Abstract
Vegetation growth is often limited by the availability of soil mineralized N (Nm), and Nm dynamics are important to forest productivity and succession. However, current dynamic global vegetation models (DGVMs) do not fully mechanistically represent the linkage between plants, microbes, and soil. Here, we sought to develop and test a DGVM with an explicit representation of the important physiological and ecological mechanisms relating to Nm dynamics. We revised an existing DGVM (FORCCHN2) to predict soil net N mineralization, nitrification, N losses, and plant N uptake. This new model couples soil microbial biomass and fine roots to quantify Nm balance for forests. We applied the model at a temperate forest in central Massachusetts, USA (Harvard Forest). FORCCHN2 reproduced more than half of the monthly variation (i.e. R2 > 50%) of net Nm as ammonium (NH4+-N), and nitrate (NO3−-N) increment over seven years. Furthermore, the model captured the dynamics of soil water and respiration well (R2 = 81% and 72%, respectively). Our results provide a mechanistic framework of Nm dynamics by incorporating Nm production, transformations, losses, and related biomass growth processes into a DGVM. This study suggests that DGVMs should consider coupling fine root growth, microbial growth, and soil processes to represent the detailed Nm dynamics, which could improve the understanding of the forest N cycle in the background of environmental changes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mechanistic understanding of the dynamics and interactions of the carbon (C) and nitrogen (N) cycles in forests has become more important with increased anthropogenic perturbations (Meyerholt et al. 2020). Processes involved in the N-cycle, notably soil N-mineralization and plant N-uptake, can strongly affect C-sequestration in forest ecosystems (Niu et al. 2016). A relatively small percentage of soil total N (primarily the soil inorganic N) is available for uptake by the vegetation. Lack of nitrogen often limits plant growth, and the processes involved in generating plant-available N in the soil are thus extremely important (Oren et al. 2001; Cleveland et al. 2013; Menge et al. 2019). Soil nitrogen mineralization is enmeshed, either directly or indirectly, in forest responses to changes in environmental factors (e.g. CO2 concentration, climate) and the soil and plant N-cycle (Rustad et al. 2001; Wang and Houlton 2009).
Soil inorganic N mainly derives from microbial mineralization, which involves internal ecosystem processes and their interactions with multiple environmental variables. Soil water content, soil temperature, and substrate concentration influence the production and losses of mineralized N (Nm) through the metabolic activity of microorganisms and plants. Due to variations in climate, plants, and soil conditions that vary spatially and temporally (Bardgett et al. 2013), experimental observations have been the principal sources used in previous studies to better understand and evaluate the factors that influence the soil Nm. However, current site scale measurements of Nm remain highly variable, and scaling these measurements to ecosystems and regions is difficult (Zhang et al. 2017). Therefore, generalizing from local field experiments to larger spatial scales or future environmental scenarios of Nm generally relies on simulation using ecological models. Constructing site-scale models validated against measurements is the logical next step in providing global estimations.
The relative incomplete formulation of previous modeling efforts also suggests that integrating the dynamics of plants and microbes into a process-based model is key to predicting soil Nm at different temporal scales. Currently, various models have been used to simulate the dynamic of Nm, generally grouped into the two categories of statistical and process-based models. The relative influence of different environmental factors on current N-mineralization is often explained using applied statistical models. Stanford et al. (1974), Dou et al. (1996), and Clivot et al. (2017) developed statistical models based on laboratory incubations and predicted potential nitrogen mineralization at the site scale. From these studies, Chapman et al. (2013) and Liu et al. (2017) collected multi-site experimental datasets and developed regional models of N-mineralization. However, static statistical models may not capture changes in forest structure or composition when evaluating or predicting values of N-mineralization over the longer term. As an alternative, dynamic models based on the interactions among N-fluxes and C-fluxes in the vegetation and organic soil components of the forest ecosystem have been developed in recent decades. These models provide useful estimations at different temporal and spatial scales though they may have large divergences or limitations (Zaehle et al. 2014). For example, Comins and McMurtrie (1993) applied their Generic Decomposition And Yield (G’DAY) model to simulate the N cycle in nutrient-limited forests, although the model does not include a description for N losses. Detailed soil N-mineralization processes, including nitrification, leaching, etc., also influence the accuracy of the Nm estimations, which are needed to improve these models. The Carnegie-Ames-Stanford (CASA) Biosphere model estimates global Nm based on some detailed constituent processes (Potter et al. 1993). The nitrification and denitrification modules of this model are mainly driven by theoretical values, and the model does not incorporate soil microbial dynamics, such as the growth and mortality of microorganisms. The Denitrification-Decomposition (DNDC) model has also been used to model the N cycle in forests (Li et al. 2000; Zhang et al. 2017). However, the application of this model is limited as it lacks an accurate description of plant growth.
