Abstract
Benthic biogeochemistry and macrofauna were investigated six times over 1 year in a shallow sub-tropical embayment. Benthic fluxes of oxygen (annual mean −918 μmol O2 m−2 h−1), ammonium (NH4 +), nitrate (NO3 −), dissolved organic nitrogen, dinitrogen gas (N2), and dissolved inorganic phosphorus were positively related to OM supply (N mineralisation) and inversely related to benthic light (N assimilation). Ammonium (NH4 +), NO3 − and N2 fluxes (annual means +14.6, +15.9 and 44.6 μmol N m−2 h−1) accounted for 14, 16 and 53 % of the annual benthic N remineralisation respectively. Denitrification was dominated by coupled nitrification–denitrification throughout the study. Potential assimilation of nitrogen by benthic microalgae (BMA) accounted for between 1 and 30 % of remineralised N, and was greatest during winter when bottom light was higher. Macrofauna biomass tended to be highest at intermediate benthic respiration rates (−1,000 μmol O2 m−2 h−1), and appeared to become limited as respiration increased above this point. While bioturbation did not significantly affect net fluxes, macrofauna biomass was correlated with increased light rates of NH4 + flux which may have masked reductions in NH4 + flux associated with BMA assimilation during the light. Peaks in net N2 fluxes at intermediate respiration rates are suggested to be associated with the stimulation of potential denitrification sites due to bioturbation by burrowing macrofauna. NO3 − fluxes suggest that nitrification was not significantly limited within respiration range measured during this study, however comparisons with other parts of Moreton Bay suggest that limitation of coupled nitrification–denitrification may occur in sub-tropical systems at respiration rates exceeding −1,500 μmol O2 m−2 h−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Processing of organic matter (OM) and nutrients in the sediments constitutes an important component of internal cycling within coastal ecosystems. Our understanding of benthic nutrient cycling has been modified extensively from early models that assumed sediments were simply sites of heterotrophic remineralisation to more recent models that include the impacts of benthic production on nutrient assimilation and sediment biogeochemistry (An and Joye 2001; Anderson et al. 2003; Ferguson et al. 2004b; Eyre and Ferguson 2005), nitrogen fixation (Herbert 1999; Paerl et al. 1996) and bioturbation (Aller 1982; Kristensen 1988; Rysgaard et al. 2000; Ferguson et al. 2003). The relative importance of denitrification as a sink for bio-available nitrogen has been increasingly recognised (Piña-Ochoa and Álvarez-Cobelas 2006; Seitzinger et al. 2006). More recently, knowledge of controls over denitrification has been advanced to include interactions between benthic production and bioturbation (Rysgaard et al. 2000; Sundbäck et al. 2000; Risgaard-Petersen 2003).
Shallow coastal systems that receive sufficient light to the sediments commonly sustain high rates of productivity by BMA (MacIntyre et al. 1996). Nutrient assimilation, autochthonous carbon and oxygen production by BMA can all have profound impacts on sediment biogeochemistry. In cases where light is sufficient to maintain a net autotrophic benthic community, sediments can become a net sink of nutrients from the water column (Ferguson et al. 2004a; Engelsen et al. 2008), or even a source of nitrogen to the system via N fixation (Howarth et al. 1988). Even where light limitation or high OM supply dictate a net heterotrophic community, BMA can still significantly influence nutrient cycling and attenuate fluxes (Risgaard-Petersen 2003; Ferguson et al. 2007). In particular, competition for dissolved inorganic nitrogen can effectively starve denitrifiers of NO3 − thereby limiting denitrification as a sink of N, and retaining it in the microbial loop (Sundbäck and Miles 2000). Autochthonous carbon production by BMA also provides a rich source of labile OM that may stimulate bacterial and metazoan productivity (Middelburg et al. 2000), both of which may influence fluxes. Finally, the impacts of diel oxygen production by BMA can have significant effects on sediment biogeochemistry (e.g. nitrification) due to changes in oxygen availability and the redox status of sediments (Risgaard-Petersen et al. 1994).
There is now a large body of evidence showing the potential impacts of bioturbation and bioirrigation by macrofauna on sediment biogeochemical processes (Kristensen and Kostka 2005). These impacts vary dynamically over seasonal cycles. Infauna respond opportunistically to seasonal variation in OM supply (Dauwe et al. 1998), however diversity and abundance are also sensitive to feedbacks from excessive OM loadings which can lead to an imbalance in anaerobic microbial activity and associated toxicity due to the build up of reduced chemical species (Vistisen and Vismann 1997). In the recovery phase after OM delivery to sediments, bioturbation can significantly impact on benthic biogeochemistry through the modification of sediment redox conditions (Aller 1994), the turnover of OM, the regeneration of inorganic nutrients via excretion, and the immobilisation or export of nutrients in biomass. Given this interdependence between OM delivery and microbial/metazoan ecology, it is useful to regard benthic biogeochemistry from a systems level (Marinelli and Williams 2003; Hooper et al. 2005; Thrush et al. 2006).
However, there are inherent difficulties in replicating these interactions in manipulative experimental studies commonly used to investigate macrofaunal controls over sediment biogeochemical processes. Controlled experimental studies have investigated species-specific effects on benthic fluxes in highly modified experimental conditions (e.g. Banta et al. 1999), and others have described the effects of experimental additions of macrofauna to organically enriched sediments [e.g. (Hansen and Kristensen 2002)]. While these studies are useful in understanding the direct effects of macrofauna on processes, it is hard to extrapolate results to an ecosystem level due to the dynamic feedbacks between macrofauna and sediment biogeochemistry that exist in the natural world. Field studies in temperate systems have characterised relationships between macrofauna and benthic nutrient cycling, however these are commonly limited by poor temporal resolution and therefore do not adequately describe feedbacks between OM supply and macrofaunal populations (e.g. Pennifold and Davis 2001; Thouzeaua et al. 2007; Engelsen et al. 2008). It is clear therefore, that seasonal surveys of benthic processes and metazoan communities can provide important insights to improve our understanding of this area (Mortimer et al. 1999), however this type of study is relatively rare and very few have been carried out to date in sub-tropical systems.
