Abstract
Aims
We aim to show the seasonality of fine root dynamics and examine the relationship between root respiration (Rr) and fine root dynamics. In addition, we try partitioning Rr into growth (Rg) and maintenance (Rm) components.
Methods
Soil respiration (Rs), fine root biomass (B), and fine root production (P) were measured simultaneously over a growing season in adjoining deciduous (DF) and evergreen (EF) forests. The Rr was separated from Rs by the trenching method, and Rr was partitioned into Rg and Rm using an empirical model.
Results
The seasonality of P was almost the same in both forests, though that of B was different. The Rr showed a positive correlation with P in both sites. Annual Rr was estimated to be 610 (DF) and 393 (EF) g C m−2 year−1. Annual Rg and Rm were 121 and 166 (DF), and 86 and 182 (EF) g C m−2 year−1, respectively.
Conclusions
We found a clear seasonal pattern in P and a positive linearity between Rr and P. Despite considerable uncertainty due to the small sample size, presence of larger roots, and measurement uncertainty, the results suggest that our approach is capable of partitioning Rr.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Terrestrial ecosystems have sequestered carbon dioxide (CO2) at a rate of 3.2 ± 0.8 Pg C year−1 during the last decade until 2016, accounting for 30% of total CO2 emissions from fossil fuels, industry, and land-use change (Le Quéré et al. 2018). Forests, one of the carbon-richest ecosystems, cover about 30% of the land surface (Bonan 2008; Keenan et al. 2015) and was estimated to be a large CO2 sink of 2.4 ± 0.4 Pg C year−1 from 1990 to 2007 (Pan et al. 2011). Therefore, forests are crucial ecosystems for mitigating climate change mostly owing to capturing atmospheric CO2 during growth.
Belowground biological processes, such as root respiration and microbial decomposition of soil organic matter, greatly contribute to the carbon balance of forest ecosystems (Davidson et al. 2006; Janssens et al. 2001). In particular, fine roots, which are commonly defined as thin roots less than 2 mm in diameter, are known for their vital role in global biogeochemical cycles (McCormack et al. 2015). Fine roots function to absorb nutrients and water from the soil and have a shorter life cycle through production, mortality, and littering with average turnover rates of about one year in temperate forests (Brunner et al. 2013; Finér et al. 2011b). In addition, despite their small biomass the contribution of fine roots to the net primary production (NPP) of forests is relatively high at 32% for boreal forest (Yuan and Chen 2010), and 22% globally (McCormack et al. 2015). Also, fine root litter is a major carbon source in forest soils (Richter et al. 1999). Therefore, fine root dynamics are key processes that govern forest productivity and biogeochemical cycles.
Fine root phenology strongly influences belowground carbon dynamics and allocation, whereas seasonality and variability in the production and respiration of fine roots are not well understood among forest types (Abramoff and Finzi 2015; McCormack et al. 2014; Radville et al. 2016). Moreover, the underlying mechanisms controlling fine root dynamics remain limited because of the lack of experimental evidence (Liu et al. 2018; Radville et al. 2016). In particular, the dynamic functions of fine roots, such as respiration, are not quantitatively understood, which has restrained the improvement of terrestrial biosphere models to predict the responses of forest ecosystems to environmental changes (McCormack et al. 2015; Warren et al. 2015). Therefore, field experiments which simultaneously measure the production and respiration of fine roots in different forest types over a growing season will help improve our qualitative understanding of the role of fine roots in forest carbon cycles.
Empirical models exist that partition plant respiration into growth and maintenance requirements (Amthor 2000; McCree 1974; Penning de Vries 1974; Thornley 1970), plus the respiratory cost of ion uptake by fine roots (Chapin III et al. 2011; Johnson 1990; Lambers et al. 2008). In these models, growth and maintenance respirations correlate linearly with production (growth) and biomass, respectively. Problems with plant respiration models occur in the quantification of respiration partitioning (Gifford 2003), which lack a mechanistic basis (Sweetlove et al. 2013). Nevertheless, laboratory experiments suggested that the models were useful for understanding the carbon balance of plants, though the partitioning would differ by plant species and environmental conditions (Lambers et al. 2008). Also, the partitioning of respiration into these functional components will help in the understanding of the ecological controls over plant respiration (Chapin III et al. 2011). These models have been adopted by terrestrial biosphere models, such as Biome-BGC (Thornton and Rosenbloom 2005), to explicitly represent autotrophic respiration as the sum of growth and maintenance respirations. However, the responses of the models’ rate coefficients to abiotic and biotic factors have not been well understood (Hopkins et al. 2013) owing to the lack of field experiments (Amthor 2000). So far, there have been no comparisons of model predictions to field conditions, except for (George et al. 2003) partitioning fine root respiration in field conditions. They separately estimated fine root respiration for growth, maintenance, and ion uptake (only nitrogen) in evergreen and deciduous forests using short-term chamber measurements and literature information.
In this study, root respiration and fine root dynamics (production and biomass) were measured simultaneously in adjoining deciduous and evergreen forests in a cool temperate climate over a growing season in order to: 1) quantify root respiration with reliability, 2) compare fine root phenology between the different forest types, and 3) examine the relationship between root respiration and fine root dynamics. In addition, we try partitioning the root respiration into growth and maintenance components on an annual basis using an empirical model. After the growth and maintenance respirations of fine roots are quantified in the two forests, the model’s rate coefficients are compared between the forests and to coefficients reported from laboratory and greenhouse experiments.
Materials and method
Study site
The study was conducted in two adjacent sites (deciduous and evergreen forests) in southern Hokkaido, northern Japan (42° 44′ N, 141° 31′ E, 125 m above sea level). The soil type was volcanogenous regosol with a high water permeability. In both sites, below the leaf litter and a 4 cm thick root mat, there is a 10 cm thick surface soil layer (A horizon) and an unweathered pumice layer (C horizon); a B horizon is lacking (Hirano et al. 2003a).
The deciduous forest (DF) was established in 1957 as a Japanese larch (Larix kaempferi) plantation. However, it was severely devastated by typhoon Songda in 2004. The typhoon blew down about 90% of the trees (Sano et al. 2010). In 2005 the fallen tree stems were removed; afterwards the plantation has not been managed (Hirano et al. 2017). In 2015 the dominant tree species was still Japanese larch, followed by Ulmus davidiana var. japonica (Japanese elm), Acer pictum subsp. dissectum (maple), and Quercus crispula (oak), with a density of 2360 stems ha−1 for trees taller than 2 m. The forest floor is covered with Dryopteridaceae sp. (fern) and shrub species, including Rubus idaeus (raspberry) (Yazaki et al. 2016).
The evergreen forest (EF) was a Japanese spruce (Picea glehnii) plantation established next to the deciduous forest in 1979. Spruce saplings were planted in a double row 1.3 m wide at intervals approximately 6 m between rows. Almost no trees fell during typhoon Songda. Tree density was 2250 stems ha−1 in 2015. Understory vegetation was sparse.
