Abstract
The interface between lotic and lentic ecosystems is often a zone of intense metabolic activity, as primary production in streams and rivers can be light limited whereas nutrients often limit primary production in lake ecosystems. Our objective was to model the influence that rivermouths (the lotic-lentic interface) could have on the loads of soluble reactive phosphorus (SRP) and dissolved inorganic nitrogen (N) passing from the tributary to the nearshore zone of a lake. To achieve this objective, we modeled the combined role of water column nutrient transformation rates with sediment nutrient flux rates. For sensitivity analysis, we picked plausible parameter ranges based on values previously measured in the Fox rivermouth (a tributary to Lake Michigan). Sensitivity analysis of the model demonstrated that overall the importance of water column processing rates increases with increasing nutrient concentration and discharge. We then applied the model to the Fox rivermouth, simulating the change in nutrients on four dates where all of the necessary parameters had been estimated. This modeling suggests that the Fox rivermouth is often a net sink for SRP and source for ammonia (NH4), with water column processing driving SRP removal and both water column and sediment flux driving NH4 dynamics. Removal of SRP in the water column means conversion to particulate and/or organic P, and those P pools are generally considered to be less bioavailable than SRP, so it may be that rivermouths disconnect upstream sources of nutrients from nearshore food webs. These results demonstrate that the interface zone between lotic and lentic systems has the potential to substantially alter the load and character of nutrients as river waters pass through rivermouths to adjacent nearshore areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Interface zones between different ecosystem types often create conditions favorable for rapid processing of nutrients and other bioreactive substances (McClain et al. 2003; Schade et al. 2005). Rapid processing occurs when nutrients can be found in excess of biological demand in one ecosystem, but in limiting quantities in the adjacent ecosystem (Schade et al. 2005). For example, production in streams is often limited by light, disturbance and turbidity (Vannote et al. 1980; Hilton et al. 2006), whereas lentic ecosystem production is primarily limited by the availability of nutrients (Schindler 2012; Paerl et al. 2016). Therefore on a landscape scale, interfaces between lotic and lentic waters are likely biogeochemical hotspots and interact to create a mosaic of biogeochemical processing environments (Kling et al. 2000; Larson et al. 2007).
Rivermouths, like saltwater estuaries, are the interface between lotic and lentic waters. As river waters enter rivermouth zones, they interact with lake waters and create a physiochemical environment that is distinct from either the upstream river or the open waters of the adjacent lentic ecosystem (Elliott and Whitfield 2011; Larson et al. 2013b). Tributary nutrient loads delivered by rivers may be retained or transformed in rivermouths prior to reaching lentic waters (Downer 1985; Krieger 2003; Morrice et al. 2004; Garnier et al. 2010; Gilbert et al. 2013; Weinke et al. 2014; Larson et al. 2016), essentially weakening the connection between upstream landscapes and the nearshore zone (Larson et al. 2013a, 2014). Estimates of nutrient loading to large lakes usually take place at water discharge gages that are placed well upstream of lake influence to simplify loading estimates (e.g., Robertson et al. 2018), but these estimates obviously do not account for these rivermouth-induced changes.
Processes that remove or transform nutrients may occur in the water column (Reisinger et al. 2015) and the sediments (Steinman et al. 2006, 2009) and include both biotic and abiotic processes (Fig. 1). Collectively, these processes result in changes in the amount and timing of nutrient fluxes entering the lake. Estimating these effects within rivermouths is complicated by seiches (i.e., backflows) that occur during storm events, and the presence of multiple surface inflows and groundwater inputs (Trebitz 2006; Morrice et al. 2011). In addition, rivermouths are often the location of intense human development, so point source inputs such as wastewater, stormwater and industrial pollution are frequently concentrated in populated rivermouths (“The Cadmus Group” 2012; Larson et al. 2013b). Therefore, mass balance approaches to estimate rivermouth effects have generally been limited to simpler rivermouths and estuaries (Krieger 2003; Robson et al. 2008; Larson et al. 2016) or have used statistical associations to identify rivermouth effects on nutrients (e.g., Larson et al. 2013a). Although there have been many studies measuring individual processing rates of nutrient transformation in river, rivermouth and estuarine ecosystems (e.g., Steinman et al. 2009; Reisinger et al. 2015; Larson et al. 2019), there have been few attempts to estimate the collective role of these processes (e.g., McCarthy et al. 2007a, b).
Conceptual model showing some important nutrient transformation and processing steps that occur in rivermouths. The most bioavailable forms of nutrients are typically their dissolved inorganic forms. More detailed descriptions are included in the introduction. Volatization is probably limited to a few forms of N (N2, ammonium, N2O)
The key processes differ for different nutrients as well. For example, soluble reactive phosphorus (SRP) delivered by river tributaries may be taken up by algae, bacteria and plants in both the water column and benthos, transforming SRP to particulate phosphorus (PP) and dissolved organic phosphorus (DOP). This newly created PP and DOP may eventually decompose and re-mineralize P, which creates a future source of recycled SRP, or it may be buried in sediments for long periods (Klump et al. 1997). Other sources of PP and DOP include inorganic and detrital sediments from upstream. Benthic and suspended sediments may release or adsorb SRP abiotically due to dis-equilibrium with surface water conditions or periods of low oxygen and biotically via bacterial decomposition and remineralization (Orihel et al. 2017).
