Abstract
Background and aims
Climate warming and elevated CO2 can modify nutrient cycling mediated by enzymes in soils, especially in cold-limited ecosystems with a low availability of nutrients and a high temperature sensitivity of decomposition and mineralization.
Methods
We estimated responses of soil extracellular enzyme activities (EEAs) to 6 years of soil warming and 9 years of CO2 enrichment at an Alpine treeline site. EEAs were measured in the litter (L), fermentation (F) and humified (H) horizons under Larix decidua and Pinus uncinata trees.
Results
Soil warming indirectly affected EEAs through altered soil moisture, fine root biomass, and C:N ratio of the organic horizons. Warming increased β-glucosidase and β-xylosidase activities in the F horizon but led to reduced laccase activity in the L horizon, probably caused by drying of the litter horizon associated with the treatment. In the H horizon, previous CO2 enrichment altered the activity of leucine amino peptidase, N-acetylglucosaminidase, and phosphatase. No interactive effects between warming and CO2 enrichment were detected. Warming affected the temperature sensitivity of β-xylosidase but not of the other enzymes.
Conclusions
Altered EEAs after six years of soil warming indicate a sustained stimulation of carbon, nitrogen and nutrient cycling under climatic warming at the alpine treeline.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Responses of soils are one of the key uncertainties in predicting impacts of global warming and elevated atmospheric CO2 on ecosystems worldwide (Henry et al. 2005; Davidson and Janssens 2006). Soils in cold environments are characterized by low availability of labile organic C and N, as a result of low temperature constraints on decomposition and mineralization (Yang et al. 2016; Bowman et al. 2001). Therefore, such environments are expected to be particularly responsive to climate warming (Smith et al. 2009). As temperature affects mineralization kinetics (Cornelissen et al. 2007; Kammer et al. 2009), increased soil temperatures tend to accelerate mineralization and may lead to a change in microbial community composition in ecosystems that are not limited by a shortage or excess of water. Whereas warming is generally expected to increase extracellular enzyme activities (EEAs) in soils, a concomitant decrease in soil moisture can reduce it (see review by Henry 2012).
A meta-analysis of studies examining responses of potential EEAs to elevated CO2 indicated that soil and ecosystem type were strongly related to how EEAs changed (Kelley et al. 2011). A consistent increase in activity was only found for chitinase, an enzyme that degrades N- and C-containing building blocks of chitin and therefore is an important source of organic N. This result was only seen in field experiments, which often run for longer periods of time than lab studies and therefore may experience more nutrient limitation induced by elevated CO2 (Kelley et al. 2011). However, interactive effects of multiple global change factors on EEAs in the field remain understudied (Henry 2012). Such effects on soil enzymes are important, given that global warming and rising atmospheric CO2 concentrations are closely linked and both affect plant inputs to soil (Henry 2012). It is, therefore, expected that the impacts of environmental changes on nutrient fluxes will be mediated by changes in the expression and activity of enzymes, primarily by those produced by soil microorganisms (Bardgett et al. 2008).
The decomposition of soil organic matter (SOM) yields nutrients that are readily available to microorganisms and plants and is largely dependent on the production of extracellular enzymes (Sinsabaugh et al. 2009). These enzymes are produced in the soil by a diverse group of heterotrophic microbes (Sinsabaugh et al. 2009) as well as by plant roots (Gramss et al. 1999). Extracellular enzyme activities (EEAs) can be considered a useful indicator of microbial functioning and resource requirements (Sinsabaugh et al. 2009) and of nutrient availability to plants. EEAs are essential for the maintenance of soil fertility (N and P mineralization) as well as for ecosystem productivity and C balance (Sinsabaugh et al. 2009). A previous field study demonstrated that the most immediate effect of an increase in soil temperature on EEAs is to accelerate the rate at which enzymes come into contact with and break down substrates (Brzostek and Finzi 2011). EEA patterns among sites (or experimental treatments) can provide insight into biochemical controls on soil carbon storage because most differences in digestive enzyme activity among sites (or experimental treatments) are probably the result of differences in SOM content or microbial community composition and biomass (Sinsabaugh et al. 2009). Regarding the temperature dependency of EEAs, enzymes produced in cold soils reach their peak activity at lower temperatures than those produced in warmer soils and therefore tend to exhibit greater temperature sensitivity (Fenner et al. 2005; Koch et al. 2007).
Climate change is expected to impact treeline ecotones (Harsch et al. 2009), yet potential changes in EEAs have never been studied in these ecosystems. In this study, we investigated responses of nutrient-specific enzymatic processes to soil warming (spring 2006 to summer 2012) and residual effects of free-air CO2 enrichment (spring 2001 to autumn 2009) at a treeline site in the Swiss Alps in 2012, the final year of the 12-year experiment. Specifically, we determined the impacts of soil warming and prior CO2 enrichment on the functioning of the soil microbial community in the rhizosphere of Larix decidua and Pinus uncinata, two treeline-forming trees. We hypothesized that (1) soil warming increased potential EEAs in the soil; (2) warming negatively affected the temperature dependency of EEAs; and (3) the residual effect of the elevated CO2 treatment, which was terminated 3 years before the end of the soil warming experiment, led to a lasting increase in N-acquiring enzyme activities.
