Abstract
The effects of anthropogenic pressures in coastal areas are extensively studied in temperate but not in tropical zones, where their impact might be amplified by high water temperatures and upwelling phenomena. Sedimentary features and benthic metabolism were studied during the non upwelling (NUPW) and upwelling (UPW) seasons in Taganga Bay (Colombia). The bay is impacted by a submarine outfall of virtually untreated, organic and nutrient-rich wastewater. Samplings were performed in November 2017 (NUPW) and in January–February 2018 (UPW) at 4 stations located in the proximity and 100, 750 and 1800 m far from the outfall, respectively, at depths between 22 and 28 m. Aerobic respiration, denitrification, dissimilative nitrate reduction to ammonium (DNRA) and nutrient fluxes were measured. The influence of the outfall was detectable 750 and 1800 m away from the point pollution source, where δ13C data suggested that ~ 40 and ~ 20% of organic inputs were terrigenous, respectively. In the proximity of the outfall benthic oxygen demand peaked and the presence of Beggiatoa mats suggested reoxidation of sulphides, that were abundant in pore water. Under sulfidic conditions, DNRA was the major driver of nitrate demand, whereas at stations far from the outfall, denitrification dominated nitrate consumption. Organic matter and nitrate inputs to the bay during the UPW season enhanced the effects of the outfall by increasing aerobic respiration and DNRA. Higher N availability during the UPW season reversed fluxes of molecular nitrogen and turned the sediments of 3 out of 4 sites from net sinks to net N2 sources. Results from this study suggest that the analysis of sediments allows tracing the impact of the outfall and that such impact is enhanced during the upwelling season. In tropical areas, marine outfalls and upwelling may act in synergy and contribute to ecosystem deterioration due to high temperatures, increase of microbial respiration, sulphide toxicity and benthic biodiversity loss.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic matter mineralization in coastal sediments provides an important ecosystem service such as benthic-pelagic coupling; nutrient regeneration from sediments may support 15–32% of nitrogen (N) and 17–100% of phosphorus (P) demand by phytoplankton (Aller 1988; Bonaglia et al. 2014; Carstensen et al. 2014; Mermillod-Blondin et al. 2004; Boynton et al. 2018). Sediments may also act as nutrient traps, however the supply of organic matter to the benthic compartment and the availability of oxygen are considered primary factors in controlling the direction and magnitude of solute fluxes (Banta et al. 1995; Hopkinson et al. 2001; Smith et al. 2012). Excess inputs of organic matter to sediments may uncouple microbial respiration and oxygen availability leading to anoxia, buildup of free sulphides (S2− + HS− + H2S), simplification of benthic macrofauna communities and loss of biogeochemical services as denitrification or P-retention (Rosenberg and Loo 1988; Grall and Chauvaud 2002; Howarth et al. 2011). Oxygen shortage produces profound changes in aquatic communities, affecting energy transfer across trophic levels and biogeochemical cycles (Diaz 2001; Testa and Kemp 2011). Under hypoxic/anoxic conditions, the accumulation of toxic, reduced anaerobic metabolites within sediments and/or water depresses the activity of microbes, plants and benthic fauna (Testa and Kemp 2011). In turn, this creates a positive feedback, as bioturbation and bioirrigation by infauna has a key role in organic matter degradation and solute transport (e.g. O2, NO3−) in sediments (Aller 1994; Bonaglia et al. 2014; Carstensen et al. 2014). Strictly anoxic and chemically reduced sediments favor P mobility and its availability in water column and reduce P burial via precipitation or co-precipitation with iron (Fe) and manganese (Mn) oxides and hydroxides and organic matter (Testa and Kemp 2011; Zilius et al. 2014). Under sulphidic conditions, the activity of denitrifers is inhibited, whereas that of nitrate ammonifiers is stimulated, and the role of sediments switches form net N sink to net N source (Carstensen et al. 2014). Such switch is increasingly reported and has deep implications for benthic N cycling (An and Gardner 2002; De Brabandere et al. 2015; Hall et al. 2017).
Superimposed to or interacting with the effects of organic matter enrichment are the seasonal variations of physical variables in water column such as temperature, vertical stratification and circulation patterns. These factors, that include upwelling phenomena, are important as they control the vertical distribution and the supply of dissolved oxygen, nutrients and oligoelements in the water mass (Testa and Kemp 2011). There is a general scarcity of studies targeting benthic metabolism measurements and the potential impact of land-use alteration, nutrient and organic enrichment on sediments biogeochemistry in low latitude tropical coastal zones (Downing et al. 1999; Boynton et al. 2018). Downing et al. (1999) suggested that alterations in the N-cycle will probably have a greater impact on tropical systems than those observed in temperate areas, due to higher water temperatures and heterotrophic activity, and to lower oxygen solubility. Upwelling phenomena represent another potentially important, understudied factor regulating these dynamics, as they may alter locally the temperature of the water mass and its physico-chemical composition (e.g. chlorophyll-a, suspended solids, oxygen, macro- and micronutrient content). Upwelling may either smooth the effects of organic enrichment or enhance them, as it represents in many areas a natural eutrophication phenomenon.
In this study, the combined effect of anthropogenic organic input associated to untreated sewage outfall and of natural upwelling on benthic biogeochemistry was analyzed in Taganga Bay, a tropical system located in Santa Marta Coastal Area (SMCA), Caribbean Colombia. There is a strong interest in understanding the effects of multiple stressors, including wastewater discharge, and seasonal upwelling on the functioning of SMCA, and in particular on the health of coral meadows, as the area is considered a hotspot of biodiversity in the Colombian Caribbean (Bayraktarov et al. 2013, 2014; Bayraktarov and Wild 2014). Despite the importance of preserving ecosystem services in the area, no biogeochemical studies targeting the impact of wastewater outfall, alone or in combination with the seasonal upwelling phenomena were carried out to date. In particular, the sediment compartment was never studied in Taganga Bay, despite its memory for past and ongoing pollution and its importance in regulating dissolved oxygen and nutrient content in the water column.
Sediment properties (organic matter quantity, C, N and P pools and δ13C and δ15N) and benthic metabolism (aerobic respiration, denitrification, nitrate ammonification and nutrient recycling) were analyzed at increasing distances from a marine outfall and along the non upwelling and upwelling seasons in SMCA. Carbon and N stable isotopes were used to determine the organic source in sediments (marine and terrestrial) and the extent of the organic pollution. The study had a threefold aim: to trace the spatial impact of the outfall on the sediments, to quantify its effects on benthic respiration and nutrient recycling and to analyze whether such effects are enhanced by the natural upwelling. We hypothesized a large stimulation of heterotrophic activity due to the organic inputs from the outfall and a suppression of biogeochemical sediment services as N removal via denitrification, sulphide precipitation and nutrient retention. We also hypothesized that organic inputs from marine upwelling act in synergy with those from the outfall, enhancing the risk of sediment anoxia and nutrient regeneration.
Materials and methods
Study area
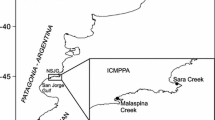
Taganga Bay has an open conformation, subject to oceanic and continental influences, and is impacted by multiple pressures including a submarine wastewater outfall, the harbor activity, the Manzanares and Gaira river discharge and by seasonal surface runoff (Escobar 1988; Ramos-Ortega et al. 2008; Mancera-Pineda et al. 2013) (Fig. 1). River discharge and surface runoff are low or null in the dry seasons (December–April and July–August) and peak in the wet seasons (May–June and September–November) (Arévalo-Martínez and Franco-Herrera 2008).
The Santa Marta sewage outfall (SMSO, Fig. 1) discharges on average 1 m3 s−1 of untreated wastewater derived from ~ 500,000 inhabitants (García 2013). The city nearly doubles its resident population during high tourist season, peaking in January and resulting in increased wastewater discharge (Díaz-Rocca and Causado-Rodríguez 2007). The outlet is located between Santa Marta and Taganga bays (11.26 Lat and − 74.22 Lon) (García 2013). The sewage outfall consists of 1 m diameter tubing and extends 428 m along the coastline (García et al. 2012). Sewage only receives a preliminary treatment for large solids removal (Díaz-Rocca and Causado-Rodríguez 2007). During 2006, the loadings of total N, P and total suspended solids (TSS) released to the bay from the outfall were estimated in ~ 1100 T N yr−1, ~ 500 T P yr−1 and ~ 12,300 T TSS yr−1, respectively (García 2013). The maximum extension of the area affected by discharge from SMSO was estimated at 600 m from the outfall from water column fecal coliforms concentration, whereas sediments were not analyzed (García 2013).