In addition to SRP, loads of labile inorganic nitrogen (N) are also thought to be important for promoting ecosystem productivity (Paerl et al. 2016). As with SRP, rivermouth biota take up ammonium (NH4) and nitrate (NOx), essentially converting it into organic forms, which are often less bioavailable and may then decompose or may be buried for longer time periods. Unlike P, many forms of N can be lost to the aquatic ecosystem via volatilization. The fate of these labile N forms could ultimately be N2, the (relatively) inert gas that makes up a large portion of the atmosphere (Schlesinger and Bernhardt 2013). The conversion of NO3 to N2, or incompletely to N2O, is referred to as denitrification, and is often responsible for large losses of N from aquatic ecosystems (Seitzinger et al. 2006; Kreiling et al. 2011; Small et al. 2016). Denitrification is often associated with sediments, as interfaces between oxic/anoxic conditions facilitate the process (Seitzinger et al. 2006). Sediment denitrification rates can be high enough to reduce overall N availability across large ecosystems (McCarthy et al. 2007b), and thus fluxes of N into the sediment could represent permanent losses to the system.
Our primary objective was to integrate estimates of water column processing and sediment flux rates to assess the degree to which an entire rivermouth can alter the riverine loads of labile N and P. To accomplish this objective, we built a simple mass-balance model that uses estimates of processing rates and water residence time to calculate estimates of the mass change that occurs to riverine loads of a nutrient over some time increment (e.g., each day). We then used this model and previously published measurements of nutrient processing and hydrology from the Fox tributary of Lake Michigan (Green Bay, WI) to assess how much the Fox rivermouth likely alters the delivery of SRP, NH4 and NOX to Lake Michigan. To implement this model for a real-world system, we incorporated variation by drawing processing rates from a range of potential values and generating a corresponding distribution of overall rivermouth effects.
Methods
Model of rivermouth nutrient transformations
In this model, we are estimating how much change in nutrient mass would occur for a certain volume of water as it passes through the rivermouth (in this case, the amount of water that enters via river discharge over a single day; Fig. 2). This model calculates the change in mass due to water column processing, the change in mass due to sediment flux and adds them together to estimate a total rivermouth effect (detailed description below and in Fig. 2). The same approach could be used to break the rivermouth into smaller segments or shorter time periods, which would require detailed information about water movement through those individual segments. For the sake of clarity, we are referring to the mass of water that enters the rivermouth from the river as ‘river water.’ These river waters and their associated load are the exclusive focus of this model, although in real life groundwater, precipitation, surface runoff and seiches will contribute additional water (and nutrients) to varying degrees in different rivermouths and at different times. These other sources of water and nutrients would change the nutrient concentration beyond what is depicted in this model.
Description of our model for the change in mass of a dissolved inorganic nutrient as it passes through the rivermouth. This model focuses on how the concentration of a nutrient within a certain volume of water changes after it passes through the rivermouth. This model is explained in more detail in the text
The concentration of nutrient in rivermouth waters after water column processing is given by the following equation (from Reisinger et al. (2015), solved for the final concentration):
where CR and CO* are the concentrations (mg L−1) of the nutrient in the river water before and after the time required to pass through the rivermouth (the water residence time), respectively. KN is the first-order water column nutrient loss rate (h−1; Reisinger et al. 2015). WRT is the residence time of the rivermouth (in hours). By using the water residence time to estimate the change in concentration, we are restricting these estimates to only the period of time when the river waters are in the rivermouth, before they reach the lake.
The next step is to translate these changes in concentration to changes in mass of that nutrient (ΔNWC; in mg). At the most basic level, we need to multiply the volume (V) by the change in concentration to get the change in mass:
where the difference in concentrations is contained within the first set of parentheses. The volume is simply the volume of water passing through the rivermouth that we are considering. This could be any amount, but for the sake of this exercise, we are using the volume of water that enters the rivermouth (from the river) over a certain period of time, so that this can be connected to the sediment flux rates (i.e. 1 day). Here, volume is estimated by multiplying discharge by time (i.e. \(V = {\text{Discharge}}~ \times {\text{Time}}\) ). In this arrangement, ∆NWC is positive when water column processing increases the concentration of that nutrient in the water column.
As mentioned, this is a very simple model that assumes processing rates remain fixed throughout the time the water is in the rivermouth. To apply this model to an actual rivermouth, we incorporated variability by iteratively running the model (thousands of times), drawing KN from distributions of real-world measurements (i.e., the posterior distribution of estimates of K generated by Larson et al. 2019). This approach is computationally simple, while still incorporating the considerable real-world variation that has been observed in these nutrient processing rates.
Sediment contributions (ΔNSED) to outflowing nutrient load is given by multiplying the sediment flux rate (FN; mg m−2 d−1) by the sediment surface area of the rivermouth (A; m2) and; the time period of interest (t; here 1 day).
As with water column processing rates, using this equation to model real-world data can use an iterative process to incorporate known variability in sediment flux rates by drawing from a distribution of potential FN values.
The total change in nutrient mass caused by the rivermouth (ΔNRivermouth) for a given time period can then be estimated by adding the results of Eq. 2 and Eq. 3 (\(\Delta {\text{N}}_{{{\text{Rivermouth}}}} = {\text{ }}\Delta {\text{N}}_{{{\text{WC}}}} + {\text{ }}\Delta {\text{N}}_{{{\text{SED}}}}\)).
Study site
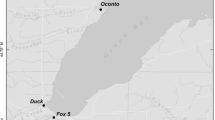
Although the model we used here could be employed in the description of any rivermouth system, we used nutrient processing rates and flows from the Fox rivermouth to provide a realistic scenario, since we have processing rate estimates from this rivermouth (Fig. 3). The Fox rivermouth has been the subject of two previous effort to directly measure nutrient processing rates (Larson et al. 2019, 2020). The Fox rivermouth occurs within an Area of Concern due to legacy contamination and pollution associated with agricultural runoff (https://dnr.wi.gov/topic/greatlakes/greenbay.html). The Fox River drains an agricultural watershed (32% row crops and 10% pasture), and also passed through one of the largest inland lakes in the Great Lakes basin (Lake Winnebago). Here we treat the rivermouth as extending upstream from the De Pere Dam (44.448256 N, 88.064133 W) and downstream to where the channelized portion of the rivermouth intersects the coastline (44.539571 N, 88.004666 W; Fig. 3). In total, this is 11.9 km longitudinally with a surface area of 4.86 km2.