Materials and methods
Study site
Our study area was located at Stillberg, close to Davos, Switzerland in the upper part of an afforestation experiment just above the current treeline (Barbeito et al. 2012). The soils were rankers and weakly developed podzols on siliceous paragneiss parent material. The organic horizon was 5–20 cm thick, with a pH of 4 and a C:N ratio of ca. 27 (Streit et al. 2014). The mean annual precipitation for the period of 1975–2012 was 1165 mm, and the mean annual air temperature was 2.1 °C; for the same period, the main growing season months (June–August) had a mean precipitation of 444 mm and a mean air temperature of 9.2 °C (Dawes et al. 2015). Scattered individuals of Larix decidua Mill. and Pinus uncinata DC., common treeline species in this region, made up the tree layer. The trees were ca. 40 years old and had average heights of 2.6 m (Larix) and 1.5 m (Pinus). A dense understorey vegetation layer was dominated by ericoid dwarf shrubs (Vaccinium myrtillus L., Vaccinium gaultherioides Bigelow (group Vaccinium uliginosum agg.) and Empetrum nigrum ssp. hermaphroditum (Hagerup) Böcher).
Experimental design
The soil warming and free-air CO2 enrichment (FACE) experiment was carried out in an area of 2500 m2 on a northeast-exposed slope (inclination: 25–30°) at 2180 m a.s.l. (Hättenschwiler et al. 2002). Within this area, 40 experimental plots were established. They were hexagonal in shape, measuring 1.1 m2 in area, and each had a single tree of one of two species in the centre. Twenty plots contained Pinus and 20 contained Larix.
CO2 enrichment
The plots were arranged into 10 groups, each containing two plots with Larix and two with Pinus. Of the 10 groups, five were randomly assigned to an elevated CO2 (ca. 580 ppm) treatment and five were maintained at ambient CO2 (ca. 380 ppm). CO2 was measured continuously with an infrared gas analyzer (IRGA, LI-800; Li-Cor Inc. Lincoln, NE, USA), which was used to automatically regulate the CO2 supply to each plot. CO2 enrichment was applied during the photosynthetically active period (i.e., day-time and snow-free periods, usually lasting from early June to the beginning of October) for about 9 years (June 2001 to October 2009) (details of the CO2 enrichment treatment are given in Hättenschwiler et al. 2002, Dawes et al. 2013).
Soil warming
Starting in 2007, soil warming was applied to one plot with Pinus and one with Larix within each of the 10 groups. As a result, five individuals of each of the two species received the soil heating treatment and the same number served as a control for the heating experiment in both elevated and ambient CO2 treatments. In each plot, 26 m of heating cable (420 W) was placed on the ground surface in the entire plot area, below the understorey vegetation, in spirals around the tree. Soil warming was accomplished by switching the power supply on and off at 1-min intervals (Hagedorn et al. 2010). Each year, warming was started directly after snow melt (usually end of May) and was maintained during the snow-free period. Mean soil temperatures at a depth of 5 cm increased by an average of 3.6 K over the six warming seasons (Dawes et al. 2015). Air temperature increased within the dwarf shrub canopy (0.9 K at 20 cm above ground) but not within the tree canopy (Hagedorn et al. 2010). The soil matric water potential (Ψsoil; hPa) was measured throughout the 2010 snow-free period using tensiometers installed in a subset of the plots at depths of 5 cm and 20 cm below the surface (Dawes et al. 2014).
Soil sampling and processing
In early August 2012, six soil cores per plot were collected from throughout the entire area of each plot and separated into the L (litter) horizon, F (fermentation; with partly decomposed plant residues) horizon, and the top 5 cm of the H (humified; with more than 70% fine-particle substances without recognizable plant structures) horizon (see Hagedorn et al. 2010). Samples from each horizon were pooled at the plot level. The samples were homogenized by sieving through a 4 mm mesh and roots were extracted. Subsamples of 1 g were taken and stored at 4 °C for EEA analyses. Other subsamples were used to determine gravimetric soil moisture (g/g moist soil) by measuring the decrease in weight after drying the soil at 105 °C. For determining the C:N mass ratio, subsamples were dried at 40 °C, sieved through a 2 mm mesh and milled prior to elemental analyses using the NC 2500 elemental analyzer (CE instruments, Wigan, UK). Extracted roots were washed, separated into size classes (<0.5 mm, 0.5–2 mm, 2–5 mm, >5 mm), dried and weighed.
Enzyme assays
Calibration solutions, buffers and substrates
Calibration solutions from 4-methylumbelliferone (MU) and aminomethylcoumarin (AMC) stock solutions were prepared in 2-methoxyethanol and further diluted with sterile H2O to different gradient concentrations, depending on the fluorescence intensity of the sample. Fluorogenic substrates were prepared according to Hoppe 1983. Diammonium 2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonate (ABTS) was dissolved in rinsing buffer (pH 4.5). The incubation buffer used for soil assays was sterile water. The following enzymes were tested: Laccase (Lac), Cellobiohydrolase (Cel), β-Glucuronidase (Glr), β-Glucosidase (Gls), Leucine amino peptidase (Leu), N-Acetylglucosaminidase (Nag), Acid phosphatase (Pho) and β-Xylosidase (Xyl) (Online Resource 1). Calibration solutions, buffers and substrates of enzyme assays were prepared following the procedure described in Pritsch et al. (2011) and are presented in Online Resource 2.