A relatively recent dynamic global vegetation model, FORCCHN Version 2.0 (FORCCHN2), is based on the allocation of tree non-structural carbohydrates (NSCs). This model has been applied in modeling the growth of plant biomass, especially for the growth of leaves and fine roots (Fang et al. 2020). However, the original FORCCHN2 model uses static parameters and a simple description of Nm based on the processes of the gross N mineralization and the N immobilization. In this study, several key plant-soil-microbe N processes were integrated into the FORCCHN2 model (Table S1). The new aspects of this work were as follows: (1) predicting daily Nm (NH4+-N: ammonium, and NO3−-N: nitrate) dynamics by mineralization, nitrification, denitrification, and N losses (including ammonia volatilization, nitrate leaching, and gas emissions during nitrification); (2) modeling the nitrification and denitrification process by predicting the dynamics of microorganisms; and (3) connecting soil N input with litterfall and plant N uptake with biomass of fine roots. We conducted sensitivity analysis and tested this model against measurements at a temperate forest, Harvard Forest.
Materials and methods
Study site
We tested the model with data from Harvard Forest, Massachusetts, USA (42° 48′ N, 72° 18′ W). Harvard Forest is predominantly a mid-successional stand of mixed hardwoods dominated by Acer and Quercus. Harvard Forest has a mean annual temperature of 8.7 °C, mean annual precipitation of 1300 mm, and a soil pH of 5.2 (see Table S2 for detailed environment information).
Measurements
The data used for model testing was downloaded from the Harvard Forest Data Archive: HF018 (http://harvardforest.fas.harvard.edu:8080/exist/apps/datasets/) (Frey and Melillo 2021). Data included soil net N mineralization, nitrification, soil respiration, and foliar N content from 2003 to 2009. The available N data were taken from field experiments with the data from control plots reflecting ambient conditions. Soil net N mineralization and nitrification were measured for the forest floor and mineral soil using an on-site buried bag incubation. Incubations were incubated for 5 weeks, April- November, and for 5 months through the winter (once each month, November through March) (Butler et al. 2012). Soil water content was measured as the volumetric water content with 15 cm time delay reflectometry (TDR, ECH20 probes, Decagon Devices, Inc., Pullman, WA) probes inserted vertically in the soil. The daily soil respiration data was measured using four methods (soda-lime systems, static chamber systems, dynamic chamber systems, and automated chamber systems), which are all based on PVC or aluminum collars inserted in the soil (dataset: HF194) (Davidson et al. 2014). The measured soil respiration was the sum of belowground autotrophic (roots and associated mycorrhizae) and heterotrophic (mainly microbes, microfauna, and mesofauna) respiration (Giasson et al. 2013).
Model initialized and driven data
We used the maximum leaf area index (LAI) from the GLASS dataset in 2003 to initialize the vegetation data (http://www.glass.umd.edu/Download.html). Meteorological and CO2 concentration data for this site were taken from the Harvard Forest flux tower observation data (FLUXNET2015 dataset: https://fluxnet.fluxdata.org/). Soil organic carbon (kg m−2), organic nitrogen (kg m−2), soil pH, soil field capacity (mm), wilting point (mm), bulk density (kg m−3), sand content (%), silt content (%), and clay content (%) were all extracted from the Harmonized World Soil Database (https://iiasa.ac.at/web/home/research/researchPrograms/water/HWSD.html) (Fischer et al. 2008). Due to the resolution of HWSD (1 km × 1 km), we chosen the grid covering the Harvard Forest as the unit area for basic soil data. These data had been successfully used previously by the original FORCCHN model to simulate the global forest carbon cycle (Ma et al. 2017).
Model description
FORCCHN2 was initialized from the LAI or tree inventory data at the plot scale (Fang et al. 2020). The model was run with a daily time step and was driven by daily meteorological data. On each day, NSCs produced by photosynthesis was used as the basic material for the life activities of individual trees (including respiration and growth). Part of the structural carbon formed by growth entered the soil as litter fall. Individuals of different tree species influenced soil organic C and N dynamics through differences in growth rates and litterfall rates as encoded in the physiological and ecological parameters for each species (Method S1 and Table S3). Species differences in litter composition affected both litter decomposition and soil turnover times, with the differing nutrient compositions of leaves and fine roots also producing differences in the respective lignin to N and C to N (C:N) ratios. Litter on the ground was divided into seven pools: above-ground metabolic, above-ground structural, below-ground metabolic, below-ground structural, fine woody, coarse woody, and below-ground coarse litter. Soil organic matter (SOM) was divided into three pools: active SOM, slow SOM, and resistant SOM, all with different decomposition rates (Method S1 and Table S4).