Taganga Bay is highly influenced by the Southern Caribbean upwelling system (Rueda-Roa and Muller-Karger 2013). It is subjected to strong seasonality caused by the Caribbean Low—level Jet of Northeast (NE) Trade Winds and the oscillations of the Intertropical Convergence Zone (Andrade and Barton 2005). Major (December–April) and minor (July–August) upwelling events occur during the dry season while minor (May–June) and major (September–November) no-upwelling periods occur during the rainy season (Fajardo 1979; Andrade and Barton 2005; Arévalo-Martínez and Franco-Herrera 2008). Seasonal upwelling leads to changes in physicochemical variables such as temperature decrease (from 30 to 21 °C), salinity increase (from 33 to 38), oxygen subsaturation conditions (< 91%) (Ramírez 1981; Salzwedel and Müller 1983; Bayraktarov et al. 2014), and increase of nitrate and chlorophyll-a concentration, turning the system from oligotrophic to mesotrophic (Arévalo-Martínez and Franco-Herrera 2008; García-Hoyos et al. 2010; Paramo et al. 2011).
Water and sediment sampling was carried out at four stations (S1 to S4, located in the proximity and at 100, 750 and 1800 m from the outfall, respectively) (Fig. 1). Specifically, S1 was located near the outfall diffusers whereas S4 was close to Punta Venado, in a sandy area with low coral covering (Martínez and Acosta 2005; Vega-Sequeda et al. 2008). On every sampling occasion the physico-chemical features of the water overlying sediments (temperature, salinity and dissolved oxygen) were analyzed with a YSI 560 multiple probe.
Sediment cores collection
Sediments were sampled with minimum disturbance by scuba divers using transparent plexiglass liners (internal diameter = 4 cm, length = 20 cm), in order to have approximately equal heights (8–9 cm) of sediment and water column. Sampling was conducted during major non upwelling rainy season—NUPW—(S1: 27 November, S2: 28 November, S3: 30 November and S4: 2 December 2017) and during major upwelling dry season—UPW—(S1: 26 January, S2: 28 January, S3: 31 January and S4: 4 February 2018). Twenty sediment cores were collected at each station. The cores were intentionally scattered along a 22–28 m depth profile, due to the variable topography of the bottom and in order to have overlapping depths among sites. Once collected, the cores were bottom and top capped and brought to the boat where the top lid was removed and the cores were submersed in a tank containing in-situ water cooled with ice packs (Fig. S1, Supplementary Material). Different tanks were used for the different stations. Bottom water (~ 40 L per site) was collected through four large PVC pipes equipped with valves at both ends. Pipes were submersed and the valves were closed near the bottom by divers. Cores were stored vertically in the dark, and immediately transferred to the laboratory where they were equipped with magnetic stirrers (more details provided in next paragraphs). During the overnight preincubation the cores were maintained submersed with the top open and the stirring on; the water in the tank was maintained at in situ temperature and at 100% oxygen saturation.
Net flux measurement at the sediment–water interface
The incubations were carried out at the chemistry laboratory of the Jorge Tadeo Lozano University, campus Santa Marta, in a temperature-controlled room maintained at 29 and 23 °C during the NUPW and UPW season, respectively. The day after sampling, dark incubations began by sealing each core with gas-tight lids. During the preincubation and incubation periods the temperature of the incubation tank varied by less than 0.3 °C. The water inside the cores was gently stirred by Teflon- coated magnetic bars without suspending the sediment (Fig. S1, supplementary material). The magnetic bars were driven by an external magnet rotated by a motor at 40 rpm. Five cores per station were used to measure the fluxes of dissolved gas (O2:Ar, N2:Ar and CH4), metals (Fe2+ and Mn2+) and inorganic nutrients (NH4+, NO2−, NO3−, PO43− and SiO2) (Table S1, Supplementary Material)). Solutes concentrations were measured at the beginning and at the end of the incubation, assuming that their uptake or release rate during the incubation was linear. At time zero, four water samples (ca. 60 ml) were collected from the incubation tank before cores were capped. Sediment incubation time was maximum 6 h to keep oxygen within 20–30% of the initial value. Incubation time was set with pilot incubations carried out before starting the experiments. At the end of the incubation, lids were removed and water samples were collected from each core. Subsamples of 12 ml were transferred into exetainers, poisoned with 200 µl of ZnCl2 solution (7 M) and later analyzed for O2:Ar and N2:Ar ratios and for CH4 concentrations with a Membrane Inlet Mass Spectrometer (MIMS) equipped with a furnace with a copper reduction column (Bay instruments). Subsamples of 15 ml were transferred to glass vials, acidified with 50 µl of concentrated HNO3 and analyzed for Fe2+ and Mn2+ with a Varian 240FS AA. Subsamples of 20 ml were filtered (Whatman, GF/F), refrigerated and stored at − 20 °C for later nutrient analysis. Nutrients were measured with a continuous-flow analyzer (San++, Skalar) according to Grasshoff et al. (1983). Detection limits were 0.1 µM for NH4+ and NO3−, 0.05 µM for NO2− and PO43− and 0.3 µM for SiO2. The Dissolved Inorganic Nitrogen (DIN) was calculated as DIN = NH4+ + NO2− + NO3−. Dissolved oxygen (DO) was also measured in the incubation tank and in the cores with a calibrated oxygen sensor (Orion Star Water™ A326). Solute fluxes across the sediment–water interface were calculated according to Eq. (1) (Dalsgaard et al. 2000):
where F is flux of measured solutes (μmol m−2 h−1), C0 is concentration at time zero (μmol l−1), Cf is concentration at the end of incubation (μmol l−1), V is the volume of water in the core (L), A is area of sediment surface in the core (m2) and t is the incubation time (h). Fluxes directed from the sediment to water column were considered as positive.
Denitrification and DNRA measurements
Sequential to net fluxes, the isotope pairing technique ITP (Nielsen 1992) was used to measure the dark rates of denitrification and nitrate ammonification (DNRA). The IPT allows partioning NO3− source for denitrification in the contribution of nitrate diffusing to the anoxic sediment from the water column (Dw) and denitrification of nitrate produced within the sediment due to nitrification (Dn). Besides denitrification (and DNRA) the concentration series approach we adopted allows to test for the occurrence of the anaerobic oxidation of NH4+ to N2 (ANAMMOX) (Dalsgaard et al. 2005; Trimmer et al. 2006; Burgin and Hamilton 2007; Koop-Jakobsen and Giblin 2009). Different amounts of 15NO3− from a 30 mM Na15NO3 solution (> 98% Sigma Aldrich) were added to the water column of each of the 12 replicate cores to perform a concentration series experiment (Dalsgaard et al. 2000). Three levels of 15NO3− concentration (20, 40 and 60 µM, each level with 4 replicates) were created adding increasing amounts of the stock 15NO3− solution to the cores water phase (Table S1, Supplementary Material). The incubation length was similar to that for fluxes, in order to keep oxygen variations within 20–30% of the initial value. At the end of the incubation the sediment and water were gently mixed. An aliquot of the slurry was transferred to a 12 ml Exetainer and poisoned with 200 µl of ZnCl2 solution (7 M); 14N15N and 15N15N abundance in N2 were analyzed by MIMS. As calculated 28N2 production was independent from the level of 15NO3− addition (no detectable ANAMMOX), denitrification rates were calculated according to the equations and assumptions of Nielsen (1992). Another aliquot of the slurry (20 ml) was transferred in 50 ml falcon tubes, treated with KCl (1 M), shaken for 1 h in the dark, centrifuged, filtered and frozen for later analysis of the 15NH4+ fraction in the ammonium pool. 15NH4+ was determined via chemical oxidation to 29N2 and 30N2 by alkaline hypobromite addition as described in Warembourg (1993). DNRA was then calculated as described in Bonaglia et al. (2014). All cores were finally sieved (mesh size 500 µm) to check for the occurrence of large macrofauna.
Sedimentary features
After incubations, the analysis of sedimentary features was conducted on subsamples from the upper sediment layer (0–3 cm) in four cores per site (Table S1, Supplementary Material). Such sediment horizon was decided on the basis of the homogeneous color of sediment (Fig. S1, Supplementary Material) and in order to get information about the most reactive, upper layer. Each sediment slice was homogenized and a subsample was taken using a cut-off 5 ml syringe. Water content and porosity were determined after drying at 60 °C until constant weight. Then each sample was homogenized and ground with a porcelain mortar and pestle prior analysis of total organic carbon (TOC), total nitrogen (TN), isotopic measurements (δ13C and δ15N), total phosphorus (TP), total inorganic phosphorus (TIP) and organic material content (OM). TOC, TN, δ13C and δ15N were measured with a Thermo Finnigan EA/NA-1100 CHN elemental analyzer coupled with an isotope ratio mass spectrometer after removing carbonates with HCl (25% v/v) and subsequent rinsing with deionized water to neutralize and dry (30 °C). The samples were run in duplicate and the analytical precision was ± 0.2% for TOC and ± 0.01% for TN. TOC and TN percent measured on sediment was corrected by the weight change due to the procedure (Tucker and Giblin 2010). For δ13C, the reference was Vienna Pee Dee Belemnite (VPDB), and for δ15N, it was atmospheric nitrogen. Isotopic ratios were expressed in the usual δ-notation (part per mill, ‰). Samples replicate analyses were within ± 0.2% for δ13C and ± 0.3% for δ15N. Organic matter content (%) was measured by loss on ignition (LOI) of 0.2 g sediment at 350 °C in a muffle furnace. TP was extracted from ashes with 37% HCl whereas TIP was extracted after treating 0.2 g of dry sediment with 1 M HCl. Both TP and TIP were determined spectrophotometrically (Aspila et al. 1976). Total Organic phosphorus (TOP) was estimated as difference between TP and TIP. Redox Potential (Eh) was measured with a Thermo Fisher ORP sensor Orion Star™ A326, inserted within the gently homogenized sediment slices. The sensor response was checked against a standard redox solution (200 mV). As the sediment slice was exposed to air the measured Eh value can be slightly overestimated. The porewater from the same slice was extracted from the sediment via centrifugation (4000 rpm, 5 min) in glass vials filled with N2 and analyzed for sulphide concentration by spectrophotometry (Cline 1969).