Sediment nutrient processing rates
To use the model in the Fox rivermouth, we used previously published measurements of sediment nutrient flux from sampling in 2016 (see Larson et al. 2020 for methodological details of these measurements and associated data at https://doi.org/10.5066/P9LVTWS8). Briefly, 3 replicate intact sediment core samples were collected at each station in April, June, August and September (2016). Intact cores were incubated in controlled temperature conditions and keep aerated to prevent hypoxia. Overlying water was sampled for changes in nutrient concentration for 24–72 h (depending on the specific nutrient). For more details, see Larson et al. (2020). For each date, the estimated mean and standard deviation were used to describe a normal distribution of sediment flux. For each simulation, we then drew an estimate from this distribution.
Water column nutrient processing rates
We used previously published measurements of water column nutrient processing from sampling in 2016 (Larson et al. 2019). Water column incubations were completed using methods that were loosely based on Reisinger et al. (2015). Briefly, whole water was incubated in clear bags in the field for 6–12 h (during April, June, August and September, 2016), with nutrient concentrations measured repeatedly over the course of the incubation. Dissolved inorganic nutrients decreased in concentration within bags due to biotic uptake or sorption to particulate material. These incubations were performed at 3 locations (Fox 2, Fox 4 and Fox 5). From these measurements, we used a Bayesian model-fitting procedure to generate posterior distributions for K. For more details, see Larson et al. (2019).
Fox rivermouth hydrology
Water residence time (WRT) was calculated on the lower Fox River rivermouth by relating mean channel velocity measured at USGS gage 040851385 Fox River at Oil Tank Depot to velocity longitudinal profiles done on the 12-km stretch of the river from the Depere Dam to Green Bay. The longitudinal profiles were divided into 11 sections and each section was compared to the velocity being measured every 5 min at 040851385. Mean downstream velocity in each of the 11 was regressed (using ordinary least squares linear regression) to the continuous velocity measurements at the gage. The result was the ability to calculate velocity in every section of the river for each reading taken at the gage. WRT was then calculated every 5 min by calculating travel time through each section of river, for the period from April through October 2016. WRT ranged from 10 h during high incoming flows at the De Pere dam to 80 h during low incoming flows. For the purposes of this manuscript, we were primarily interested in the range and median water residence time and discharge data.
Simulations of the Fox rivermouth at the time of nutrient processing experiments
All of the data needed to calculate ΔNRivermouth were generated on four occasions, and we’ve focused on four dates when experiments occurred (April 21, June 17, August 3 and September 20, 2016). During a 2-week period around each of these dates, measurements of sediment and water column processing rates were available as described above. Initial concentrations (CR) were from initial conditions in either the water column incubation experiments (Larson et al. 2019) or the sediment incubation experiments (Larson et al. 2020) at the site closest to the De Pere Dam (the upstream end of the rivermouth), which was Fox 3 (for April) or Fox 1 (for all other dates). We used the individual retained estimates of KN derived during our previous analysis (i.e., the individual runs from the Bayesian analysis; Larson et al. 2019) as the potential distribution of values for KN. We included all KN estimates from experiments where the initial concentration was above the detection limit. Estimates of ΔNSED (mean and standard deviation) for the Fox rivermouth were previously published in Larson et al. (2020). Here we used those previously reported mean and standard deviation estimates to describe a normally distributed range of potential ΔNSED values. For each of these four dates, the ΔNRivermouth was estimated 10,000 times, with each iteration drawing a value for KN and ΔNSED from these distributions (see Statistical Appendix for R code). For the August sampling, NOX concentrations were below the detection limit, which prevented good estimates of 1st order loss rates in the water column, and so ΔNOXRivermouth was not calculated for August.
While the model here describes the processing of labile nutrient forms, there are external sources of labile nutrients that are not accounted for in this model. Two major surface water sources merge with the Fox rivermouth below the De Pere dam (Ashwaubenon Creek and the East River) and at least ten other point source inputs of P are present (The Cadmus Group 2012). Therefore this model is not designed to predict the actual changes in concentration that may occur in the Fox rivermouth, but rather how the rivermouth would process Fox River inputs prior to them reaching Lake Michigan (Green Bay).
Sensitivity analysis of rivermouth processing
To determine which parameters most strongly influence ΔNRivermouth, we systematically varied the values of concentration, discharge, WRT, KN and FN. This sensitivity analysis was done using plausible ranges of values for the Fox rivermouth as a model system. Rivermouths with dramatically different fundamental characteristics (e.g., much longer residence times, much smaller river inputs, etc.) would probably require different methods to estimate processing rates, or would need to be broken up into different segments or time-steps, although those systems could be modeled with this approach as well. For example, our water column processing rates were estimated over 6–12 h, and the Fox rivermouth has a water residence times that ranges between 10 and 50 h. A different method for measuring water column processing rates would probably be needed to estimate what these water column processing rates would be in a rivermouth where water residence time was measured in weeks, rather than hours.
For the Fox rivermouth, WRT is strongly driven by the incoming discharge, but also driven by wind-driven seiche events. For the purpose of this sensitivity analysis, we regressed all of our observed 2016 discharge measurements and WRT estimates to create a log-normal regression relating discharge to WRT (R2 0.83, see Figure S1). We then used this relationship to estimate what the WRT would be given the discharges we used in the sensitivity analysis. This empirically-derived relationship would be different for rivermouths with different morphologies and watershed properties, and does not account for stochastic events that create backflows (seiches). The lack of data on water residence time limits our ability to model other rivermouths of similar fundamental size and discharge in the Great Lakes or elsewhere. All of the exact values used in this sensitivity analysis are described in the statistical appendix R code (https://doi.org/10.5066/P9PNDSXR) and correspond to the ranges of KN and FN observed in previous studies of the Fox rivermouth (Larson et al. 2019, 2020).