This study describes interactions between benthic microalgae and macrofauna in the control of benthic processes over an annual cycle in a nearshore environment of a sub-tropical coastal embayment (Moreton Bay). We hypothesised that benthic metabolism and nutrient cycling would broadly respond to climatic controls over OM supply (e.g. seasonal variation in temperature and light, and the episodic nature of freshwater inputs to the system), but that feedbacks from benthic microalgae and macrofauna were likely to impart strong secondary controls over the seasonal variation in benthic processes.
Methods
Study area
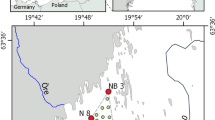
Moreton Bay is a semi-enclosed embayment located adjacent to the city of Brisbane in southeast Queensland, Australia (Fig. 1). The bay is separated from the Pacific Ocean by Moreton Island and North Stradbroke Island, and has a surface area of approximately 1,775 km2, a depth of up to 29 m (average = 6.25 m), and a volume of approximately 1.11 km3. Water exchange with the ocean occurs via a 16 km wide opening to the north, Pumicestone Passage (0.5 km) in the north-west and two minor openings to the east and south; south passage (3.5 km), and Jumpinpin (1 km). The bay receives terrestrial runoff from four river catchments (Logan, Brisbane, Pine and Caboolture) which have a combined area of 19,255 km2. The region receives a summer-dominated (December–February) rainfall associated with tropical depressions that move on to the catchment from the north. Moreton Bay receives an annual average rainfall of 1,177 mm (Brisbane International Airport station no. 40223; n = 49 years) and a mean evaporation that is greater than the mean rainfall (Epan = 1,961 mm; n = 12 years). Approximately 28 % of the catchment remains undisturbed and the Moreton Bay region is home for approximately two million people (Dennison and Abal 1999; Eyre and McKee 2002).
Site map showing location of water quality sample sites and benthic flux sampling sites. Also included are the locations of other bays in western Moreton Bay used for comparisons in Fig. 8
Study site and experimental design
Deception Bay is located in north western Moreton Bay and bounded to the north by Pumicestone Passage and to the south by Redcliffe Peninsula. The bay receives terrestrial runoff from the Caboolture River, a number of smaller tidal creeks and urban stormwater drains along the southern fringes. Deception Bay currently supports a large prawn fishery during the summer and autumn months of the year. The benthic processes study area consisted of three main sites located 500 m apart in a northeastern transect away from the Caboolture River mouth (Fig. 1). Each main site consisted of two sub-sites located 50 m apart along the northeastern transect (6 sub-sites in total). All sites were in water approximately 4 m deep (mean high tide).
Background environmental data
Water quality data was supplied by the Queensland Environmental Protection Agency as part of the Ecosystem Health Monitoring Program administered by the Moreton Bay Waterways and Catchments Partnership. Water quality was sampled monthly for physicochemical properties, secchi depth and dissolved/particulate organic and inorganic nutrients at the eight sites shown in Fig. 1. Details of sample collection and analysis can be found in (Dennison and Abal 1999). Daily solar radiation (satellite derived MJ m−2), air temperature, rainfall, wind speed and wind direction data from Brisbane Airport and Caboolture Post Office (rainfall only) was supplied by the National Bureau of Meteorology.
Benthic fluxes
Samples were collected six times between April 2003 and May 2004. At each sample time, six replicate sediment cores were collected by divers at each sub-site (36 cores in total) in presoaked 95 mm I.D. × 500 mm long clear Plexiglas tubes, retaining approximately 200 mm sediment and 300 mm (2.5 L) overlying water. Care was taken to retain only cores with an undisturbed sediment surface. Cores were transported at in situ temperature and light to the laboratory within 2 h of collection, where they were placed uncapped into an incubator with 150 L of recirculated and aerated site water at collection temperature. The in situ diel light–dark climate was maintained throughout the incubation, and PAR irradiance at the sediment surface was set at the daily mean measured at the site of collection using a LI-COR 2 pi underwater PAR sensor. Cores were equipped with self-stirrers set at 10 cm above the sediment surface and stirring rates set to just below resuspension. A 24 h equilibration period was allowed (Eyre et al. 2002), and flux incubations were started at approximately 2 h after sunset the following evening. The flux incubation schedule consisted of a 9 h dark incubations, followed by flushing with site water for 3 h, followed by a 9 h light incubation. Cores were capped and dissolved oxygen (O2) concentrations (±0.01 mg L−1) and pH (±0.001) were measured electro-chemically and samples were taken for the analysis of N2, Ar, NH4 +, NO x , dissolved organic nitrogen (DON), dissolved inorganic phosphorus (DIP), DOP (dissolved organic phosphorus) every 3 h. An equal volume of site water replaced withdrawn sample water, so that no head space was introduced into the cores. O2 concentration typically only decreased about 20 % during the dark incubation. Samples for N2:Ar were allowed to flow into gas-tight glass vials and preserved with HgCl2 and stored submerged at 2 °C below ambient temperature until analysis. Samples for nutrient analysis were filtered immediately through 0.45 μm cellulose acetate filters and stored at −20 °C until analysis. Following the benthic flux incubation, cores were uncapped and flushed with site water for 6 h before labelled 15NO3 − was added to a concentration of 100 μmol. An equilibration time of 3 h was observed before cores were capped and the isotope pairing (IP) incubation was commenced. Cores for analysis of 15NO3 −:14NO3 − were sacrificed every 3 h. Full details of the IP methods are given in Ferguson and Eyre (2007).
Sediment properties and macrofauna
Sediment cores were processed for sediment properties (chlorophyll-a, pheophytin, total organic carbon, and nitrogen) immediately upon completion of benthic flux incubations. Cores were carefully drained under subdued light ensuring no disturbance of surface sediments, and the top 3 mm of sediment was removed by scalpel. A 1 cm3 sub-sample was placed immediately into 9 mL of 93 % acetone (giving a final extraction concentration of 90 % acetone assuming mean sediment porosity = 0.3) for chlorophyll analysis and the remainder placed in a polyethylene vial for total organic C and N analysis. All samples were stored in the dark at −20 °C until analysis. Chlorophyll analysis was carried out within 1 week of collection. The remainder of the sediment core was transferred to a calico bag and stored in 70 % ethanol until sieving through a 1 mm mesh size sieve for macrofauna.