Decadal mean annual air temperature and precipitation from 2008 to 2017 were 8.3 ± 0.3 °C and 1312 ± 172 mm (mean ± 1 standard deviation (SD)), respectively, at a meteorological station (Tomakomai) 14 km from the study site. The lowest and highest monthly mean air temperatures were − 3.9 °C in January and 20.8 °C in August, respectively. About 85% of the annual precipitation falls in the snow-free period from April to November. Snow usually covers the ground from early December through early April.
Experimental design
Field experiments were conducted from September 2014 to November 2015. In September 2014, to measure soil CO2 efflux, a set of four 0.5 m × 0.5 m aluminum collars were installed, where one collar was used for a control, another for root sampling, the third for litter removal, and the fourth for trenching. Five replications were established in each site for partitioning total soil respiration (Rs) into leaf litter decomposition (Rl), belowground heterotrophic respiration (Rh), and root respiration (Rr). To keep root density similar, four collars in each set were placed on a circle with a 2 m radius around larch trees in the DF site, or were aligned 2 m away from a tree row in the EF site (Fig. 1). Collar spacing was 20 cm, except for the trenching collars where it was 40 cm to ease the trenching effect on the next collar. Collars were inserted only 1 to 2 cm into the soil to minimize damaging surface roots (Wang et al. 2005). To avoid lateral air leak under the collars, each collar was surrounded with a bank of soil. For control collars (CC), no treatment was applied. In sampling collars (SC), the fine root experiments (described later) were conducted. In litter removal collars (LC), 1 mm meshed screens were set between the litter layer and soil surface in early November 2014. The mesh was temporarily removed with the overlying litter, and then soil CO2 efflux was measured without leaf litter. After the measurement, the mesh and litter were immediately put back on the collar. The trenching method was applied to remove Rr from Rs. For trenching collars (TC), four PVC boards 4 mm thick were vertically inserted to a depth of 0.3 m along a square collar in November 2014 to prevent roots from intruding. The decomposition of dead roots (Rd) left in TC was estimated by the root litter bag method (described later). Rl and Rr were calculated using the following equations:
where Rs is soil CO2 efflux measured in CC or SC, and RLC and RTC are CO2 effluxes from LC and TC, respectively.
Soil CO2 efflux
Soil CO2 efflux was measured once or twice a month from September 2014 to November 2015, except during the snow season. Before the treatments, CO2 efflux was also measured in September and October 2014 to check for initial spatial variations among CC, SC, LC, and TC collars. The measurements were conducted manually on each collar between 10:00 and 16:00 using a closed chamber system with two 50 cm tall cubic chambers made of transparent acrylic plastic (Sun et al. 2017). CO2 concentration and air temperature inside the chamber were measured every 5 s with an infrared gas analyzer (LI820; Li-Cor Inc., Lincoln, NB, USA) and a thermocouple probe (MHP; Omega Engineering, Stamford, CA, USA). Outputs were recorded using a data logger (CR1000; Campbell Scientific Inc., Logan, UT, USA). Each chamber was closed for 180 s. Despite the short closing, soil CO2 efflux might have been underestimated by about 5% because of lateral diffusion in relatively porous root mats (Hutchinson and Livingston 2001). Immediately after measurement, soil temperature (Ts) at a depth of 5 cm and volumetric soil water content (SWC) of the top 5 cm layer were measured, respectively, in each collar with a thermocouple probe (MHP; Omega Engineering) and a soil moisture sensor (SM150; Delta-T Devices Ltd., Cambridge, UK). Sprouts in each collar were picked carefully before each measurement. In addition, Ts at a depth of 5 cm were monitored hourly in two CC at each site using button-type temperature loggers (Thermochron SL type; KN laboratories, Osaka, Japan). SWC at a depth of 3 cm were also monitored every half-hour in four replications at a nearby re-growing forest site using TDR sensors (CS615; Campbell Scientific, Inc.) along with air temperature and precipitation (Hirano et al. 2017).
The relationship between soil CO2 efflux (R, μmol m−2 s−1) and Ts (°C) was analyzed for each collar using the following exponential equation:
where a and b are fitting parameters. To analyze the relationship with SWC, temperature-normalized soil CO2 efflux (Rb, μmol m−2 s−1) was calculated using the following equation (Hirano et al. 2003b):
where Tb is base temperature set as the mean Ts at each site. Also, bivariate models combining Eq. 3 for Ts with various equations for SWC (Reichstein and Janssens 2009) were applied to estimate soil CO2 efflux. CO2 efflux was calculated hourly from the monitoring data of Ts and SWC using a best-fit model and converted into annual CO2 emission between November 2014 and November 2015.
Dead root decomposition in trenched collars
The root litter bag method was applied to assess CO2 emissions (Rd) through the decomposition of dead roots left in TC (Epron et al. 1999; Gholz et al. 2000; Silver and Miya 2001). To determine the initial dry weight of dead roots (X0, g DM m−2), live roots were extracted in the same manner as in the fine root experiment (described later) from 30 cm thick surface soil samples in a 0.3 m × 0.3 m area near each TC (n = 5) at each site in early November 2014. The roots were separated into three classes by diameter: fine (< 2 mm), medium (2–10 mm), and coarse roots (> 10 mm). The root samples were air-dried, and parts of the samples were oven-dried at 70 °C for 48 h to determine the water content of each root class. From the water content, the dry weights of the remaining air-dried samples were determined. Air-dried samples were put into 2 mm meshed bags that were 10 cm × 10 cm; samples were 1, 3, and 5 g in dry weight, respectively, for fine, medium, and coarse roots. In each site, 25 bags for each class were buried in the A horizon near TC in late November 2014. Five bags were collected from each class five times until May 2017, and the dead roots remaining in the bags were oven-dried. The decomposition constant k (year−1) was determined using the following equation (Wieder and Lang 1982):
where Y is the dry weight of remaining dead roots (g DM bag−1) at time t (year), and Y0 is the initial dry weight of root samples (g DM bag−1). From k and X0, annual Rd (g C m−2 year−1) from November 2014 was calculated for each root class using a conventional conversion factor of 0.5 from root dry matter to carbon (Chapin III et al. 2011; Ravindranath and Ostwald 2008).
Fine root experiment
The sequential soil core method was applied to measure fine root biomass (B; g DM m−2) and its temporal variation (ΔB; g DM m−2 period−1). Three soil cores were sampled from each SC in mid-November 2014, early May, mid-June, early August, late September, and mid-November 2015. To confirm the trenching effect on fine root existence, soil cores were also sampled similarly from TC in early May and mid-June 2015. Using a perforated board for positioning, core sampling was made in each SC at three points, which were randomly selected from a grid with 8 cm spacing. Soil cores down to 14 cm were taken using a stainless-steel edged tube with an inner diameter of 2.4 cm. The total sampling area was 13.6 cm2 for each collar each time, which accounted for 0.5% of the collar area (0.25 m2). The collected core samples were first divided into the root mat (top 4 cm) and A horizon (lower 10 cm), and then washed several times with tap water to remove soil particles and fragmented litter. Next, living fine roots were extracted and were visually separated into tree and herbaceous roots in the DF site, where herbaceous plant roots were not negligible. The fine roots were dried for 48 h at 70 °C and weighed. Tree fine roots originated mainly from larch trees and shrub species in the DF site, but were almost entirely from spruce trees in the EF site.