Results
Sensitivity of model outcomes to different variables
For the sensitivity analysis, we calculated the change in a hypothetical nutrient (in terms of mass change per day) across a broad range of first-order loss rates (K values), sediment flux rates, discharge and water residence times. Most of the range of K values that we considered suggested that the water column is a net sink for a nutrient, while most of the range of sediment flux rates we considered suggest that sediments are a net source of nutrients. The ranges we considered are based on the measurements we collected in the Fox and Duck rivermouths. Especially for sediment flux rates, those values seem plausible for other systems as well (as discussed in Larson et al. 2020). The ranges of values observed for the individual nutrients SRP, NH4 and NOX in the Fox rivermouth are highlighted in Fig. 4 as well. Across the range in discharge that is present in the Fox rivermouth, we looked at three different nutrient concentrations, one most applicable to SRP (0.005 mg L−1), one most applicable to NH4 (0.035 mg N L−1), and one most applicable to NOX (0.64 mg N L−1; Fig. 4).
Sensitivity analysis of rivermouth nutrient retention or release model. Individual panels are arrayed in a grid with increasing discharge (left to right) and increasing nutrient concentration (going down). The three rows of nutrient concentrations are close to observed values for soluble reactive phosphorus (SRP; A–C), ammonium (NH4; D–F) and nitrate (NOX; G–I). The discharge ranged from the minimum to the maximum observed in the Fox River during 2016 and surface area was held constant at 4.86 km2 (surface area present in the Fox rivermouth). Boxes indicate the range of sediment flux and first−order water column loss (K) observed in experiments from Larson et al. (2019, 2020). The SRP box is solid, while the NOX box is dotted
Across all potential K and sediment nutrient flux rates considered here, low nutrient concentrations correspond to a stronger influence of sediment processes and high nutrient concentrations correspond to a stronger influence of water column processes (Fig. 4). In Fig. 4, this basically means at low nutrient concentrations the contour lines are more vertical (indicating moving along the K axis doesn’t change the rivermouth effect) and at high concentrations, the contour lines are more horizontal (indicating moving along the sediment flux axis doesn’t change the rivermouth effect). Increasing discharge expands the importance of water column processes, but this seems to have a minor influence at high and low nutrient concentrations (e.g., Fig. 4D–F).
Within the specific ranges of nutrient processing rates we observed in the Fox for SRP, NH4 and NOX, more specific conclusions can be drawn. At low discharge, the rivermouth is likely a source of NH4 across the range of observed KNH4 and sediment NH4 flux (Fig. 4D–F), but as discharge increases, water column losses may result in the rivermouth becoming a net sink for NH4 at times when sediment flux is low and the absolute magnitude of KNH4 is relatively high (Fig. 4D–F). Overall, discharge was less important for the rivermouth effect on both SRP (Fig. 4A–C) and NOX (Fig. 4G–I).
At higher nutrient concentrations, those more relevant to NOX at many times during the year, the role of sediment nutrient flux becomes greatly diminished (Fig. 4). The almost horizontal contour lines on Fig. 4G–I demonstrate that across the entire range of sediment flux values considered, there is only small change in the magnitude of the rivermouth effect. Therefore, at these higher nutrient concentrations the role of water column processing is substantially more important than sediment flux in dictating whether the rivermouth is a net source or sink of nutrients and the magnitude of the rivermouth effect (Fig. 4G–I).
These model outcomes are sensitive to the WRT, but this sensitivity does little to alter the conditions that drive the direction of the rivermouth effect (net loss or net gain) and instead has more influence on the magnitude of the rivermouth effect (Figure S2). As a result, identifying parameter spaces where the rivermouth is a net source or sink is only slightly influenced by WRT. Increasing the surface area of the rivermouth without changing residence time or discharge increases the relative importance of sediment flux (Figure S3).
Simulating labile nutrient dynamics over four days in the Fox rivermouth
Within the Fox rivermouth our measured KNOX rates were highly variable, and so changes in NOX due to the water column were highly variable, and do not indicate a clear directionality within the rivermouth (Table 1). Because the concentrations of NOX were relatively high, this translated into water column effects that swamp out any sediment flux effect in the model. A clear majority of simulations suggested the rivermouth was a sink for NOX in April (82.1% of simulations) and a lesser majority indicated the rivermouth was a sink in June (67.3% of simulations; Table 1). In September, the simulations were nearly split on the directionality of the rivermouth effect, with 54.6% of simulations indicating the rivermouth was a sink (Table 1).
For SRP, the mass of nutrient transformed in the water column was 3–10 times higher than the mass of SRP flux into or out of the sediment (Table 1), so water column processing was also much more important than sediment processing for determining the overall rivermouth effect on these dates. In June, August and September, the majority of simulations indicated the rivermouth was a net sink for SRP (69.6, 86.8 and 99.9% of simulations), with water column removal of SRP occurring at a greater rate than sediment flux of SRP into the water column (Table 1). In April, the water column appeared more likely to be a source of SRP (72.6% of simulations had a net increase).
Unlike SRP and NOX, NH4 sediment fluxes were substantial enough to have a strong influence on the overall rivermouth effect. Sediment and water column NH4 effects were usually on the same order of magnitude, although in opposite directions (Table 1). In April, ~ 65% of simulations suggested the rivermouth was a NH4 sink, whereas in June, August and September, ~ 70% of simulations suggested the rivermouth was a net source of NH4.