Contribution of terrestrial organic matter to Taganga Bay sediments
The contribution of terrestrial and marine organic matter to sediment organic carbon was estimated using a two-end-member mixing model based on the equation derived by Calder and Parker (1968) taken from Schlunz et al. (1999). This approach has been widely applied in organic matter pollution studies of marine sediments (Li et al. 2016; Zhou et al. 2006). The terrestrial organic carbon contribution was calculated using Eq. (2):
The contribution of marine organic matter to the TOC was then estimated from Eq. (3):
where δ13Cmarine is the δ13C of marine end member, δ13Cterrestrial is the δ13C of terrestrial end member and δ13Corg is the measured value in sediment samples. A δ13Cterrestrial value of − 27.0‰ was used as end member based on the low δ13C values of sediment samples in the study area (Li et al. 2016). For the marine end member (δ13Cmarine) a value of − 20.3 ‰ was used as this is a typical carbon isotopic composition for diatoms in temperate marine ecosystems (Gearing et al. 1984). Furthermore, Gearing et al. (1984) indicated that planktonic isotope ratios varied little with water temperature (0 to 20 °C). A prevalence of diatoms and dinoflagellates has been found in the Santa Marta and Taganga bays (Ramírez-Barón et al. 2010; Garcés-Ordóñez et al. 2016).
Statistical Analysis
A two-way analysis of variance (2-way ANOVA) was carried to test the significance of the factors season (NUPW versus UPW period), sampling site and of their interactive effects on sedimentary properties, stable carbon and nitrogen isotopes (δ13C and δ15N), sedimentary fluxes and microbial N transformations. Rcmdr package of the R-Project for Statistical Computing (R version 3.5.1) was used to fit different models to the data (Fox 2005). Kolmogorov–Smirnov’s test and Levene’s tests were employed to assess the data normality (p > 0.05) and variance homogeneity (p > 0.05), respectively. In the case of heteroscedasticity, data were 1/x, \(\sqrt{\text{x}}\), ln(x), log(x2) or log(x) transformed. A goodness of fit was obtained after transformations. For significant factors, post hoc pairwise comparisons were performed using the Tukey HSD test. Significance was accepted for p < 0.05.
Correlations between sediment properties and nutrient fluxes were analyzed using simple and multiple lineal regression models, Pearson—test analysis (significance level p < 0.0001) and redundancy analysis (RDA), respectively. RDA was performed in the R environment using the vegan package. Before RDA analysis, a data exploratory multivariate analysis through Principal Component Analysis (PCA) (results not shown) was made on sedimentary properties (explanatory variables) and nutrient fluxes to detect and remove redundant variables lead to multicollinearity and affect data interpretation. Then, in the RDA analysis, one replicate of nutrient fluxes was randomly deleted to have a balanced arrangement along with sedimentary properties (4 replicates). This process was repeated three times to verify the validity of RDA results. The significance of sedimentary properties was tested against 999 permutation of ANOVA function included in vegan package (p < 0.05). RDA results were interpreted based on the scaling used. The angles vectors representing sedimentary properties and nutrient fluxes reflect their linear correlation (scaling 1). Angles between all vectors reflect linear correlation (scaling 2) (Legendre and Legendre 1998).
Two ways PERMANOVA was applied to analyze the multivariate variance to the raw variables of nutrient fluxes, denitrification and DNRA. The adonis function within vegan package was used to perform PERMANOVA test against 999 permutation. Post hoc pairwise comparisons were made using the function pairwise adonis in R. Significance was accepted for p < 0.05.
Results
Water and sedimentary features
The upwelling altered the bottom water physico-chemical conditions in Taganga Bay. It resulted in a nearly 7 °C decrease of average water temperatures, from 29.16 ± 0.25 to 22.58 ± 0.21 °C, in a decrease of DO concentration and saturation rates, from 235 ± 10 µM (123% saturation) to 208 ± 15 µM (106% saturation) and in an increase of salinity, from 35.98 ± 0.19 to 37.05 ± 0.02.
Sediments from the four stations were sandy, with comparable density, porosity and water content but with marked differences in terms of OM, C, N and P content, and redox status (Table 1). There was a clear gradient of impact from the sediments closest to the outfall (S1), that were more organic, sulphidic and covered by Beggiatoa mats, to the outer station (S4) that had less organic loaded and more oxidized sediments. The two-way ANOVA adopted for the analysis of sediment features showed a good fit to experimental data (R2 > 75%, Table 2). The season, station and interaction (season x station) terms were statistically significant for all parameters except for TOP. The effect of station was higher for TOC and TN than that of season while both effects were strong for OM, TP and TIP (Table 2). Sediment C, N, P, and OM content were in general highly correlated across the stations (Table S2, Supplementary Material).
Organic carbon and nitrogen isotopic composition in the Taganga Bay sediments
The sedimentary C and N isotopic composition displayed significant spatial and seasonal variations (Fig. 2). The most and last depleted average δ13C values were measured in S1 and in S4, respectively, both during the UPW season (Table 1). The seasonal effect was considerably stronger than the spatial variation and the interaction (Table 2). The pairwise comparisons between S1 and S2 did not show a clear distinction, but differences between these stations and stations S3 and S4 were significant. Most and least depleted values of δ13C were negatively correlated with TOC, OM, TN, TP, and TIP but not with TOP, or δ15N (Table S2, Supplementary Material).
Signatures of δ13C(V-PDB) and δ15N(AIR) in surface sediments (0–3 cm) of 4 stations (S1 to S4) located along increasing distances from a sewage outfall. Station S1 is the closest to the outfall. Measurements were performed in November 2017 and in January–February 2018, during non-upwelling (NUPW) and upwelling season (UPW), respectively
Values of δ15N also displayed high spatial and temporal variability with the more depleted values measured in S1 and S2 during the NUPW season (Table 1). Average δ15N values increased from the NUPW to UPW season, in particular at S2, and the effect of season was greater than the effect of station and of the interaction. Pairwise comparisons suggested significant differences between δ15N values measured at S1–S3 and S3–S4 (Table 2). Outcomes of pairwise and Pearson’s coefficient indicate that spatial distribution of δ15N did not correspond to that of TN. Furthermore, there were no significant correlations with other sedimentary properties.
The molar ratio TOC:TN calculated at the different sites and over the two sampling seasons varied by an order of magnitude, from 3 to 24 (Table 1). In general, TOC:TN molar ratios were lower at S1 and S3 in both seasons; the highest ratio was found at S4 during the NUPW season.
The relative contribution of terrestrial organic carbon ranged from 13.0 to 94.9%, with the highest average contribution calculated for S1 during the UPW season (86.8 ± 8.4%) and declining from S1 to S4 (Fig. 3). At S3, 750 m far from the outfall, the average terrestrial contribution of organic C nearly halved as compared to S1 (45.5 ± 7.3%, n = 8) while at S4, 1.8 km far from the outfall, it was minor (18.9 ± 5.7%, n = 8) and marine sources prevailed. There was an increase of terrestrial TOC average values from the NUPW to UPW season for S1 and S2, whereas the opposite occurred at S3 and S4.
Relative contribution of terrestrial versus marine organic carbon in surface sediments (0–3 cm depth) calculated for 4 stations along increasing distance from a sewage outfall in Taganga Bay—Colombian Caribbean. Measurements were carried out during the non-upwelling (NUPW) and upwelling (UPW) season. Averages ± standard deviations (n = 4) are reported
Aerobic and anaerobic metabolism
Oxygen uptake rates displayed clear spatial and seasonal trends, with decreasing respiration along the transect S1–S4 and with higher rates measured at S1 and S2 during the UPW as compared to the NUPW season (Table 3 and Fig. 4). The effects of season and sampling station were highly significant but there was an interaction between the two factors (Table 3). Rates of oxygen consumption ranged from − 1018.4 to − 4519.0 µmol O2 m−2 h−1 (NUPW season) and from − 1269.6 to − 6311.0 µmol O2 m−2 h−1 (UPW season). In both investigated seasons the average O2 uptake rate measured at S1 was nearly 3.6 times higher than that at S4. During the UPW season oxygen demand increased significantly (S1 and S2, by 24 and 62%, respectively) or tended to increase (S3 and S4, by 4 and 11%, respectively) as compared to the NUPW season. Variation coefficients were generally low (< 15%) at all stations and sampling seasons. Sediment sieving at the end of the incubations revealed the occasional presence of a few, small oligochaetes.