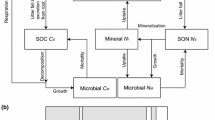
Inorganic N dynamics The soil mineralized N pool (NPmin) included two sections: the NH4+-N pool (Ammonium: Amm) and the NO3−-N pool (Nitrate: Nit) (Fig. 1). The main control process of NPmin was defined by the following functions:
Mineralization, immobilization, volatilization, nitrification, denitrification, tree uptake, and leaching were assumed to determine the mineralized N pool (NPmin) (Niu et al. 2016), with nitrate derived from soil available ammonium through nitrification (nitri). The soil ammonium and nitrate pool were calculated by:
where Ngross is the soil gross N mineralization rate; Immamm is the immobilized N, which represents transitions from mineralized ammonium to the active SOM pool; vol is the flux of ammonia volatilization; uptake is the plants N uptake; gasnitri is the gas emissions during the nitrification process; denit is the amount of denitrification, and leaching is the amount of N leaching. The soil net mineralization rate is equal to the gross N mineralization rate minus mineralized N loss rate (i.e. immobilization, volatilization, and leaching).
Gross N mineralization and N immobilization We assumed that N mineralization was in the form of ammonium and that mineralized N processes only occurred in the active SOM pool (Kirschbaum and Paul, 2002). We followed Kirschbaum and Paul (2002) to model the soil gross N mineralization rate and immobilization rate:
where Minamm is the amount mineralized ammonium rate; Pa,n and Pa,c is the amounts of C and N in the active SOM pool (i.e. calculated by Method S1); RCN is the critical C:N ratio of the active SOM pool for N mineralization. RCN is determined by the initial soil organic carbon (CSOM,0) and nitrogen (NSOM,0) content in the soil active pool (Ma et al. 2017):
Ammonium volatilization Previous studies have shown that ammonium storage is decreased when the soil pH value (pH) becomes large. Here, we used the soil pH to determine the ammonium volatilization (Lin et al. 2000):
where a and b are the parameters of soil pH function.
Ammonium nitrification Nitrification is the key process in the N cycle whereby ammonium is converted into nitrate by nitrobacteria (van Kessel et al. 2015). Nitrification always progresses with emissions of gases (gasnitri) such as nitric oxide. Zhang et al. (2017) used the Monod kinetics and soil pH to model the nitrification. We assumed that soil water content and soil temperature also influence N nitrification (nitri) because these environmental factors can affect the activity of nitrifiers (Lin et al. 2000; Yang et al. 2017). We modified the equation as:
where nitri is the loss of ammonium to nitrification; Bnitri is the biomass of nitrifiers (kg m−2); nmax is the maximum nitrification rate (day−1); COEnr is the nitrification coefficient; CN is the soil C:N; wfps is the fraction of soil pore space filled with water; anitri and bnitri are the parameters for water influence; T is the soil temperature; and kgas is the parameter for gas emissions during nitrification.
Nitrate denitrification We assumed that this process depends on the biomass of denitrifiers (Bdenit), soil pH, and soil temperature as:
where kdenit is the coefficient of nitrate consumption; Rnit is the relative growth rate of denitrifiers (h−1) (see Eq. 27); EFnit is the efficiency parameter for nitrate denitrifiers; Mnit is the maintenance coefficient of nitrate (h−1); Ntotal is the total N of all the oxynitride (kg m−2); Topt,denit is the optimum temperature of denitrification; Q10,nitri is the temperature sensitivity of denitrification.
Nitrate Leaching We assumed that nitrogen leaching only happens in the nitrate pool. Leaching of soil nitrate (leaching) was calculated as the product of water content (WS) (Lin et al. 2000):
where al and bl are the parameters; WF is the field capacity.
Tree nitrogen uptake We used the approach known as the Michaelis–Menten function to represent mineralized nitrogen competition between trees and other inorganic nitrogen consumers (Ghimire et al. 2016). Tree uptake was determined by tree fine root biomass (Cfineroot):
where Nmax is the maximum nitrogen uptake per root biomass at 25 °C; kmin is the concentration of available nitrogen at which nitrogen uptake proceeds at one-half of its maximum rate; aup and bup are the parameters of water influence; Q10,up is the temperature sensitivity of uptake; T is the soil temperature; Topt,nitri is the optimum temperature of uptake.
Nitrifiers and denitrifiers The net incremental biomass of nitrifiers is determined by the growth and mortality rates and the biomass of nitrifiers. Both rates are modified by soil dissolved organic carbon (DOC) and water content. DOC is the main substrate for microbial growth and water availability inhibits the activity of microbial enzymes. DOC is calculated by the FORCCHN2 model (Eqn S26). The net increment of nitrifiers biomass (NB,nitri) is determined by growth (Gnitri) and mortality (Dnitri) rates (Li et al. 2000; Zhang et al. 2017):
where Gmax and Dmax maximum growth and death rate of nitrifiers, respectively.
The net increment of denitrifiers biomass (NB,denit) is determined by growth (Gdenit) and mortality (Ddenit) rates. The growth rate of denitrifiers (Gdenit) is related to soil temperature and pH:
where MC is the maintenance coefficient on carbon; YC is the maximum growth rate of denitrifiers on soluble carbon.
We used the Michaelis–Menten equation of two substrates to model the relative growth rate of denitrifiers (Rnit):
where MUnit is the maximum growth rate of denitrifiers; aDOC and anit are the half-saturation of DOC and nitrate, respectively.