A substantial proportion of the inferred load of these nutrients are transformed during passage through the rivermouth. The load of river nutrients in this analysis is inferred from the initial volume and the initial concentration (Fig. 2). The median proportion of the nutrients brought into the rivermouth by the river that were gained or lost ranged from −0.32 to 0.74 in our SRP models, −0.06 to 0.57 in our NH4 models and −0.28 to −0.01 in our NOX models (Table 1). This is a very crude method for estimating load, and our inferred loads are much lower than the loads reported at the outflow of the Fox River (below the rivermouth; Robertson et al. 2018), which we would expect given the other inputs to the Fox rivermouth that are outside the framework of this model (“The Cadmus Group” 2012).
Discussion
Our model combines areal sediment flux rates and volume-based water column transformation rates into a single mass estimate of the rivermouth’s effect on riverine nutrient loads. This model demonstrates that across a broad range of conditions, variation in water column processes drive variation in the rivermouth effect when concentrations are high, and variation in sediment flux rates is more important when nutrient concentrations are low. The relative importance of water column and sediment processing are thought to vary among ecosystems, with small headwater streams dominated by benthic processes (Mulholland and Webster 2010) and lentic systems experiencing a mix of benthic and pelagic processes (Wetzel 2001; Price and Carrick 2011). The simple model we indicated that over a large parameter space, it is necessary to understand both processes to assess how rivermouths alter riverine nutrient loads.
In our example with the Fox rivermouth, we treated the entire rivermouth as a single section and modeled mass changes occurring in a single day, but this same approach could be used to model individual rivermouth sub-sections over shorter time periods. One aspect that could be improved beyond simply modeling at a different spatio-temporal scale is the interactions between drivers of rivermouth nutrient effects. For example, phytoplankton and bacteria are able to rapidly vary their community composition and nutrient uptake rate in response to variation in nutrient concentrations (McCarthy et al. 2007a; Cáceres et al. 2019) and variation in discharge is likely not independent of variation in processing rates (Gardner and Doyle 2018).
The application of this model to the Fox rivermouth yielded some interesting insights to both this particular ecosystem and how the naturally occurring ranges of real-world processing rates fit within the overall parameter space we explored in the sensitivity analysis. Previous research in this system suggested that sediments could contribute a meaningful percentage of the total SRP and NOX load of the Fox River (Larson et al. 2020), and sediment flux can strongly influence the rivermouth effect across a wide range of potential parameter space in the model. However, our on-site data in the Fox occurred in parameter spaces where sediment flux had a small effect on SRP and NOX compared to the dynamics occurring in the water column. This is consistent with estimates of metabolism in Green Bay, with sites near the Fox River having much more pelagic than benthic primary production (Althouse et al. 2014).
Although our model suggests that water column processes are as important or more important than sediment flux rates over a wide range of plausible conditions in rivermouths similar to the Fox, the influence of these water column ‘losses’ on nearshore productivity is not clear. Loss of inorganic nutrients in the water column indicates a conversion to particulate or organic forms. Relative to dissolved forms of inorganic P, particulate P is often considered to be less bioavailable (Baker et al. 2014; Qin et al. 2015) and particulate P associated with inorganic sediment may be bound to iron or aluminum complexes, making the P difficult for phytoplankton to access. However, pelagic conversion of SRP to particulate P is most likely dominated by microbial uptake (including bacteria, cyanobacteria and phytoplankton). As these microbes die and lyse, the organic compounds that contain P are still relatively bioavailable, so much of this particulate P could be cycled in the water column or decompose and remineralized after sedimentation (Orihel et al. 2017). Although some of the microbially-held particulate P will sink into the sediments and be buried in Green Bay itself (Klump et al. 1997), it is not clear whether the decrease in SRP observed in the rivermouth due to water column processing translates into a similar decrease in the ability of the total P pool to sustain water column primary production in the nearshore.
Previously, management agencies in other areas have attempted to reduce sediment P release by adding alum (which binds strongly with P), and those efforts seem to reduce P flux rates (Steinman and Ogdahl 2012; Steinman et al. 2018). Sediment P flux in the Fox rivermouth appears relatively high relative to measurements made in other ecosystems (Orihel et al. 2017; Larson et al. 2020), so perhaps sediment manipulation could be a strategy to reduce P availability in Green Bay. However, wind and flow-driven resuspension and redistribution of sediments might complicate sediment amendments or movements. Even with the high P flux rates that were observed in the Fox rivermouth, our models suggest that passage of SRP through the rivermouth is driven primarily by variation in water column processes.
As with SRP, the water column removal of inorganic N is probably mostly driven by uptake by biota and results in plankton incorporating N into their tissues. This organic N can be released while biota still live, and also after they die and begin to decompose, so a substantial portion of the ‘removed’ N will also likely become available again over time. Some of this organic N may sink and be buried, and in the sediments some of that may be denitrified into N2 gas (Schlesinger and Bernhardt 2013), while other N will be released back into the water column in inorganic forms (as we observed in Larson et al. 2020).
In our model, there was some uncertainty about the overall effect of the Fox Rivermouth on the inorganic N load, but most of the summer and late summer simulations suggested an overall increase in the preferred NH4 (and possibly a reduction in NO3). The form of N strongly influences its ability to support aquatic primary producers. The general consensus is that reduced, inorganic N (NH4) is the preferred form of N for cyanobacterial species, and the ratio between oxidized (NO3) and reduced N is hypothesized to strongly influence competitive interactions between cyanobacteria and algal species (Glibert 2017). To use NO3 or urea, phytoplankton must first turn on additional enzymatic machinery that converts those N species into NH4, which requires the acquisition of trace metals and higher energetic costs (Rees and Bekheet 1982; Glass et al. 2012; Oliver et al. 2012; Glibert 2017).