The ingrowth core method (e.g. (Vogt et al. 1998)) was applied to measure fine root production (P; g DM m−2 period−1). Ingrowth cores were made of plastic cylindrical frames wrapped in a 2 mm meshed sheet. The cores were 14 cm long and had a diameter of 2.3 cm. Soils were taken from the A horizon in September 2014, and roots were removed using a 2 mm mesh after air drying. The cores were filled with root-free, air-dried soil and inserted vertically into three pits after the soil core sampling at each SC. The ingrowth cores were replaced simultaneously with the sequential core sampling. Soil samples in the collected ingrowth cores were first divided into the top 4 cm layer (root mat) and below 10 cm (A horizon), and washed to extract living fine roots. The fine roots were weighed after drying for 48 h at 70 °C. The dry matter is equivalent to P through each sampling interval, and annual P was calculated as the sum of periodic P over a year. Using a conventional factor of 0.5, dry matter was converted to carbon. Annual mortality (M; g DM m−2 year−1) was calculated as a difference between annual P and annual ΔB. In addition, fine-root turnover rates were calculated as the ratio of annual P and mean B (Brunner et al. 2013).
Growth and maintenance respirations
The simple equation of growth (Rg) and maintenance respiration (Rm) partitioning follows (Amthor 2000) and (Thornley 1970):
where gR is the growth respiration coefficient, and mR is the maintenance respiration coefficient. To adopt the equation for field data, it was modified into:
where c is gR (g C g DM−1), d is base respiration rate per unit biomass at 0 °C (g C g DM−1 day−1), f is a temperature coefficient (°C−1), Ts is soil temperature at a depth of 5 cm (°C), and h is residual (g C m−2 day−1), which includes the respiration of ion uptake, herbaceous roots, and thicker roots. d·exp(f·Ts) is equivalent to mR (g C g DM−1 day−1) at Ts. The parameters of c, d, f, and h were determined by curve fitting to the data set of Rr (g C m−2 day−1), P (g DM m−2 day−1), B (g DM m−2), and Ts, which were calculated at the same intervals. The Rr and Ts were averaged over each interval of P measurement, and B was the mean of two measurements at the beginning and end of an interval. Next, Rr, P, and B were spatially averaged for each site (n = 5). Thus, the number of the data set for the four variables (Rr, Ts, P, and B) was equivalent to the frequency of P measurement (n = 5).
Statistical analysis
ANOVA and post hoc Tukey’s HSD were applied to compare environmental factors, fine root biomass, fine root production, and soil CO2 effluxes among treatments. t-test was applied to compare mean values between the sites. Parameters in the equations were determined by curve fitting to the data set (n = 5) for each forest. In addition, uncertainties (± 1 SD) of annual Rg and Rm were estimated from those of the parameters and measurements using the law of error propagation. Data analyses were conducted with a software package (Origin Pro 2015 J; Origin Lab Corporation, Northampton, MA, USA).
Results
Environmental conditions
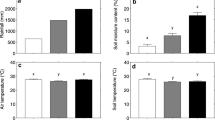
Mean air temperature at 1.5 m aboveground and total precipitation measured in the nearby re-growing forest site during the growing season from May to October 2015 were 14.0 °C and 822 mm, respectively, which were within the range of 1 SD from their decadal means of 14.4 ± 0.5 °C and 949 ± 188 mm between 2006 and 2015. Daily mean Ts peaked in August (Fig. 2a). Mean Ts was 8.8 °C in the DF site and 7.5 °C in the EF site from November 2014 to November 2015. The higher Ts in the DF site was mainly caused by more incident solar radiation on the forest floor through the defoliated canopy in spring. In 2015, SWC showed a seasonal variation with a minimum in August, which was opposite to Ts (Fig. 2b).
Mean Ts was not significantly different among four treatments at both sites; however, it was significantly lower in the EF site (Table 1). In contrast, mean SWC significantly increased after trenching (TC), though no significant difference was found between the sites.
Decomposition of dead roots
Dead roots decayed exponentially. The decomposition constant k was higher in the DF site than in the EF site and was highest for fine roots, followed by medium and coarse roots in both sites (Table 2). Using Eq. 6, annual Rd was calculated for each root class using the k values and initial root biomass and totaled 131 and 89 g C m−2 year−1, respectively, in the DF and EF sites (Table 2), accounting for 20% and 16% of the initial carbon content. The contribution of fine root decomposition to the total Rd was 34% and 52%, respectively, in the DF and EF sites.
Soil CO2 efflux
Soil CO2 efflux was measured before the treatment and showed no significant difference among four collars in all five replications in each site (ANOVA, P > 0.05), which indicates that correction for spatial variation in CO2 efflux was unnecessary. Also, no significant difference was found between CO2 effluxes from CC and SC collars in 2015 even after the start of soil core sampling (paired t-test, P > 0.05); mean CO2 effluxes (± 1 SD) were 4.97 ± 4.30 (CC) and 4.77 ± 3.34 (SC) μmol m−2 s−1 in the DF site, 3.91 ± 2.76 (CC) and 4.02 ± 2.69 (SC) μmol m−2 s−1 in the EF site. These results indicate that the effect of core sampling on CO2 efflux was negligible. Thus, CO2 efflux from SC was used as Rs to minimize spatial discrepancy between flux measurement and root sampling.
Soil CO2 efflux showed a similar seasonal variation in both sites with a peak in late July (Fig. 3). Soil CO2 efflux was significantly correlated to Ts (P < 0.001) in an exponential manner (Eq. 3) on every collar (R2 > 0.55). Figure 4 shows the mean relationships for SC and TC. The Q10 values (mean ± 1 SD, n = 5) calculated using the fitting parameter (b) were 2.7 ± 0.5 (SC) and 3.0 ± 0.5 (TC) in the DF site, and 2.4 ± 0.2 (SC) and 2.4 ± 0.2 (TC) in the EF site. The results were significantly higher (P < 0.05) in the DF site but not significantly different (P > 0.05) between SC and TC. Soil CO2 efflux was normalized for mean Ts (Rb) according to Eq. 4 using Tb of 14 °C and 12 °C, respectively, in the DF and EF sites (Table 1). In both sites, Rb had no significant relationship with SWC ranging between 0.1 and 0.6 m3 m−3 (Fig. 5). Also, adding SWC did not improve adjusted R2 than Ts alone. Thus, using Eq. 3, hourly CO2 efflux was estimated from Ts monitoring data for each collar and summed up over an annual period starting in November 2014. The annual values (n = 5) were 1255 ± 401 (SC), 1044 ± 334 (LC), and 776 ± 83 (TC) g C m−2 year−1 in the DF site, and 1139 ± 386 (SC), 1027 ± 323 (LC), and 835 ± 172 (TC) g C m−2 year−1 in the EF site. The SD was lower in TC than in SC, because spatial variations in root respiration were excluded in TC. Annual Rs, Rl, Rh, and Rr were calculated from the annual soil CO2 effluxes and Rd (Table 3). Here, Rr includes not only the respiration of tree fine roots but also those of tree medium and coarse roots, and herbaceous roots. The contributions of Rr to Rs were 49% and 35%, respectively, in the DF and EF sites. Also, Rd accounted for 10% and 8% of Rs, respectively, in the DF and EF sites.