Fluorescence and absorbance readings
The fluorescence and absorbance intensities were measured with a computerized microplate fluorimeter and colorimeter (Tecan, Infinite M200, software Megellan). Fluorescence measurements were carried out at 368 nm excitation and 465 nm emission. Absorbance measurements for ABTS were made at 425 nm.
Profiling of soil extracellular enzyme activities
As activities measured at low substrate concentrations underestimate potential enzyme activities (Huang et al. 1998) and as the assay duration should produce a linear response with time (German et al. 2011), preliminary tests were made to establish the saturating assay conditions by incubating the soil solution with different substrate concentrations for each enzyme and at diverse incubation times. The enzyme assays were carried out by using water as the diluent for sample homogenates, which allowed the sample itself to control the pH of the assay (Zantua and Bremner 1975). An incubation time of 4 h proved to be optimal.
Temperature dependence of enzyme activity
To test temperature dependence, it was necessary to run the assays at a range of incubation temperatures, including the temperature of the sampling location and that of optimum enzyme activity (Wallenstein et al. 2009; German et al. 2011). Subsamples of each soil sample were simultaneously incubated for 4 h at three different temperatures in the laboratory: 10 °C and 14 °C, which correspond roughly to the mean daylight temperature in the control and warmed plots at 5 cm depth during the growing season (Hagedorn et al. 2010), and 25 °C, which is the optimum temperature used for enzyme assays. See Online Resource 2 for the microplate set-up and assay procedures and for details of calculations used in fluorescence assays.
Statistical analyses
We applied linear mixed effects models fitted with the restricted maximum likelihood estimation method (REML) to assess effects of treatments on enzyme activities. The three organic horizons (L, F and H) were always tested in separate models. For all linear mixed effects models, the random effects structure reflected the experimental design, with enzymatic measurements in 40 individual plots nested within 20 soil warming treatment sub-groups nested within 10 CO2 treatment groups. Statistical models included warming treatment (unwarmed or warmed), prior CO2 enrichment (ambient or elevated), plot tree species (Larix or Pinus) and their interactions as fixed effects and were tested for significance using Type I conditional F tests (Pinheiro and Bates 2000). We included incubation temperature (10 °C, 14 °C and 25 °C) as a fixed effect and tested for interactions between incubation temperature and other fixed factors, including appropriate aspects of the random effects structure to account for repeated measures. All linear mixed effects models were run using the nlme package in R (Pinheiro et al. 2016).
In addition, we conducted path analyses to examine the potential causal relationships between variables of environment (CO2 enrichment, soil warming and soil moisture), vegetation growth (tree height, shrub biomass and fine root biomass), C:N ratio of the organic horizons, and the measured activities of Lac, Pho and Gls/(Nag + Leu), the quotient of activities of the C-degrading enzyme (Gls) over N-acquiring enzymes (Leu and Nag). Average enzyme activities were calculated from values of the L-F-H horizons at 25 °C in order to assess the overall potential responses of EEAs. We used the average CO2 concentrations that the experimental plots were exposed to during the CO2 experiment. We included manual measurements of soil temperature (average across the organic horizons of n = 5 measurement times in each plot in 2012) and the gravimetric soil water content measured once in August 2012 (Online Resource 3) and the soil water content of every plot in 2012 was normalized by the average soil water content in each soil horizon (Online Resource 3).
Estimates of tree height and shrub biomass in the final harvest (Dawes et al. 2015), as well as the total fine root biomass (<2 mm) summed over all horizons and the soil C:N ratio in the L-F-H horizons, measured in the same samples for which EEA analyses were performed, were all included in the path analyses. Tree heights were distinct for the two tree species (Dawes et al. 2015) (Online Resource 3) and therefore this parameter mainly reflected influences of tree species identity. The calculations of the C:N ratio in the L-F-H horizons provide information about organic matter quality at the time of the enzyme assays. Path analysis was performed using the lavaan package in R (Rosseel 2012). The adequacy of the hypothesized structural relationships was verified using χ2 tests and AIC. All variables were standardized to (0, 1). Prior to analyses, we transformed data where necessary to meet assumptions of normality. The chosen p-value for detecting statistical differences was P < 0.05. For all statistical analyses, we used R version 3.2.5 (R Core Team 2016). To assess the effects of the experimental treatments, soil organic horizons and tree species on EEA profiles, principal component analysis (PCA) was performed using Canoco 5 (Microcomputer Power, Ithaca, NY, USA). The activities of the eight enzymes measured at the incubation temperature of 25 °C were used, resulting in a data matrix of eight columns (8 enzymes) and 120 rows (3 soil horizons × 40 plots). Enzyme activities were log-transformed. The response data columns were centred and rows standardized by norm. Treatments were introduced as supplementary environmental factors and fitted on ordination scores using multiple regression.
Results
Soil warming affected enzyme activities, with specific responses for the different soil horizons and plot tree species, most likely indirectly via soil moisture, litter quality and plant productivity. Activities generally decreased with warming in the L horizon and increased in the F horizon, with no effect in the H horizon. Residual CO2 enrichment effects, on the contrary, were mainly seen in the H horizon, again in a tree species-dependent manner.
