Abstract
Nutrient transformation processes such as assimilation, dissimilatory transformation, and sorption to sediments are prevalent in benthic zones of headwater streams, but may also occur in the water column. The river continuum concept (RCC) predicts that water column processes become increasingly important with increasing stream size. We predicted that water column nutrient uptake increases with stream size, mirroring carbon/energy dynamics predicted by the RCC. We measured water column uptake of ammonium (\({\text{NH}}_{4}^{ + }\)), nitrate (\({\text{NO}}_{3}^{ - }\)), and soluble reactive phosphorus (SRP) in 1st through 5th order stream and river reaches (discharge: 50–68,000 L s−1) in three watersheds ranging from <1 to >70 % developed lands. We found that water column volumetric uptake (U vol ) of \({\text{NH}}_{4}^{ + }\), \({\text{NO}}_{3}^{ - }\), and SRP did not significantly differ among watersheds and we did not find any longitudinal patterns for U vol . Uptake velocity (v f ) of \({\text{NH}}_{4}^{ + }\) increased with stream size, whereas \({\text{NO}}_{3}^{ - }\) and SRP v f did not differ with stream size or among watersheds. Both U vol and v f were related to water column metabolism and material suspended in the water column, but specific relationships differed among solutes and uptake metrics. Median water column v f across 15 sites was 4, 9, and 19 % of median whole-stream \({\text{NH}}_{4}^{ + }\), \({\text{NO}}_{3}^{ - }\), and SRP v f based upon a previous meta-analysis. Thus, although we could not demonstrate a generalized longitudinal pattern in water column nutrient uptake, water column processes can be important. An improved mechanistic understanding of the controls on uptake and the ultimate fate of nutrients will facilitate effective management and restoration for mitigating downstream nutrient export.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
River networks regulate nutrient export from the terrestrial landscape to receiving waters. These networks transition from headwater streams to mid-order non-wadeable rivers, and end in large rivers. Nutrients entering streams in the headwaters are processed by biofilms colonizing benthic (i.e., bottom) surfaces through a combination of assimilatory and dissimilatory microbially-mediated processes which subsequently influence downstream export, either temporarily or permanently (Peterson et al. 2001; Hall and Tank 2003; Mulholland et al. 2008). We already recognize that small streams are important for nutrient uptake at the watershed scale (Bernhardt et al. 2003; Alexander et al. 2007), and there is substantial research on the export of nutrients from coastal rivers to oceans (Meybeck 1982; Howarth et al. 1996; Caraco and Cole 1999). Yet the contribution of rivers to watershed nutrient dynamics and the mechanisms controlling riverine nutrient uptake are less clear (Ensign and Doyle 2006; Tank et al. 2008).
In headwater streams, a portion of nutrients removed from the water column are removed permanently (i.e., via denitrification), but the majority are only delayed temporarily (e.g., via assimilatory uptake; Mulholland et al. 2008), and eventually are available for export to larger rivers downstream. Larger rivers are more efficient at removing nutrients per unit stream length compared to headwater streams (Seitzinger et al. 2002). However, specific mass loss rates are expected to decline with increasing water depth because of reduced contact between the benthos and the overlying water column (Alexander et al. 2007). This contradictory evidence has led to conclusions that the efficiency of riverine nutrient processing could be higher (Seitzinger et al. 2002; Wollheim et al. 2006) or lower (Alexander et al. 2007) than headwaters.
As streams increase in size and become rivers, the river continuum concept (RCC) predicts that energy inputs shift in predictable patterns related to physical changes along the longitudinal gradient (Vannote et al. 1980). For example, in medium to large rivers, there is greater light availability associated with an open canopy, but also increased turbidity which limits light penetration to the stream bottom. Therefore, water column processes should drive whole-ecosystem metabolism in medium to large rivers. Indeed, a shift from heterotrophy to autotrophy occurred at a 5th order river in a study of energy dynamics along the RCC (Webster 2007). In contrast to predictions about metabolism along the stream size gradient, nutrient dynamics were not explicitly mentioned in the RCC, although the authors did predict that the biological community in would be dominated by planktonic species in large rivers, thereby “reflecting the semi-lentic nature of such waters” (Vannote et al. 1980). Gross primary production (GPP) and the ratio of production to respiration generally increase moving downstream (McTammany et al. 2003), likely due to the presence of potamoplankton in larger rivers (Descy and Gosselain 1994). Higher GPP should increase nutrient demand in the water column due to the stoichiometric requirements of autotrophs, reflecting metabolic patterns documented at the reach scale (Hall and Tank 2003). We expect that the water column will be biologically-active in rivers, and as such, assimilatory nutrient uptake in rivers may shift from the benthic zone to the water column paralleling the metabolic predictions of the RCC (Vannote et al. 1980).
Studies of whole-stream nutrient uptake reflect the combination of water column and benthic uptake, but benthic processes are assumed to drive uptake rates in streams (Peterson et al. 2001), and water column uptake is often assumed to be negligible. This assumption may be because a seminal paper on phosphorus (P) uptake in a forested stream concluded that phytoplankton contributed <5 % of total phosphate uptake throughout the year (Mulholland et al. 1985). However, a few studies of water column nutrient processing have shown that water column P uptake increases with stream order in a forested watershed (Corning et al. 1989), and phytoplankton uptake constituted 16 % of total P uptake in a eutrophic river (Barlow-Busch et al. 2006). In addition to assimilatory nutrient uptake, denitrification can occur within anoxic microsites associated with suspended sediments in river water (Liu et al. 2013), providing another mechanism for water column nutrient uptake in rivers. These individual studies suggest that water column nutrient dynamics may contribute significantly to riverine nutrient uptake and supplement benthic nutrient processing in certain ecosystems, but field-based research across stream size gradients is needed to provide a mechanistic understanding of where and when water column processes constitute a substantial nutrient uptake pathway.
Nutrient removal throughout a river network protects water quality by either permanently removing nutrients prior to downstream export or by modifying the timing of nutrient export due to assimilation into biomass, which represents temporary nutrient removal. Understanding mechanisms of nutrient removal is especially important given the well-known influence of human land use on watershed nutrient export to sensitive downstream ecosystems such as the Gulf of Mexico (Alexander et al. 2008) or Lake Erie (Baker and Richards 2002). Anthropogenic point and non-point sources have increased nitrogen (N) and P loads throughout river networks globally (Carpenter et al. 1998; Seitzinger et al. 2005). Although small increases in nutrients can increase nutrient uptake in headwater streams (Mulholland et al. 2008), uptake efficiencies decrease with increasing nutrient enrichment (O’Brien et al. 2007; Mulholland et al. 2008) resulting in increased nutrient export to downstream ecosystems despite increased headwater nutrient uptake rates. Although nutrient uptake occurs in larger downstream river segments, the effect of stream size on nutrient uptake is variable (Hall et al. 2013). Factors that co-vary with stream size (e.g., canopy cover, temperature, width, depth, velocity) are known to influence nutrient removal from the water column, yet studies of nutrient uptake along stream/river size gradients are limited and further study is needed.