Relationship between soil CO2 efflux and soil temperature at a depth of 5 cm for SC and TC collars in a) DF and b) EF sites. Mean ± 1 SD are shown (n = 5). An exponential equation (Eq. 3) is fitted significantly (P < 0.001)
Fine root dynamics
Tree fine root biomass (B) showed a clear seasonal variation with a peak in September in the DF site (Fig. 6a), whereas seasonality was indeterminable in herbaceous fine root biomass. In the EF site, B had less seasonality (Fig. 6c). No herbaceous fine root biomass was found in the EF site. Peak B was 482 and 559 g DM m−2, respectively, in the DF and EF sites (Fig. 6b and c). On average, 56% and 49% of B existed in the root mat in the DF and EF sites, respectively. In TC, B in May and June 2015 were 32.0 ± 6.6 and 11.6 ± 1.4 g DM m−2, respectively, in the DF site (n = 5), and 41.8 ± 10.2 and 16.4 ± 3.0 g DM m−2, respectively, in the EF site (n = 5). B decreased significantly between May and June (P < 0.001) in both sites. In June 2015, B in the trenched collars (TC) decreased to 3% in comparison with the non-trenched collars (SC) in both sites.
No herbaceous fine root was found in the ingrowth cores even in the DF site, probably because herbaceous plants were picked out periodically. Tree fine root production (P) showed a distinct seasonality with a peak between 2.3 to 2.5 g DM m−2 day−1 in summer (mid-June to early August) in both sites (Fig. 6d and e). The seasonal variations of B and P were similar in the DF site (Fig. 6b and d). Even during winter and early spring between November 21, 2014 and May 1, 2015, P was measured at 0.14 ± 0.08 and 0.22 ± 0.14 g DM m−2 day−1 in the DF and EF sites, respectively; P was not significantly different between the sites (P = 0.26). The P was positively correlated (P < 0.05) with mean Ts during core-sampling intervals (Fig. 7) but showed no significant relationship with SWC (P > 0.05, data not shown). In both sites, Rr was significantly correlated with P (Fig. 8, P < 0.05), but not with B (P > 0.05, data not shown).
Annual P was not significantly different between the DF and EF sites (Table 4). Although the root mat was only 4 cm thick, P in the root mat accounted for 45 to 48% of total P. Mean B was significantly larger in the EF site (P < 0.01), although the difference was not significant if herbaceous fine root biomass was included (P > 0.05). In the DF site, herbaceous fine root biomass accounted for 25% (110 g DM m−2) of total B. In November 2014, fine root accounted for 20% and 33% of total tree root biomass, respectively, in the DF and EF sites (Table 2). The tree fine root turnover rate was 1.08 ± 0.36 year−1 in the DF site, which was significantly larger than 0.68 ± 0.12 year−1 in the EF site (P < 0.05).
Partitioning root respiration into growth and maintenance components
Rr was partitioned into Rg and Rm using Eq. 8. In both sites, no significant correlation was found between P and B (P > 0.05). Despite a relatively high R2 (≥ 0.87), the fitting was not significant (P ≥ 0.065) because of the small number of samples (n = 5) for both sites (Table 5). All four parameters were not significantly different between the sites (P ≥ 0.55). The Q10 of Rm calculated from f (temperature coefficient) were 3.3 and 2.6, respectively, for DF and EF. Using these parameters, annual Rg and Rm were calculated (Table 6). The annual totals (Rg + Rm + h) were 638 and 396 g C m−2 year−1, respectively, in the DF and EF sites. These totals exceeded Rr by 28 and 3 g C m−2 year−1, respectively, in the DF and EF sites. The contribution of Rg to the total was almost the same (19 vs. 22%), though Rm was larger in the EF site (26 vs. 46%). As a result, h contributed more in the DF site.
Discussion
Soil respiration and root respiration
Root respiration (Rr) was calculated as the residual of total soil respiration (Rs) after subtracting leaf litter decomposition (Rl) and soil heterotrophic respiration (Rh) (Eq. 2). Thus, Rr corresponds to mycorrhizosphere respiration, which consists of not only respiration of living root tissue but also rhizomicrobial and mycorrhizal respiration (Moyano et al. 2009). There was no significant difference in soil CO2 efflux between control (CT) and sampling collars (SC) even after soil sampling. Thus, soil CO2 efflux on SC was treated as Rs, because it enabled us to directly compare soil CO2 efflux and fine root dynamics in the same collar.
Trenching is a commonly used method to estimate soil heterotrophic respiration, although it potentially causes biases owing to dead root decomposition, lack of water uptake by roots, and lack of root litter input (Epron et al. 1999; Hanson et al. 2000; Subke et al. 2006). In this study, dead root decomposition (Rd) was quantified by the litter bag method. Soil water content (SWC) was significantly higher in TC (Table 1), but no relationship with soil CO2 efflux was found using bivariate models and temperature-normalized CO2 efflux (Fig. 5), suggesting that an increase in SWC minimally affected soil CO2 efflux. Tree fine root biomass (B) in TC was only 3% in comparison with SC in June 2015, which indicates that trenching worked effectively. In contrast, belowground microbial respiration was probably underestimated to a small extent due to the lack of root litter input (Subke et al. 2006). Also, trenching killed ectomycorrhizal fungi and consequently might have enhanced the decomposition of soil organic matter by saprotrophic fungi (Gadgil effect) (Fernandez and Kennedy 2016).
The annual Rs of 1255 and 1139 g C m−2 year−1 (Table 3) estimated from hourly Ts, were relatively large but within the range of reported values from temperate forests (Subke et al. 2006), which were 828 ± 267 g C m−2 year−1 for coniferous forests and 976 ± 514 g C m−2 year−1 for deciduous forests, respectively (mean ± 1 SD, n = 28). Our results were also larger than the annual Rs of 760 ± 40 g C m−2 year−1 measured for 8 years in a Japanese larch plantation in central Japan (Teramoto et al. 2017), which had an annual air temperature (8.6 °C) similar to our site (8.3 °C). Before the typhoon disturbance, annual Rs was 934 g C m−2 year−1 in the DF site in 2003 (Liang et al. 2010), which was smaller by 321 g C m−2 year−1 than in 2015 despite having similar annual mean soil temperatures (8.6 °C in 2003 and 8.8 °C in 2015). The larger Rs in 2015 was partly due to higher soil temperature during the summer. In 2015, soil temperature in July and August were higher on average by 2.4 °C because of a sparser canopy. The annual contributions of Rr to Rs (Rr / Rs) were 0.49 and 0.35, respectively, in the DF and EF sites (Table 3). These ratios were compatible with results from a meta-analysis (Subke et al. 2006), where Rr / Rs was calculated to be 0.43 ± 0.21 for deciduous forests and 0.49 ± 0.14 for coniferous forests (n = 28, respectively).