Soil warming
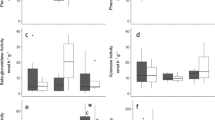
Soil warming decreased the Lac activity in the L horizon, more so under the deciduous Larix (−98%) than under the evergreen Pinus (−65%) (F 1,8 = 7.3693, P = 0.0265; Fig. 1a). No soil warming effect on Lac activity was detected in the F or H horizons.
Warming effects on extracellular soil enzyme activities in the organic soil horizons at the alpine treeline (Davos, Switzerland): a laccase (Lac) activity in the litter (L) horizon (F 1,8 = 7.3693, P = 0.0265); b β-glucosidase (Gls) activity in the F horizon (F 1,8 = 10.413, P = 0.0121); c β-xylosidase (Xyl) in the F horizon (F 1,8 = 5.748, P = 0.0433); d N-Acetylglucosaminidase (Nag) activity in the F horizon (F 1,16 = 5.647, P = 0.0303). Values represent means and standard errors (±1 SE) for enzyme activities exposed to different warming treatments (warmed or unwarmed) in plots containing a Larix (left) or Pinus (right) tree (n = 5), after incubation one of three temperatures (T10 = 10 °C, T14 = 14 °C, T25 = 25 °C). Only significant warming effects are shown (see complete set of statistical results in Online Resource 4)
In contrast, soil warming increased the Gls activity in the F horizon (F 1,8 = 10.413, P = 0.0121; Fig. 1b), and this effect was slightly greater under Larix (+99%) than under Pinus (+75%). Similarly, soil warming increased the Xyl activity in the F horizon (74% higher in warmed plots under Larix in comparison to 36% under Pinus (F 1,8 = 5.748, P = 0.0433); Fig. 1c). The soil warming x tree species interaction was significant for Nag activity in the F horizon (F 1,16 = 5.647, P = 0.0303; Fig. 1d). In plots with Larix, Nag activity doubled under soil warming compared to in unwarmed plots, while it decreased by 18% in warmed plots with Pinus (Fig. 1d).
In the H horizon, soil warming was never significant as a main effect on enzyme activity. However, there was a significant soil warming x incubation temperature interaction for the Xyl activity in the H horizon (F 1,72 = 4.3956, P = 0.0395). The Xyl activity showed greater temperature sensitivity in unwarmed plots compared to in warmed plots, irrespective of plot tree species (Online Resource 4). Generally, and as expected, higher incubation temperatures increased enzyme activities, but treatment effects were already significant at the lower and ecologically relevant soil temperatures (Figs 1 and 2).
Extracellular soil enzyme activities tested for residual CO2 effects in the organic soil horizon (H) at the alpine treeline (Davos, Switzerland): a leucine amino peptidase (Leu) (F 1,16 = 5.0072, P = 0.0398); b N-Acetylglucosaminidase (Nag) (F 1,16 = 5.772, P = 0.0288); c phosphatase (Pho) (F 1,16 = 5.6340, P = 0.0305). Values represent means and standard errors (±1 SE) for enzyme activities exposed to different CO2 treatment levels (ambient or elevated) in plots containing a Larix (left) or Pinus (right) tree (n = 5), after incubation at one of three temperatures (T10 = 10 °C, T14 = 14 °C, T25 = 25 °C). Only significant CO2 effects are shown (see complete set of statistical results in Online Resource 4)
Residual effects of CO2 treatment
Significant CO2 x tree species interactions were found in the H horizon for three enzymes, in all cases indicating greater CO2-related increases in activity under Pinus and smaller increases or even decreases under Larix. Leu: Pinus + 50% vs. Larix + 10%; F 1,16 = 5.0072, P = 0.0398; Fig. 2a); Nag: Pinus + 91% vs. Larix − 30% (F 1,16 = 5.772, P = 0.0288; Fig. 2b); Pho: Pinus + 22% vs. Larix − 40% (F 1,16 = 5.6340, P = 0.0305; Fig. 2c).
Effects of litter and soil moisture, vegetation and SOM
The path analyses indicated that, in this study, Lac activity was directly influenced by changes in litter water content. Similarly, the analysis indicated a direct effect of the prior CO2 treatment on Lac activity (Fig. 3a). In contrast, Gls/(Nag + Leu) was primarily driven by the C:N ratio of organic horizons, which was strongly dependent on plot tree species (Fig. 3b). Pho activity was affected by the soil C:N ratio and was positively related to the fine root biomass, which, in turn, was reduced by increased soil temperatures (Fig. 3c). For this model, only the F and H horizons were used because the Pho activity was strongly associated with the rhizosphere, which is not present in the L horizon. This was supported by the statistical model fit for Pho, which was strongly improved with the exclusion of the L horizon (not the case for the other two models). Generally, the soil EEA profiles depended most strongly on soil organic horizon. They were variably affected by the soil warming and residual CO2 treatments, with warming effects more strongly related to plots with Larix and CO2 enrichment effects to plots with Pinus, as revealed by ordination analysis (Online Resource 5).
