A, Frossard E (eds) Phosphorus in action: biological processes in soil phosphorus cycling. Springer, Berlin, Heidelberg, pp 339–369
Reed SC, Yang X, Thornton PE (2015) Incorporating phosphorus cycling into global modeling efforts: a worthwhile, tractable endeavor. New Phytol 208:324–329. https://doi.org/10.1111/nph.13521
Reich PB, Hobbie SE, Lee T et al (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925. https://doi.org/10.1038/nature04486
Reich PB, Hobbie SE, Lee TD (2014) Plant growth enhancement by elevated CO2 eliminated by joint water and nitrogen limitation. Nat Geosci 7:920–924. https://doi.org/10.1038/ngeo2284
Robinson J, Munnich D, Simpson P, Orchard P (1993) Pasture associations and their relation to environment and agronomy in the Goulburn District. Aust J Bot 41:627. https://doi.org/10.1071/BT9930627
Ross GM, Horn S, Macdonald CA et al (2020) Metabarcoding mites: three years of elevated CO2 has no effect on oribatid assemblages in a Eucalyptus woodland. Pedobiologia 81:150667
Sato T, Ezawa T, Cheng W, Tawaraya K (2015) Release of acid phosphatase from extraradical hyphae of arbuscular mycorrhizal fungus Rhizophagus clarus. Soil Sci Plant Nutr 61:269–274. https://doi.org/10.1080/00380768.2014.993298
Sharma SB, Sayyed RZ, Trivedi MH, Gobi TA (2013) Phosphate solubilizing microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. Springerplus 2. https://doi.org/10.1186/2193-1801-2-587
Shipley B, Meziane D (2002) The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct Ecol 16:326–331. https://doi.org/10.1046/j.1365-2435.2002.00626.x
Smith SE, Jakobsen I, Grønlund M, Smith FA (2011) Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition1. Plant Physiol 156:1050–1057. https://doi.org/10.1104/pp.111.174581
Soepadmo E (1993) Tropical rain forests as carbon sinks. Chemosphere 27:1025–1039. https://doi.org/10.1016/0045-6535(93)90066-E
Soka G, Ritchie M (2014) Arbuscular mycorrhizal symbiosis and ecosystem processes: prospects for future research in tropical soils. Open J Ecol 04:11–22. https://doi.org/10.4236/oje.2014.41002
Soong JL, Marañon-Jimenez S, Cotrufo MF et al (2018) Soil microbial CNP and respiration responses to organic matter and nutrient additions: evidence from a tropical soil incubation. Soil Biol Biochem 122:141–149. https://doi.org/10.1016/j.soilbio.2018.04.011
Souza RC, Solly EF, Dawes MA et al (2017) Responses of soil extracellular enzyme activities to experimental warming and CO2 enrichment at the alpine treeline. Plant Soil 416:527–537. https://doi.org/10.1007/s11104-017-3235-8
Sun Y, Peng S, Goll DS et al (2017) Diagnosing phosphorus limitations in natural terrestrial ecosystems in carbon cycle models. Earth’s Future 5:730–749. https://doi.org/10.1002/2016EF000472
Talbot JM, Allison SD, Treseder KK (2008) Decomposers in disguise: mycorrhizal fungi as regulators of soil C dynamics in ecosystems under global change. Funct Ecol 22:955–963. https://doi.org/10.1111/j.1365-2435.2008.01402.x
Tarnawski S, Aragno M (2006) The influence of elevated [CO2] on diversity, activity and biogeochemical functions of rhizosphere and soil bacterial communities. In: Nösberger J, Long SP, Norby RJ et al (eds) Managed ecosystems and CO2. Springer, Berlin/Heidelberg, pp 393–412
Tate KR (1984) The biological transformation of P in soil. Plant Soil 76:245–256. https://doi.org/10.1007/BF02205584
Terrer C, Vicca S, Hungate BA et al (2016) Mycorrhizal association as a primary control of the CO2 fertilization effect. Science 353:72–74. https://doi.org/10.1126/science.aaf4610
Terrer C, Jackson RB, Prentice IC et al (2019) Nitrogen and phosphorus constrain the CO2 fertilization of global plant biomass. Nat Clim Chang 9:684–689. https://doi.org/10.1038/s41558-019-0545-2