To improve our understanding of nutrient dynamics across river networks, we quantified water column uptake of ammonium (\({\text{NH}}_{4}^{ + }\)), nitrate (\({\text{NO}}_{3}^{ - }\)), and soluble reactive phosphorus (SRP) along a stream size gradient in three watersheds with contrasting land-use. Using in situ chamber measurements of water column nutrient uptake, we addressed the following questions: (1) how does water column nutrient uptake vary within and among river networks? and (2) what processes control water column nutrient uptake? We predicted that (1) water column nutrient uptake and demand would increase with stream size due to increased suspended material in larger streams and rivers (Vannote et al. 1980; Dodds and Whiles 2004); (2) water column nutrient processing would be higher in watersheds with anthropogenic land use due to increased nutrient availability; and (3) water column metabolism and concentration of suspended materials would drive water column uptake.
Methods
Site description
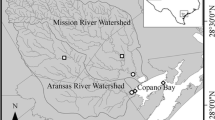
We performed this study using a size gradient of streams to rivers with watershed areas ranging from 39 to 2200 km2 within three contrasting watersheds: the Snake River Watershed located in western Wyoming, the Manistee River Watershed located in northern lower Michigan and the Elkhart Branch of the St. Joseph River Watershed located in northern Indiana (Fig. 1). Although these sites spanned 1st through 5th order, the headwater sites have larger watershed areas than in previous RCC studies (e.g., Minshall et al. 1983), due to the topography, geology, and land use within the watersheds, particularly in the Midwestern United States. We conducted our research during summer baseflow conditions, sampling Manistee and St. Joseph sites in 2011 and Snake sites in either 2010 or 2013. For the Snake, we initially sampled 4th and 5th order sites as part of a separate project in 2010, and expanded the work by sampling 1st through 3rd order sites in 2013 when we realized that including a more nearly pristine watershed like the Snake would provide a logical comparison for this study of water column uptake along river continua.
The 15 study streams were located in three separate watersheds, the Snake River Watershed (SNK) in western Wyoming, the Manistee River Watershed (MAN) in northern Michigan, and the St. Joseph River Watershed (STJ) of northern Indiana and southern Michigan. Stream order of each site is denoted in the site name
We characterized each study watershed using ArcGIS 10.0 (Esri Corporation, Redlands, CA, USA). First we used the hydrology tools in the spatial analyst toolbox to delineate watersheds using USGS digital elevation models (30 m2 resolution) accessed using The National Map (Dollison 2010; http://www.nationalmap.gov). We then used the delineated watersheds to extract land use characteristics from the 2006 National Land Cover Database, also accessed via The National Map (Dollison 2010). We report human influence on watersheds as % developed lands, which is the summation of urban/suburban and agricultural lands in the watershed.
Water column nutrient uptake incubations
We measured water column nutrient uptake using in situ chamber incubations at 5 sites (1st–5th order reaches) in each watershed for a total of n = 15 sites. At each site, we filled replicate 2.8 L clear polycarbonate chambers (n = 15 chambers) with depth-integrated river water collected by wading as close to the thalweg as possible. Although we were unable to sample the thalweg in the largest river reaches, samples were always collected in areas of high flow. For Snake and Manistee sites, we amended each incubation chamber with either \({\text{NH}}_{4}^{ + }\), \({\text{NO}}_{3}^{ - }\), or SRP to raise ambient concentrations by 35 µg N or P L−1; this amendment was necessary because ambient nutrient concentrations were near our analytical detection limits in comparison to St. Joseph sites which had sufficiently high ambient concentrations and therefore did not require nutrient amendments (Table 1). Increasing nutrient concentrations can influence nutrient uptake by saturating nutrient demand (Dodds et al. 2002). Any saturation of nutrient demand would decrease our estimate of nutrient uptake; therefore our estimates of nutrient uptake in the two low-nutrient watersheds are conservative. Nevertheless, experimental increases in nutrient concentration were minimal and incubation periods were short, making community or biophysical responses to the nutrient amendments unlikely.
At each site, we attached replicate chambers to metal posts installed in the stream bed, suspending chambers near the water surface to provide a locally representative light and temperature regime throughout each incubation (15 total chambers per site, n = 5 replicates for each of 3 solutes). We conducted incubations at approximately the same time of day at each site, and we always performed incubations in full sunlight. For each chamber, we collected water samples hourly over a minimum of 4 h. Chambers were continuously agitated by turbulence at the water surface during field deployment, and we agitated each chamber prior to each sampling to ensure homogeneity and minimize sediment deposition over the incubation. We collected water samples by removing 60 mL of water from each chamber, initially filtering 20 mL through a 0.2 µm SUPOR filter (Pall Corp., Port Washington, NY, USA) into either a 60 mL acid-washed Nalgene bottle (Thermo Scientific, Waltham, MA, USA) or a sterile 50 mL centrifuge tube (VWR, Radnor, PA, USA) as a rinse, followed by the remaining 40 mL of sample. We stored samples on ice until returning to the laboratory, where samples were frozen until water chemistry analysis. We quantified dissolved \({\text{NH}}_{4}^{ + } {-} {\text{N}}\) using the phenol-hypochlorite method (Solorzano 1969), \({\text{NO}}_{3}^{ - } {-} {\text{N}}\) using the cadmium reduction method (APHA 1995) and SRP using the ascorbic acid method (Murphy and Riley 1962) on a Lachat Flow Injection Autoanalyzer (Lachat Instruments, Loveland, CO, USA).
Calculation of water column nutrient uptake
Although water column nutrient uptake is often estimated using Michaelis–Menten dynamics in lake and marine studies (Aksnes and Egge 1991; Dodds et al. 1991), stream nutrient spiraling studies and the SPARROW water quality model both assume first-order dynamics (Newbold et al. 1983; Stream Solute Workshop 1990; Smith et al. 1997). For this study, we assumed first-order dynamics and calculated water column nutrient uptake based on nutrient spiraling theory (Stream Solute Workshop 1990) to compare water-column uptake to whole-stream uptake. First, we calculated the nutrient loss rate within each chamber (k; h−1) using the decline in nutrient concentration over time and then fitting a line to the natural log (loge) transformed data:
where C 0 and C t are the concentrations of N or P as either \({\text{NH}}_{4}^{ + }\), \({\text{NO}}_{3}^{ - }\), or SRP in the chamber at time 0 and t, respectively. We set k to equal zero for any chamber that did not show a significant decline in nutrient concentration over time. It is likely that mineralization and nitrification may have occurred at low rates, and these mechanisms may have offset nutrient uptake in certain chambers with low assimilatory activity.
After calculating k, we calculated solute uptake flux per unit volume of river water as:
where U vol is volumetric uptake rate (mg m−3 h−1) and C b is the background nutrient concentration (mg m−3). This approach is consistent with whole-stream nutrient uptake studies. To account for variation in C b , we also calculated uptake velocity (v f ) via:
where v f represents biological demand relative to ambient nutrient concentration (m h−1 or mm min−1), and d is mean stream/river depth (m). Operationally, v f is ideal for comparing nutrient demand across sites with different ambient nutrient concentrations (Stream Solute Workshop 1990).