Conclusion
Given the huge area of the watershed and the widely dispersed nature of nutrient sources, an interesting result of this modeling effort is that a 4.86 km2 rivermouth appears to be capable of substantially altering SRP and inorganic N concentrations in river water inputs. For example, in our August 3rd model, 84.1 kg of SRP were delivered to the rivermouth from the Fox River and our median estimate suggested 49.7 kg were transformed into particulate P in the water column before reaching the coast of Lake Michigan. Although this seems like a dramatic change, very labile nutrient forms are often cycled rapidly in productive ecosystems. For example, in a study on the eutrophic Grand River (a Lake Erie tributary), Barlow-Busch et al. (2006) measured gross uptake rates of phosphate and found that the range of turnover times for PO43− was 0.45–15 h. Nutrient loads are directly implicated in the intensity, severity and toxicity of cyanobacterial blooms and other eutrophication problems in Green Bay (Althouse et al. 2014), and therefore watershed-wide management of urban and agricultural sources of nutrients are a primary research and management focus (“The Cadmus Group” 2012; Kreiling et al. 2018).
Rivermouths are often small relative to the watersheds they drain, but the modeling here supports other evidence that suggests they can mediate (to some degree) the relationship between the watershed and the nearshore zone (Larson et al. 2013a, 2014, 2016). By using the Fox rivermouth as a test location for exploring this model, we may have focused on an unrepresentative example of a rivermouth (at least among other Great Lakes rivermouths). The Fox River is one of the largest tributaries of the Great Lakes and the rivermouth is heavily developed (i.e., channelized and disconnected from riparian areas), which decreases water residence time and opportunity for nutrient transformation. Other Great Lakes rivermouths may have configurations that result in more complete transformation of labile nutrients to organic form or particulate forms, essentially more effectively disconnecting the incoming labile nutrient concentrations from the nearshore zone. For example, the Muskegon rivermouth (also a Lake Michigan tributary) has a residence time of 21 days (Biddanda et al. 2018), compared to the 10–50 h common in the Fox rivermouth, and large rivermouths effects have been observed in the Muskegon (Marko et al. 2013). Although this model can be used for systems with very different underlying structures than the Fox, we would recommend parameterizing the models using different methods for estimating water column processing (and likely sediment nutrient flux) to account for those longer residence times. For example, a 12 h water column incubation probably isn’t a useful way of estimating the net effect of water column processing over 21 days.
There are other rivermouths that are similar in many respects to the Fox, most notably the Maumee rivermouth (Lake Erie), which also drains an agricultural watershed, is among the largest tributaries in the Great Lakes and has a heavily developed rivermouth. The movement and fate of labile nutrients is particularly relevant in the Maumee system, as there is a watershed-wide focus on reducing not just phosphorus loads, but specifically SRP (Annex 4 Task Team 2015). However, if the Maumee rivermouth functions much like the Fox, then we might expect to see rapid changes in the availability of SRP just within the rivermouth, independent of what is happening in the watershed.
Data Availability
Data used in this paper is available at ScienceBase (https://doi.org/10.5066/P9PNDSXR).
Code availability
All code associated with this paper is available at ScienceBase (https://doi.org/10.5066/P9PNDSXR).
References
Althouse B, Higgins S, Vander Zanden MJ (2014) Benthic and planktonic primary production along a nutrient gradient in Green Bay, Lake Michigan, USA. Freshw Sci 33:487–498. https://doi.org/10.1086/676314
Annex 4 Task Team (2015) Recommended phosphorus loading targets for Lake Erie. https://www.epa.gov/glwqa/report-recommended-phosphorus-loading-targets-lake-erie. Accessed 7 Dec 2021
Baker DB, Confesor R, Ewing DE et al (2014) Phosphorus loading to Lake Erie from the Maumee, Sandusky and Cuyahoga rivers: the importance of bioavailability. J Great Lakes Res 40:502–517. https://doi.org/10.1016/J.JGLR.2014.05.001
Barlow-Busch L, Baulch HM, Taylor WD (2006) Phosphate uptake by seston and epilithon in the Grand River, southern Ontario. Aquat Sci 68:181–192. https://doi.org/10.1007/s00027-006-0806-9
Biddanda BA, Weinke AD, Kendall ST et al (2018) Chronicles of hypoxia: time-series buoy observations reveal annually recurring seasonal basin-wide hypoxia in Muskegon Lake – a Great Lakes estuary. J Great Lakes Res 44:219–229. https://doi.org/10.1016/J.JGLR.2017.12.008
Cáceres C, Spatharis S, Kaiserli E et al (2019) Temporal phosphate gradients reveal diverse acclimation responses in phytoplankton phosphate uptake. ISME J 13:2834–2845. https://doi.org/10.1038/s41396-019-0473-1
Downer RGH (1985) Lipid metabolism. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology biochemistry and pharmacology. Pergamon Press, Elmsford, pp 77–114