Analytical methods
All nutrient analyses were carried out colourmetrically using Lachat™ Flow Injection Analysis. Analytical errors (in brackets) were determined as the average %CV of the triplicates. Because the variance of the analytical procedures propagates additively, the variance associated with the nutrient forms calculated by difference was estimated as the sum of the variances of the two measured nutrient forms used in the calculation (Eyre 1995). Analytical accuracy for nutrient analysis was maintained using standard additions of certified laboratory standards in both Milli-Q and low nutrient seawater. Nitrite (NO2 −) was determined using sulphanilamide (2.8 %), oxidised nitrogen (NO x ) was determined by cadmium reduction (3.6 %) and nitrate (NO3 −) was determined as the difference between NO x and NO2 − (6.2 %). NH4 + was determined using hypochlorite/phenolate (5.1 %) and dissolved inorganic nitrogen (DIN) was determined as the sum of NO x and NH4 + (11.3 %). Total dissolved nitrogen (TDN) was determined by persulfate digestion and sulphanilamide (4.1 %) (Valderrama 1981) and DON determined as TDN minus DIN (15.4 %). Dinitrogen gas (N2) was determined from N2:Ar ratios measured using membrane inlet mass spectrometry (MIMS) with O2 removal (0.01 %) (Eyre et al. 2002). 15NO3:14NO3 were also determined using MIMS with O2 removal (Eyre et al. 2002). Chlorophyll was measured using 90 % acetone extraction, and chlorophyll-a and pheophytin were estimated using equations given in (Jeffrey and Welschmeyer 1997). Total sediment organic carbon and nitrogen were determined by a LECO CNS analyser. Carbonates were removed using 2 M HCl prior to analysis. Macrofauna were identified to species level.
Flux calculations
Fluxes across the sediment–water interface were calculated by linear regression of the concentration data, corrected for the addition of replacement water and changes in the blank, as a function of incubation time, core water volume and surface area. Dark flux rates were calculated using concentration data from the first 9 h of the incubation and light flux rates were calculated using concentration data from the second 9 h of the incubation. Positive fluxes denote efflux from the sediment to the water, and negative fluxes denote uptake by sediments from the water. Net flux rates are calculated from the dark flux*dark hours plus the light flux*light hours divided by 24.
Statistical analysis
Spatial and temporal variability in sediment properties, macrofauna and benthic metabolism was investigated using ANOVA (SPSS v11), including independent variables of sample event, light treatment, sub-site and site. Interactions between factors (e.g. sample time × site) identified by the ANOVA were investigated using t tests specific to an interaction.
Results
Environmental conditions
A summary of environmental forcings and bay water quality averaged over the 2 week period preceding each sample time is given in Table 1. Freshwater inflows to the bay were greatest during autumn 2003 followed by autumn 2004 (sample times 1 and 5 respectively), with the difference between these years reflecting the inter-annual variation in rainfall patterns characteristic of the sub-tropical east Australian coast (Murphy and Timbal 2008). Wind speeds tended to be greatest during summer, while wind direction was predominantly from the southwest during winter shifting to the southeast during summer (Table 1; Fig. 1). Water column chlorophyll-a was greatest during summer 2004 (sample time 4), with lowest values recorded during late autumn 2004 and winter 2003 (sample times 2 and 5 respectively). Organic carbon supply to the bay is dominated by phytoplankton production (Ferguson and Eyre 2010), hence sediment TOC tracked the seasonal cycle of water column chlorophyll-a (r 2 = 0.66). In contrast, water clarity (indicated by secchi depth) was poorest during summer and best during winter. The seasonal trend in water clarity was closely related to wind speed and direction (wind driven resuspension) and water column chlorophyll-a (contributions of phytoplankton to attenuation). Benthic chlorophyll-a closely followed the seasonal trend in water clarity (r 2 = 0.86). The benthic light climate varied between 1 and 13 % of surface irradiance.
Benthic metabolism
O2 uptake during the dark displayed significant variation (p < 0.001) between the smallest uptake occurring in late autumn 2004 (sample time 6) and largest uptake occurring during late spring 2003 (sample time 3; Fig. 2a). There was a significant reduction in O2 uptake during the light (p < 0.001). Net O2 fluxes were always uptake throughout the study (Table 2). Gross benthic O2 productivity (GBP) varied significantly among sample times (p < 0.001) ranging from a minimum during autumn 2004 (sample time 5) to a maximum during late spring 2003 (sample time 3; Table 2). GPB was positively related to benthic respiration throughout the study (r 2 = 0.52; p < 0.001). Estimations of benthic p/r indicated net heterotrophic metabolism at all sites during the study, with mean values ranging between 0.01 during autumn 2004 (sample time 5) and 0.27 during sample time 6 (Table 2).
a Benthic O2 fluxes, b–d dissolved nitrogen fluxes, e N2 fluxes, f isotope pairing estimates of denitrification, g percentage of direct denitrification, and h DIP fluxes. The fluxes are shown for the light incubations (white boxes) and dark incubations (grey boxes) for each sample time (6 sample sites pooled; n = 36). Isotope pairing estimates are shown for each sample time (6 sample sites pooled; n = 6). The upper and lower limits of the boxes indicate the 75th and 25th percentiles respectively, the line within the box indicates the median, whiskers indicate 90th and 10th percentiles, and circles indicate 95th and 5th percentiles
Benthic nutrient fluxes
NH4 + fluxes varied significantly between sample times (p < 0.001), with highest fluxes occurring in late spring 2003 and summer 2004 (sample times 3 and 4) and lowest fluxes during winter 2003 (sample time 2; Fig. 2b). There was a significant increase in NH4 + fluxes during the light (p < 0.023). Nitrate (NO3 −) fluxes varied significantly between all sample times (p < 0.001), with largest fluxes occurring in summer 2004 (sample time 4) and smallest fluxes during winter 2003 (sample time 2; Fig. 2c). There was a significant increase in NO3 − fluxes during the light (p < 0.001). DON fluxes ranged from uptake during autumn 2003 (sample time 1) and release during spring 2003 (sample time 3). DON fluxes were more variable than inorganic fluxes throughout the study, especially during the first three sample times when there was significant spatial and light/dark variation (Fig. 2d).