The decomposition of dead tree roots was well explained with the rate constant of k (Table 2). The k was higher in the DF site than in the EF site (0.16–0.42 vs. 0.10–0.29 year−1) and highest for fine roots. A study conducted in a beech forest in France showed k values of 0.38 year−1 for fine roots and 0.22 year−1 for coarse roots (> 2 mm) (Epron et al. 1999), which is similar to the result in the DF site. Also, a meta-analysis reported that mean k values for fine roots were 0.44 and 0.17 year−1, respectively, for broadleaf and coniferous forests, and k values became lower with root diameter (Silver and Miya 2001). Annual Rd were 131 and 89 g C m−2 year−1, respectively, for DF and EF sites (Table 3), which accounted for 10% and 8% of annual Rs, respectively. If the Rd was neglected in Eq. 2, Rr would be calculated to be 479 and 304 g C m−2 year−1, respectively, for DF and EF sites, which would underestimate Rr by 21% and 23%. In a beech forest, (Epron et al. 1999) reported that Rr was 40% underestimated by annual Rd of 160 g C m−2 year−1.
Fine root dynamics
We applied the sequential coring method for tree fine root biomass (B) and the ingrowth core method for tree fine root production (P) for a total soil depth of 14 cm. In this area 83–85% of fine roots existed in the surface 15 cm of the soil layer (Sakai et al. 2007), suggesting underestimation of B and P in this study. The ingrowth core method has been widely applied mainly because of its easy applicability (Addo-Danso et al. 2016). However, the ingrowth core method tends to underestimate P in comparison with the minirhizotron method, which yields more reliable estimates (Addo-Danso et al. 2016; Finér et al. 2011b; Hendricks et al. 2006; Majdi et al. 2005). Thus, our P results could be underestimated.
In both sites, P showed a clear seasonality with a peak in summer and was found to be 0.14–0.22 g DM m−2 day−1 even during a period from winter through early spring (Fig. 6). The period ranged from November 21, 2014 to May 1, 2015, in which albedo indicated that snow covered the ground from December 2 to April 4 in a nearby re-growing forest site (Hirano et al. 2017). Mean soil temperature in the period was 1.4 and 0.4 °C, respectively, in the DF and EF sites. A delay in temperature rise in April in the EF site (Fig. 2) was caused by delayed thaw due to a dense canopy, indicating that snow covered the ground until April 20 in the EF site. In addition, larch trees were still leafless on May 1, 2015. In this area larch trees usually begin to leaf out in early May (Hirano et al. 2003a). Therefore, despite including early spring after thaw the measured P suggests that tree fine roots grew during winter (Radville et al. 2016).
The seasonal P was well explained by soil temperature (Ts) (Fig. 7). Similar seasonal variations were reported from many boreal and temperate forests (Brassard et al. 2009; Fukuzawa et al. 2013; Noguchi et al. 2005; Steinaker et al. 2010; Tierney et al. 2003). Also, B increased in summer in the DF site, whereas no clear seasonal variation was found in the EF site (Fig. 6). Although summer B was comparable, winter B was greater in the EF site. The discrepancy in seasonality was probably attributed to the life span of fine roots. Mean life span, as the reciprocal of a turnover rate, was 0.93 and 1.47 year, respectively, in the DF and EF sites (Table 4). In the EF site, the longer life span may not have decreased B even in winter. In boreal and temperate forests, some studies reported seasonal variations similar to the DF site (Brassard et al. 2009; Coleman et al. 2000), but other studies found no seasonality (Fukuzawa et al. 2013; Makkonen and Helmisaari 1998; Noguchi et al. 2005).
Annual mean B was 340 and 516 g DM m−2, respectively, in the DF and EF sites (Table 4). These values are smaller than mean values of 505 g DM m−2 in deciduous broadleaf forests and 607 g DM m−2 in evergreen conifer forests in temperate regions (Finér et al. 2011a). The reasons for the smaller B are possibly because of the thin surface soil in both sites, tree thinning by the typhoon in the DF site, and possibly limited sampling depth. Annual P in the two sites were almost the same (368 vs. 352 g DM m−2 year−1) despite the large difference of B. As a result, turnover rates, which are defined as the ratio of annual P and mean B, were significantly higher in the DF site (1.09 year−1) than in the EF site (0.68 year−1). If the maximum B is used instead of mean B (Brunner et al. 2013), turnover rates of the two sites are almost identical (0.30 vs. 0.31 year−1). Although the ingrowth core method was used in this study, the annual P was similar to the mean P of temperate forests (337 g DM m−2 year−1), which was measured by various methods, especially using the sequential coring method (Finér et al. 2011b). Also, the P of this study was slightly larger than that measured by the minirhizotron method from a cedar plantation (320 g DM m−2 year−1) in central Japan (Noguchi et al. 2005). A review paper showed that the turnover rates from mean B were 1.10 year−1 for both deciduous Fagus sylvatics and evergreen Picea abies (Brunner et al. 2013). These turnover rates are almost identical with the DF turnover rate. The turnover rate of the EF site was smaller than that of Picea abies.
Growth and maintenance respiration
Rr was partitioned into growth (Rg) and maintenance respirations (Rm) of tree fine roots using a model (Eq. 8) by multiple regression. The defects of the model include the lack of respiration due to root-derived compounds (rhizomicrobial and mycorrhizal respiration) and ion uptake. In addition, respiration except tree fine roots were not included in the analyses. Moreover, B and P were probably underestimated because of the limited sampling depth of 14 cm and the use of the ingrowth core method. These facts certainly resulted in considerable uncertainty. Rhizomicrobial and mycorrhizal respiration may be related to P and B, because they depend on rhizodeposits and carbohydrates derived from roots (Moyano et al. 2009). Ion uptake respiration is expected to correlate to tree photosynthesis (Lambers et al. 2008). Although we did not consider ion uptake respiration, gross primary production (GPP) of the larch forest growing in the DF site before the typhoon disturbance peaked in June–July (Hirano et al. 2003a; Hirata et al. 2007). Also, in an evergreen conifer forest of Pinus resinosa in Japan, GPP peaked in June–August (Mizoguchi et al. 2012). These seasonal variations of GPP are like those of P with a peak in summer (Fig. 6). Thus, ion uptake respiration was probably partly included in Rg. The residual (h) was much larger in the DF site (Table 6), which is consistent with the fact that B accounted for only 20% of total tree root biomass (Table 2), and herbaceous root biomass accounted for 24% of total fine root biomass (Table 4).