Environmental characteristics
After completing the incubations for water column nutrient uptake, we sampled each replicate chamber for total suspended solids (TSS), suspended organic material as ash-free dry mass (AFDM), and suspended chlorophyll a using the remaining water in each chamber. We first sampled for TSS and AFDM by filtering a sub-sample of water within the chamber onto a pre-ashed, pre-weighed 1.0 µm glass-fiber (GF) filter (Pall Corp., Port Washington, NY, USA) which we returned to the laboratory, dried at 60 °C for at least 48 h and weighed for TSS. We then ashed TSS filters at 500 °C for 4 h and re-weighed, with the difference between dry and ashed weights providing AFDM and the mass remaining after ashing providing suspended inorganic material (Steinman et al. 2006). We measured chlorophyll a by filtering a separate subsample onto a 1.0 µm GF filter (Pall Corp., Port Washington, NY, USA), which we subsequently placed on ice in the dark, returned to the laboratory and stored frozen until we analyzed filters for chlorophyll a using the cold-methanol fluorometric technique (Wetzel and Likens 2001).
We measured water column GPP and ecosystem respiration (ER) at each site using a light–dark bottle incubation technique (Bott 2006). We filled 50 mL polypropylene centrifuge tubes (VWR International, LLC, Radnor, PA, USA) (n = 5) with stream water which had been previously measured for temperature, pH, and dissolved oxygen (DO; both in mg L−1 and % saturation). We sealed centrifuge tubes underwater to ensure that no air bubbles were present, and then incubated centrifuge tubes underwater for at least 2 h in ambient light and temperature conditions. We measured DO after 2 h, with the change in DO providing an estimate of water column net ecosystem productivity (NEP). We repeated this process for an additional 2 h but covered the centrifuge tubes with aluminum foil sleeves to ensure no light penetration and the subsequent DO change in the dark provided ER. We then calculated GPP as:
where |ER| is the absolute value of ER.
Given the wide range of discharge (Q) across our study sites, we estimated Q three ways. For wadeable streams, we used a Marsh-McBirney Flo-Mate 2000 (Marsh-McBirney, Inc., Frederick, MD, USA) to calculate Q using the partial summation midsection method (Gore 2006). For non-wadeable rivers, we located our study sites near USGS gaging stations which provided Q. For intermediate sites that were too deep to wade, and did not have a USGS gage nearby, we used a watershed-specific relationship between watershed area and Q estimated at other sites within the watershed using the two methods above to estimate site-specific Q. These watershed-specific relationships (p < 0.01, r2 > 0.95 for each) were only used to estimate Q at site 3 in the Manistee and site 4 in the St. Joseph (Fig. 1).
Statistical analysis
We used linear mixed-effects models (LMMs) to test the influence of stream size and watershed identity on water column nutrient uptake. We tested (1) how water column nutrient uptake varied across sites and watersheds, and (2) how water column nutrient demand relative to concentration (as v f ) differed across sites and watersheds. Additionally, we compared these patterns across solutes by running separate models for the above questions for each solute resulting in a total of six models. We tested for significance (1) using U vol as the response variable and (2) using v f as the response variable. Our models were similar to those constructed by Nelson et al. (2013), taking the general form:
where y ij is the response variable from chamber j at site i, μ is the intercept, β1–3 are the parameter estimates associated with each independent, fixed effect (watershed area, watershed identity, and their interaction), b i is the random effect associated with study site, and ε ij is the residual error. We used watershed area as our stream size metric and watershed identity as a categorical variable to encompass general differences across watersheds (e.g., land use, geology, climate, etc.). Watershed area was generally comparable across watersheds, although Manistee was larger than St. Joseph or Snake at 4th and 5th order sites. We use the terms ‘stream size’ and ‘watershed’ when reporting our results to refer to watershed area and watershed identity, respectively. We used the lmer function in package lme4 in the statistical software R (version 3.0.2, R Core Development Team) to run the LMMs. We then used package lmerTest to calculate significance probabilities (p) from the LMMs. Finally, we calculated the marginal and conditional R2 for each LMM according to Nakagawa and Schielzeth (2013). The marginal R2 (\({\text{R}}_{{{\text{LMM}}({\text{m}})}}^{2}\)) is the variance explained by the fixed factors alone (i.e., not accounting for the variance explained by the random effect), whereas the conditional R2 (\({\text{R}}_{{{\text{LMM}}({\text{c}})}}^{2}\)) is the variance explained by the entire model (Nakagawa and Schielzeth 2013) (see Supplementary Material for example R code). We used mixed-effects models to fully account for within-site variation, reflecting a more conservative statistical approach than using the mean of five replicate chambers, which would be required to meet the assumptions of analysis of co-variance.
In addition to using LMMs to examine the effect of stream size and watershed on water column uptake, we explored which biotic and abiotic factors were related to water column nutrient uptake across the land-use and stream size gradients using simple linear regression (SLR) and variables measured at both the chamber and reach scale. To be consistent with our analyses across independent variables, for any chamber-specific measurement (e.g., chlorophyll a, AFDM, TSS) we calculated the chamber average for each solute to provide a single value for each independent variable at each site (n = 15 sites). All data other than water column GPP and ER were non-normally distributed, so we log10-transformed all other data prior to any statistical analyses, which were performed in the statistical software R (version 3.0.2, R Core Development Team).
Results
Environmental characteristics
Both abiotic and biotic material suspended in the water column differed among watersheds and were consistently lowest in Snake, intermediate in Manistee, and highest in St. Joseph (Table 1); differences were reflected in the watershed means for water column chlorophyll a, AFDM, and TSS for the Snake, Manistee, and St. Joseph watersheds, respectively (Table 1). Dissolved solutes also followed similar patterns among watersheds where dissolved inorganic N (DIN) was lowest in Snake, intermediate in Manistee, and highest in St. Joseph as reflected in watershed means for \({\text{NH}}_{4}^{ + } {-} {\text{N}}\) and \({\text{NO}}_{3}^{ - } {-} {\text{N}}\).
Differences in particles and dissolved nutrients among watersheds were likely driven by patterns of human land use, with Snake having little to no area under human development (0.0–0.7 % developed), Manistee with increased agricultural and urban catchment area (9.1–19.4 % developed), and St. Joseph with high amounts of row-crop agriculture and urban lands (74.3–83.4 % developed). Patterns of inorganic P concentrations, however, did not follow this pattern, with SRP being lowest in Manistee (7 µg L−1), intermediate in Snake (16 µg L−1), and highest in St. Joseph (20 µg L−1). In general, longitudinal patterns of water chemistry and suspended particles were less distinct than the watershed effect. Water column chlorophyll a increased with stream size in the Snake (SLR; p < 0.001, r2 = 0.30) and Manistee (SLR; p < 0.001, r2 = 0.71) but not in the St. Joseph (SLR; p = 0.15). In contrast, TSS and AFDM of suspended particles within each watershed were similar across the stream size gradient (Table 1). Finally, although \({\text{NO}}_{3}^{ - } {-} {\text{N}}\) increased with stream size at Manistee (SLR; p < 0.001, r2 = 0.79), other dissolved inorganic nutrients did not vary consistently across the size gradient.