N2 fluxes displayed no significant seasonal trends (Fig. 2e). Denitrification rates based on IP measurements ranged between 6.5 ± 2 μmol m−2 h−1 during winter 2003 (sample time 2) and 32 ± 5 μmol m−2 h−1 during summer 2004 (sample time 4). Coupled nitrification–denitrification (D n ) dominated throughout the study (annual mean 96 %), and even during periods of peak OM supply (i.e. summer 2004) D n remained at >90 % of total denitrification.
DIP fluxes increased significantly from winter 2003 (sample time 2) to summer 2004 (sample time 4; p < 0.001). The ratio of DIN fluxes to DIP fluxes (DIN:DIP) ranged from −5 during winter 2003 (sample time 2) to over 40 during summer 2004 (sample time 4). There was a significant increase in DIN:DIP flux ratios during the light (p > 0.015).
Macrofauna
Total macrofauna biomass displayed no clear seasonal trends (Fig. 3a). Biomass throughout the study was dominated by bristle crabs (Pilumnidae; 63 %) followed by bivalves (26 %), polychaetes (6 %), and brittle stars (Ophiactidae; 5 %; Fig. 3b). Contributions of smaller predator organisms (Sipunculida, Nematoda, and Nermatina) and protozoa (Foraminiferida) increased during sample time 3, however their overall contributions were more than two orders of magnitude smaller than bristle crabs. Polychaete biomass comprised variable contributions from surface deposit feeders (Trichobranchidae and Capitellidae) and burrowing scavengers (Nereididae: Table 3), with much smaller contributions from various predator species. There was a dramatic drop in total biomass during autumn 2004 driven by a reduction in bristle crab abundance. Species richness was closely related to overall organism density which was highest during winter and late spring 2003, and was driven by a higher diversity of polychaetes, amphipods, and bivalves, combined with higher densities of brittle stars, smaller predators and protozoa (Fig. 3c, d). The relationships between total biomass, species richness and density and benthic community respiration are presented in Fig. 4. Total macrofauna biomass was poorly related to respiration, tending to be highest at intermediate respiration rates and decreased at higher respiration rates (Fig. 4a). Both species richness and density tended to increase with respiration (Figs. 4b, c), reflecting an increase in the abundance of surface deposit feeders (Table 3).
Relationships between N fluxes and benthic metabolism
The relative importance of different net N fluxes during each sample time is shown in Table 4. Potential N mineralisation was calculated from net O2 flux assuming the breakdown of OM with Redfield C:N (6.68). This was considered appropriate since phytoplankton is estimated to dominate OM supply to sediments in Deception Bay (Ferguson and Eyre 2010), however it is possible that benthic microalgae detritus C:N ~ 9, (Sundbäck et al. 2000) may have also influenced the labile OM pool. The N accounted for by measured processes (Naccounted = DIN + N2 + BMA assimilation) represented between 64 and 101 % of the total N mineralisation based on Redfield material. N2 was consistently the dominant N flux (35–70 % of Naccounted), with NH4 + and NO3 − fluxes contributing considerably less (−8 to 22 and 7–24 % of Naccounted respectively). BMA assimilation accounted for between 1 and 30 % of Naccounted. Denitrification efficiency (DE) [DE = N2 flux/(N2 flux + DIN flux)] varied between 44 % (sample time 3) and 105 % (sample time 2). There was a significant drop in DE during the summer (sample times 3 and 4) relative to the autumn and winter (sample times 1, 2, 5 and 6).
Inorganic nitrogen fluxes are plotted against dark O2 consumption as an indication of OM mineralisation in Fig. 5. All N fluxes increased with OM mineralisation, although there was considerable variation in N fluxes at intermediate OM mineralisation rates (500–1,000 μmol O2 m−2 h−1). The upper limits of NH4 + fluxes increased with OM mineralisation, with a marked increase in NH4 + flux rate above 1,000 μmol O2 m−2 h−1 (Fig. 5a). The lower limits of NH4 + fluxes (uptake) tended to be greatest at intermediate OM mineralisation rates. The upper limits of NO3 − fluxes increased with OM mineralisation, however there was a marked decline in the upper limits of NO3 − fluxes above approximately 1,000 μmol O2 m−2 h−1 (Fig. 5b). The upper limits of N2 fluxes peaked at intermediate OM mineralisation rates (500–1,000 μmol O2 m−2 h−1) and declined markedly with increasing respiration above approximately 1,000 μmol O2 m−2 h−1 (Fig. 5c). In contrast, denitrification rates estimated using IP showed no clear reduction above 1,000 μmol O2 m−2 h−1. Estimated direct denitrification (D w = direct denitrification of NO3 − from the overlying water column) displayed similar trends to N2 fluxes. The difference between denitrification estimates (N2 flux–IP rate) was approximately 10 μmol N2 m−2 h−1 at the minimum respiration rate, with upper limits (>35 μmol N2 m−2 h−1) peaking at 800 μmol O2 m−2 h−1 and declining markedly to zero at maximum respiration.