The sums of Rg and Rm, which were rough estimates of tree fine root respiration, were similar between the DF and EF sites (287 vs. 268 g C m−2 year−1; Table 6), although their contributions to total root respiration were different (45 vs. 68%). (George et al. 2003) used short-term chamber measurements and published information to partition fine root respiration into growth, maintenance, and ion uptake (only nitrogen) components in deciduous (Liquidambar styraciflua) and evergreen (Pinus taeda) forests. They reported that annual fine root respiration in the deciduous and evergreen forests were 245 and 639 g C m−2 year−1, respectively, and maintenance respiration accounted for 86 and 98% of the total fine root respiration, respectively. Although annual P (345 g DM m−2 year−1) of the deciduous forest was almost identical to that (368 g DM m−2 year−1) of the DF site (Table 4), growth respiration (24 g C m−2 year−1) was only 20% of the DF’s Rg (Table 6). Their ion uptake respiration was less than 4% of total fine root respiration. When limited to the growing season between May and November, tree fine root respiration (Rg plus Rm) were calculated to be 260 and 230 g C m−2, respectively, in the DF and EF sites. These values are similar to those estimated from chamber measurements for Quercus rubra (229 g C m−2), Tsuga canadensis (242 g C m−2), and Fraxinus alba (270 g C m−2) in the Harvard Forest (Abramoff and Finzi 2016).
The Rg accounted for 42 and 32% of the total fine root respiration in the DF and EF sites, respectively (Table 6). The higher contribution in the DF site might be due to its higher turnover rate (Lambers et al. 2008). Growth (gR) and maintenance (mR) coefficients (Eq. 7) of whole root respiration for some species have been determined from growth chamber or greenhouse experiments. Using the units of mmol O2 (g DM)−1 for gR and nmol O2 (g DM)−1 s−1 for mR, and explicitly including the respiration of ion uptake in gR, whole root gR and mR, respectively, were 11 and 26 for Dactylis glomerata, 19 and 21 for Festuca ovina, 12 and 6 for Quercus suber, and 18 and 22 for Triticum aestivum (Lambers et al. 2008). For Eucalyptus sp. cuttings, gR and mR of whole root respiration were 5.2 mmol CO2 (g DM)−1 and 9.7 nmol CO2 (g DM)−1 s−1, respectively, at 22 °C (Thongo M’Bou et al. 2010), in which ion uptake respiration was not separated from Rg. As for fine roots in field conditions (George et al. 2003), gR coefficients were 5.1 (P. taeda) and 5.8 mmol CO2 (g DM)−1 (L. styraciflua), and mR coefficients were 8.9 (P. taeda) and 10.2 nmol CO2 (g DM)−1 s−1 (L. styraciflua) at 25 °C. In our study for tree fine roots, gR (= c) were 26.7 and 20.0 mmol CO2 (g DM)−1, respectively, in the DF and EF sites. In addition, mR (= d·exp(f·Ts)) were 5.5 (DF) and 2.7 (EF) nmol CO2 (g DM)−1 s−1 at 22 °C, and 7.9 (DF) and 3.6 (EF) nmol CO2 (g DM)−1 s−1 at 25 °C. The mR increases with Ts according to Q10 of 3.3 and 2.6, respectively, in the DF and EF sites. Although the respiratory quotient should be considered, gR of our study was relatively higher than those of the other species. However, our study’s mR were lower. Most of the differences in the coefficients possibly arose from differences in chemical composition of the roots, different rates of alternative pathway respiration, and different methods used (Lambers et al. 2008). In addition, differences between whole root respiration and fine root respiration would be another reason for the different coefficients.
Conclusions
We conducted a year-round field experiment to measure the production and biomass of tree fine roots, and soil respiration simultaneously in adjoining deciduous and evergreen forests on the same soil type. The periodic measurement of the three items was made in the same collar to minimize inconsistency among items by spatial positions. Using the field data, we partitioned tree fine root respiration into its growth and maintenance components on an annual basis by multiple regression using an empirical model. Growth (gR) and maintenance (mR) coefficients of the model were not significantly different between the forests. In comparison with reported values from laboratory or greenhouse experiments, in our study gR was higher, but mR was lower. Considerable uncertainty in the partitioning arose from a small sample size, the existence of thicker roots, and uncertainty in field measurement. However, the results suggest the availability of the approach using the model in the field. To obtain more reliable results, ion uptake respiration should be incorporated into the model.
References
Abramoff RZ, Finzi AC (2015) Are above- and below-ground phenology in sync? New Phytol 205:1054–1061. https://doi.org/10.1111/nph.13111
Abramoff RZ, Finzi A (2016) Seasonality and partitioning of root allocation to rhizosphere soils in a midlatitude forest. Ecosphere 7:1–19. e01547. https://doi.org/10.1002/ecs2.1547
Addo-Danso SD, Prescott CE, Smith AR (2016) Methods for estimating root biomass and production in forest and woodland ecosystem carbon studies: a review. Forest Ecol Manag 359:332–351. https://doi.org/10.1016/j.foreco.2015.08.015
Amthor JS (2000) The McCree-de wit-Penning deVries-Thornly respiration paradigms: 30 years later. Ann Bot-London 86:1–20
Bonan G (2008) Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320:1444–1449
Brassard BW, Chen HYH, Bergeron Y (2009) Influence of environmental variability on root dynamics in northern forests. Crit Rev Plant Sci 28:179–197. https://doi.org/10.1080/07352680902776572
Brunner I, Bakker MR, Björk RG, Hirano Y, Lukac M, Aranda X, Børja I, Eldhuset TD, Helmisaari HS, Jourdan C, Konôpka B, López BC, Miguel Pérez C, Persson H, Ostonen I (2013) Fine-root turnover rates of European forests revisited: an analysis of data from sequential coring and ingrowth cores. Plant Soil 362:357–372. https://doi.org/10.1007/s11104-012-1313-5
Chapin FS III, Matson PA, Vitousek PM (2011) Plant respiration. Principles of terrestrial ecosystem ecology, 2nd edn. Springer, New York
Coleman MD, Dickson RE, Isebrands JG (2000) Contrasting fine-root production, survival and soil CO2 efflux in pine and poplar plantations. Plant Soil 225:129–139
Davidson EA, Richardson AD, Savage KE, Hollinger D (2006) A distinct seasonal pattern of the ratio of soil respiration to total ecosystem respiration in a spruce-dominated forest. Glob Chang Biol 12:230–239. https://doi.org/10.1111/j.1365-2486.2005.01062.x
Epron D, Farque L, Lucot E, Badot PM (1999) Soil CO2 efflux in a beech forest: the contribution of root respiataion. Ann For Sci 56:289–295