Volumetric uptake in the water column
Water column k of \({\text{NH}}_{4}^{ + }\) ranged from 0.001 to 0.058 h−1, \({\text{NO}}_{3}^{ - }\) k ranged from 0.001 to 0.131 h−1, and SRP k ranged from 0.000 to 0.118 h−1 (Table 2), but k did not differ across stream size or watershed for any solute (statistical results not shown). For chambers with significant declines in concentration, the first-order decay model fit the data well, with mean r2 values of 0.65, 0.63, and 0.69 for \({\text{NH}}_{4}^{ + }\), \({\text{NO}}_{3}^{ - }\), and SRP, respectively. Of the 90 chambers measured for each solute, we were unable to measure significant uptake in 16 \({\text{NH}}_{4}^{ + }\), 33 \({\text{NO}}_{3}^{ - }\), and 34 SRP chambers. Chambers with no significant \({\text{NO}}_{3}^{ - }\) uptake were most common in the St. Joseph watershed, whereas chambers with no significant SRP uptake were most common in the Snake watershed.
Water column volumetric uptake (U vol ) varied both within and among solutes. Volumetric uptake of SRP (U vol-SRP ; 0.000–2.33 mg SRP m−3 h−1) and \({\text{NH}}_{4}^{ + }\) (\({U_{{vol{\text{-}}{NH}}_{4}}}\); 0.004–4.15 mg \({\text{NH}}_{4}^{ + } {-} {\text{N}}\) m−3 h−1) were less variable than U vol of \({\text{NO}}_{3}^{ - }\) (\({U_{{vol{\text{-}}{NO}}_{3} }}\) ), which spanned more than 5 orders of magnitude (0.001–363 mg \({\text{NO}}_{3}^{ - } {-} {\text{N}}\) m−3 h−1; Fig. 2). Variability in \({U_{{vol{\text{-}}{NO}}_{3} }}\) was strongly influenced by ambient nutrient concentration, which is used in the calculation of U vol . For example, sites in the highly agricultural St. Joseph watershed had very high background \({\text{NO}}_{3}^{ - }\) concentrations and also showed volumetric uptake rates that were often >100 times higher than at sites in the Snake.
Water column volumetric uptake (U vol ) of a \({\text{NH}}_{4}^{ + }\), b \({\text{NO}}_{3}^{ - }\), and c SRP did not differ across watershed area or among watersheds in the Manistee (gray circles), St. Joseph (black circles), or Snake (white circles) watersheds. Error bars represent one SE. Note the logarithmic scales on both axes
After accounting for random variation in U vol among sites (using the random effect in our models), U vol did not differ across stream size (p = 0.61, 0.69, 0.44; Table 3) or among watersheds (p = 0.32, 0.14, 0.36; Table 3), and there was no interaction between stream size and watershed (p = 0.39, 0.52, 0.46; Table 3) for \({\text{NH}}_{4}^{ + }\), \({\text{NO}}_{3}^{ - }\), or SRP, respectively. Although fixed factors in the LMMs were often not significant, these models explained a large proportion of the variation in U vol , with \(R_{LMM(m)}^{2}\) ranging from 0.13 for U vol-SRP to 0.76 for \({U_{{vol{\text{-}}{NO}}_{3} }}\). These results suggest that although individual parameters were not statistically significant, 13–77 % of the variation in U vol was explained by the combination of watershed, stream size and the interaction term. In addition to the fixed factors, \(R_{LMM(c)}^{2}\), which includes the random effect in our model, ranged from 0.28 for U vol-SRP to 0.91 for \({U_{{vol{\text{-}}{NH}}_{4} }}\) (Table 3). The difference between \(R_{LMM(m)}^{2}\) and \(R_{LMM(c)}^{2}\) indicates that the random factor of study site substantially improved the model.
Water column nutrient demand
Water column uptake velocity (v f ), which represents nutrient demand relative to concentration, of \({\text{NH}}_{4}^{ + }\) (0.008–0.975 mm min−1), \({\text{NO}}_{3}^{ - }\) (0.013–2.11), and SRP (0.000–2.63 mm min−1) was similar across solutes (Fig. 3). Decreased variability in v f relative to U vol further suggests that much of the variability in U vol reflected differences in the concentration of ambient nutrients, whereas concentration is not included in the calculation of v f.
Water column uptake velocity (v f ) for a \({\text{NH}}_{4}^{ + }\) increased with watershed area in the Manistee (gray circles), St. Joseph (black circles), and Snake (white circles), whereas b \({\text{NO}}_{3}^{ - }\), and c SRP v f did not differ across watershed area. No differences were seen among watersheds for any solute. Error bars represent one SE. Note the logarithmic scale on the x axis
After incorporating random variation in v f among sites, the demand for \({\text{NH}}_{4}^{ + }\) (\({v_{{f{\text{-}}{NH}}_{4} }}\)) increased with stream size (p = 0.02; Fig. 3) but demand for \({\text{NO}}_{3}^{ - }\) and SRP did not (Table 3). Water column nutrient demand did not differ across watersheds and there was no significant interaction between stream size and watershed for any solute (Table 3). Similar to U vol , the fixed factors in these models were often not significant, but the suite of LMMs explained a large amount of the variation in v f among solutes (Table 3): \(R_{LMM(m)}^{2}\) ranged from a low of 0.12 for v f-NO3 to a high of 0.43 for \({v_{{f{\text{-}}{NH}}_{4} }}\), and \(R_{LMM(c)}^{2}\) ranged from 0.59 for \({v_{{f{\text{-}}{NO}}_{3} }}\) to 0.82 for \({v_{{f{\text{-}}{NH}}_{4} }}\) (Table 3), suggesting somewhat less variation in uptake velocity was accounted for by the LMMs, compared to volumetric uptake.
Environmental controls on water column nutrient dynamics
Our goal was to use environmental data to explain variation in nutrient uptake metrics across the size gradient, among watersheds of varying land use, and among solutes; we found that LMMs consistently explained a substantial portion of the variance in the data. Nevertheless, the fixed factors were not consistently significant, suggesting that some factor(s) other than stream size and watershed controlled variation in water column nutrient dynamics. Chlorophyll a, GPP, and ER each were each related to \({U_{{vol{\text{-}}{NH}}_{4} }}\) (r 2 = 0.52, 0.27, and 0.57, respectively; Table 4). Both chlorophyll a and GPP were positively related to \({U_{{vol{\text{-}}{NH}}_{4} }}\), whereas ER was negatively related to \({U_{{vol{\text{-}}{NH}}_{4} }}\). Similar to \({\text{NH}}_{4}^{ + }\), chlorophyll a and GPP each were related to \({U_{{vol{\text{-}}{NO}}_{3} }}\) (r2 = 0.57 and 0.43), as was suspended inorganic material (r2 = 0.50; Table 4). In contrast to either N species, ER was the only factor significantly related to U vol-SRP (r2 = 0.43; Table 4), and this was a negative relationship.
Site or chamber specific factors also influenced water column uptake velocities of \({\text{NH}}_{4}^{ + }\), \({\text{NO}}_{3}^{ - }\), and SRP. Uptake velocity of \({\text{NH}}_{4}^{ + }\) was positively related to suspended organic matter (as AFDM) and ER (r2 = 0.33 for both; Table 4). In contrast to \({\text{NH}}_{4}^{ + }\), \({v_{{f{\text{-}}{NO}}_{3} }}\) was positively related to suspended inorganic material (r2 = 0.32; Table 4). Similar to \({\text{NH}}_{4}^{ + }\), suspended AFDM and ER both were related to v f-SRP (r2 = 0.33 and 0.58, respectively), as was background SRP concentration (r2 = 0.44; Table 4). Both ER and background SRP were negatively related to v f-SRP , whereas suspended AFDM was positively related to v f-SRP .