Relationships between macrofauna and N fluxes
Total macrofauna biomass was positively related to increased NH4 + flux during the light relative to the dark (Fig. 6a). There was a weak negative relationship between total macrofauna biomass and DE. However, stronger trends occurred between polychaetes biomass and DE, with the direction of the influence family specific: Capitellid and Trichobranchid polychaetes were associated with a reduction in DE, while larger Nereidid polychaetes (predominant during low OM supply in autumn and winter) were associated with a marked increase in DE (Fig. 6b, c).
a The difference between light and dark NH4 + fluxes plotted as a function of total macrofauna biomass (symbols are sample time means ± SD; n = 36). b Denitrification efficiency plotted as a function of Capitellid and Trichobranchid polychaete biomass expressed as a percentage of total polychaetes biomass (symbols are sample time means ± SD; n = 36). c Denitrification efficiency plotted as a function of Nereidid polychaete biomass expressed as a percentage of total polychaetes biomass
Fates of remineralised N
There was considerable variation in the relative contribution of each fate across the range of total N remineralisation (Naccounted; Fig. 7), however when seasonal variation in climatic factors (e.g. light climate and OM supply) and interaction between the primary fates are considered together this variation is more easily interpreted. The co-variation between benthic respiration and GBP resulted in positive relationships between BMA assimilation and benthic respiration, however the difference in light climate between summer (low) and winter (high) resulted in distinctly different seasonal groupings (Fig. 7a). The percentage of DIN release broadly increases with benthic respiration (Fig. 7b) due to factors such as increased OM mineralisation and decreased denitrification (Fig. 7c). However within individual seasonal groupings there was a negative slope between the DIN release fraction and respiration due to the secondary influence of BMA assimilation (Fig. 7d). BMA assimilation was also negatively related to the N2 fraction, indicating likely competition between BMA and denitrifiers for NO3 − (Fig. 7e). However the % N2 flux was considerably higher relative to BMA assimilation during winter compared with summer (Fig. 7e). Hence, despite the negative influence of BMA assimilation over denitrification, DE was actually highest during winter when BMA assimilation also peaked (Fig. 7f). This can be explained by the stimulation of DE by Nereidid polychaetes which tended to dominate during winter (Fig. 6c).
a BMA assimilation, b DIN flux, and c N2 flux expressed as a percentage of Naccounted and plotted against dark O2 consumption. Also shown are decreases in the percentage contributions of d DIN flux, and e N2 flux as a function of BMA assimilation. f While BMA limited both DIN and N2 flux, there was a broad increase in denitrification efficiency with BMA assimilation, indicating that denitrification was maintained at higher rates of BMA assimilation while DIN release was reduced to zero
Discussion
Net rates of NH4 + regeneration during this study were within the seasonal range reported for other shallow embayments of western Moreton Bay (Dennison and Abal 1999; Ferguson et al. 2007), and within the expected range (relative to net benthic O2 flux) for sub-tropical systems in the general region (Fig. 8). The upper limits of O2:NH4 + flux ratio (Fig. 8a) displays a marked increase at net O2 respiration rates greater than 800 μmol m−2 h−1. The consistency of this trend across the sub-tropical systems suggests an OM loading threshold beyond which fundamental changes in benthic O2 and N cycling occur. In the current study, this threshold coincided with decreases in burrowing macrofauna and a reduction in the relative contributions of NO3 − flux. Hence, the increase in O2:NH4 + flux ratio may have resulted from a limitation of nitrification, due to reduced O2 supply and/or sulfide inhibition, or alternatively due to increases in the relative importance of dissimilatory nitrate reduction to ammonium which is expected to be favoured over nitrification as respiration increases (Christensen et al. 1990). The implications of this for denitrification efficiency are discussed below.
a The relationship between net metabolism (O2) and NH4 + flux in various sub-tropical and temperate systems. b The relationship between a proxy for light climate (secchi depth/total depth) and net NH4 + flux in various sub-tropical and temperate systems (means). c The relationship between net metabolism and N2 flux in Deception Bay and other parts of western Moreton Bay (see Fig. 1 for locations)
Influences of benthic productivity and macrofauna over DIN flux
Our estimations indicate that BMA assimilation can account for a significant portion of remineralised N, especially during winter when light climate improves and BMA standing crop is greatest. However, light climate at our study site was consistently near the compensation depth for BMA (Fear et al. 2004), and there was no discernable reduction (or reversal) in DIN efflux during the light as might be expected where BMA productivity occurs (Tyler et al. 2003). Using secchi depth/total depth as a proxy scale for potential light limitation, observations from sub-tropical systems show that despite high variation in OM mineralisation rate, no efflux of NH4 + occurs from sediments where secchi depth exceeds total water depth (Fig. 8b). Hence, while benthic productivity may still proceed in low light situations, its influence over DIN uptake may become light-limited (Sundbäck et al. 2004). According to this scale, Deception Bay sediments during the study were moderately light limited (Fig. 8b), which may help explain the lack of measured DIN uptake by sediments from the water column. However, while sediments never became a net sink of DIN from the water column, BMA assimilation formed an important control over the release of DIN to the water column and constituted an important seasonal control over pelagic-benthic coupling in this system (Ferguson and Eyre 2010).
The lack of any discernable influence of BMA over light DIN flux and the observed increase in NH4 + efflux during the light in this study may have been in part due to a masking effect caused by increased macrofaunal activity (and hence increased OM mineralisation and excretion) during the light (Fig. 6a). Increased rates of infauna feeding have been shown to peak during the day in association with BMA production (Buffan-Dubau and Caarman 2000), while increases in NH4 + efflux associated with bivalves may be largely due to excretion (Yamada and Kayama 1987). Also consistent with excretion is the significant increase in DIN:DIP flux ratio observed during the light (Karlson et al. 2007). However, despite some apparent influences of macrofauna over diurnal variation in fluxes during this study, there was no clear associations between macrofauna and net fluxes as observed in many studies (Henriksen et al. 1983; Kristensen et al. 1991; Clavero et al. 1992). Our results suggest more subtle, indirect impacts due to macrofauna associated with the modification of sediment properties during the seasonal transition between maximum and minimum OM supply. This is discussed in relation to denitrification below.
Controls over denitrification
Denitrification was the dominant fate for remineralised N during this study (annual mean = 54 %; Table 4) and accounted for between 6 and 20 % of N released during OM mineralisation (refer to potential N mineralisation in Table 4), which is similar to bare sediments (i.e. no macrophytes) in southern Moreton Bay (6–26 %; Eyre et al. 2011) and within the range reported for coastal environments (Seitzinger 1988; Middelburg et al. 1996). The dominance of D n at our study site is consistent with trends observed in nearby parts of western and southern Moreton Bay (Ferguson et al. 2007; Eyre et al. 2011) and oligotrophic systems with low NO3 − concentrations in the water column (Sundbäck and Miles 2000), but in contrast to systems where direct denitrification of water column nitrate (D w) is at least seasonally important (Christensen et al. 1990; Risgaard-Petersen et al. 1994; Rysgaard et al. 1995; Dong et al. 2000; Eyre and Ferguson 2005).