Path model depicting the potential causal relationships among variables of environment (CO2 enrichment, soil warming and soil moisture), vegetation growth (tree height, dwarf shrub above-ground biomass and fine root biomass), C:N ratio of the organic horizons, and the measured activities of a laccase (χ2 7 = 4.153, P = 0.762), b C/N acquiring enzymes (χ2 11 = 12.229, P = 0.347) defined as the quotient of activities of the C-degrading enzyme (Gls) over activities of the N-acquiring enzymes (Leu and Nag), and c phosphatase activity (χ2 7 = 5.293, P = 0.624). Numbers with the arrows represent standardized path coefficients. Arrows with thick lines represent significant relationships (P < 0.05), whereas grey lines represent relationships that were not significant (P > 0.05). Black = positive relationship, Red = negative relationship. Percentages indicate the variance explained by each variable
Discussion
We found complex responses of EEAs to experimental warming and elevated atmospheric CO2 treatments, with the enzyme activity responses varying among soil horizons and between plots with the different tree species. The hypothesis that soil warming increased EEAs was partially supported, as we observed both positive and negative effects of the soil warming. In addition, the hypothesis that warming decreased the temperature sensitivity of EEAs was only confirmed for one hemicellulose-degrading enzyme in the H horizon, suggesting little acclimatization of EEA to warming. The hypothesis that CO2 enrichment led to increased EEAs was also partially supported, as activities of protein-, chitin- and P-compound degrading enzymes in the H horizon were related to previous CO2 enrichment, albeit in a tree-species dependent manner.
Effects of warming
Laccase (Lac) is an important lignin-degrading enzyme commonly synthesized by fungi (Blackwood et al. 2007). In the present study, the activity of Lac was strongly reduced by the soil warming treatment in the L horizon, where a concomitant soil drying by 50% was observed with higher temperatures. We measured potential EEA, and therefore our results indicate that the Lac activity was significantly reduced either by a changed microbial community composition or by reduced enzyme production by the same microbes. Whether the change in Lac activity was caused by warming alone or in combination with reduced moisture is unclear, but path analyses suggest that an indirect effect of warming via reduced soil moisture was important. Water plays a key role in regulating soil microbial activity (Schindlbacher et al. 2012) including decomposition rates (Davidson et al. 1998). Lignin restricts access by enzymes to cell wall polysaccharides, and thus a reduction in lignin degradation by Lac in the L horizon may also restrict the availability of labile C sources to soil microbes (Talbot et al. 2012). At Stillberg, this might have contributed to the observed shift towards microbial use of older SOM substrate in deeper soil horizons under warming, shown in a 13C labelling study within the same experiment (Streit et al. 2014).
Increased temperatures alone are expected to lead to substantial increases in soil enzyme activities (Wallenstein et al. 2009) if other factors, such as moisture, are not limiting (Criquet et al. 2004; Sardans and Peñuelas 2005). Warming in our experiment enhanced the activities of a cellulase (Gls) and a hemicellulase (Xyl) under both Larix and Pinus in the F horizon. The accelerated cellulose and hemicellulose degradation in response to warming was greater under Larix than under Pinus. The litter of Pinus had a higher C:N ratio compared to that of Larix, as well as a greater percentage of non-structural carbohydrates, sugars and starch (Hagedorn and Machwitz 2007). The path analyses indeed indicated that these treespecific differences in litter quality most likely are important factors explaining the differences in EEA responses to warming. A similar pattern was seen for the N-acquiring enzyme chitinase (Nag), which liberates N-acetylglucosamine from the cell wall of fungi and from exoskeletons of arthropods. We found that EEAs decreased under Pinus and increased under Larix in the F horizon of the warmed plots. Besides the C:N ratio of the soil organic matter, variation in fungal biomass may influence extracellular enzyme activities (Šnajdr et al. 2011) and the distribution of soil enzymes (Baldrian et al. 2010).
Warmer soils can also have an indirect effect on enzyme activities by increasing the availability of mineral N in soils, which can affect both fungal composition (Sterkenburg et al. 2015) and microbial activity (Keeler et al. 2009). N availability indeed increased in warmed soils in this treeline experiment (Dawes et al. 2016). In a long-term N fertilization experiment at temperate forest and grassland sites, added N caused a general stimulation of the activities of cellulose-degrading and N- and P-acquiring enzymes in the soil organic horizon at a depth of 10 cm, probably because N is an important component in enzyme production and stimulates microbial activity, particularly in N-limited systems (Keeler et al. 2009).
In summary, our results support a warming-related increase in SOM mineralization (Hagedorn et al. 2010) and an increase in soil microbial metabolic activity (Streit et al. 2014) at our site. We expect that the global warming will increase EEAs in soil horizons not affected by reduced soil moisture, which may in turn cause greater SOC decomposition and heterotrophic respirational C losses in in alpine treeline ecosystems.
Temperature sensitivity of enzyme activity
We observed a warming-induced decrease in the temperature sensitivity of β-xylosidase activity in the H horizon, but this effect was absent for the other enzymes and soil horizons. Cold-adapted microorganisms tend to respond more efficiently to increased temperature than warm-adapted ones (Brzostek and Finzi 2011). The temperature sensitivity of enzymes is related to the production of isoenzymes by microorganisms (Wallenstein 2011). Cold-adapted enzymes have a very low optimum temperature for reaction (Huston et al. 2000; Coker et al. 2003), a relatively high affinity for substrate (Fenner et al. 2005) and a high catalytic potential (Bradford et al. 2010) at low temperatures. Although soil warming decreased temperature sensitivity of β-xylosidase in our study, there was no evidence of shifts in the temperature sensitivity of other EEAs, probably because the warming effects were not large enough to substantially stimulate the production of warm-adapted enzymes by microorganisms.