Discussion
Water column nutrient processes in lotic ecosystems have been overlooked largely due to the lack of empirical data on riverine nutrient uptake in sites where the water column would most likely contribute to nutrient uptake, such as non-wadeable rivers (Tank et al. 2008). In addition, there has been a disciplinary focus on the importance of benthic nutrient processing in headwaters (Peterson et al. 2001; Alexander et al. 2007). While the assimilation of P by phytoplankton was negligible in a forested headwater stream (Mulholland et al. 1985), the roles of both assimilatory (Corning et al. 1989) and dissimilatory (Liu et al. 2013) processes in the water column likely increase in large streams and rivers. Using empirical measurements of water column nutrient uptake in flowing waters across river continua of contrasting land use, we found that water column nutrient uptake occurred in the majority of chambers we deployed across all sites, ranging from headwaters to mainstem rivers and from watersheds with low human development to highly-agricultural systems. The only example of non-detectable uptake was for SRP at the fifth site in the Snake, which had no significant SRP uptake in any of the five replicate mesocosms (Table 2).
Although water column nutrient uptake was detectable in all watersheds, the influence of stream size varied among solutes and uptake metrics, and there were no statistically significant differences in water column nutrient uptake across watersheds. As predicted, the concentration of suspended organic material (water column chlorophyll a and AFDM), water column metabolism, and suspended inorganic material often were related to water column nutrient uptake and demand, suggesting a combination of abiotic and biotic processes can drive water column uptake of dissolved nutrients.
Water column nutrient uptake varies within and among river networks
We expected water column nutrient uptake to increase longitudinally and to differ across watersheds due to human land use which causes alterations in canopy cover as well as nutrient availability. However, U vol did not differ from headwaters to rivers for any solute, suggesting that the water column plays a role in processing nutrients across a wide range of stream and river sizes and volumetric uptake rates are consistent throughout a watershed. It is possible that the lack of a stream-size response is because we did not include small enough streams or large enough rivers in our study design. The smallest sites in our study had watersheds more than 10× the size of those included in previous studies, whereas the largest site we included in this study is the fifth order river in the Snake, which is in the middle of the stream-order gradient considered by the RCC (Vannote et al. 1980), but in the range of (although slightly smaller than) rivers included in previous empirical tests of the RCC (Minshall et al. 1983; Rosi-Marshall and Wallace 2002; McTammany et al. 2003).
A study of energy dynamics along the Little Tennessee River continuum demonstrated a transition from heterotrophy to autotrophy at 5th order (Webster 2007), which is the approximate size of our largest study sites. The lack of both very small headwater streams and very large rivers in the current study, combined with a lack of support for a trend of increasing water column uptake along the river continuum, suggests that our study may be missing the transition from benthic-dominated to water column-dominated ecosystems. Yet nutrient uptake in the water column of rivers is similar to lake, estuary, and marine estimates (Reisinger 2015), showing that this transition does indeed occur.
In addition to the lack of a stream size effect, U vol did not differ across watersheds. This was surprising, particularly for \({U_{{vol{\text{-}}{NO}}_{3} }}\), due to large differences in ambient \({\text{NO}}_{3}^{ - }\) concentration across watersheds. It is likely that the lack of a significant effect of watershed is due to variability of \({U_{{vol{\text{-}}{NO}}_{3} }}\) among sites (Fig. 2), as the full LMM explained >90 % of the total variation. The fact that U vol did not differ across stream size for any solute could be due to the dependence of U vol on ambient concentration, which may conceal any biological patterns that vary with ecosystem size. For example, a site with high nutrient concentrations but low suspended algae may still have higher U vol than a site with more suspended chlorophyll a but lower nutrient concentrations. Therefore, a high U vol may result from increased biological activity in the water column, high ambient concentrations, or both, and isolating the influence of these two drivers is challenging.
While U vol is scaled per unit volume of water, it is conceptually analogous to gross nutrient uptake (U), which is commonly used in stream spiraling studies to scale nutrient uptake to the area of the stream bottom (Stream Solute Workshop 1990). A well-known complication in comparing U among streams has been that ambient nutrient concentration is included in the U calculation (Stream Solute Workshop 1990). In addition, we acknowledge that uptake rates can saturate with increasing concentration, thus areal or volumetric uptake should either scale linearly or via Michaelis–Menten dynamics in response to increasing amendment concentration within a site (Dodds et al. 2002). We suggest that saturation was unlikely due to short incubation times and the minimal amount of nutrient amendment added to each chamber.
Despite differences across sites and watersheds in ambient \({\text{NH}}_{4}^{ + }\) and material suspended in the water column, water column \({v_{{f{\text{-}}{NH}}_{4} }}\) was remarkably consistent among watersheds and increased with stream size (Fig. 3a). The consistent increase in \({v_{{f{\text{-}}{NH}}_{4} }}\) suggests that physical changes in the stream, such as increases in depth or reductions in canopy cover with increasing river width, increase the amount of \({\text{NH}}_{4}^{ + }\) removed by the water column, as we predicted based on the conceptual framework outlined in the RCC (Vannote et al. 1980).
As observed for \({\text{NH}}_{4}^{ + }\), \({v_{{f{\text{-}}{NO}}_{3} }}\) also did not differ among watersheds. If high background \({\text{NO}}_{3}^{ - }\) saturated nutrient uptake, we would expect the agricultural St. Joseph watershed to have decreased \({v_{{f{\text{-}}{NO}}_{3} }}\). However, we found no difference in \({v_{{f{\text{-}}{NO}}_{3} }}\) across watersheds, suggesting that \({\text{NO}}_{3}^{ - }\) demand relative to concentration was not saturated by \({\text{NO}}_{3}^{ - }\) in this range, which contrasts with previous results for whole-stream v f (Hall et al. 2009a). In contrast to either N species, the trends for v f-SRP along the stream size gradient were different in Midwestern watersheds (Manistee and St. Joseph) compared to the Western watershed (Snake), with v f-SRP tending to increase with stream size in Midwestern watersheds, but decreasing with size in the Snake (Fig. 3c). Contrasting patterns among regions may reflect higher availability of P (relative to N) in the Snake compared to Manistee or St. Joseph watersheds.
Although water column nutrient uptake occurred across a range of stream sizes and nutrient concentrations, it is possible that these rates are negligible when compared to benthic nutrient uptake. By comparing our water column v f measurements to reach-scale v f from a recently compiled meta-analysis (Hall et al. 2013), we find that water column demand is lower than reach-scale demand, but the ranges overlapped for each solute (Fig. 4). Median water column \({v_{{f{\text{-}}{NH}}_{4} }}\) from this study represented 4 % of the median reach-scale \({v_{{f{\text{-}}{NH}}_{4} }}\), whereas the median water column \({v_{{f{\text{-}}{NO}}_{3} }}\) was 9 % of the reach scale median and water column v f-SRP was 19 % of the reach scale median. The fact that the median water column v f from 15 reaches of varying size can represent up to ~1/5th of reach-scale v f of previously published reach-scale measurements confirms the need to incorporate water column dynamics in the conceptual framework of nutrient processing in lotic ecosystems (Fig. 5).