Model initialization and coupling
The FORCCHN2 model used the maximum LAI of the first year and the forest types to initialize the vegetation information, which included tree size and biomass (see Method S2). The forest type of Harvard Forest was the deciduous broadleaf forest and the maximum LAI of this forest was 5.7 m2 m−2. The FORCCHN2 assumed that fine root growth was related to leaf growth (Method S1) and that the growth processes of other organs were as described by Fang et al. (2020). For soil initialized conditions, the initial value of the mineralized N pool was assumed to be 0.3% of the soil organic N pool (Table S2). The initial value of the active C pool was assumed to be 0.065% of the soil organic C pool (Pa,c0). The initial value of the active N pool (Pa,n0) was assumed as Pa,c0 divided by the RC,N. We allocated the organic C and N into 11 pools by using the initialized allocation parameters (Table S5). After setting these initial soil parameters, we conducted a spin-up run (∼ 100 years) using multiyear mean climate data from 2003 to 2009. The resulting allocation ratios of the 11 pools were considered as the new allocation parameters for our simulations. We coupled the tree and soil dynamics through litter and fine root-related processes through Eq. 18 (Fig. 1). All variables and parameters are summarized in Tables 1 and 2.
Before validation, we obtained model parameters from the literature and conducted a sensitivity analysis to test the parameters of Table 2. Here, we used the sensitivity index to quantify parameter sensitivity in this study. This sensitivity index (SI) is expressed as the ratio of predicted soil net mineralization (g m−2 day−1) by the original parameter and the relative change parameter (Lenhart et al. 2002):
where n is the number of total days during 2003–2009; j is the jth day; y0j is the predicted soil net mineralization at the jth day; x0 is the value of the original parameter; △x is 20% of x0; y2j is the predicted soil net mineralization when the parameter equal to x0 plus △x at the jth day; y1j is the predicted soil net mineralization when the parameter equal to x0 minus △x at the jth day. Note that the larger absolute value of SI implies a higher parameter sensitivity, and Lenhart et al. (2002) ranked the sensitivity into four classes based on SI: (1) 0 ≤|SI|< 0.05, this means small to negligible sensitivity; (2) 0.05 ≤|SI|< 0.2, this means medium sensitivity; (3) 0.2 ≤|SI|< 1, this means high sensitivity; (4) |SI|≥ 1, this means very high sensitivity.
We used correlation coefficient (r), model efficiency (E), root mean square error (RMSE), and mean absolute error (MAE) to evaluate predictions against measurements (Method S3).
Results
Sensitivity analysis
Overall, the sensitivity index of each parameter varied within a range from -0.6 to 0.6 (Fig. 2). Some parameters showed a large effect on the predicted results of net mineralization (i.e. kmin, Q10,denit, Nmax, Q10,up and aup, |SI|> 0.2), while some parameters showed an inconsequential effect on the predicted results of net mineralization as the sensitivity index was small (e.g. a, bnitri, kgas, and EFnit, |SI|< 0.05). Based on the equations in this model, we found that the parameters with high sensitivity were critical components of the N uptake and denitrification. Indicating that all parameters exhibited a sensitivity index of less than 0.6 and could not be better constrained using the datasets in this study. Therefore, we set the model parameters to their original constant values in this study (Table 2).
Soil respiration
Soil autotrophic and heterotrophic respiration represented the dynamics of soil organic carbon, which was affected by soil organic nitrogen storage changes (Fig. 1). The model performed well in simulating the intra-annual variability of daily soil respiration for Harvard Forest (Fig. 3 and Table 3, r = 0.9, E = 0.76). Soil respiration was unsurprisingly the lowest during winter and the highest during the warmest days. However, model predictions generally underestimated the value of soil respiration in non-growing seasons (RMSE = 1.16 g C m−2 day−1, MAE = 0.85 g C m−2 day−1).
Soil water and microbes dynamics
In spring and winter, the lower evapotranspiration maintained higher soil water content than in summer. We used the water sub-module of FORCCHN2 to predict the dynamics of water content in the soil (Fig. 4a) and assumed that soil water content inhibited the activity of microbes (Eq. 21). The model was able to reproduce the dynamics of soil water content (r = 0.85, E = 0.70). Water dynamics also influenced the activity of nitrifiers. For example, the nitrifiers showed a negative growth rate between days 210–270 due to the lowest water content (Fig. 4b). The activity of denitrifiers was assumed to be highly influenced by temperature (Fig. 4c): denitrifiers decreased in winter and early spring, and then grew rapidly in the transition time between spring and summer.