Residual effects of CO2 enrichment
CO2 enrichment increased the activities of N- and P-acquiring enzymes under Pinus and had both positive and negative impacts in soils with Larix. Increased EEAs probably resulted from the stimulated microbial production of the chitinases, peptidases and phosphatases, suggesting that rhizosphere microbes allocated C towards producing enzymes to acquire N and P under elevated CO2 (Hungate et al. 1997; Hamilton and Frank 2001; Ebersberger et al. 2003). The increase in EEAs is in agreement with observations that elevated CO2 stimulated the activities of N-acquiring enzymes in a temperate forest stand at low elevation dominated by Pinus taeda L. (Phillips et al. 2011). In addition, the increases in EEAs under elevated CO2 support the findings of an earlier study at our site, where dissolved organic C (DOC) concentration increased in the soil solution during the CO2 treatment (Hagedorn et al. 2008); after termination of CO2 enrichment, DOC continued to be greater in previously enriched plots with Pinus but not in plots with Larix (Dawes et al. 2013). Soil respiration at our site was stimulated by the CO2 enrichment but there were no changes in the composition of soil microbial communities (Hagedorn et al. 2013). In combination, these findings indicate accelerated SOM turnover (Dawes et al. 2013), which was probably catalysed by the activities of chitinase, peptidase and phosphatase, although the residual CO2 effects on the activities of these enzymes were relatively small in 2012.
We attribute the slight shifts in the EEAs in plots previously exposed to elevated CO2 to changes in how much C plants transferred to soil microorganisms. It is also possible that a C-enriched substrate in plots exposed to elevated CO2 increased the microbial demand for N- and P-compounds, particularly under Pinus. This tree species showed a somewhat greater starch accumulation in elevated CO2 plots (Dawes et al. 2013). Moreover, the different responses of EEAs below Pinus or Larix might be related to indirect effects via understorey vegetation. Understorey species contributed most of the fine roots in our plots, irrespective of warming or CO2 treatment (Dawes et al. 2013 and 2015), which likely influenced soil organic matter quality. The path analyses indicated that the activities of C- and N-acquiring enzymes were mainly driven by soil quality (i.e. the C:N ratio), whereas the activity of phosphatase was additionally dependent on fine root biomass. This suggests that either root-associated fungi or the plants themselves contribute significantly to the activity of this enzyme.
Conclusions
Our experimental simulation of climate change induced changes in EEAs, which likely impacted C, N, and P dynamics at the alpine treeline. Overall, enzyme activities responded more markedly to the 6-year soil warming treatment than to the past CO2 enrichment that lasted 9 years. Warming might have affected EEAs not only directly, but also indirectly through changes in soil water availability, organic matter quality or fine root biomass. The divergent warming effects among EEAs and the horizon-specific responses indicate that 6 years of warmer soils induced a sustained alteration in belowground carbon and nutrient dynamics. While the suppressed laccase activity in the litter horizon under warming may cause an accumulation of recalcitrant lignin, the overall stimulation of the other enzymes in the F horizon indicates an accelerated SOM mineralization and an associated release of N and P. In conjunction with the small warming effects on the temperature dependency of EEAs, these responses show that there was little thermal adaptation to the 6-year warming treatment. In turn, our results suggest that substantial carbon losses from alpine treeline soils can be expected under warmer soils of the future.
References
Baldrian P, Vě M, Cajthaml T, Petránková M, Šnajdr J (2010) Small-scale distribution of extracellular enzymes, fungal, and bacterial biomass in Quercus petraea forest topsoil. Biol Fertil Soils 46:717–726
Barbeito I, Dawes M, Rixen C, Senn J, Bebi P (2012) Factors driving mortality and growth at treeline: a 30-year experiment of 92000 conifers. Ecology 93:389–401
Bardgett RD, Freeman C, Ostle NJ (2008) Microbial contributions to climate change through carbon cycle feedbacks. ISME J 2:805–814
Blackwood CB, Waldrop MP, Zak DR, Sinsabaugh RL (2007) Molecular analysis of fungal communities and laccase genes in decomposing litter reveals differences among forest types but no impact of nitrogen deposition. Environ Microbiol 9:1306–1316
Bowman W, Seastedt T, Program L-TER (2001) Structure and function of an alpine ecosystem: Niwot ridge, Colorado. Oxford University Press, USA
Bradford MA, Watts BW, Davies CA (2010) Thermal adaptation of heterotrophic soil respiration in laboratory microcosms. Glob Chang Biol 16:1576–1588
Brzostek E, Finzi A (2011) Substrate supply, fine roots, and temperature control proteolytic enzyme activity in temperate forest soils. Bull Ecol Soc Am 92:892–902