Terrer C, Phillips RP, Hungate BA et al (2021) A trade-off between plant and soil carbon storage under elevated CO2. Nature 591:599–603. https://doi.org/10.1038/s41586-021-03306-8
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355. https://doi.org/10.1111/j.1469-8137.2004.01159.x
Treseder KK, Allen MF (2002) Direct nitrogen and phosphorus limitation of arbuscular mycorrhizal fungi: a model and field test. New Phytol 155:507–515. https://doi.org/10.1046/j.1469-8137.2002.00470.x
Treseder KK, Cross A (2006) Global distributions of arbuscular mycorrhizal fungi. Ecosystems 9:305–316
van Groenigen KJ, De Graaff M-A, Six J et al (2006) The impact of elevated atmospheric [CO2] on soil C and N dynamics: a meta-analysis. Managed ecosystems and CO2. Springer, Berlin, pp 373–391
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen–phosphorus interactions. Ecol Appl 20:5–15. https://doi.org/10.1890/08-0127.1
Watt M, Evans JR (1999) Linking development and determinacy with organic acid efflux from proteoid roots of white lupin grown with low phosphorus and ambient or elevated atmospheric CO2 concentration. Plant Physiol 120:705–716
Wei L, Vosátka M, Cai B et al (2019) The role of arbuscular mycorrhiza fungi in the decomposition of fresh residue and soil organic carbon: a mini-review. Soil Sci Soc Am J 83:511–517. https://doi.org/10.2136/sssaj2018.05.0205
Willis A, Rodrigues BF, Harris PJC (2013) The ecology of arbuscular mycorrhizal fungi. CRC Crit Rev Plant Sci 32:1–20. https://doi.org/10.1080/07352689.2012.683375
Xu J, Liu S, Song S et al (2018) Arbuscular mycorrhizal fungi influence decomposition and the associated soil microbial community under different soil phosphorus availability. Soil Biol Biochem 120:181–190. https://doi.org/10.1016/j.soilbio.2018.02.010
Yang X, Thornton PE, Ricciuto DM, Hoffman FM (2016) Phosphorus feedbacks constraining tropical ecosystem responses to changes in atmospheric CO2 and climate. Geophys Res Lett 43:7205–7214. https://doi.org/10.1002/2016GL069241
Yuan Y, Li Y, Mou Z et al (2021) Phosphorus addition decreases microbial residual contribution to soil organic carbon pool in a tropical coastal forest. Glob Change Biol 27:454–466. https://doi.org/10.1111/gcb.15407
Zhang Q, Wang YP, Matear RJ et al (2014) Nitrogen and phosphorous limitations significantly reduce future allowable CO2 emissions. Geophys Res Lett 41:632–637. https://doi.org/10.1002/2013GL058352
Zhou J, Zang H, Loeppmann S et al (2019) Arbuscular mycorrhiza enhances rhizodeposition and reduces the rhizosphere priming effect on the decomposition of soil organic matter. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2019.107641
Acknowledgements
We thank Gavin McKenzie and Goran Lopaticki for their help with the chamber set up, maintenance and troubleshooting for the duration of this experiment. Thanks to Pushpinder Matta and Christopher Mitchell for their help with nutrient analyses and to Johanna Pihlblad and Johanna Wong-Bajracharya for their assistance during the harvest of this experiment and sample processing.
Funding
No external funding was received for this project.
Author information
Authors and Affiliations
Contributions
LCG conceived the original idea, set up and harvested the experiment, carried out the laboratory and statistical analyses and wrote the manuscripts with input from YC, JRP, and EP.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there are no conflict of interest.
Additional information
Responsible Editor: Marie-Anne de Graaff.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Castañeda-Gómez, L., Powell, J.R., Pendall, E. et al. Phosphorus availability and arbuscular mycorrhizal fungi limit soil C cycling and influence plant responses to elevated CO2 conditions. Biogeochemistry 160, 69–87 (2022). https://doi.org/10.1007/s10533-022-00939-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-022-00939-3