Fluxes of ferrous iron, manganous manganese and methane were below the limit of detection of our methods (< 1 µmol Fe2+ or Mn2+ m−2 h−1 and < 0.1 µmol CH4 m−2 h−1) and are not reported.
Net N 2 fluxes and rates of denitrification and nitrate ammonification
The net fluxes of N2 were characterized by small scale variability, as suggested by high variation coefficients, and by marked seasonal differences, depending upon the interaction with the factor site (Fig. 5 and Table 3). Rates were both positive, suggesting the dominance of denitrification over N2 fixation, and negative, suggesting the dominance of N2 fixation. In the transition from the NUPW to the UPW season negative N2 fluxes measured in S1, S2 and S3 became positive whereas at station S4 net fluxes were negative in both seasons. Net N2 fluxes ranged from − 109.6 to 67.6 µmol N m–2 h–1 during the NUPW season and from 50.0 to 186.3 µmol N m–2 h–1 during the UPW season.
Calculated rates of 28N2 production from the IPT concentration series were always independent from the level of 15NO3− added to the core water phase, suggesting that ANAMMOX was not contributing to N2 production at the sampling stations. Rates of total denitrification were very low at three out of four stations (2 < x < 4 µmol N m−2 h−1) with S3 as only exception (8 < x < 11 µmol N m−2 h−1). At all sampling sites denitrification was mostly sustained (90%) by production of nitrate within sediments by nitrification (Fig. 6). Total denitrification (Dtot) showed high significant spatial and temporal variations (Table 3). Lowest average values of Dtot were found at S1 during the UPW season while highest values were observed at S3 during the NUPW season. Dtot decreased at S1 (by ~ 35%) and S3 (by ~ 25%) whereas rates remained virtually unchanged at S2 and S4 between the NUPW and UPW season. During the same time lag, average rates of Dw increased in all stations. At S1, S2 and S3 Dw increased by a factor of ~ 2.4 whereas at S4 rates increased by a factor of ~ 3.
Rates of DNRA were consistently higher during the UPW season, except at S4, and during both seasons DNRA rates decreased from S1 to S4 (Table 3 and Fig. 7). More in detail, S1 and S2 displayed similar rates, higher than those in S3 and S4, during the NUPW season, whereas during the upwelling period DNRA rates decreased from S1 to S4. Significant positive correlations were found between DNRA and TOC and Dw whereas negative correlations were observed between DNRA and Dn. (Table S2, Supplementary Material). The comparison of the share of nitrate consumption between Dtot and DNRA indicated that the latter process was responsible for a major, similar fraction of nitrate consumption (60 to 65%) at stations S1 and S2 in both UPW and NUPW seasons. The contribution of DNRA to nitrate uptake was smaller and consistent in the two seasons at station S3 (15–17%) and S4 (29–31%).
Nutrient fluxes at the sediment–water interface
Dissolved inorganic N, P and Si fluxes were low as compared to what expected from oxygen demand and the elemental composition of sediments and did not follow clear patterns (Fig. 8). Fluxes of NO3− and NH4+ were significantly different between sampling periods and among stations (Table 3). The effect of season was stronger for NO3− while the effect of the interaction was important for NH4+. NO3− flux dominated DIN fluxes at S1 in both seasons, and at S2 and S4 only in the UPW season. Significant positive correlations were found between NO3− and O2 fluxes (Table S2, Supplementary Material). Ammonium fluxes dominated inorganic N exchanges at S3 in both seasons, while at S2 and S4 they were relevant only in November. Fluxes of NO2− were negligible and are not reported.
DIP fluxes were generally low and ranged from − 0.56 to 0.17 µmol P m−2 h−1. They displayed a significant temporal variation whereas there was no significant difference among stations (Table 3). Significant spatial and seasonal effects were determined also for SiO2 fluxes, that ranged from − 3.19 µmol Si m–2 h–1 at S3 during the UPW season to 7.04 µmol Si m–2 h−1 measured at S3 during the NUPW season. Sediments were sources of SiO2 at S1 and S2 in both seasons and at S3 and S4 during the NUPW season, whereas SiO2 was taken up at S3 and S4 during the UPW season.
Multivariate analysis
The PCA analysis allowed to identify a subset of sedimentary properties (TOC, δ13C and δ15N) and of nutrient fluxes (O2, N2, NH4+, NO3−, DIP, SiO2, Dw, Dn and DNRA) with essential information for the RDA analysis. TOC, δ13C and δ15N were the most significant variables explaining 41.90% of changes in the nutrient fluxes in the two axes of RDA that were statistically significant (p < 0.001, Table S3 and Table S4 in Supplementary Material). Fluxes of NH4+, SiO2 and DNRA were directly correlated with the increase of TOC while Dn and NO3− were inversely correlated (Fig. 9 and Fig. S2 in Supplementary Material). TOC and δ13C were not correlated with δ15N, whereas there was a strong negative correlation between TOC and δ13C. δ15N showed slightly higher correlation with N2, DIP and Dw than with other fluxes. Results from the RDA align with what described in previous paragraphs and confirm that δ15N is a poor predictor of the effects of the outfall on the benthic compartment. Two-way PERMANOVA showed highly significant differences over the stations, seasons and interaction on nutrient fluxes, Dw, Dn and DNRA. Pairwise test pointed out that stations and interaction were significantly different from each other (Table 4 and Table S5 in Supplementary Material).
Discussion
Sedimentary features
Carbon isotopic signature along the transect as tracer of anthropic impact
Carbon isotopic signature along the analyzed transect traced the spatial variation of anthropogenic TOC, TP and TIP in sediments, as reported elsewhere by Church et al. (2006), Gao et al. (2008) and Dang et al. (2018). In Taganga Bay the most depleted average δ13C values were found in the sediments with the highest oxygen average uptake, chemically reduced conditions (very negative redox potential and buildup of free sulphides in the pore water, and presence of Beggiatoa mats), suggesting a large, measurable impact of the organic matter delivered from the outfall. The average δ13C values at S1 and S2 were similar to or more depleted than values reported in marine sediments impacted by untreated and primary treated sewage effluents in Portugal, Canada and China (− 24.0 to − 25.4‰, Sampaio et al. 2010; Burd et al. 2013; Li et al. 2016). In contrast, the less impacted sediments of station S4 showed the most enriched average δ13C values, lowest oxygen average uptake, oxidized sediments without free sulphides in the upper horizon and higher relative percentage of marine material.
The sedimentary gradients determined along the transect of anthropogenic organic matter input are consistent with those indicated by Hargrave et al. (2008). These authors provide a nomogram for benthic organic enrichment zonation based on Eh and total free sulphides, conforming to our findings, with sediments at S1 and S2 chemically reduced (Eh < − 150 mv), grossly polluted (~ 75% of terrigenous OC) and with Beggiatoa mats clearly visible on the sediment surface. The appearance of white Beggiatoa mats on sediments is a consequence of the increase (S2− above 500–1000 µM) and vertical migration of free sulfides in pore water (Hargrave et al. 2008) which may explain the production of NH4+, due to DNRA (Preisler et al. 2007). Sediments from station S3 possibly showed a transitory organic enrichment, oxic type B (~ − 50 < Eh < 100) with a lower relative proportion of terrigenous OC. Sediments from station S4 had positive redox potential and there were no indications of anaerobic metabolism end-products accumulation in the pore water, suggesting low impact.
The influence of sewage effluent on sedimentary features, as evidenced by carbon isotopes signature, was clearly detectable at 750 m (S3) and 1800 m (S4) far from the outfall (~ 45% and ~ 20% contribution of terrestrial organic carbon, respectively). These results suggest that the analysis of sediments reveals a much more spatially extended impact of the outfall in the study area than previously evaluated, at least by a factor of 3. In fact, according to García (2013), the maximum extension of an area affected by SMSO discharge is 600 m, if the concentration of fecal coliforms in the water column is considered. Terrigenous OC at S4 may possibly reflect a high background value in Taganga Bay, due to the influence and/or mixing of wastewater outfall with other anthropogenic sources such as riverine inputs, surface runoff and harbor activities due to currents and transport process.
The significant interactions between the effects produced by the submarine outfall and the upwelling might be partly due to the overlapping of the UPW and the high touristic season, when the organic and nutrient loads generated by the town of Santa Marta peak. It can be expected that the further increase of the loads generated by the outfall may act in synergy with the effects of the upwelling on particulate matter, nutrient and oligoelements mobilization. The two-end-member mixing model we employed supports this statement as it suggests during the UPW season an increase of terrestrial OC at S1 and S2 and an increase of marine OC at S3 and S4. Our interpretation for what observed close to the outfall is that the increase of the sewage loads generated during the highest tourist season of Santa Marta city (December–January) produces a much large, local effect than that generated by the UPW. On the contrary, the marine OC increment calculated for the outer stations is the result of the dominant net effect of the UPW-associated particulate transport.