Coker JA, Sheridan PP, Loveland-Curtze J, Gutshall KR, Auman AJ, Brenchley JE (2003) Biochemical characterization of a ß-galactosidase with a low temperature optimum obtained from an Antarctic arthrobacter isolate. J Bacteriol 185:5473–5482
Core Team R (2016) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Cornelissen JH, Van Bodegom PM, Aerts R, Callaghan TV, Van Logtestijn RS, Alatalo J, Stuart Chapin F, Gerdol R, Gudmundsson J, Gwynn-Jones D, Hartley AE, Hik DS, Hofgaard A, Jónsdóttir IS, Karlsson S, Klein JA, Laundre J, Magnusson B, Michelsen A, Molau U, Onipchenko VG, Quested HM, Sandvik SM, Schmidt IK, Shaver GR, Solheim B, Soudzilovskaia NA, Stenström A, Tolvanen A, Totland Ø, Wada N, Welker JM, Zhao X, Team M (2007) Global negative vegetation feedback to climate warming responses of leaf litter decomposition rates in cold biomes. Ecol Lett 10:619–627
Criquet S, Ferre E, Farnet A, Petit Null JL (2004) Annual dynamics of phosphatase activities in an evergreen oak litter: influence of biotic and abiotic factors. Soil Biol Biochem 36:1111–1118
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Davidson EA, Belk E, Boone RD (1998) Soil water content and temperature as independent or confounded factors controlling soil respiration in a temperate mixed hardwood forest. Glob Chang Biol 4:217–227
Dawes MA, Hagedorn F, Handa IT, Streit K, Ekblad A, Rixen C, Körner C, Hättenschwiler S (2013) An alpine treeline in a carbon dioxide-rich world: synthesis of a nine-year free-air carbon dioxide enrichment study. Oecologia 171:623–637
Dawes MA, Zweifel R, Dawes N, Rixen C, Hagedorn F (2014) CO2 enrichment alters diurnal stem radius fluctuations of 36-yr-old Larix decidua growing at the alpine tree line. New Phytol 202:1237–1248
Dawes MA, Philipson CD, Fonti P, Bebi P, Hättenschwiler S, Hagedorn F, Rixen C (2015) Soil warming and CO2 enrichment induce biomass shifts in alpine tree line vegetation. Glob Chang Biol 21:2005–2021
Dawes MA, Schleppi P, Hättenschwiler S, Rixen C, Hagedorn F (2016) Soil warming opens the nitrogen cycle at the alpine treeline. Glob Chang Biol 23:421–434
Ebersberger D, Niklaus PA, Kandeler E (2003) Long term CO2 enrichment stimulates N-mineralisation and enzyme activities in calcareous grassland. Soil Biol Biochem 35:965–972
Fenner N, Freeman C, Reynolds B (2005) Observations of a seasonally shifting thermal optimum in peatland carbon-cycling processes; implications for the global carbon cycle and soil enzyme methodologies. Soil Biol Biochem 37:1814–1821
German DP, Weintraub MN, Grandy AS, Lauber CL, Rinkes ZL, Allison SD (2011) Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol Biochem 43:1387–1397
Gramss G, Voigt K-D, Kirsche B (1999) Oxidoreductase enzymes liberated by plant roots and their effects on soil humic material. Chemosphere 38:1481–1494
Hagedorn F, Machwitz M (2007) Controls on dissolved organic matter leaching from forest litter grown under elevated atmospheric. Soil Biol Biochem 39:1759–1769
Hagedorn F, van Hees PAW, Handa IT, Hättenschwiler S (2008) Elevated atmospheric CO2 fuels leaching of old dissolved organic matter at the alpine treeline. Glob Biogeochem Cycles 22:GB2004
Hagedorn F, Martin M, Rixen C, Rusch S, Bebi P, Zürcher A, Siegwolf R, Wipf S, Escape C, Roy J, Hättenschwiler S (2010) Short-term responses of ecosystem carbon fluxes to experimental soil warming at the Swiss alpine treeline. Biogeochemistry 97:7–19
Hagedorn F, Hiltbrunner D, Streit K, Ekblad A, Lindahl B, Miltner A, Frey B, Handa IT, Hättenschwiler S (2013) Nine years of CO2 enrichment at the alpine treeline stimulates soil respiration but does not alter soil microbial communities. Soil Biol Biochem 57:390–400
Hamilton WE, Frank DA (2001) Can plants stimulate soil microbes and their own nutrient supply? Evidence from a grazing tolerant grass. Ecology 82:2397–2402
Harsch MA, Hulme PE, McGlone MS, Duncan RP (2009) Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecol Lett 12:1040–1049
Hättenschwiler S, Handa IT, Egli L, Asshoff R, Ammann W, Körner C (2002) Atmospheric CO2 enrichment of alpine treeline conifers. New Phytol 156:363–375
Henry HA (2012) Soil extracellular enzyme dynamics in a changing climate. Soil Biol Biochem 47:53–59
Henry HAL, Juarez JD, Field CB, Vitousek PM (2005) Interactive effects of elevated CO2, N deposition and climate change on extracellular enzyme activity and soil density fractionation in a California annual grassland. Glob Chang Biol 11:1808–1815
Hoppe H (1983) Significance of exoenzymatic activities in the ecology of brackish water: measurements by means of methylumbelliferyl-substrates. Mar Ecol Prog Ser 11:299–308
Huang P, Senesi N, Buffle J (1998) Structure and surface reactions of soil particles. John Wiley & Sons Ltd, Chichester
Hungate BA, Lund CP, Pearson HL, Chapin FS (1997) Elevated CO2 and nutrient addition after soil N cycling and N trace gas fluxes with early season wet-up in a California annual grassland. Biogeochemistry 37:89–109