Similar to other N fluxes, denitrification rates based on IP measurements and net N2 fluxes both increased with OM mineralisation (Fig. 6c). This is consistent with the model results of Middelburg et al. (1996), who suggested that OM supply is the primary controlling factor over denitrification in shelf sediments. They proposed that as the percentage of aerobic respiration increased with diminishing OM supply, the distance between zones of nitrification and denitrification increased, resulting in a decrease in the amount of labile OM that escaped aerobic mineralisation and reached the denitrification zone. In contrast to this model, the N2 proportion of Naccounted in our study is greatest at low respiration rates and decreases with increasing respiration (Fig. 7c). Further, our results show that the positive relationship between OM mineralisation and denitrification is better described by a form of envelope function, with the upper limits of the denitrification envelope peaking at respiration rates of approximately 1,000 μmol m−2 h−1, and declining (net N2 fluxes) or plateauing (IP) above this rate (Fig. 5c, d). The drop in denitrification at higher respiration rates is consistent with overall trends in N2 fluxes measured using N2:Ar observed in nearby sub-tropical rivers and estuaries (Cook et al. 2004; Eyre and Ferguson 2005), with Cook et al. (2004) suggesting that the decline in total denitrification above respiration rates of 1,000 μmol m−2 h−1 is due to limitation of the D n pathway. However, our data show that D w actually declined while NO3 − effluxes were still relatively high above this threshold (Fig. 7b), suggesting that other controls may also be responsible for the decline in denitrification at higher respiration rates in Deception Bay.
We propose that the additional variation in DE across the OM mineralisation range is due to increases in the volume of potential denitrification sites within the sediment profile imparted by interactions between BMA and bioturbation. IP rates were consistently lower than measured N2 flux throughout the study (Fig. 3), suggesting that the IP measurement underestimates actual denitrification rates at this site. This underestimation by IP may be due to incomplete mixing of the added 15NO3 − substrate in sediments where bioturbation by polychaetes and BMA production caused a complex heterogenous redox structure in surface sediments (Ferguson and Eyre 2007). Denitrification potential is significantly enhanced by an overlap of aerobic and anaerobic respiration zones (caused primarily by the existence of anaerobic micro-niches with the oxic zone, and oxidised burrow structures penetrating into the anaerobic zone (Middelburg et al. 1996; Ferguson and Eyre 2007), which improves both OM and NO3 − supply to denitrifiers. Differences between N2:Ar and IP estimates of denitrification peaked at moderate OM loading (1,000 μmol m−2 h−1) with good light climate and relatively high Nereidid polychaete biomass, reflecting the stimulation of denitrification due to 3D redox complexity imparted by autotrophic production and burrow structures. This effect may explain the higher rates of denitrification per unit BMA assimilation observed during winter sample times (Fig. 7e).
The difference between denitrification estimates (N2:Ar–IP) decreased as respiration increased above 1,000 μmol m−2 h−1, with estimates converging at maximum respiration (1,500 μmol m−2 h−1). At high respiration rates (>1,000 μmol m−2 h−1) burrowing deposit-feeding macrofauna (e.g. Nereidid polychaetes) were absent, with macrofauna dominated by surface deposit feeders (e.g. brittle stars and amphipods). The absence of deeper burrowing macrofauna most likely resulted in a more 2D sediment profile with less effective overlap between nitrification and denitrification zones and reduced areal extent of oxic-anoxic boundaries. Hence, the reduction in N2 fluxes above 1,000 μmol m−2 h−1 can be seen to result from a progression towards minimum complexity in surface sediment redox zonation.
Synthesis
Benthic N cycling during this study was primarily controlled by the balance between autotrophic and heterotrophic processes, however our data suggest that feedbacks from macrofauna imparted significant impacts on denitrification efficiency. High nitrification efficiency and resultant dominance of D n may be a distinctive characteristic of sub-tropical coastal systems under low to moderate OM loadings. Decreases in denitrification efficiency were evident at net O2 fluxes >1,000 μmol m−2 h−1, which coincides with potential decreases in O2 supply and 3D complexity associated with lower densities of deeper burrowing macrofauna. While nitrification was not completely shut down within the respiration range measured in this study, comparison with other parts of western and southern Moreton Bay, and other Australian estuaries, suggest that limitation of D n associated with reduced O2 supply may be the primary control over regional trends in denitrification at higher respiration rates exceeding 1,500 μmol m−2 h−1 [Fig. 7c; (Ferguson et al. 2007; Eyre and Ferguson 2009)].