Soil inorganic N dynamics
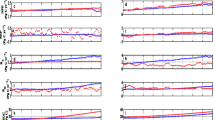
To directly compare predictions with measurements (limited to the available data), the daily predictions of mineralized N data were accumulated to monthly values. All of the simulated and measured results (i.e. net soil N mineralization rate, net soil ammonium increment rate, and net soil nitrification rate) from Harvard Forest were qualitatively similar in their patterns of monthly fluctuations (Fig. 5a, b, c and Table 3, r > 0.7). Most minimum and maximum measured values were seen in January and July, respectively. The predictions had less inter-annual variability than the Harvard Forest measurements.
For the net soil N mineralization rate, simulated results were quite similar to the Harvard forest experimental results though there were marked differences in 2006 and 2007 (Fig. 5a, r = 0.84, E = 0.55). The model overestimated the net soil N mineralization rate in 2006 and 2007, whereas it was underestimated slightly in the summer of other years (RMSE = 2.35 g N m−2 day−1, MAE = 1.61 g N m−2 day−1). For the net soil ammonium increment rate, patterns were similar to the net soil N mineralization rate because the soil ammonium was mainly based upon N mineralization (Fig. 5b, r = 0.82, E = 0.52). The model also overestimated soil ammonium increment rate in 2006 and 2007 (RMSE = 2.23 g N m−2 day−1, MAE = 1.51 g N m−2 day−1). The model overestimated the net soil nitrification rate in most years (Fig. 5c, RMSE = 0.24 g N m−2 day−1, MAE = 0.18 g N m−2 day−1). These results showed the poorest simulated performance (r = 0.76, E = 0.18). In 2004, the model overestimated the value of the net soil nitrification rate.
For the dynamics of daily mineralized N, there was insufficient available data to robustly test the model. Here, we calculated all the details of daily mineralized N’s dynamics including inorganic N production (i.e. soil net N mineralization) (Fig. 6a), total N losses (Fig. 6b) (i.e. equal to the sum of N losses of ammonium and nitrate), N uptake (Fig. 6a), and soil inorganic N pool (Fig. 6c). All of the results changed with the microbes and the concentration of the substrates. The soil inorganic N pool had regular seasonal variations during a given year: in the spring, soil inorganic N pool changed around the initial value due to the low gross N mineralization rate and low tree N uptake. In the summer, the soil inorganic N pool increased because the gross N mineralization rate was more than N uptake and N losses. Then, soil inorganic N pools decreased slowly in the autumn and winter. The results showed that the N mineralization rate and tree N uptake had increased at the end of the year because the model assumed a part of litter fell onto the soil in the fall (i.e. controlled by phenology). The predicted results supported the hypothesis that N losses were consistent with the corresponding substrate concentrations and environment factors, such as leaching of nitrate with changes of soil water content calculated by Eq. 17 and volatilization with ammonium dynamics calculated by Eq. 8. Note that the total N losses consumed a small part of produced inorganic N (Fig. 6a: Gross mineralization and Total losses) in accordance with observations from this site. Site observations indicated no significant leaching or gaseous losses of N (Butler et al. 2012). For tree N uptake, the results showed a strong seasonal pattern (Fig. 6a: Tree uptake): in early spring, trees only needed a small amount of inorganic nitrogen because of slow fine root growth. The peak N uptake occurred in summer because of the creation of a large biomass of fine roots. In the FORCCHN2, the fine root was assumed to begin growing at the time when the environmental conditions reached the requirement of leaf phenology (Fig. S1).
a Daily dynamics of gross N mineralization, N losses and tree N uptake at Harvard Forest during 2003, b N losses; further subdivided into, c the nitrate losses; and d the ammonium losses, e the daily dynamics of soil inorganic N pool with the black line indicating the initial value. Negative values indicate soil N losses
Discussion
FORCCHN2 was able to reproduce the temporal changes in soil respiration and N mineralization rates at Harvard Forest. Although there was not enough measured data to run a strong test of the daily dynamics of every detailed process, the model yielded plausible results for each sub-process.
Soil respiration includes underground autotrophic and soil heterotrophic respiration, which is correlated with biotic and abiotic factors such as temperature and soil water content. Coupled with the predictions of litterfall and root biomass, this model was able to simulate daily soil respiration at this site. However, there were some major discrepancies between predictions and observations in the non-growing season, likely resulting from three factors; first, our predictions assumed that surface soil has little litter input in the non-growing season because the deciduous trees are leafless (Butler et al. 2012). Second, the temperature in the non-growing season is low and far below the assumed optimum temperature of plant root and microbial respiration. For the optimum temperature, we used the globalized parameters instead of localized parameters because few localized optimum temperature data were available for this site (Giasson et al. 2013). Third, the heterotrophic respiration in the non-growing season may dependent upon DOC availability or concentration (Bowering et al. 2020), but our model has not established a direct relationship between the respiration and DOC. Thus, model accuracy could likely be improved with accurate localized temperature parameters, DOC relationship, and litterfall information.