Fernandez CW, Kennedy PG (2016) Revisiting the 'Gadgil effect': do interguild fungal interactions control carbon cycling in forest soils? New Phytol 209:1382–1394. https://doi.org/10.1111/nph.13648
Finér L, Ohashi M, Noguchi K, Hirano Y (2011a) Factors causing variation in fine root biomass in forest ecosystems. Forest Ecol Manag 261:265–277. https://doi.org/10.1016/j.foreco.2010.10.016
Finér L, Ohashi M, Noguchi K, Hirano Y (2011b) Fine root production and turnover in forest ecosystems in relation to stand and environmental characteristics. Forest Ecol Manag 262:2008–2023. https://doi.org/10.1016/j.foreco.2011.08.042
Fukuzawa K, Shibata H, Takagi K, Satoh F, Koike T, Sasa K (2013) Temporal variation in fine-root biomass, production and mortality in a cool temperate forest covered with dense understory vegetation in northern Japan. Forest Ecol Manag 310:700–710. https://doi.org/10.1016/j.foreco.2013.09.015
George K, Norby RJ, Hamilton JG, DeLucia EH (2003) Fine-root respiration in a loblolly pine and sweetgum forest growing in elevated CO2. New Phytol 160:511–522. https://doi.org/10.1046/j.1469-8137.2003.00911.x
Gholz HL, Wein DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Glob Chang Biol 6:751–765
Gifford RM (2003) Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research. Funct Plant Biol 30:171. https://doi.org/10.1071/fp02083
Hanson PJ, Edwards NT, Garten CT, Andrews JA (2000) Separation root and soil microbial contributions to soil respiration: a review of mehotds and observations. Biogeochemistry 48:115–146
Hendricks JJ, Hendrick RL, Wilson CA, Mitchell RJ, Pecot SD, Guo D (2006) Assessing the patterns and controls of fine root dynamics: an empirical test and methodological review. J Ecol 94:40–57. https://doi.org/10.1111/j.1365-2745.2005.01067.x
Hirano T, Hirata R, Fujinuma Y, Saigusa N, Yamamoto S, Harazono Y, Takada M, Inukai K, Inoue G (2003a) CO2 and water vapor exchange of a larch forest in northern Japan. Tellus B 55:244–257. https://doi.org/10.1034/j.1600-0889.2003.00063.x
Hirano T, Kim H, Tanaka Y (2003b) Long-term half-hourly measurement of soil CO2 concentration and soil respiration in a temperate deciduous forest. J Geophys Res-Atmos 108. https://doi.org/10.1029/2003JD003766
Hirano T, Suzuki K, Hirata R (2017) Energy balance and evapotranspiration changes in a larch forest caused by severe disturbance during an early secondary succession. Agric For Meteorol 232:457–468. https://doi.org/10.1016/j.agrformet.2016.10.003
Hirata R, Hirano T, Saigusa N, Fujinuma Y, Inukai K, Kitamori Y, Takahashi Y, Yamamoto S (2007) Seasonal and interannual variations in carbon dioxide exchange of a temperate larch forest. Agric For Meteorol 147:110–124. https://doi.org/10.1016/j.agrformet.2007.07.005
Hopkins F, Gonzalez-Meler MA, Flower CE, Lynch DJ, Czimczik C, Tang J, Subke J-A (2013) Ecosystem-level controls on root-rhizosphere respiration. New Phytol 199:339–351. https://doi.org/10.1111/nph.12271
Hutchinson GL, Livingston GP (2001) Vents and seals in non-steady-state chambers used for measuring gas exchange between soil and the atmosphere. Eur J Soil Sci 52:675–682
Janssens IA, Lankreijer H, Matteucci G, Kowalski AS, Buchmann N, Epron D, Pilegaard K, Kutsch W, Longdoz B, Grunwald T, Montagnani L, Dore S, Rebmann C, Moors EJ, Grelle A, Rannik U, Morgenstern K, Oltchev S, Clement R, Gudmundsson J, Minerbi S, Berbigier P, Ibrom A, Moncrieff J, Aubinet M, Bernhofer C, Jensen NO, Vesala T, Granier A, Schulze ED, Lindroth A, Dolman AJ, Jarvis PG, Ceulemans R, Valentini R (2001) Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob Chang Biol 7:269–278
Johnson IR (1990) Plant respiration in relation to growth, maintenance, ion uptake and nitrogen assimilation. Plant Cell Environ 13:319–328
Keenan RJ, Reams GA, Achard F, de Freitas JV, Grainger A, Lindquist E (2015) Dynamics of global forest area: results from the FAO global Forest resources assessment 2015. Forest Ecol Manag 352:9–20. https://doi.org/10.1016/j.foreco.2015.06.014
Lambers H, Chapin III FS, Pons TL (2008) The role of respiration in plant carbon balance. Plant Physiological Ecology. 2nd edn. Springer, New York
Le Quéré C, Andrew RM, Friedlingstein P, Sitch S, Hauck J, Pongratz J, Pickers PA, Korsbakken JI, Peters GP, Canadell JG, Arneth A, Arora VK, Barbero L, Bastos A, Bopp L, Chevallier F, Chini LP, Ciais P, Doney SC, Gkritzalis T, Goll DS, Harris I, Haverd V, Hoffman FM, Hoppema M, Houghton RA, Hurtt G, Ilyina T, Jain AK, Johannessen T, Jones CD, Kato E, Keeling RF, Goldewijk KK, Landschützer P, Lefèvre N, Lienert S, Liu Z, Lombardozzi D, Metzl N, Munro DR, Nabel JEMS, S-i N, Neill C, Olsen A, Ono T, Patra P, Peregon A, Peters W, Peylin P, Pfeil B, Pierrot D, Poulter B, Rehder G, Resplandy L, Robertson E, Rocher M, Rödenbeck C, Schuster U, Schwinger J, Séférian R, Skjelvan I, Steinhoff T, Sutton A, Tans PP, Tian H, Tilbrook B, Tubiello FN, van der Laan-Luijkx IT, van der Werf GR, Viovy N, Walker AP, Wiltshire AJ, Wright R, Zaehle S, Zheng B (2018) Global carbon budget 2018. Earth Syst Sci Data 10:2141–2194. https://doi.org/10.5194/essd-10-2141-2018
Liang N, Hirano T, Zheng ZM, Tang J, Fujinuma Y (2010) Soil CO2 efflux of a larch forest in northern Japan. Biogeosciences 7:3447–3457. https://doi.org/10.5194/bg-7-3447-2010
Liu S, Luo D, Yang H, Shi Z, Liu Q, Zhang L, Kang Y (2018) Fine root dynamics in three Forest types with different origins in a subalpine region of the eastern Qinghai-Tibetan plateau. Forests 9:517. https://doi.org/10.3390/f9090517
Majdi H, Pregitzer K, Morén A-S, Nylund J-E, Ågren IG (2005) Measuring fine root turnover in Forest ecosystems. Plant Soil 276:1–8. https://doi.org/10.1007/s11104-005-3104-8
Makkonen K, Helmisaari HS (1998) Seasonal and yearly variations of fine-root biomass and necromass in a scots pine (Pinus sylvestris L.) stand. Forest Ecol Manag 102:283–290
McCormack ML, Adams TS, Smithwick AH, Eissenstat DM (2014) Variability in root production, phenology, and turnover rate among 12 temperate tree species. Ecology 95:2224–2235
McCormack ML, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo D, Helmisaari HS, Hobbie EA, Iversen CM, Jackson RB, Leppalammi-Kujansuu J, Norby RJ, Phillips RP, Pregitzer KS, Pritchard SG, Rewald B, Zadworny M (2015) Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol 207:505–518. https://doi.org/10.1111/nph.13363
McCree KJ (1974) Equations for the rate of dark respiration of white clove and grain sorghum, as functions of dry weight, photosynthetic rate, and temperature. Crop Sci 14:509–514