Elliott M, Whitfield AK (2011) Challenging paradigms in estuarine ecology and management. Estuar Coast Shelf Sci 94:306–314. https://doi.org/10.1016/j.ecss.2011.06.016
Gardner JR, Doyle MW (2018) Sediment–water surface area along rivers: Water column versus benthic. Ecosystems 21:1505–1520. https://doi.org/10.1007/s10021-018-0236-2
Garnier J, Billen G, Némery J, Sebilo M (2010) Transformations of nutrients (N, P, Si) in the turbidity maximum zone of the Seine estuary and export to the sea. Estuar Coast Shelf Sci 90:129–141. https://doi.org/10.1016/J.ECSS.2010.07.012
Gilbert M, Needoba J, Koch C et al (2013) Nutrient loading and transformations in the Columbia River estuary determined by high-resolution in situ sensors. Estuaries Coasts 36:708–727. https://doi.org/10.1007/s12237-013-9597-0
Glass JB, Axler RP, Chandra S, Goldman CR (2012) Molybdenum limitation of microbial nitrogen assimilation in aquatic ecosystems and pure cultures. Front Microbiol 3:1–11. https://doi.org/10.3389/fmicb.2012.00331
Glibert PM (2017) Eutrophication, harmful algae and biodiversity — challenging paradigms in a world of complex nutrient changes. Mar Pollut Bull 124:591–606. https://doi.org/10.1016/j.marpolbul.2017.04.027
Hilton J, O’Hare M, Bowes MJ, Jones JI (2006) How green is my river? a new paradigm of eutrophication in rivers. Sci Total Environ 365:66–83. https://doi.org/10.1016/j.scitotenv.2006.02.055
Kling GW, Kipphut GW, Miller MM, O’Brien WJ (2000) Integration of lakes and streams in a landscape perspective: the importance of material processing on spatial patterns and temporal coherence. Freshw Biol 43:477–497. https://doi.org/10.1046/j.1365-2427.2000.00515.x
Klump JV, Edgington DN, Sager PE, Robertson DM (1997) Sedimentary phosphorus cycling and a phosphorus mass balance for the Green Bay (Lake Michigan) ecosystem. Can J Fish Aquat Sci 54:10–26. https://doi.org/10.1139/f96-247
Kreiling RM, Richardson WB, Cavanaugh JC, Bartsch LA (2011) Summer nitrate uptake and denitrification in an upper Mississippi River backwater lake: The role of rooted aquatic vegetation. Biogeochemistry 104:309–324. https://doi.org/10.1007/s10533-010-9503-9
Kreiling RM, Thoms MC, Richardson WB (2018) Beyond the edge: linking agricultural landscapes, stream networks, and best management practices. J Environ Qual 47:42. https://doi.org/10.2134/jeq2017.08.0319
Krieger K (2003) Effectiveness of a coastal wetland in reducing pollution of a Laurentian Great Lake: hydrology, sediment, and nutrients. Wetlands 23:778–791
Larson JH, Frost PC, Zheng Z et al (2007) Effects of upstream lakes on dissolved organic matter in streams. Limnol Oceanogr 52:60–69
Larson JH, Richardson WB, Vallazza JM, Nelson JC (2013a) Rivermouth alteration of agricultural impacts on consumer tissue δ(15)N. PLoS ONE 8:e69313. https://doi.org/10.1371/journal.pone.0069313
Larson JH, Trebitz AS, Steinman AD et al (2013b) Great Lakes rivermouth ecosystems: scientific synthesis and management implications. J Great Lakes Res 39:513–524. https://doi.org/10.1016/j.jglr.2013.06.002
Larson JH, Frost PC, Xenopoulos MA et al (2014) Relationships between land cover and dissolved organic matter change along the river to lake transition. Ecosystems 17:1413–1425. https://doi.org/10.1007/s10021-014-9804-2
Larson JH, Frost PC, Vallazza JM et al (2016) Do rivermouths alter nutrient and seston delivery to the nearshore? Freshw Biol 61:1935–1949. https://doi.org/10.1111/fwb.12827
Larson JH, Evans MA, Fitzpatrick FA et al (2019) Water column nutrient processing rates in rivermouths of Green Bay (Lake Michigan). Biogeochemistry 142:73–93. https://doi.org/10.1007/s10533-018-0517-z
Larson JH, James WF, Fitzpatrick FA et al (2020) Phosphorus, nitrogen and dissolved organic carbon fluxes from sediments in freshwater rivermouths entering Green Bay (Lake Michigan; USA). Biogeochemistry 147:179–197. https://doi.org/10.1007/s10533-020-00635-0
Marko KM, Rutherford ES, Eadie BJ et al (2013) Delivery of nutrients and seston from the Muskegon River Watershed to near shore Lake Michigan. J Great Lakes Res 39:672–681. https://doi.org/10.1016/j.jglr.2013.08.002
McCarthy M, Gardner W, Lavrentyev P et al (2007a) Effects of hydrological flow regime on sediment-water interface and water column nitrogen dynamics in a Great Lakes coastal wetland (Old Woman Creek, Lake Erie). J Gt Lakes 33:219–231
McCarthy MJ, Lavrentyev PJ, Yang L et al (2007b) Nitrogen dynamics and microbial food web structure during a summer cyanobacterial bloom in a subtropical, shallow, well-mixed, eutrophic lake (Lake Taihu, China). Hydrobiologia 581:195–207. https://doi.org/10.1007/s10750-006-0496-2
McClain ME, Boyer EW, Dent CL et al (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6:301–312. https://doi.org/10.1007/s10021-003-0161-9
Morrice JA, Kelly JR, Trebitz AS et al (2004) Temporal dynamics of nutrients (N and P) and hydrology in a lake superior coastal wetland. J Great Lakes Res 30:82–96. https://doi.org/10.1016/S0380-1330(04)70379-2
Morrice JA, Trebitz AS, Kelly JR et al (2011) Determining sources of water to Great Lakes coastal wetlands: a classification approach. Wetlands 31:1199–1213. https://doi.org/10.1007/s13157-011-0232-1
Mulholland PJ, Webster JR (2010) Nutrient dynamics in streams and the role of J-NABS. J North Am Benthol Soc 29:100–117. https://doi.org/10.1899/08-035.1