The isotopic signature of N in sediments suggests multiple co-occurring processes
The nonlinear variation of δ15N along the analyzed transect likely reflects the complexity of the biogeochemical N-cycle (Bedard-Haughn et al. 2003; Meyers 1997). Such complexity might be exacerbated in impacted ecosystems, where the level of fractionation can be affected by short-term temporal or spatial variability of dominant microbial N transformations (Bedard-Haughn et al. 2003). The average δ15N values at S1 and S2 were similar or more enriched than values reported in other impacted areas in Portugal, Canada and China (2.1 to 5.2‰, Sampaio et al. 2010; Burd et al. 2013; Li et al. 2016). δ15N-enriched values were also observed at S3, where total denitrification rates were nearly 3–4 times higher than at the other stations. This is in agreement with the preferential consumption of 14N by denitrifiers (Mariotti et al. 1981; Bedard-Haughn et al. 2003; Alkhatib et al. 2012; Rooze and Meile 2016). Slightly depleted δ15N values at S4 could be due to N2 fixation rates (Bedard-Haughn et al. 2003).
What emerges from our seasonal results is that processes as denitrification, leading to higher δ15N signatures, and N2-fixation, leading to lower δ15N signatures, may vary over relatively short time and spatial scales. Net N2 fluxes in fact were mostly negative during the NUPW season, when the benthic system need to import reactive nitrogen for microbial growth, to sustain heterotrophic processes in organic-enriched sediments. During the UPW season, only a few months later, nitrate concentrations in the water column nearly doubled (from 1.10 to 1.89 µM) and organic inputs from terrestrial and marine sources increased. Under these circumstances the net fluxes of N2 were reversed in 3 out of 4 stations, suggesting that denitrification exceeded-fixation.
The stoichiometry of organic C and N
The sedimentary ratios of TOC and TN have been widely used as proxies to elucidate the source and fate of organic matter in marine sediments (Meyers 1997; Li et al. 2016; Wang et al. 2018). Generally, TOC:TN ratios higher than 15 characterize terrigenous material (Meyers 1997; Wang et al. 2018) whereas values close to 6–8 are generally associated to fresh phytoplankton detritus (e.g. from upwelling waters, as determined in our study area by Bayraktarov and Wild 2014). Ratios at S1, S2 and S3 indicate a mixture of terrestrial and marine sources, whereas at S4 the high TOC:TN ratio is unexpected. S4 is far from the outfall and has the lowest relative proportion of terrigenous organic carbon; its high TOC:TN ratio can be explained by settling of micro- and macroalgae growing on reef substrate due to anthropogenic nutrient input and upwelling, as reported by Vega-Sequeda et al. (2008) at Punta Venado, near station S4. High TOC:TN ratios in algal organic matter can be due to N-limitation, supported by negative N2 fluxes, enhanced synthesis of lipid-rich organic matter or enhanced preservation of carbon-rich components and degradation of nitrogen-rich components (Meyers 1997). Similarly to δ15N, TOC:TN ratios have limitations as tracers of organic pollution and do not allow to discriminate organic matter sources to marine sediments due to the complexity of N-cycle biogeochemical processes (Bedard-Haughn et al. 2003; Meyers 1997).
Metabolic rates
Oxygen and nutrient fluxes
Regardless the origin of the organic matter to sediments and despite marked decrease of bottom water temperatures during the UPW season, the OC input from the marine side stimulated or maintained elevated oxygen uptake in all stations. Bayraktarov and Wild (2014) found that from 75 to 79% of the annual organic matter input to the sediments of Santa Marta coastal area was supplied during UPW, with higher inputs at exposed than at sheltered sites. They also reported higher oxygen uptake at sheltered sites due to lower water currents promoting particles sedimentation, which is consistent with oxygen uptake rates at S4 that were 26 and 23% higher than at S3 in the NUPW and UPW seasons, respectively.
Sedimentary oxygen uptake peaked at S1 with rates comparable or even higher than those reported for estuaries and marine ecosystems that receive high loadings of organic matter (Table 5). At station S1 rates of oxygen and nitrate consumption were nearly 4 and 12 times higher than those measured far from the outfall, respectively. As sediment sieving revealed the absence of significant contribution of macrofauna to benthic processes, we speculate that most oxygen consumption was microbial or chemical and that most small scale variability was due to the distribution of Beggiatoa colonies, patchily colonizing surface sediments at S1 and S2.
Inorganic nutrient fluxes (ammonium, reactive Si and P) were generally low when compared to fluxes measured in different impacted coastal areas (Table 5) and did not show a clear spatial gradient as it was observed for sedimentary features, oxygen uptake rate and nitrate fluxes. The absence of a consistent trend of inorganic nutrient regeneration between stations proximal to and distant from the outfall, suggests mechanisms that are uncoupled to oxygen uptake and not redox-dependent. Alternatively, decomposition might be incomplete and lead to the release of dissolved organic forms of N and P, which were not measured in the present study. Limited efflux of inorganic N, P and Si might be a consequence of poor bioturbation by macrofauna in the Taganga Bay sediments, in turn due to organic-impacted and sulphidic sediments. Measured inorganic N, Si and P regeneration might be low as compared to expected rates (i.e. from oxygen uptake and sedimentary C:N or C:P ratios) due to the refractory macromolecular quality of the terrestrial organic carbon and to inefficient anaerobic mineralization rates. The presence of Beggiatoa mats at S1 and S2 and the absence of measurable Fe2+, Mn2+ and CH4 fluxes suggests that most of the organic matter is mineralized via suphate reduction and that large fraction of the oxygen uptake is due to reoxidation processes.
As the sewage outfall has an installed capacity of 2.5 m3 s−1 (García 2013) the loads of organic matter and nutrients from the outfall are expected to increase. Under this scenario, sedimentary features and fluxes should be continuously monitored as saturation of processes retaining nutrients or replacement of denitrification by DNRA may turn sediments permanent sources of N and P to the water column. We do not think that present analytical techniques allow to trace significant increase of nutrient concentration due to sediment regeneration in the water mass, as the dilution is very large. Attempts, made in the proximities of floating fish cages, revealed no differences in nutrient concentrations along increasing distances from the cages. However, bioassays based on phytoplankton revealed much higher primary production (eutrophication, sensu Nixon 1995) in the proximity of cages. This ultimately means that sediments provide much evident and measurable impact of the outfall than the water column dissolved nutrients, that sedimentary nutrient fluxes need to me monitored and that phytoplankton may respond to very small increase of water column nutrients (Dalsgaard and Krause-Jensen 2006).
The recent increase of phytoplankton blooms in the Santa Marta coastal area, including potentially toxigenic microalgae, such as Pseudo-nitzschia, Anabaenopsis, Gonyaulax, Gyrodinium, Gymnodinium, Prorocentrum, Scripsiella y Cochlodinium represents a warning signal about the effects of anthropogenic nutrient inputs to this system (Garcés-Ordóñez et al. 2016). Such blooms suggest subtle changes in water (and sediment) chemistry that likely affect nutrient concentrations and stoichiometry and that should be carefully considered.
Microbial N transformations
Discrepancies between measurements based on N2/Ar and IPT have been widely discussed; N2/Ar might overestimate true rates of denitrification due to reactions between O2 and N2 in the mass spectrometer ion source. As during dark incubations O2 decreases, such reactions may lead to an apparent increase of N2 (Eyre et al. 2002, 2013; Lunstrum and Aoki 2016). As the MIMS employed in our study was equipped with a copper reduction column maintained at 600 °C we think that most oxygen interferences were removed. Also the IPT presents some limitations depending on site-specific sediment features. At S1and S2 for example visible mats of Beggiatoa might have affected the results of our denitrification measurements (Song et al. 2013). Beggiatoa have large intracellular pools of nitrate which prevent correct evaluation of 15NO3− to 14NO3− ratio in the denitrification zone, possibly leading to a slight underestimation of the nitrate reduction rates. We acknowledge that future application of the IPT in the Taganga Bay sediments with Beggiatoa will require to determine the vertical distribution and intracellular NO3− content of these microbes, together with additional experiments specifically targeting rates in individual cells (see the review by Robertson et al. (2019) on how to tackle IPT limitations).
Results from N2/Ar ratio measurements suggest a seasonal shift between the dominance of N-fixation and denitrification in 3 out of 4 sites, that may be related to increased availability of water column nitrate or ammonium from mineralization processes during the UPW season. A similar dynamic, regulated by large seasonal availability of reactive N, is reported by Newell et al. (2016) in the Waquoit Bay and by Zilius et al. (2018) in a coastal lagoon.