Uptake velocity of \({\text{NH}}_{4}^{ + }\), \({\text{NO}}_{3}^{ - }\), and SRP between reach scale measurements (gray boxes) and water column measurements from this study (white boxes) overlapped, with median water column v f representing 4–19 % of median reach-scale v f . Reach-scale studies were compiled from Hall et al. (2013) and included any non-isotope study which reported v f . The median (horizontal line in the boxes), 25th and 75th (edges of boxes), 10th and 90th (whiskers) and 5th and 95th (dots) percentiles are presented. Sample size is provided above each box
Water column nutrient uptake incorporated into a conceptual model of nutrient uptake along a longitudinal continuum from headwaters to large rivers. The longitudinal transition from headwaters to rivers is accompanied by an increase in depth, represented by the increasing thickness of the water column (light gray). This increase in depth may result in a greater concentration of water column biota (solid circles) and a decrease in benthic biota (dark gray line) overlying the stream bed (black). Dissolved nutrients can be taken up by either benthic or water column biota (dark arrows), with a portion of these nutrients being mineralized (light arrows). All processes that influence nutrient uptake in the benthic zone (e.g., assimilation, denitrification, nitrification, sorption) also occur in the water column
The first and second Lotic Intersite Nitrogen eXperiments (LINXI and LINXII) substantially improved our understanding of nitrogen processing in headwater streams (Peterson et al. 2001; Webster et al. 2003; Mulholland et al. 2008). The dynamic balance of \({\text{NH}}_{4}^{ + }\) and \({\text{NO}}_{3}^{ - }\) concentrations in streams is controlled by input, assimilatory and dissimilatory uptake, abiotic sorption, and mineralization (Peterson et al. 2001; Mulholland et al. 2008; Hall et al. 2009a). Yet, the results from the LINX projects focused primarily on benthic processes, typical of the field of stream biogeochemistry for over 20 years. While this view is accurate and appropriate for headwater streams, increasing our knowledge of mechanisms driving nutrient uptake in rivers is a key priority (Mulholland and Webster 2010), and the data presented here demonstrate that this priority requires an additional focus on the water column.
Biotic and abiotic properties control water column nutrient dynamics
The three watersheds we studied ranged from the mostly pristine Snake (<1 % developed lands) to the agricultural St. Joseph (>70 % developed lands), and these contrasting land uses were mirrored by differences in DIN concentrations, suspended organic and inorganic material, and suspended algal biomass, which also increased with stream size (Table 1). The patterns in suspended chlorophyll a in minimally altered watersheds were consistent with predictions from the RCC, which predicts increased phytoplankton down the river continuum (Vannote et al. 1980). In the agriculturally dominated St. Joseph watershed, water column chlorophyll a did not differ across stream order, suggesting that anthropogenic land-use alters patterns predicted by the RCC.
In addition to increasing nutrient concentrations (Carpenter et al. 1998), anthropogenic land use increases suspended sediments (Wood and Armitage 1997), which were positively related with U vol and v f for \({\text{NO}}_{3}^{ - }\) and v f for SRP. The relationship between suspended inorganic sediments and v f-SRP may reflect sorption dynamics. However, the relationship between \({U_{{vol{\text{-}}{NO}}_{3} }}\) and suspended inorganic matter was not likely due to sorption as \({\text{NO}}_{3}^{ - }\) does not typically bind with sediment under standard conditions of temperate freshwaters (Schlesinger and Bernhardt 2013). Instead, it is possible that biotic assimilation by biofilms on suspended sediments drives uptake, or there are low oxygen microsites on particles that promote microbial denitrification, as occurs in the oxic water column of the ocean (Michotey and Bonin 1997). Although typically assumed to occur in the benthic zone of freshwaters, water column denitrification can occur in the water column and increases linearly with suspended sediment in river water (Liu et al. 2013). Therefore, there are two direct mechanisms for human land use to alter water column nitrate uptake—via increased ambient nutrients, and/or elevated suspended sediment.
Whole-stream metabolism in part controls reach-scale nutrient uptake (Hall and Tank 2003; Hall et al. 2009a). Therefore, we expected that water column metabolism should control water column nutrient uptake. As expected, both suspended chlorophyll a and water column GPP were significantly related to U vol for both \({\text{NH}}_{4}^{ + }\) and \({\text{NO}}_{3}^{ - }\). However, N concentrations may be a common driver of chlorophyll a, GPP, and U vol rather than autotrophic processes directly controlling water column uptake. We predicted that the relationships between water column metabolism and water column nutrient uptake should extend to v f as well. Interestingly, water column v f of \({\text{NH}}_{4}^{ + }\) and \({\text{NO}}_{3}^{ - }\) were not related to any autotrophic metric, but were related to suspended organic (\({\text{NH}}_{4}^{ + }\)) or inorganic (\({\text{NO}}_{3}^{ - }\)) material in the water column, suggesting that water column demand for N (v f ) was primarily a heterotrophic process. Future research is needed that can partition the role of autotrophic and heterotrophic processes and would increase our understanding of the ultimate fate of nutrients processed in the water column.
Abiotic and biotic factors predicted processing of N and P in the water column, suggesting that both biological and physical processes drive water column nutrient dynamics. Both biotic assimilation and abiotic sorption to sediments represent temporary nutrient removal by converting bioavailable dissolved nutrients to less available forms. Abiotic sorption can account for up to 70 % of P uptake in streams (Aldridge et al. 2010), and if sorption to sediments is the primary uptake mechanism, then this mechanism may result in pulsed export during floods. For example, most total P export from Kansas streams occurred during spates, likely due to sediment export (Banner et al. 2009).
In streams, assimilatory uptake by biota represents the majority of nutrient removal from the water column (Mulholland et al. 2008), yet the ultimate fate of assimilated nutrients is not well documented. Although assimilation does not represent a permanent sink for nutrients (sensu denitrification), assimilatory uptake alters the timing (i.e., delays downstream transport) and the bioreactivity of nutrients (Hall et al. 2009a, b). For example, previous research has shown that particles are repeatedly deposited and resuspended into the water column as they move downstream (Thomas et al. 2001; Newbold et al. 2005; Fig. 5). Additionally, assimilated nutrients, now in organic form, can be re-mineralized (Ashkenas et al. 2004), enter hyporheic storage (Triska et al. 1989), be deposited on benthos (Bernot and Dodds 2005), enter floodplains (Hall et al. 2009b), or be transferred to higher trophic levels via consumption (Dodds et al. 2004). Although the duration of the delay resulting from water column assimilation relative to downstream transport is likely shorter than the delay due to benthic assimilation, it is probable that some proportion of nutrients assimilated in the water column will enter longer flowpaths in benthic, hyporheic, or floodplain storage. Identifying and quantifying the fate of assimilatory pathways beginning in the water column would improve our understanding of downstream nutrient export, and therefore should be a focus of future research.