Comparing the process responses of this model against the field experimental measured data provided essential information on model validity. Our predictions of N mineralization showed a strong seasonality, which conforms to the observed variations. Fluctuations can be largely attributed to the seasonal variation of temperature and soil water content that not only affects soil microorganism activities but also the amount of litterfall (Sierra 1997; Pendall et al. 2008). Similar fluctuations also occurred in the simulated results of net ammonium and nitrate increment, because the simulated N-mineralization produces ammonium some of which is converted to nitrate (Zhu et al. 2013).
Other discrepancies between simulated results and measurements could be partially explained by the uncertainties inherent in soil measurements (Jastrow et al. 2005). For example, soil respiration in winter was hard to measure because of the non-turbulent transport of CO2 (Giasson et al. 2013). The lack of detailed in situ soil information may also be a source of error in modeled C and N dynamics. The initialized soil parameters for this work were obtained from large spatial scale datasets (1 km × 1 km in this study), but the soil environmental variables can vary widely over short distances (Lucas-Borja et al. 2012). Potential errors could also have arisen from our use of the LAI data, and not the actual tree inventory data (i.e. the LAI data could not provide accurate information of biomass and species). Prior studies have indicated that different tree species could directly influence the N-mineralization rate (Van Der Krift and Berendse 2001), but the experiments available for model testing did not detail the actual vegetation information in this site. Further, we only considered some bacteria instead of all microorganisms that involved soil C and N. For example, we modeled the plant N uptake from the fine roots and did not express the competition from other N consumers explicitly. We need to further explore the mechanisms from other microorganisms in our future work.
In addition, the parameters and processes in the model did not completely explain the actual vegetation and soil conditions, for example the litterfall rate may have been slightly overestimated and thus increased the amount of simulated net mineralized N. We did not consider external N inputs in the model because some recent studies have suggested that the role of external N inputs on forest N-mineralization remains uncertain (Rodriguez et al. 2014). However, the experimental processes in situ have natural external N inputs (including the N deposition, biological N fixation, etc.) and many studies indicate that these N inputs have a strong influence on soil N-mineralization (Nave et al. 2009; Prescott 2010). Furthermore, the net N-mineralization rate in 2005 has three obvious peak values (Harvard forest), which may arise from the pretreatment of a new soil warming and nitrogen addition experiment initiated in 2005 (Record et al. 2010). At this time, this model does not incorporate such case-specific exogenous interventions. This could be added as a case study in future but our intent here is to use a global parameterization and test its generality.
The results showed a better performance for net N-mineralization and net ammonium increment and relatively poorer performance for the net nitrate increment. First, although the parameters of nitrification and denitrification have achieved the desired results, these parameters may also generate potential uncertainties, such as the COEnr and kdenit. However, these parameters are difficult to measure directly and have not been well studied (Zhang et al. 2017). Using observations to constrain and calibrate the nitrification and denitrification parameters is effective, and more observed sites are needed to calibrate these more difficult parameters. Second, we predicted nitrification and denitrification based on the dynamics of microbes. Soil substrate concentration, temperature, and moisture are the main drivers for soil bacteria activities and soil microbial biomass including growth and mortality. Among these factors, water availability inhibits the nitrifiers by reducing intracellular water potential and restraining the enzymatic activity (Stark and Firestone 1995; Zhang et al. 2017). The seasonal variation in soil temperature is a major factor in affecting the activities of denitrifiers through inhibiting the activity of enzymes. However, the ability of this model to explain these factors is limited. For example, soil water freezes in winter and early spring killing soil microbes and reducing plant uptake, which can cause an overestimation or underestimation of nitrification and denitrification (Gao et al. 2018). Thus, further research is needed to better constrain the potential impact of such events on nitrate dynamics.
In summary, we predicted the dynamics of mineral N by coupling the carbon dynamics and the detailed N cycle process es within an updated model. This model was able to generate day-to-day inorganic N production from N-mineralization, N losses for leaching and gaseous emissions, and plant N uptake. We view this model development as a new step toward a more mechanistic treatment of terrestrial N cycling based on the dynamics of plant biomass, soil substrate, and microbes. This model would help us to better understand and predict ecosystem N cycling in forest soils. However, further improvements in the prediction of mineralized N include: using more model validation sites to further verify the general applicability of the model, exploring the nitrification process more clearly in future experiments, and increasing the scope of N inputs considered by the model.
Data availability
The nitrogen measured data used in this study are publicly available through the Harvard Forest Data Archive HF018. The soil respiration data used in this study are publicly available through the Harvard Forest Data Archive HF194.