Results from the IPT suggest the co-occurrence of DNRA and denitrification in sediments, as also reported elsewhere (Burgin and Hamilton 2007; Gardner and McCarthy 2009; Newell et al. 2016; Bonaglia et al. 2014). The seasonal increase of water column nitrate was addressed to the upwelling (Arévalo-Martínez and Franco-Herrera 2008; Ramírez-Barón et al. 2010) and not to the outfall, due the huge dilution factor. Such increase was likely responsible for higher nitrate demand by organic sediments via DNRA or it may have favored the growth of primary producers competing with bacteria and potentially impacting corals (Carstensen et al. 2000; Diaz-Pulido and Garzón-Ferreira 2002; Dalsgaard 2003; Franco-Herrera et al. 2006). In Taganga Bay DNRA increased from the NUPW to UPW season in all stations.
DNRA increases with salinity and eutrophication likely due to higher sulphate reduction rates and sulphide accumulation, as shown in the present study and by others (Gardner et al. 2006; Dong et al. 2011; Bernard et al. 2015). The predominance of DNRA over denitrification in tropical estuaries may be due to both an energetic advantage of nitrate ammonifiers over denitrifiers when competing for limited nitrate, and to higher affinity for nitrate by the nitrate ammonifiers at tropical temperatures permanently > 24 °C. These explanations can be realistic for our case study, with rates of DNRA having similar patterns as those of oxygen demand and sulphide concentration in pore water.
Station S3 displayed the highest rates of denitrification in the two campaigns, likely due to moderate carbon enrichment, lower microbial oxygen consumption, absence of free sulphides in porewater and a molar ratio TOC:TN similar to Redfield ratio (8 ± 3). Ammonium availability and oxygen penetration likely drove nitrification in surface sediments, leading to high rates of coupled denitrification. At S4 denitrification rates were low likely due to the scarce availability and high competition for reactive nitrogen, resulting in net import and minimum loss.
Expected increase of organic load to the Taganga Bay sediments from the outfall and during the UPW/touristic season will turn the benthic system more and more sulphidic. The suppression of denitrification and the increase of DNRA may ultimately lead to lower net N losses, higher NH4+ mobility and stimulation of algal growth, as reported in Bernard et al. (2015). Results from this study support the evidence of strong and complex relationships between variations in oceanographic conditions and biological process in the near coastal zone and of positive feedback between upwelling and impacts associated to the outfall and the touristic season (Paramo et al. 2011).
Conclusions
Sediments seem to track past and ongoing impacts from a submarine outfall better than the water column. Taganga Bay sediments allow to trace the organic pollution from a sewage outfall and reveal that the spatial extent of the impact is much larger, by a factor of 3, than what hypothesized from water column analysis of fecal coliforms. Seasonal analysis of sediment features and metabolic rates suggest also an interaction between the effects of submarine outfall and those of upwelling, due to additional nutrient and organic matter inputs from the open sea to the coastal area. The cascading effects of organic inputs, increasing respiration, sulphide build up, suppression of denitrification and increase of ammonification may dramatically impact the macrofauna community, leading to species loss over increasing areas, and stimulate the growth of algae, with negative implications for corals, water transparency and tourism. This is a real concern, as the loads released by the outfall are expected to increase. Tropical areas as Taganga Bay seem intrinsically more vulnerable to these impacts than temperate areas due to the different temperature regimes, which may easily uncouple microbial respiration and oxygen availability.
References
Alkhatib M, Lehmann MF, Del Giorgio PA (2012) The nitrogen isotope effect of benthic remineralization-nitrification- denitrification coupling in an estuarine environment. Biogeosciences 9(5):1633–1646. https://doi.org/10.5194/bg-9-1633-2012
Aller RC (1988) Benthic fauna and biogeochemical processes in marine sediments: the role of burrow structures BT—nitrogen cycling in coastal marine environments. In: Blackburn TH, Sørensen J (eds) Nitrogen cycling in coastal marine environments. Wiley, New York, pp 301–338
Aller RC (1994) Bioturbation and remineralization of sedimentary organic matter: effects of redox oscillation. Chem Geol 114(3–4):331–345. https://doi.org/10.1016/0009-2541(94)90062-0
An S, Gardner WS (2002) Dissimilatory nitrate reduction to ammonium (DNRA) as a nitrogen link, versus denitrification as a sink in a shallow estuary (Laguna Madre/Baffin Bay, Texas). Mar Ecol Prog Ser 237:41–50. https://doi.org/10.3354/meps237041
Andrade CA, Barton ED (2005) The Guajira upwelling system. Cont Shelf Res 25(9):1003–1022. https://doi.org/10.1016/j.csr.2004.12.012
Arévalo-Martínez DL, Franco-Herrera A (2008) Características oceanográficas de la surgencia frente a la ensenada de Gaira, Departamento de Magdalena, época seca menor de 2006. Boletín de Investigaciones Marinas y Costeras 37(2):131–162
Aspila K, Agemian H, Chau ASY (1976) A semi-automated method for the determination of inorganic, organic and total phosphate in sediments. Analyst 101:187–197
Banta GT, Giblin AE, Hobbie JE et al (1995) Benthic respiration and nitrogen release in Buzzards Bay, Massachusetts. J Mar Res 53:107–135
Bayraktarov E, Wild C (2014) Spatiotemporal variability of sedimentary organic matter supply and recycling processes in coral reefs of Tayrona National Natural Park, Colombian Caribbean. Biogeosciences 11(11):2977–2990. https://doi.org/10.5194/bg-11-2977-2014
Bayraktarov E, Pizarro V, Eidens C et al (2013) Bleaching susceptibility and recovery of Colombian Caribbean corals in response to water current exposure and seasonal upwelling. PLoS ONE 8(11):1–11. https://doi.org/10.1371/journal.pone.0080536
Bayraktarov E, Bastidas-Salamanca ML, Wild C (2014) The physical environment in coral reefs of the Tayrona National Natural Park (Colombian Caribbean) in response to seasonal upwelling. Boletín de Investigaciones Marinas y Costeras 43(1):137–157
Bedard-Haughn A, Van Groenigen JW, Van Kessel C (2003) Tracing15N through landscapes: potential uses and precautions. J Hydrol 272(1–4):175–190. https://doi.org/10.1016/S0022-1694(02)00263-9
Berelson WM, Johnson K, Coale K et al (2002) Organic matter diagenesis in the sediments of the San Pedro Shelf along a transect affected by sewage effluent. Cont Shelf Res 22:1101–1115
Bernard RJ, Mortazavi B, Kleinhuizen AA (2015) Dissimilatory nitrate reduction to ammonium (DNRA) seasonally dominates NO3− reduction pathways in an anthropogenically impacted sub-tropical coastal lagoon. Biogeochemistry 125(1):47–64. https://doi.org/10.1007/s10533-015-0111-6
Bonaglia S, Nascimento FJA, Bartoli M et al (2014) Meiofauna increases bacterial denitrification in marine sediments. Nat Commun 5(5133):1005–1011. https://doi.org/10.1038/ncomms6133
Boynton WR, Ceballos MAC, Bailey EM et al (2018) Oxygen and nutrient exchanges at the sediment-water interface: a global synthesis and critique of estuarine and coastal data. Estuar Coasts 41(2):301–333. https://doi.org/10.1007/s12237-017-0275-5
Burd B, Macdonald T, Bertold S (2013) The effects of wastewater effluent and river discharge on benthic heterotrophic production, organic biomass and respiration in marine coastal sediments. Mar Pollut Bull 74(1):351–363. https://doi.org/10.1016/j.marpolbul.2013.06.029
Burgin AJ, Hamilton SK (2007) Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Front Ecol Environ 5(2):89–96. https://doi.org/10.1890/1540-9295(2007)5[89:HWOTRO]2.0.CO;2
Calder JA, Parker PL (1968) Stable carbon isotope ratios as indexes of petrochemical pollution of aquatic systems. Environ Sci Technol 2(7):535–539
Carstensen J, Conley DJ, Bonsdorff E, Gustafsson BG et al (2014) Hypoxia in the Baltic Sea: biogeochemical cycles, benthic fauna, and management. Ambio 43(1):26–36. https://doi.org/10.1007/s13280-013-0474-7
Church TM, Sommerfield CK, Velinsky DJ et al (2006) Marsh sediments as records of sedimentation, eutrophication and metal pollution in the urban Delaware Estuary. Mar Chem 102(1–2):72–95. https://doi.org/10.1016/j.marchem.2005.10.026