References
Aller RC (1982) The effects of macrobenthos on chemical properties of marine sediment and overlying water. In: McCall PL, Tevesz JS (eds) Animal sediment relations: the biogenic alteration of sediments. Plenum Press, New York, pp 53–102
Aller RC (1994) Bioturbation and remineralization of sedimentary organic matter: effects of redox oscillation. Chem Geol 114:331–345
An S, Joye SB (2001) Enhancement of coupled denitrification by benthic photosynthesis in shallow subtidal estuarine sediments. Limnol Oceanogr 46:62–74
Anderson IC, McGlathery KJ, Tyler AC (2003) Microbial mediation of ‘reactive’ nitrogen transformations in a temperate lagoon. Mar Ecol Prog Ser 246:73–84
Banta GT, Holmer M, Jensen MH, Kristensen E (1999) Effects of two polychaete worms, Nereis diversicolor and Arenicola marina, on aerobic and anaerobic decomposition in a sandy marine sediment. Aquat Microb Ecol 19:189–204
Buffan-Dubau E, Caarman KR (2000) Diel feeding behaviour of meiofauna and their relationship with microalgal resources. Limnol Oceanogr 45:381–395
Christensen PB, Nielsen LP, Sørensen J, Revsbech NP (1990) Denitrification in nitrate-rich streams: diurnal and seasonal variation related to benthic oxygen metabolism. Limnol Oceanogr 35:640–651
Clavero V, Fernandez JA, Niell FX (1992) Bioturbation by Nereis sp. and its effects on the phosphate flux across the sediment–water interface in the Palmones River estuary. Hydrobiologia 235(236):387–392
Cook PLM, Eyre BD, Leeming R, Butler ECV (2004) Benthic fluxes of nitrogen in the tidal reaches of a turbid, high-nitrate sub-tropical river. Estuar Coast Shelf Sci 59:675–685
Dauwe B, Herman PMJ, Heip CHR (1998) Community structure and bioturbation potential of macrofauna at four North Sea stations with contrasting food supply. Mar Ecol Prog Ser 173:67–83
Dennison WC, Abal EG (1999) Moreton Bay study: a scientific basis for the healthy waterways campaign. South East Queensland Regional water Quality Management Strategy, Brisbane
Dong LF, Thornton DCO, Nedwell DB, Underwood GJC (2000) Denitrification in sediments of the River Colne estuary, England. Mar Ecol Prog Ser 203:109–122
Engelsen A, Hulth S, Pihl L, Sundback K (2008) Benthic trophic status and nutrient fluxes in shallow water sediments. Estuar Coast Shelf Sci 78:783–795
Eyre BD (1995) A first-order nutrient budget for the tropical Moresby estuary and catchment North Queensland, Australia. J Coast Res 11:717–732
Eyre BD, Ferguson AJP (2005) Benthic metabolism and nitrogen cycling in a sub-tropical east Australian estuary (Brunswick): temporal variability and controlling factors. Limnol Oceanogr 50:81–96
Eyre BD, Ferguson AJP (2009) Denitrification efficiency for defining critical loads of carbon in shallow coastal ecosystems. Hydrobiologia 629:137–146
Eyre BD, McKee LJ (2002) Carbon, nitrogen and phosphorus budgets for a shallow sub-tropical coastal embayment (Moreton Bay, Australia). Limnol Oceanogr 47:1043–1055
Eyre BD, Rysgaard S, Dalsgaard T, Christensen PB (2002) Comparison of isotope pairing and N2/Ar methods for measuring sediment denitrification—assumptions, modifications and implications. Estuaries 25:1077–1087
Eyre BD, Ferguson AJP, Webb A, Maher D, Oakes JM (2011) Denitrification, N-fixation and nitrogen and phosphorus fluxes in different benthic habitats and their contribution to the nitrogen and phosphorus budgets of a shallow oligotrophic sub-tropical coastal system (southern Moreton Bay, Australia). Biogeochemistry 102:111–133
Fear J, Gallo T, Hall N, Loftin J, Paerl H (2004) Predicting benthic microalgal oxygen and nutrient flux responses to a nutrient reduction management strategy for the eutrophic Neuse River Estuary, North Carolina, USA. Estuar Coast Shelf Sci 61:491–506
Ferguson AJP, Eyre BD (2007) Seasonal discrepancies in denitrification measured by isotope pairing and N2: Ar techniques. Mar Ecol Prog Ser 350:19–27
Ferguson AJP, Eyre BD (2010) Carbon and nitrogen cycling in a shallow productive sub-tropical coastal embayment (western Moreton Bay, Australia). Ecosystems 13:1127–1144
Ferguson AJP, Eyre BD, Gay JM (2003) Organic matter and benthic metabolism in euphotic sediments along shallow sub-tropical estuaries, northern NSW, Australia. Aquat Microb Ecol 33:137–154
Ferguson AJP, Eyre BD, Gay JM (2004a) Benthic nutrient fluxes in euphotic sediments along shallow sub-tropical estuaries, northern NSW, Australia. Aquat Microb Ecol 37:219–235
Ferguson AJP, Eyre BD, Gay JM (2004b) Nutrient cycling in the sub-tropical Brunswick estuary, Australia. Estuaries 27:1–17
Ferguson AJP, Eyre BD, Gay JM, Emtage N, Brooks L (2007) Benthic metabolism and nitrogen cycling in a sub-tropical coastal embayment: spatial and seasonal variation and controlling factors. Aquat Microb Ecol 48:175–195
Hansen K, Kristensen E (2002) Impact of macrofaunal recolonisation on benthic metabolism and nutrient fluxes in a shallow marine sediment previously overgrown with macroalgal mats. Estuar Coast Shelf Sci 45:613–628
Henriksen K, Rasmussen MB, Jensen A (1983) Effect of bioturbation on microbial nitrogen transformations in the sediment and fluxes of ammonium and nitrate to the overlaying water. Ecol Bull 35:193–205
Herbert, RA (1999) Nitrogen cycling in coastal marine ecosystems. FEMS Microbiol Rev 23:563–590
Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setälä H, Symstad AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Howarth RW, Marino R, Lane J, Cole JJ (1988) Nitrogen fixation in freshwater, estuarine, and marine ecosystems. 1 Rates and importance. Limnol Oceanogr 33:669–687
Jeffrey SW, Welschmeyer NA (1997) Spectrophotometric and fluorometric equations in common use in oceanography. In: Jeffrey SW, Mantoura RFC, Wright SW (eds) Phytoplankton pigments in oceanography: guidelines to modern methods. UNESCO, Paris, pp 597–615
Karlson K, Bonsdorff E, Rosenberg R (2007) The impact of benthic macrofauna for nutrient fluxes from Baltic Sea sediments. Ambio 36:161–167