References
Bardgett RD, Manning P, Morrien E, Vries FT (2013) Hierarchical responses of plant–soil interactions to climate change: consequences for the global carbon cycle. J Ecol 101:334–343
Bowering KL, Edwards KA, Prestegaard K, Zhu X, Ziegler SE (2020) Dissolved organic carbon mobilized from organic horizons of mature and harvested black spruce plots in a mesic boreal region. Biogeosciences 17:581–595
Butler SM, Melillo JM, Johnson JE, Mohan J, Steudler PA, Lux H, Burrows E, Smith RM, Vario CL, Scott L, Hill TD, Aponte N, Bowles F (2012) Soil warming alters nitrogen cycling in a New England forest: implications for ecosystem function and structure. Oecologia 168:819–828
Chapman LY, McNulty SG, Sun G, Zhang Y (2013) Net nitrogen mineralization in natural ecosystems across the conterminous US. Int J Geosci 4:1300–1312
Cleveland CC, Houlton BZ, Smith WK, Marklein AR, Reed SC, Parton W et al (2013) Patterns of new versus recycled primary production in the terrestrial biosphere. Proc Natl Acad Sci 110:12733–12737
Clivot H, Mary B, Valé M, Cohan J-P, Champolivier L, Piraux F, Laurent F, Justes E (2017) Quantifying in situ and modeling net nitrogen mineralization from soil organic matter in arable cropping systems. Soil Biol Biochem 111:44–59
Comins H, McMurtrie R (1993) Long-Term response of nutrient-limited forests to CO" 2 enrichment; equilibrium behavior of plant-soil models. Ecol Appl 3:666–681
Davidson E, Ellison A, Finzi A, Hadley J, Melillo J, Munger W, Ollinger S, Tang J, Varner R (2014) 20-Year synthesis of soil respiration data at harvard forest 1991–2008. Harvard Forest Data Archive: HF194
Dou Z, Toth JD, Jabro JD, Fox RH, Fritton DD (1996) Soil nitrogen mineralization during laboratory incubation: dynamics and model fitting. Soil Biol Biochem 28:625–632
Fang J, Lutz J, Wang L, Shugart H, Xiaodong Y (2020) Using climate-driven leaf phenology and growth to improve predictions of gross primary productivity in North American forests. Glob Change Biol 26:6974–6988
Fischer G, Nachtergaele F, Prieler S, Van Velthuizen HT, Verelst L, Wiberg D (2008) Global agro-ecological zones assessment for agriculture (GAEZ 2008). IIASA, Laxenburg
Frey S, Melillo J (2021) Barre woods soil warming experiment at harvard forest since 2001. Harvard Forest Data Archive: HF018.
Gao D, Zhang L, Liu J, Peng B, Fan Z, Dai W, Jiang P, Bai E (2018) Responses of terrestrial nitrogen pools and dynamics to different patterns of freeze-thaw cycle: a meta-analysis. Glob Change Biol 24:2377–2389
Ghimire B, Riley WJ, Koven CD, Mu M, Randerson JT (2016) Representing leaf and root physiological traits in CLM improves global carbon and nitrogen cycling predictions. J Adv Model Earth Syst 8:598–613
Giasson M-A, Ellison AM, Bowden RD, Crill PM, Davidson EA, Drake JE, Frey SD, Hadley JL, Lavine M, Melillo JM (2013) Soil respiration in a northeastern US temperate forest: a 22-year synthesis. Ecosphere 4:1–28
Jastrow JD, Michael Miller R, Matamala R, Norby RJ, Boutton TW, Rice CW, Owensby CE (2005) Elevated atmospheric carbon dioxide increases soil carbon. Glob Change Biol 11:2057–2064
Kirschbaum MU, Paul KI (2002) Modelling C and N dynamics in forest soils with a modified version of the CENTURY model. Soil Biol Biochem 34:341–354
Lenhart T, Eckhardt K, Fohrer N, Frede HG (2002) Comparison of two different approaches of sensitivity analysis. Phys Chem EarthParts a–c 27:645–654
Li C, Aber J, Stange F, Butterbach-Bahl K, Papen H (2000) A process-oriented model of N2O and NO emissions from forest soils: 1. Model development. J Geophys Res 105:4369–4384
Lin B-L, Sakoda A, Shibasaki R, Goto N, Suzuki M (2000) Modelling a global biogeochemical nitrogen cycle in terrestrial ecosystems. Ecol Model 135:89–110
Liu Y, Wang C, He N, Wen X, Gao Y, Li S, Niu S, Butterbach-Bahl K, Luo Y, Yu G (2017) A global synthesis of the rate and temperature sensitivity of soil nitrogen mineralization: latitudinal patterns and mechanisms. Glob Change Biol 23:455–464
Lucas-Borja M, Candel Pérez D, López Serrano F, Andrés M, Bastida F (2012) Altitude-related factors but not Pinus community exert a dominant role over chemical and microbiological properties of a Mediterranean humid soil. Eur J Soil Sci 63:541–549
Ma J, Shugart HH, Yan X, Cao C, Wu S, Fang J (2017) Evaluating carbon fluxes of global forest ecosystems by using an individual tree-based model FORCCHN. Sci Total Environ 586:939–951. https://doi.org/10.1016/j.scitotenv.2017.02.073
Menge DN, Chisholm RA, Davies SJ, Abu Salim K, Allen D, Alvarez M et al (2019) Patterns of nitrogen-fixing tree abundance in forests across Asia and America. J Ecol 107:2598–2610