Mizoguchi Y, Ohtani Y, Takanashi S, Iwata H, Yasuda Y, Nakai Y (2012) Seasonal and interannual variation in net ecosystem production of an evergreen needleleaf forest in Japan. J Forest Res-Jpn 17:283–295. https://doi.org/10.1007/s10310-011-0307-0
Moyano F, Atkin OK, Bahn M, Bruhn D, Burton AJ, Heinemeyer A, Kutsch W, Wieser G (2009) Respiration from roots and the mychorrhizosphere. In: Kutsch W, Bahn M, Heinemeyer A (eds) Soil carbon dynamics. Cambridge University Press, Cambridge
Noguchi K, Sakata T, Mizoguchi T, Takahashi M (2005) Estimating the production and mortality of fine roots in a Japanese cedar (Cryptomeria japonica D. Don) plantation using a minirhizotron technique. J Forest Res-Jpn 10:435–441. https://doi.org/10.1007/s10310-005-0163-x
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG, Ciais P, Jackson RB, Pacala SW, McGuire AD, Piao S, Rautiainen A, Sitch S, Hayes D (2011) A large and persistent carbon sink in the world's forests. Science 333:988–993. https://doi.org/10.1126/science.1201609
Penning de Vries FWT (1974) Substrate utilization and respiration in relation to growth and maintenance in higher plants. Neth J Agric Sci 22:40–44
Radville L, McCormack ML, Post E, Eissenstat DM (2016) Root phenology in a changing climate. J Exp Bot 67:3617–3628. https://doi.org/10.1093/jxb/erw062
Ravindranath NH, Ostwald M (2008) Carbon inventory methods: handbook for greenhouse gas inventory, carbon mitigation and Roundwood production. Springer, Projects
Reichstein M, Janssens IA (2009) Semi-empirical modeling of the response of soil respiration to environmental factors in laboratory and field conditions. In: Kutsch W, Bahn M, Heinemeyer A (eds) Soil carbon dynamics. Cambridge University Press, Cambridge
Richter DD, Markewitz D, Trumbore SE, Wells CG (1999) Rapid accumulation and turnover of soil carbon in a re-establishing forest. Nature 400:56–58
Sakai Y, Takahashi M, Tanaka N (2007) Root biomass and distribution of a Picea—Abies stand and a Larix—Betula stand in pumiceous Entisols in Japan. J Forest Res-Jpn 12:120–125. https://doi.org/10.1007/s10310-006-0270-3
Sano T, Hirano T, Liang N, Hirata R, Fujinuma Y (2010) Carbon dioxide exchange of a larch forest after a typhoon disturbance. Forest Ecol Manag 260:2214–2223. https://doi.org/10.1016/j.foreco.2010.09.026
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419. https://doi.org/10.1007/s004420100740
Steinaker DF, Wilson SD, Peltzer DA (2010) Asynchronicity in root and shoot phenology in grasses and woody plants. Glob Chang Biol 16:2241–2251. https://doi.org/10.1111/j.1365-2486.2009.02065.x
Subke J-A, Inglima I, Francesca Cotrufo M (2006) Trends and methodological impacts in soil CO2 efflux partitioning: a metaanalytical review. Glob Chang Biol 12:921–943. https://doi.org/10.1111/j.1365-2486.2006.01117.x
Sun LF, Teramoto M, Liang N, Yazaki T, Hirano T (2017) Comparison of litter-bag and chamber methods for measuring CO2 emissions from leaf litter decomposition in a temperate forest. J Agric Meteorol 73:59–67. https://doi.org/10.2480/agrmet.D-16-00012
Sweetlove LJ, Williams TC, Cheung CY, Ratcliffe RG (2013) Modelling metabolic CO(2) evolution--a fresh perspective on respiration. Plant Cell Environ 36:1631–1640. https://doi.org/10.1111/pce.12105
Teramoto M, Liang N, Zeng J, Saigusa N, Takahashi Y (2017) Long-term chamber measurements reveal strong impacts of soil temperature on seasonal and inter-annual variation in understory CO 2 fluxes in a Japanese larch ( Larix kaempferi Sarg.) forest. Agric For Meteorol 247:194–206. https://doi.org/10.1016/j.agrformet.2017.07.024
Thongo M’Bou A, Saint-André L, de Grandcourt A, Nouvellon Y, Jourdan C, Mialoundama F, Epron D (2010) Growth and maintenance respiration of roots of clonal Eucalyptus cuttings: scaling to stand-level. Plant Soil 332:41–53. https://doi.org/10.1007/s11104-009-0272-y
Thornley JHM (1970) Respiration, growth and maintenance in plants. Nature 227:304–305
Thornton PE, Rosenbloom NA (2005) Ecosystem model spin-up: estimating steady state conditions in a coupled terrestrial carbon and nitrogen cycle model. Ecol Model 189:25–48. https://doi.org/10.1016/j.ecolmodel.2005.04.008
Tierney G, Fahey TJ, Groffman PM, Hardy JP, Fitzhugh RD, Driscoll CT, Yavitt JB (2003) Environmental control of fine root dynamics in a northern hardwood forest. Glob Chang Biol 9:670–679
Vogt KA, Vogt DJ, Bloomfield J (1998) Analysis of some direct and indirect methods for estimating root biomass and production of forests at an ecosystem level. Plant Soil 200:71–89
Wang W, Zu Y, Wang H, Hirano T, Takagi K, Sasa K, Koike T (2005) Effect of collar insertion on soil respiration in a larch forest measured with a LI-6400 soil CO2 flux system. J Forest Res-Jpn 10:57–60. https://doi.org/10.1007/s10310-004-0102-2
Warren JM, Hanson PJ, Iversen CM, Kumar J, Walker AP, Wullschleger SD (2015) Root structural and functional dynamics in terrestrial biosphere models--evaluation and recommendations. New Phytol 205:59–78. https://doi.org/10.1111/nph.13034
Wieder RK, Lang GE (1982) A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 63:1636–1642
Yazaki T, Hirano T, Sano T (2016) Biomass accumulation and net primary production during the early stage of secondary succession after a severe Forest disturbance in northern Japan. Forests 7. https://doi.org/10.3390/f7110287
Yuan ZY, Chen H (2010) Fine root biomass, production, turnover rates, and nutrient contents in boreal Forest ecosystems in relation to species, climate, fertility, and stand age: literature review and meta-analyses. Crit Rev Plant Sci 29:204–221. https://doi.org/10.1080/07352689.2010.483579
Acknowledgements
This study was supported by JSPS KAKENHI (nos. 25241002 and 17 K20037) and the Environment Research and Technology Development Fund (2-1705) of the Environmental Restoration and Conservation Agency. We thank the Hokkaido Regional Office of the Forestry Agency for allowing us to use the study site, and N. Saigusa, R. Hirata and the staff of CGER for managing the site, and K. Fukuzawa and K. Takagi for teaching us how to measure fine roots.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Hans Lambers.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, L., Hirano, T., Yazaki, T. et al. Fine root dynamics and partitioning of root respiration into growth and maintenance components in cool temperate deciduous and evergreen forests. Plant Soil 446, 471–486 (2020). https://doi.org/10.1007/s11104-019-04343-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-019-04343-z