Cline JD (1969) Spectrophometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr 14(3):454–458. https://doi.org/10.4319/lo.1969.14.3.0454
Dalsgaard T (2003) Benthic primary production and nutrient cycling in sediments with benthic microalgae and transient accumulation of macroalgae. Limnol Oceanogr 48(6):2138–2150. https://doi.org/10.4319/lo.2003.48.6.2138
Dalsgaard T, Krause-Jensen D (2006) Monitoring nutrient release from fish farms with macroalgal and phytoplankton bioassays. Aquaculture 256(1–4):302–310
Dalsgaard T, Nielsen LP, Brotas V et al (2000) Protocol handbook for nitrogen cycling in estuaries. NICE, London
Dalsgaard T, Thamdrup B, Canfield DE (2005) Anaerobic ammonium oxidation (anammox) in the marine environment. Res Microbiol 156(4):457–464. https://doi.org/10.1016/j.resmic.2005.01.011
Dang DH, Evans RD, Durrieu G et al (2018) Quantitative model of carbon and nitrogen isotope composition to highlight phosphorus cycling and sources in coastal sediments (Toulon Bay, France). Chemosphere 195:683–692. https://doi.org/10.1016/j.chemosphere.2017.12.109
De Brabandere L, Bonaglia S, Kononets MY et al (2015) Oxygenation of an anoxic fjord basin strongly stimulates benthic denitrification and DNRA. Biogeochemistry 126(1–2):131–152. https://doi.org/10.1007/s10533-015-0148-6
Diaz RJ (2001) Overview of hypoxia around the world. J Environ Qual 30(2):275–281. https://doi.org/10.2134/jeq2001.302275x
Diaz-Pulido G, Garzón-Ferreira J (2002) Seasonality in algal assemblages on upwelling-influenced coral reefs in the Colombian Caribbean. Bot Mar 45(3):284–292. https://doi.org/10.1515/BOT.2002.028
Díaz-Rocca LH, Causado-Rodríguez E (2007) La insostenibilidad del desarrollo urbano: El caso de Santa Marta-Colombia. Clío América 1(1):64–100
Dong LF, Sobey MN, Smith CJ et al (2011) Dissimilatory reduction of nitrate to ammonium, not denitrification or anammox, dominates benthic nitrate reduction in tropical estuaries. Limnol Oceanogr 56(1):279–291. https://doi.org/10.4319/lo.2011.56.1.0279
Downing JA, Mcclain M, Twilley R et al (1999) The impact of accelerating land-use change on the N-cycle of tropical aquatic ecosystems: current conditions and projected changes. Biogeochemistry 46:109–148
Escobar A (1988) Estudio de algunos aspectos ecologicos y de la contaminacion bacteriana en la Bahia de Santa Marta, Caribe Colombiano. Boletin de Investigaciones Marinas y Costeras 18:39–57
Eyre BD, Rysgaard S, Dalsgaard T, Christensen PB (2002) Comparison of isotope pairing and N2:Ar methods for measuring sediment denitrification—assumptions, modifications, and implications. Estuaries 25(6A):1077–1087
Eyre BD, Maher DT, Squire P (2013) Quantity and quality of organic matter (detritus) drives N2 effluxes (net denitrification) across seasons, benthic habitats, and estuaries. Glob Biogeochem Cycles 27(4):1083–1095. https://doi.org/10.1002/2013GB004631
Fajardo G (1979) Surgencia costera en las proximidades de la península colombiana de La Guajira. Boletin Cientifico CIOH 2:7–19
Ferguson A, Eyre B (2012) Interaction of benthic microalgae and macrofauna in the control of benthic metabolism, nutrient fluxes and denitrification in a shallow sub-tropical coastal embayment (western Moreton Bay, Australia). Biogeochemistry 112(1–3):423–440. https://doi.org/10.1007/s10533-012-9736-x
Fox J (2005) The R commander: a basic-statistics graphical user interface to R. J Stat Softw 14(9):1–42
Franco-Herrera A, Castro L, Tigreros P (2006) Plankton dynamics in the south-central Caribbean Sea: strong seasonal changes in a coastal tropical system. Carib J Sci 42(1):24–38
Gao J, Wang Y, Pan S et al (2008) Spatial distributions of organic carbon and nitrogen and their isotopic compositions in sediments of the Changjiang Estuary and its adjacent sea area. J Geog Sci 18(1):46–58. https://doi.org/10.1007/s11442-008-0046-0
Garcés-Ordóñez O, Arteaga E, Obando P et al (2016) Atención a eventuales emergencias ambientales en la zona marino-costera del departamento del Magdalena. Convenio CORPAMAG-INVEMAR; código: PRY-CAM-011-14. Informe técnico final (Issue 14)
García F (2013) Modelación de los efectos del emisario submarino de santa marta sobre la calidad del agua. PhD dissertation, Universidad de Antioquia, Facultad de Ingeniería.
García F, Palacio C, Garcia U (2012) Water quality at Santa Marta Coastal Area (Colombia). Dyna 79(173):85–94
García-Hoyos LM, Franco-Herrera A, Ramire-Barón JS et al (2010) Dinámica océano-atmósfera y su influencia en la biomasa fitoplanctónica en la zona costera del epartamento del Magdalena. Boletín de Investigaciones Marinas y Costeras 39(2):307–335
Gardner WS, McCarthy MJ (2009) Nitrogen dynamics at the sediment-water interface in shallow, sub-tropical Florida Bay: why denitrification efficiency may decrease with increased eutrophication. Biogeochemistry 95(2):185–198. https://doi.org/10.1007/s10533-009-9329-5
Gardner WS, McCarthy MJ, An S et al (2006) Nitrogen fixation and dissimilatory nitrate reduction to ammonium (DNRA) support nitrogen dynamics in Texas estuaries. Limnol Oceanogr 51(1 II):558–568
Gearing JN, Gearing PJ, Rudnick DT et al (1984) Isotopic variability of organic carbon in a phytoplankton-based, temperate estuary. Geochim Cosmochim Acta 48(5):1089–1098. https://doi.org/10.1016/0016-7037(84)90199-6
Giblin AE, Hopkinson CS, Tucker J (1997) Benthic metabolism and nutrient cycling in Boston Harbor, Massachusetts. Estuaries 20(2):346–364. https://doi.org/10.1007/BF02690378
Grall J, Chauvaud L (2002) Marine eutrophication and benthos: the need for new approaches and concepts. Glob Change Biol 8(9):813–830
Grasshoff KM, Ehrhardt M, Kremling K (1983) Methods of seawater analysis, 2nd edn. Verlag Chemie, Berlin
Hall POJ, Almroth E, Bonaglia S et al (2017) Influence of natural oxygenation of baltic proper deep water on benthic recycling and removal of phosphorus, nitrogen, silicon and carbon. Front Mar Sci 4(February):1–14. https://doi.org/10.3389/fmars.2017.00027
Hargrave BT, Holmer M, Newcombe CP (2008) Towards a classification of organic enrichment in marine sediments based on biogeochemical indicators. Mar Pollut Bull 56(5):810–824. https://doi.org/10.1016/j.marpolbul.2008.02.006
Hopkinson CS, Giblin AE, Tucker J (2001) Benthic metabolism and nutrient regeneration on the continental shelf of Eastern Massachusetts, USA. Mar Ecol Prog Ser 224:1–19. https://doi.org/10.3354/meps224001
Howarth R, Chan F, Conley DJ et al (2011) Coupled biogeochemical cycles: Eutrophication and hypoxia in temperate estuaries and coastal marine ecosystems. Front Ecol Environ 9(1):18–26. https://doi.org/10.1890/100008
Koop-Jakobsen K, Giblin AE (2009) Anammox in tidal marsh sediments: the role of salinity, nitrogen loading, and marsh vegetation. Estuar Coasts 32(2):238–245. https://doi.org/10.1007/s12237-008-9131-y
Legendre P, Legendre L (1998) Numerical ecology, 2nd edn. Elsevier, Amsterdam
Li Y, Zhang H, Tu C et al (2016) Sources and fate of organic carbon and nitrogen from land to ocean: identified by coupling stable isotopes with C/N ratio. Estuar Coast Shelf Sci 181:114–122. https://doi.org/10.1016/j.ecss.2016.08.024
Lunstrum A, Aoki LR (2016) Oxygen interference with membrane inlet mass spectrometry may overestimate denitrification rates calculated with the isotope pairing technique. Limnol Oceanogr Methods 14(7):425–431. https://doi.org/10.1002/lom3.10101
Mancera-Pineda J, Pinto G, Vilardy S (2013) Patrones de distribución espacial de masas de agua en la bahía de Santa Marta, Caribe Colombiano: Importancia relativa del upwelling y outwelling. Boletín de Investigaciones Marinas y Costeras 42(2):329–360
Mariotti A, Germon JC, Hubert P et al (1981) Experimental determination of nitrogen kinetic isotope fractionation: some principles; illustration for the denitrification and nitrification processes. Plant Soil 62(3):413–430. https://doi.org/10.1007/BF02374138
Martínez S, Acosta A (2005) Cambio temporal en la Estructura de la comunidad coralina del área de Santa Marta - Parque Nacional Natural Tayrona (Caribe Colombiano). Boletin de Investigaciones Marinas y Costeras 34:161–192. https://doi.org/10.1213/ane.0b013e31816e5128