Meyerholt J, Sickel K, Zaehle S (2020) Ensemble projections elucidate effects of uncertainty in terrestrial nitrogen limitation on future carbon uptake. Glob Change Biol 26:3978–3996
Nave L, Vance E, Swanston C, Curtis P (2009) Impacts of elevated N inputs on north temperate forest soil C storage, C/N, and net N-mineralization. Geoderma 153:231–240
Niu S, Classen AT, Dukes JS, Kardol P, Liu L, Luo Y, Rustad L, Sun J, Tang J, Templer PH (2016) Global patterns and substrate-based mechanisms of the terrestrial nitrogen cycle. Ecol Lett 19:697–709
Oren R, Ellsworth DS, Johnsen KH, Phillips N, Ewers BE, Maier C, Schäfer KV, McCarthy H, Hendrey G, McNulty SG (2001) Soil fertility limits carbon sequestration by forest ecosystems in a CO2-enriched atmosphere. Nature 411:469–472
Pendall E, Rustad L, Schimel J (2008) Towards a predictive understanding of belowground process responses to climate change: have we moved any closer? Funct Ecol 22:937–940
Potter CS, Randerson JT, Field CB, Matson PA, Vitousek PM, Mooney HA, Klooster SA (1993) Terrestrial ecosystem production: a process model based on global satellite and surface data. Global Biogeochem Cycles 7:811–841
Prescott CE (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–149
Record S, Frey S, Ellison A (2010) Short-term affects on vegetation in soil warming plus nitrogen addition experiment at Harvard forest 2006. Harvard Forest Data Archive: HF163
Rodriguez A, Lovett GM, Weathers KC, Arthur MA, Templer PH, Goodale CL, Christenson LM (2014) Lability of C in temperate forest soils: assessing the role of nitrogen addition and tree species composition. Soil Biol Biochem 77:129–140
Rustad L, Campbell J, Marion G, Norby R, Mitchell M, Hartley A, Cornelissen J, Gurevitch J (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Schimel JP, Bennett J (2004) Nitrogen mineralization: challenges of a changing paradigm. Ecology 85:591–602
Sierra J (1997) Temperature and soil moisture dependence of N mineralization in intact soil cores. Soil Biol Biochem 29:1557–1563
Stanford G, Carter J, Smith S (1974) Estimates of potentially mineralizable soil nitrogen based on short-term incubations. Soil Sci Soc Am J 38:99–102
Stark JM, Firestone MK (1995) Mechanisms for soil moisture effects on activity of nitrifying bacteria. Appl Environ Microbiol 61:218–221
Thomas R, Bonan G, Goodale C (2013) Insights into mechanisms governing forest carbon response to nitrogen deposition: a model–data comparison using observed responses to nitrogen addition. Biogeosciences 10:3869–3887
Van Der Krift TA, Berendse F (2001) The effect of plant species on soil nitrogen mineralization. J Ecol 89:555–561
van Kessel MA, Speth DR, Albertsen M, Nielsen PH, Op den Camp HJ, Kartal B, Jetten MS, Lucker S (2015) Complete nitrification by a single microorganism. Nature 528:555–559
Wang YP, Houlton BZ (2009) Nitrogen constraints on terrestrial carbon uptake: Implications for the global carbon-climate feedback. Geophys Res Lett 36:45
Yang WH, Ryals RA, Cusack DF, Silver WL (2017) Cross-biome assessment of gross soil nitrogen cycling in California ecosystems. Soil Biol Biochem 107:144–155
Zaehle S, Medlyn BE, De Kauwe MG, Walker AP, Dietze MC, Hickler T, Luo Y, Wang YP, El-Masri B, Thornton P (2014) Evaluation of 11 terrestrial carbon–nitrogen cycle models against observations from two temperate Free-Air CO2 Enrichment studies. New Phytol 202:803–822
Zhang K, Peng C, Wang M, Zhou X, Li M, Wang K, Ding J, Zhu Q (2017) Process-based TRIPLEX-GHG model for simulating N2O emissions from global forests and grasslands: Model development and evaluation. J Adv Model Earth Syst 9:2079–2102
Zhu T, Meng T, Zhang J, Yin Y, Cai Z, Yang W, Zhong W (2013) Nitrogen mineralization, immobilization turnover, heterotrophic nitrification, and microbial groups in acid forest soils of subtropical China. Biol Fertil Soils 49:323–331
Acknowledgements
This study was supported by the National Natural Science Foundation of China (32101349, 31570473) and by a grant from the University of Virginia Center for Global Inquiry and Innovation. This study also was supported by the National Natural Science Foundation of China (31700462, 31870465) and the National Key Research and Development Program of China (Grant 2018YFC1509003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Susan Ziegler.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fang, J., Lutz, J.A., Shugart, H.H. et al. Predicting soil mineralized nitrogen dynamics with fine root growth and microbial processes in temperate forests. Biogeochemistry 158, 21–37 (2022). https://doi.org/10.1007/s10533-021-00883-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-021-00883-8