Huston AL, Krieger-Brockett BB, Deming JW (2000) Remarkably low temperature optima for extracellular enzyme activity from Arctic bacteria and sea ice. Environ Microbiol 2:383–388
Kammer A, Hagedorn F, Shevchenko I, Leifeld J, Guggenberger G, Goryacheva T, Rigling A, Moiseev P (2009) Treeline shifts in the Ural mountains affect soil organic matter dynamics. Glob Chang Biol 15:1570–1583
Keeler BL, Hobbie SE, Kellogg LE (2009) Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12:1–15
Kelley AM, Fay PA, Polley HW, Gill RA, Jackson RB (2011) Atmospheric CO2 and soil extracellular enzyme activity: a meta-analysis and CO2 gradient experiment. Ecosphere 2:1–20
Koch O, Tscherko D, Kandeler E (2007) Temperature sensitivity of microbial respiration, nitrogen mineralization, and potential soil enzyme activities in organic alpine soils. Global Biogeochem Cy 21:GB4017
Phillips RP, Finzi AC, Bernhardt ES (2011) Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol Lett 14:187–194
Pinheiro J, Bates D (2000) Mixed-effects models in S and S-PLUS. Springer, New York
Pinheiro J, Bates D, DebRoy S, Sarkar D (2016) Nlme: linear and nonlinear mixed effects models. R package version 3:1–128
Pritsch K, Courty P, Churin J-L, Cloutier-Hurteau B, Ali M, Damon C, Duchemin M, Egli S, Ernst J, Fraissinet-Tachet L, Kuhar F, Legname E, Marmeisse R, Müller A, Nikolova P, Peter M, Plassard C, Richard F, Schloter M, Selosse M-A, Franc A, Garbaye J (2011) Optimized assay and storage conditions for enzyme activity profiling of ectomycorrhizae. Mycorrhiza 21:589–600
Rosseel Y (2012) Lavaan: an R package for structural equation modeling. J Stat Softw 48:1–36
Sardans J, Peñuelas J (2005) Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biol Biochem 37:455–461
Schindlbacher A, Wunderlich S, Borken W, Kitzler B, Zechmeister-Boltenstern S, Jandl R (2012) Soil respiration under climate change: prolonged summer drought offsets soil warming effects. Glob Chang Biol 18:2270–2279
Sinsabaugh RL, Hill BH, Follstad Shah JJ (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–798
Smith W, Germino M, Johnson D, Reinhardt K (2009) The altitude of alpine treeline: a bellwether of climate change effects. Bot Rev 75:163–190
Šnajdr J, Dobiášová P, Větrovský T, Valášková V, Alawi A, Boddy L, Baldrian P (2011) Saprotrophic basidiomycete mycelia and their interspecific interactions affect the spatial distribution of extracellular enzymes in soil. FEMS Microbiol Ecol 78:80–90
Sterkenburg E, Bahr A, Brandström Durling M, Clemmensen KE, Lindahl BD (2015) Changes in fungal communities along a boreal forest soil fertility gradient. New Phytol 207:1145–1158
Streit K, Hagedorn F, Hiltbrunner D, Portmann M, Saurer M, Buchmann N, Wild B, Richter A, Wipf S, Siegwolf RTW (2014) Soil warming alters microbial substrate use in alpine soils. Glob Chang Biol 20:1327–1338
Talbot J, Yelle D, Nowick J, Treseder K (2012) Litter decay rates are determined by lignin chemistry. Biogeochemistry 108:279–295
Wallenstein D (2011) Controls on the temperature sensitivity of soil enzymes: a key driver of in situ enzyme activity rates enzymes: a key driver of in situ enzyme activity rates. In: Shukla G, Varma A (eds) Soil enzymology. Springer, Berlin, pp 245–258
Wallenstein MD, McMahon SK, Schimel JP (2009) Seasonal variation in enzyme activities and temperature sensitivities in Arctic tundra soils. Glob Chang Biol 15:1631–1639
Yang Z, Wullschleger SD, Liang L, Graham DE, Gu B (2016) Effects of warming on the degradation and production of low-molecular-weight labile organic carbon in an Arctic tundra soil. Soil Biol Biochem 95:202–211
Zantua M, Bremner J (1975) Comparison of methods of assaying urease activity in soils. Soil Biol Biochem 7:291–295
Acknowledgments
We are grateful to many colleagues at the SLF and WSL for contributing to the Stillberg CO2 enrichment and soil warming experiment. In particular, we thank Stephan Hättenschwiler and I. Tanya Handa for planning and initiating this climate change study. We thank Barbara Meier and Rosmarie Eppenberger at the WSL for their assistance in lab measurements. We also thank Adam Butler at the BioSS (UK) for helping with statistical analysis. Major funding sources for this 12-year study included the following: the Swiss National Science Foundation from 2001 to 2005 (grant 31-061428.00 to Stephan Hättenschwiler) and from 2007 to 2010 (grant 315200-116861 to CR); the Velux Foundation from 2007 to 2012 (grant 371 to FH); and the WSL from 2012 to 2016 (grant to CR and MD). This work is part of RCS’s Master’s thesis, which was funded by CAPES, Brazil; the Large-scale Biosphere-Atmosphere Programme of MCTI, Brazil is acknowledged for additional support.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Timothy Cavagnaro .
Rights and permissions
About this article
Cite this article
Souza, R.C., Solly, E.F., Dawes, M.A. et al. Responses of soil extracellular enzyme activities to experimental warming and CO2 enrichment at the alpine treeline. Plant Soil 416, 527–537 (2017). https://doi.org/10.1007/s11104-017-3235-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3235-8