Conclusions
We have shown that water column nutrient uptake occurs in streams of all sizes. The relative demand for \({\text{NH}}_{4}^{ + }\) in the water column increased with stream size (\({v_{{f{\text{-}}{NH}}_{4} }}\) ), whereas \({\text{NO}}_{3}^{ - }\) and SRP demand did not differ across watersheds or stream size. Volumetric uptake also did not differ across watersheds or stream size. Median water column v f at our 15 study sites represented approximately 4–19 % of median reach-scale estimates of v f , supporting the argument for incorporating water column dynamics into lotic biogeochemical theory (Fig. 5). Although water column nutrient processing may indeed be negligible in specific streams, these processes may contribute to total nutrient uptake within a watershed. The ultimate fate of nutrients removed by the water column may differ from those taken up in the benthic zone. A mechanistic understanding of the controls on nutrient uptake from streams to rivers is essential for successful mitigation of the export of anthropogenic nutrient pollution (Smil 2000; Galloway et al. 2004) and would allow for further investigation on the ultimate fate of nutrients processed in flowing waters.
References
Aksnes DL, Egge JK (1991) A theoretical model for nutrient uptake in phytoplankton. Mar Ecol Prog Ser 70:65–72
Aldridge KT, Brookes JD, Ganf GG (2010) Changes in abiotic and biotic phosphorus uptake across a gradient of stream condition. River Res Appl 26:636–649
Alexander RB, Boyer EW, Smith RA, Schwarz GE, Moore RB (2007) The role of headwater streams in downstream water quality. J Am Water Resour Assoc 43:41–59
Alexander RB, Smith RA, Schwarz GE, Boyer EW, Nolan JV, Brakebill JW (2008) Differences in phosphorus and nitrogen delivery to the Gulf of Mexico from the Mississippi River Basin. Environ Sci Technol 42:822–830
APHA (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington
Ashkenas LR, Johnson SL, Gregory SV, Tank JL, Wollheim WM (2004) A stable isotope tracer study of nitrogen uptake and transformation in an old-growth forest stream. Ecology 85:1725–1739
Baker DB, Richards RP (2002) Phosphorus budgets and riverine phosphorus export in northwestern Ohio watersheds. J Environ Qual 31:223–229
Banner EBK, Stahl AJ, Dodds WK (2009) Stream discharge and riparian land use influence in-stream concentrations and loads of phosphorus from central plains watersheds. Environ Manag 44:552–565
Barlow-Busch L, Baulch HM, Taylor WD (2006) Phosphate uptake by seston and epilithon in the Grand River, southern Ontario. Aquat Sci 68:181–192
Bernhardt ES, Likens GE, Buso DC, Driscoll CT (2003) In-stream uptake dampens effects of major forest disturbance on watershed nitrogen export. Proc Natl Acad Sci 100:10304–10308
Bernot MJ, Dodds WK (2005) Nitrogen retention, removal, and saturation in lotic ecosystems. Ecosystems 8:442–453
Bott TL (2006) Primary productivity and community respiration. In: Hauer FR, Lamberti GA (eds) Methods stream ecol, 2nd edn. Elsevier, Amsterdam, pp 663–690
Caraco NF, Cole JJ (1999) Human impact on nitrate export: an analysis using major world rivers. Ambio 28:167–170
Carpenter SR, Caraco NF, Correll DL, Howarth RW, Sharpley AN, Smith VH (1998) Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol Appl 8:559–568
Corning KE, Duthie HC, Paul BJ (1989) Phosphorus and glucose uptake by seston and epilithon in boreal forest streams. J N Am Benthol Soc 8:123–133
Descy J-P, Gosselain V (1994) Development and ecological importance of phytoplankton in a large lowland river (River Meuse, Belgium). Hydrobiologia 289:139–155
Dodds WK, Whiles MR (2004) Quality and quantity of suspended particles in rivers: continent-scale patterns in the United States. Environ Manag 33:355–367
Dodds WK, Priscu JC, Ellis BK (1991) Seasonal uptake and regeneration of inorganic nitrogen and phosphorus in a large oligotrophic lake: size-fractionation and antibiotic treatment. J Plankton Res 13:1339–1358
Dodds WK, López AJ, Bowden WB, Gregory S, Grimm NB, Hamilton SK, Hershey AN, Martí E, McDowell WH, Meyer JL, Morrall D, Mulholland PJ, Peterson BJ, Tank JL, Valett HM, Webster JR, Wollheim W (2002) N uptake as a function of concentration in streams. J N Am Benthol Soc 21:206–220
Dodds WK, Martí E, Tank JL, Pontius J, Hamilton SK, Grimm NB, Bowden WB, McDowell WH, Peterson BJ, Valett HM, Webster JR, Gregory S (2004) Carbon and nitrogen stoichiometry and nitrogen cycling rates in streams. Oecologia 140:458–467
Dollison RM (2010) The National Map: New viewer, services, and data download: U.S. Geological Survey Fact Sheet 2010-3055
Ensign SH, Doyle MW (2006) Nutrient spiraling in streams and river networks. J Geophys Res 111:G04009
Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, Karl DM, Michaels AF, Porter JH, Townsend AR, Vörösmarty CJ (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226
Gore JA (2006) Discharge measurements and streamflow analysis. In: Hauer FR, Lamberti GA (eds) Methods stream ecol, 2nd edn. Academic Press, Burlington, pp 51–77
Hall RO, Tank JL (2003) Ecosystem metabolism controls nitrogen uptake in streams in Grand Teton National Park, Wyoming. Limnol Oceanogr 48:1120–1128
Hall RO, Tank JL, Sobota DJ, Mulholland PJ, O’Brien JM, Dodds WK, Webster JR, Valett HM, Poole GC, Peterson BJ, Meyer JL, McDowell WH, Johnson SL, Hamilton SK, Grimm NB, Gregory SV, Dahm CN, Cooper LW, Ashkenas LR, Helton AM, Crenshaw CM, Burgin AJ, Bernot MJ, Bealieu JJ, Arango CP (2009a) Nitrate removal in stream ecosystems measured by 15 N addition experiments: Total uptake. Limnol Oceanogr 54:653–665
Hall RO, Baker MA, Arp CD, Koch BJ (2009b) Hydrologic control of nitrogen removal, storage, and export in a mountain stream. Limnol Oceanogr 54:2128–2142
Hall RO, Baker MA, Rosi-Marshall EJ, Tank JL, Newbold JD (2013) Solute-specific scaling of inorganic nitrogen and phosphorus uptake in streams. Biogeosciences 10:7323–7331
Howarth RW, Billen G, Swaney D, Townsend A, Jaworski N, Lajtha K, Downing JA, Elmgren R, Caraco N, Jordan T, Berendse F, Freney J, Kudeyarov V, Murdoch P, Zhao-Lian Z (1996) Regional nitrogen budgets and riverine N & P fluxes for the drainages to the North Atlantic Ocean: natural and human influences. Biogeochemistry 35:75–139
Liu T, Xia X, Liu S, Mou X, Qiu Y (2013) Acceleration of denitrification in Turbid Rivers due to denitrification occurring on suspended sediment in oxic waters. Environ Sci Technol 47:4053–4061
McTammany ME, Webster JR, Benfield EF, Neatrour MA (2003) Longitudinal patterns of metabolism in a southern Appalachian river. J N Am Benthol Soc 22:359–370
Meybeck M (1982) Carbon, nitrogen, and phosphorus transport by world rivers. Am J Sci 282:401–450