Kristensen E (1988) Benthic fauna and biogeochemical processes in marine sediments: Microbial activities and fluxes. In: Blackburn TH, Sørensen J (eds) Nitrogen cycling in coastal marine environments, vol 33. Wiley, Chichester, pp 275–299
Kristensen E, Kostka JE (2005) Macorofauna burrows and irrigation: microbiological and biogeochemical interactions. In: Kristensen E, Haese RR, Kostka JE (eds) Interactions between macro- and microorganisms in marine sediments, vol 60. American Geophysical Union, Washington, DC, p 390
Kristensen E, Jensen MH, Aller RC (1991) Direct measurement of dissolved inorganic nitrogen exchange and denitrification in individual polychaete (Nereis virens) burrows. J Mar Res 49:355–377
MacIntyre HL, Geider RJ, Miller DC (1996) Microphytobenthos: the ecological role of the “secret garden” of unvegetated, shallow-water marine habitats. 1. Distribution, abundance and primary production. Estuaries 19:186–201
Marinelli RL, Williams TJ (2003) Evidence for density-dependent effects of infauna on sediment biogeochemistry and benthic-pelagic coupling in nearshore systems. Estuar Coast Shelf Sci 57:179–192
Middelburg JJ, Soetaert K, Herman PMJ, Heip CHR (1996) Denitrification in marine sediments: a model study. Global Biogeochem Cycles 10:661–673
Middelburg JJ, Barranguet C, Boschker HTS, Herman PMJ, Moens T, Heip CHR (2000) The fate of intertidal microphytobenthos carbon: an insitu 13C-labeling study. Limnol Oceanogr 45:1224–1234
Mortimer RJG, Davey JT, Krom MD, Watson PG, Frickers PE, Clifton RJ (1999) The effect of macrofauna on porewater profiles and nutrient fluxes in the intertidal zone of the Humber estuary. Estuar Coast Shelf Sci 48:683–699
Murphy B, Timbal B (2008) A review of recent climate variability and climate change in southeastern Australia. Int J Climatol 28:2872–2880
Paerl HW, Fitzpatrick M, et al (1996) Seasonal nitrogen fixation dynamics in a marine microbial mat: potential roles of cyanobacteria and microheterotrophs. Limnol Oceanogr 41(3):419–427
Pennifold M, Davis J (2001) Macrofauna and nutrient cycling in the Swan River Estuary, Western Australia: experimental results. Hydrol Process 15:2537–2553
Piña-Ochoa E, Álvarez-Cobelas M (2006) Denitrification in aquatic environments: a cross-system analysis. Biogeochemistry 81:111–130
Risgaard-Petersen N (2003) Coupled nitrification–denitrification in autotrophic and heterotrophic estuarine sediments: on the influence of benthic microalgae. Limnol Oceanogr 48:93–105
Risgaard-Petersen N, Rysgaard S, Nielsen LP, Revsbech NP (1994) Diurnal variation of denitrification and nitrification in sediments colonised by benthic microphytes. Limnol Oceanogr 39:573–579
Rysgaard S, Christensen PB, Nielsen LP (1995) Seasonal variation in nitrification and denitrification in estuarine sediment colonised by benthic microalgae and bioturbating infauna. Mar Ecol Prog Ser 126:111–121
Rysgaard S, Christensen PB, Sørensen MV, Funch P, Berg P (2000) Marine meiofauna, carbon and nitrogen mineralisation in sandy and soft sediments of Disko Bay, West Greenland. Aquat Microb Biol 21:59–71
Seitzinger S (1988) Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnol Oceanogr 33:702–724
Seitzinger S, Harrison JA, Böhlke JK, Bouwman AF, Lowrance R, Peterson B, Tobias C, Van Drecht G (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecol Appl 16:2064–2090
Sundbäck K, Miles A (2000) Balance between denitrification and microalgal incorporation of nitrogen in microtidal sediments, NE Kattegat. Aquat Microb Ecol 22:291–300
Sundbäck K, Miles A, Göransson E (2000) Nitrogen fluxes, denitrification and the role of microphytobenthos in microtidal shallow-water sediments: an annual study. Mar Ecol Prog Ser 200:59–76
Sundbäck K, Linares F, Larson F, Wulff A, Engelsen A (2004) Benthic nitrogen fluxes along a depth gradient in a microtidal fjord: the role of denitrification and microphytobenthos. Limnol Oceanogr 49:1095–1107
Thouzeaua G, Gralla J, Claviera J, Chauvauda L, Jeana F, Leynaerta A, Longphuirta S, Amicea E, Amourouxb D (2007) Spatial and temporal variability of benthic biogeochemical fluxes associated with macrophytic and macrofaunal distributions in the Thau lagoon (France). Estuar Coast Shelf Sci 72:432–446
Thrush SF, Hewitt JE, Gibbs MM, Lundquist C, Norkko A (2006) Functional role of large organisms in intertidal communities: community effects and ecosystem function. Ecosystems 9:1029–1040
Tyler AC, McGlathery KJ, Anderson IC (2003) Benthic algae control sediment–water column fluxes of organic and inorganic nitrogen compounds in a temperate lagoon. Limnol Oceanogr 48:2125–2137
Valderrama JC (1981) The simultaneous analysis of TP and TN in natural waters. Mar Chem 10:109–122
Vistisen B, Vismann B (1997) Tolerance to low oxygen and sulfide in Amphiura filformis and Ophiura albida (Echinodermata: Ophiuroidea). Mar Biol 128:241–246
Yamada H, Kayama M (1987) Liberation of nitrogenous compounds from bottom sediments and effect of bioturbation by small bivalve, Theora lata (Hinds). Estuar Coast Shelf Sci 24:539–555
Acknowledgments
This work was supported by an ARC Linkage Grant (LP0212075) awarded to Bradley Eyre, Angus Ferguson, and John Kirkwood and an ARC Discovery Grant (DP0342956) awarded to Bradley Eyre. The ARC Linkage Project was in partnership with the Moreton Bay Waterways and Catchments Partnership, the Queensland Department of Primary Industries and the Queensland Environmental Protection Authority who provided funding and in-kind support for the study. We would like to thank our divers and boat crew Simon Hartley, Danny Bucher and Max Egan, our field assistants Paul Kelly, Damien Maher, Jodie Walker, Jaimie Potts and Geoff Coade; and our analyst Iain Alexander. Without their invaluable support this study would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferguson, A., Eyre, B. Interaction of benthic microalgae and macrofauna in the control of benthic metabolism, nutrient fluxes and denitrification in a shallow sub-tropical coastal embayment (western Moreton Bay, Australia). Biogeochemistry 112, 423–440 (2013). https://doi.org/10.1007/s10533-012-9736-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-012-9736-x