Oliver RL, Hamilton DP, Brookes JD, Ganf GG (2012) Physiology, blooms and prediction of planktonic cyanobacteria. In: Whitten BA (ed) Ecology of cyanobacteria II. Springer, Dordrecht, pp 155–194
Orihel DM, Baulch HM, Casson NJ et al (2017) Internal phosphorus loading in Canadian fresh waters: a critical review and data analysis. Can J Fish Aquat Sci 25:1–25. https://doi.org/10.1139/cjfas-2016-0500
Paerl HW, Scott JT, McCarthy MJ et al (2016) It takes two to tango: when and where dual nutrient (N & P) reductions are needed to protect lakes and downstream ecosystems. Environ Sci Technol 50:10805–10813. https://doi.org/10.1021/acs.est.6b02575
Price K, Carrick H (2011) Meta-analytical approach to explain variation in microbial phosphorus uptake rates in aquatic ecosystems. Aquat Microb Ecol 65:89–102. https://doi.org/10.3354/ame01537
Qin C, Liu H, Liu L et al (2015) Bioavailability and characterization of dissolved organic nitrogen and dissolved organic phosphorus in wastewater effluents. Sci Total Environ 511:47–53. https://doi.org/10.1016/J.SCITOTENV.2014.11.005
Rees TAV, Bekheet IA (1982) The role of nickel in urea assimilation by algae. Planta 156:385–387. https://doi.org/10.1007/BF00393307
Reisinger AJ, Tank JL, Rosi-Marshall EJ et al (2015) The varying role of water column nutrient uptake along river continua in contrasting landscapes. Biogeochemistry 125:115–131. https://doi.org/10.1007/s10533-015-0118-z
Robertson DM, Hubbard LE, Lorenz DL, Sullivan DJ (2018) A surrogate regression approach for computing continuous loads for the tributary nutrient and sediment monitoring program on the Great Lakes. J Great Lakes Res 44:26–42. https://doi.org/10.1016/j.jglr.2017.10.003
Robson BJ, Bukaveckas PA, Hamilton DP (2008) Modelling and mass balance assessments of nutrient retention in a seasonally-flowing estuary (Swan River Estuary, Western Australia). Estuar Coast Shelf Sci 76:282–292. https://doi.org/10.1016/j.ecss.2007.07.009
Schade JD, Espeleta JF, Klausmeier CA et al (2005) A conceptual framework for ecosystem stoichiometry: balancing resource supply and demand. Oikos 109:40–51
Schindler DW (2012) The dilemma of controlling cultural eutrophication of lakes. Proc Biol Sci 279:4322–4333. https://doi.org/10.1098/rspb.2012.1032
Schlesinger WH, Bernhardt ES (2013) Biogeochemistry: An analysis of global change, 3rd edn. Academic Press, Waltham
Seitzinger S, Harrison JA, Böhlke JK et al (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl 16:2064–2090. https://doi.org/10.1890/1051-0761(2006)016[2064:DALAWA]2.0.CO;2
Small GE, Finlay JC, McKay RML et al (2016) Large differences in potential denitrification and sediment microbial communities across the Laurentian great lakes. Biogeochemistry 128:353–368. https://doi.org/10.1007/s10533-016-0212-x
Steinman AD, Ogdahl ME (2012) Macroinvertebrate response and internal phosphorus loading in a Michigan Lake after alum treatment. J Environ Qual 41:1540. https://doi.org/10.2134/jeq2011.0476
Steinman AD, Nemeth L, Nemeth E, Rediske R (2006) Factors influencing internal P loading in a western Michigan, drowned river-mouth lake. J North Am Benthol Soc 25:304–312. https://doi.org/10.1899/0887-3593(2006)25[304:FIIPLI]2.0.CO;2
Steinman A, Chu X, Ogdahl M (2009) Spatial and temporal variability of internal and external phosphorus loads in Mona Lake, Michigan. Aquat Ecol 43:1–18. https://doi.org/10.1007/s10452-007-9147-6
Steinman AD, Hassett MC, Oudsema M, Rediske R (2018) Alum efficacy 11 years following treatment: phosphorus and macroinvertebrates. Lake Reserv Manag 34:167–181. https://doi.org/10.1080/10402381.2017.1402834
“The Cadmus Group” (2012) Total maximum daily load and watershed management plan for total phosphorus and total suspended solids in the lower Fox river basin and lower Green Bay. Accessed online at: https://dnr.wi.gov/water/wsSWIMSDocument.ashx?documentSeqNo=62246254. Accessed 7 Dec 2021
Trebitz AS (2006) Characterizing seiche and tide-driven daily water level fluctuations affecting coastal ecosystems of the Great Lakes. J Great Lakes Res 32:102–116
Vannote RL, Minshall GW, Cummins KW (1980) River continuum concept. Can J Fish Aquat Sci 37:130–137
Weinke AD, Kendall ST, Kroll DJ et al (2014) Systematically variable planktonic carbon metabolism along a land-to-lake gradient in a Great Lakes coastal zone. J Plankton Res 36:1528–1542. https://doi.org/10.1093/plankt/fbu066
Wetzel RG (2001) Limnology, 3rd edn. Academic Press, Amsterdam
Acknowledgements
Thanks to Enrika Hlavacek for preparing Figure 3 and Emily Wimmer for assistance in preparing Figure 4. This research was partially funded by the Great Lakes Restoration Initiative and the U.S. Geological Survey Ecosystem Mission Area.
Funding
This research was partially funded by the Great Lakes Restoration Initiative and the U.S. Geological Survey Ecosystem Mission Area.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Jennifer Leah Tank.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Larson, J.H., Evans, M.A., Fitzpatrick, F.A. et al. Benthic and planktonic inorganic nutrient processing rates at the interface between a river and lake. Biogeochemistry 155, 189–203 (2021). https://doi.org/10.1007/s10533-021-00821-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-021-00821-8