McCarthy MJ, Newell SE, Carini SA et al (2015) Denitrification dominates sediment nitrogen removal and is enhanced by bottom-water hypoxia in the Northern Gulf of Mexico. Estuar Coasts 38(6):2279–2294. https://doi.org/10.1007/s12237-015-9964-0
Mermillod-Blondin F, Rosenberg R, Norling K et al (2004) Influence of bioturbation by three benthic infaunal species on microbial communities and biogeochemical processes in marine sediment. Aquat Microb Ecol 36:271–284. https://doi.org/10.3354/ame036271
Meyers PA (1997) Organic geochemical proxies of paleoceanographic, paleoimnologic, and paleoclimatic processes. Org Geochem 27(5–6):213–250. https://doi.org/10.1016/S0146-6380(97)00049-1
Newell SE, McCarthy MJ, Gardner WS et al (2016) Sediment nitrogen fixation: a call for re-evaluating coastal N budgets. Estuar Coasts 39(6):1626–1638. https://doi.org/10.1007/s12237-016-0116-y
Nielsen LP (1992) Denitrificaction in sediment determined from nitrogen isotope pairing. FEMS Microbiol Ecol 86:357–362
Nixon SW (1995) Coastal marine eutrophication: a definition, social causes, and future concerns. Ophelia 41(1):199–219. https://doi.org/10.1080/00785236.1995.10422044
Paramo J, Correa M, Núñez S (2011) Evidencias de desacople físico-biológico en el sistema de surgencia en la Guajira, caribe Colombiano. Rev Biol Mar Oceanogr 46(3):421–430. https://doi.org/10.4067/S0718-19572011000300011
Preisler A, De Beer D, Lichtschlag A et al (2007) Biological and chemical sulfide oxidation in a Beggiatoa inhabited marine sediment. ISME J 1(4):341–353. https://doi.org/10.1038/ismej.2007.50
Ramírez G (1981) Características Fisico-Químicas de la Bahía de Santa Marta (Agosto 1980-Julio 1981). Boletín de Investigaciones Marinas y Costeras 13:111–121
Ramírez-Barón JS, Franco-Herrera A, García-Hoyos LM, López-Cerón DA (2010) La comunidad fitoplanctónica durante eventos de surgencia y no surgencia, en la Zona Costera del Departamento del Magdalena, Caribe colombiano. Boletín de Investigaciones Marinas y Costeras 39(2):233–263
Ramos-Ortega LM, Vidal LA, Vilardy S et al (2008) Análisis de la contaminación microbiológica (coliformes totales y fecales) en la bahía de Santa Marta, caribe colombiano. Acta Biol Colomb 13(3):87–98
Robertson EK, Bartoli M, Brüchert V (2019) Application of the isotope pairing technique in sediments: use, challenges, and new directions. Limnol Oceanogr Methods 17(2):112–136. https://doi.org/10.1002/lom3.10303
Rooze J, Meile C (2016) The effect of redox conditions and bioirrigation on nitrogen isotope fractionation in marine sediments. Geochim Cosmochim Acta 184:227–239. https://doi.org/10.1016/j.gca.2016.04.040
Rosenberg R, Loo LO (1988) Marine eutrophication induced oxygen deficiency: effects on soft bottom fauna, western Sweden. Ophelia 29(3):213–225. https://doi.org/10.1080/00785326.1988.10430830
Rueda-Roa DT, Muller-Karger FE (2013) The southern Caribbean upwelling system: sea surface temperature, wind forcing and chlorophyll concentration patterns. Deep Sea Res I 78:102–114. https://doi.org/10.1016/j.dsr.2013.04.008
Salzwedel H, Müller K (1983) A summary of Meteorological and hydrological data from the Bay of Santa Marta, Colombian Caribbean. Boletin de Investigaciones Marinas y Costeras 13:67–83
Sampaio L, Freitas R, Máguas C et al (2010) Coastal sediments under the influence of multiple organic enrichment sources: an evaluation using carbon and nitrogen stable isotopes. Mar Pollut Bull 60(2):272–282. https://doi.org/10.1016/j.marpolbul.2009.09.008
Schlunz B, Schneider RR, Muller PJ et al (1999) Terrestrial organic carbon accumulation on the Amazon deep sea fan during the last glacial sea level low stand. Chem Geol 159(1–4):263–281
Smith J, Burford MA, Revill AT et al (2012) Effect of nutrient loading on biogeochemical processes in tropical tidal creeks. Biogeochemistry 108(1–3):359–380. https://doi.org/10.1007/s10533-011-9605-z
Song GD, Liu SM, Marchant H, Kuypers MMM, Lavik G (2013) Anammox, denitrification and dissimilatory nitrate reduction to ammonium in the East China Sea sediment. Biogeosciences 10(11):6851–6864. https://doi.org/10.5194/bg-10-6851-2013
Testa JM, Kemp WM (2011) Oxygen—dynamics and biogeochemical consequences. In: Testa JM, Kemp WM (eds) Treatise on estuarine and coastal science, vol 5. Elsevier, Amsterdam
Trimmer M, Risgaard-Petersen N, Nicholls JC et al (2006) Direct measurement of anaerobic ammonium oxidation (anammox) and denitrification in intact sediment cores Mark. Mar Ecol Prog Ser 326:37–47. https://doi.org/10.3354/meps326037
Tucker J, Giblin A (2010) Quality Assurance Project Plan (QAPP) for benthic nutrient flux studies: 2010. Massachusetts Water Resources Authority, Boston
Vega-Sequeda J, Rodríguez-Ramírez A, Reyes-Nivia MC et al (2008) Formaciones Coralinas Del Área De Santa Marta: Estado Y Patrones De Distribución Espacial De La Comunidad Bentonica. Boletin de Investigaciones Marinas y Costeras 37(2):87–105
Wang C, Lv Y, Li Y (2018) Riverine input of organic carbon and nitrogen in water-sediment system from the Yellow River estuary reach to the coastal zone of Bohai Sea, China. Cont Shelf Res 157:1–9
Warembourg FR (1993) Nitrogen fixation in soil and plant systems. In: Knowles R, Blackburn TH (eds) Nitrogen isotope techniques. Academic Press, San Diego, pp 127–156
Zhou J, Wu Y, Zhang J et al (2006) Carbon and nitrogen composition and stable isotope as potential indicators of source and fate of organic matter in the salt marsh of the Changjiang Estuary, China. Chemosphere 65(2):310–317. https://doi.org/10.1016/j.chemosphere.2006.02.026
Zilius M, Bartoli M, Bresciani M, Katarzyte M et al (2014) Feedback mechanisms between cyanobacterial blooms, transient hypoxia, and benthic phosphorus regeneration in shallow coastal environments. Estuar Coasts 37(3):680–694. https://doi.org/10.1007/s12237-013-9717-x
Zilius M, Vybernaite-Lubiene I, Vaiciute D et al (2018) The influence of cyanobacteria blooms on the attenuation of nitrogen throughputs in a Baltic coastal lagoon. Biogeochemistry 141(2):143–165. https://doi.org/10.1007/s10533-018-0508-0
Acknowledgements
This study was funded by COLCIENCIAS in the Call No. 714-2015—Research projects, technological development and innovation in environment, oceans and biodiversity (Contract—FP44842-020-2016). Doctoral Training Program (COLCIENCIAS—2014) supported PhD student. Prof. Marco Bartoli’s trip to Colombia to carry out field campaigns was supported by the call Fellows Colombia Program (ICETEX—2017) – Inter-institutional Ph.D Program – Marine Sciences and Universidad Nacional de Colombia, campus Medellin (Researchers Mobility Call—2017). Dr. Andres Franco and Alfonso Gamero of the Universidad Jorge Tadeo Lozano, campus Santa Marta are acknowledged for providing laboratory facilities and logistic support for this study. Prof. Bartoli is thanked by supporting chemistry analyses in Parma University (Italy), Ferrara University (Italy) and Marine Research Institute of Klaipeda University (Lithuania). Prof. Paola Iacumin and Dott. and Antonietta Di Matteo from Geology Department of Parma University and Irma Lubiene from Klaipeda University are acknowledged for the technical assistance in isotopic composition and nutrients analyses. We are grateful to two anonymous reviewers for their accurate and constructive comments. We are thankful with Johann Camil Delgado, Maximiliano Arredondo, Lina Maria Ramirez and Tayrona dive center at Taganga for their help in core collection and sampling. Fabio Suarez of Faro Tecnológico company is acknowledged by support in the field campaigns logistic.
Author information
Authors and Affiliations
Contributions
Conceptualization: [DMAG, MB and MT-B]; Methodology: [DMAG and MB]; formal analysis and investigation: [DMAG and MB]; writing—original draft preparation: [DMAG and MB]; writing—review and editing: [DMAG, MB, DGS and MT-B], Funding acquisition [DMAG, MB, DGS and MT-B], Supervision: [MB, DGS and MT-B].
Corresponding authors
Additional information
Responsible Editor: Maren Voss.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arroyave Gómez, D.M., Gallego Suárez, D., Bartoli, M. et al. Spatial and seasonal variability of sedimentary features and nitrogen benthic metabolism in a tropical coastal area (Taganga Bay, Colombia Caribbean) impacted by a sewage outfall. Biogeochemistry 150, 85–107 (2020). https://doi.org/10.1007/s10533-020-00689-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-020-00689-0