Michotey V, Bonin P (1997) Evidence for anaerobic bacterial processes in the water column: denitrification and dissimilatory nitrate ammonification in the northwestern Mediterranean Sea. Mar Ecol Prog Ser 160:47–56
Minshall GW, Petersen RC, Cummins KW, Bott TL, Sedell JR, Cushing CE, Vannote RL (1983) Interbiome comparison of stream ecosystem dynamics. Ecol Monogr 53:1–25
Mulholland PJ, Webster JR (2010) Nutrient dynamics in streams and the role of J-NABS. J N Am Benthol Soc 29:100–117
Mulholland PJ, Newbold JD, Elwood JW, Ferren LA, Webster JR (1985) Phosphorus spiralling in a woodland stream : seasonal variations. Ecology 66:1012–1023
Mulholland PJ, Helton AM, Poole GC, Hall RO, Hamilton SK, Peterson BJ, Tank JL, Ashkenas LR, Cooper LW, Dahm CN, Dodds WK, Findlay SEG, Gregory SV, Grimm NB, Johnson SL, McDowell WH, Meyer JL, Valett HM, Webster JR, Arango CP, Beaulieu JJ, Bernot MJ, Burgin AJ, Crenshaw CL, Johnson LT, Niederlehner BR, O’Brien JM, Potter JD, Sheibley RW, Sobota DJ, Thomas SM (2008) Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 452:202–205
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142
Nelson CE, Bennett DM, Cardinale BJ (2013) Consistency and sensitivity of stream periphyton community structural and functional responses to nutrient enrichment. Ecol Appl 23:159–173
Newbold JD, Elwood JW, O’Neill RV, Sheldon AL (1983) Phosphorus dynamics in a woodland stream ecosystem: a study of nutrient spiraling. Ecology 64:1249–1265
Newbold JD, Thomas SA, Minshall GW, Cushing CE, Georgian T (2005) Deposition, benthic residence, and resuspension of fine organic particles in a mountain stream. Limnol Oceanogr 50:1571–1580
O’Brien JM, Dodds WK, Wilson KC, Murdock JN, Eichmiller J (2007) The saturation of N cycling in Central Plains streams: 15 N experiments across a broad gradient of nitrate concentrations. Biogeochemistry 84:31–49
Peterson BJ, Wollheim WM, Mulholland PJ, Webster JR, Meyer JL, Tank JL, Martí E, Bowden WB, Valett HM, Hershey AE, McDowell WH, Dodds WK, Hamilton SK, Gregory S, Morrall DD (2001) Control of nitrogen export from watersheds by headwater streams. Science 292:86–90
Reisinger AJ (2015) The role of the water column in nutrient dynamics of lotic ecosystems. Dissertation. University of Notre Dame, Notre Dame, IN
Rosi-Marshall EJ, Wallace JB (2002) Invertebrate food webs along a stream resource gradient. Freshw Biol 47:129–141
Schlesinger WH, Bernhardt ES (2013) Biogeochemistry: an analysis of global change, 3rd edn. Academic Press, Oxford
Seitzinger SP, Styles RV, Boyer EW, Alexander RB, Billen G, Howarth RW, Mayer B, Breemen NV (2002) Nitrogen retention in rivers: model development and application to watersheds in the northeastern U.S.A. Biogeochemistry 57(58):199–237
Seitzinger SP, Harrison JA, Dumont E, Beusen AHW, Bouwman AF (2005) Sources and delivery of carbon, nitrogen, and phosphorus to the coastal zone: an overview of global nutrient export from watersheds (NEWS) models and their application. Glob Biogeochem Cycles 19:GB4S01
Smil V (2000) Phosphorus in the environment: natural flows and human interferences. Anu Rev Energy Environ 25:53–88
Smith RA, Schwarz GE, Alexander RB (1997) Regional interpretation of water-quality monitoring data. Water Resour Res 33:2781–2798
Solorzano L (1969) Determination of ammonia in natural waters by the phenolhypochlorite method. Limnol Oceanogr 14:799–801
Steinman AD, Lamberti GA, Leavitt PR (2006) Biomass and pigments of benthic algae. In: Hauer FR, Lamberti GA (eds) Methods stream ecol, 2nd edn. Elsevier, Amsterdam, pp 357–379
Stream Solute Workshop (1990) Concepts and methods for assessing solute dynamics in stream ecosystems. J N Am Benthol Soc 9:95–119
Tank JL, Rosi-Marshall EJ, Baker MA, Hall RO (2008) Are rivers just big streams? A pulse method to quantify nitrogen demand in a large river. Ecology 89:2935–2945
Thomas SA, Newbold JD, Monaghan MT, Minshall GW, Georgian T, Cushing CE (2001) The influence of particle size on seston deposition in streams. Limnol Oceanogr 46:1415–1424
Triska FJ, Kennedy VC, Avanzino RJ, Zellweger GW, Bencala KE (1989) Retention and transport of nutrients in a third-order stream in northwestern California: hyporheic processes. Ecology 70:1893–1905
Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Can J Fish Aquat Sci 37:130–137
Webster JR (2007) Spiraling down the river continuum: stream ecology and the U-shaped curve. J N Am Benthol Soc 26:375–389
Webster JR, Mulholland PJ, Tank JL, Valett HM, Dodds WK, Peterson BJ, Bowden WB, Dahm CN, Findlay S, Gregory SV, Grimm NB, Hamilton SK, Johnson SL, Martí E, McDowell WH, Meyer JL, Morrall DD, Thomas SA, Wollheim WM (2003) Factors affecting ammonium uptake in streams—an inter-biome perspective. Freshw Biol 48:1329–1352
Wetzel RG, Likens GE (2001) Composition and biomass of phytoplankton. In: Wetzel RG, Likens GE (eds) Limnol. Anal., vol Third. Springer Science + Business, New York, pp 139–163
Wollheim WM, Vörösmarty CJ, Peterson BJ, Seitzinger SP, Hopkinson CS (2006) Relationship between river size and nutrient removal. Geophys Res Lett 33:2–5
Wood P, Armitage P (1997) Biological effects of fine sediment in the lotic environment. Environ Manag 21:203–217
Acknowledgments
We thank E. Taylor-Salmon, N. Anderson, and Z. Volenec for help collecting field data, U. Mahl for assistance with laboratory analyses, and R. Hesselink, S. Powers, and B. Hanrahan for critical reviews of earlier versions of this manuscript. This manuscript was greatly improved by two anonymous reviewers. We also thank the University of Wyoming - National Park Service Research Station in Grand Teton National Park for logistical support and Grand Teton National Park for permission to access sites in the Snake River Watershed. Multiple land owners provided access to sites in the St. Joseph River Watershed. A.J. Reisinger was partially supported by a Schmitt Presidential Fellowship provided by the Graduate School at the University of Notre Dame, a University of Wyoming - National Parks Service Small Grant to fund Summer 2013 research, and a General Endowment Fund Award from the Society for Freshwater Science. This research was part of a larger project supported by National Science Foundation grants DEB 09-22118 awarded to JLT, DEB 09-21598 awarded to ROH, DEB 09-22153 awarded to MAB, and 10-07807 awarded to EJRM.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reisinger, A.J., Tank, J.L., Rosi-Marshall, E.J. et al. The varying role of water column nutrient uptake along river continua in contrasting landscapes. Biogeochemistry 125, 115–131 (2015). https://doi.org/10.1007/s10533-015-0118-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-015-0118-z