Abstract
Anammox bacteria are widespread in the marine environment, but studies of anammox in marshes and other wetlands are still scarce. In this study, the role of anammox in nitrogen removal from marsh sediments was surveyed in four vegetation types characteristic of New England marshes and in unvegetated tidal creeks. The sites spanned a salinity gradient from 0 to 20 psu. The impact of nitrogen loading on the role of anammox in marsh sediments was studied in a marsh fertilization experiment and in marshes with high nitrogen loading entering through ground water. In all locations, nitrogen removal through anammox was low compared to denitrification, with anammox accounting for less than 3% of the total N2 production. The highest relative importance of anammox was found in the sediments of freshwater-dominated marshes, where anammox approached 3%, whereas anammox was of lesser importance in saline marsh sediments. Increased nitrogen loading, in the form of nitrate from natural or artificial sources, did not impact the relative importance of anammox, which remained low in all the nitrogen enriched locations (<1%).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intensified scientific interest in nitrogen’s role in the marine environment over the last decades has led to the discovery of novel nitrogen-transforming microbial pathways (Brandes et al. 2007; Burgin and Hamilton 2007). Anammox (anaerobic ammonium oxidation) is a microbial pathway carried out by chemolithoautotrophic bacteria, where ammonium is oxidized under anoxic conditions using nitrite as electron acceptor (Mulder et al. 1995; Van de Graaf et al. 1995, 1996). Like denitrification, which converts nitrate into gaseous nitrogen, the end product of the anammox pathway is dinitrogen, which may be lost from the sediment to the atmosphere, and consequently, anammox is a sink for bioavailable nitrogen.
Anammox bacteria are widespread in the marine environment (Schmid et al. 2007) and account for a significant amount of the N2 production in anaerobic water columns (Dalsgaard et al. 2003; Hammersley et al. 2007; Kuypers et al. 2005) and in sediments in deeper water (Engstrom et al. 2005; Thamdrup and Dalsgaard 2002). In sediments of shallow coastal areas, the relative importance (Engstrom et al. 2005; Thamdrup and Dalsgaard 2002) of anammox is usually low compared to denitrification, accounting mainly for less than 10% of the total N2 production (Dalsgaard et al. 2005; Rich et al. 2008; Risgaard-Petersen et al. 2004; Trimmer et al. 2003). The controls on anammox in shallow sediments are not well understood, but both increased organic matter (Trimmer et al. 2003) and increased nitrate availability (Risgaard-Petersen et al. 2004) have been proposed to increase anammox activity.
Studies on anaerobic ammonium oxidation in natural wetlands are few. In mangroves, anammox was found to be of minor importance compared to denitrification, accounting for up to 10% of the total N2 production (Meyer et al. 2005), and in riverine riparian wetlands, the relative importance of anammox was judged to be insignificant (Matheson et al. 2003). However, anammox was shown to coexist with conventional coupled nitrification–denitrification in wetlands constructed for water treatment purposes, where anammox also contributed significantly to the nitrogen removal (Dong and Sun 2007; Paredes et al. 2007). High rates of denitrification have been recognized as an important process by which excess nitrogen in tidal marshes may be reduced (Hamersley 2002; Teal and Howes 2000; Valiela and Cole 2002). The rhizosphere of vegetated wetland sediments stimulate denitrification (Bodelier et al. 1996; Reddy et al. 1989; Sherr and Payne 1978). Internal oxygen transport through the aerenchyma of salt marsh grasses result in oxic micro-zones surrounding the roots and rhizomes stimulating coupled nitrification–denitrification at depth in the sediment. Furthermore, exudation of labile organic compounds from roots and rhizomes serve as electron donor for denitrification. Inorganic nitrogen in salt marsh sediment exists predominantly in the form of ammonium, and nitrate availability is usually low. This results in a tight coupling between nitrification and denitrification in the salt marsh sediment (Dollhopf et al. 2005; Hamersley 2002; Thompson et al. 1995). These environmental conditions could also provide a suitable habitat for anammox bacteria, where nitrite as an electron acceptor for the anammox process may be produced as an intermediate of both nitrification and denitrification. However, the role of anaerobic ammonium oxidation in marsh sediments is still largely unknown.
In this study, the relative importance of anammox as a nitrogen-removing pathway was surveyed in tidal marsh sediments over a salinity gradient, extending over four vegetation types characteristic for New England marshes, ranging from the Spartina alterniflora-dominated salt marsh to the oligohaline Typha angustifolia-dominated marsh. Salinity has been shown to be a significant controlling factor for nitrogen removal efficiency in estuarine sediments due to increased ammonium retention in freshwater sediments resulting in a higher turnover of nitrogen via coupled nitrification–denitrification (Seitzinger et al. 1991). The same mechanism may be advantageous to anammox bacteria as well assuring high ammonium availability. In general, anammox bacteria have been found widespread in marine as well as freshwater environments (Penton et al. 2006; Schmid et al. 2007), and they have a high ability to withstand salinity changes (Boran et al. 2006). Consequently, anammox could contribute to the nitrogen removal in the freshwater as well as in the salt marsh.
Nitrate availability has been suggested as being a significant factor affecting denitrification (Seitzinger 1988) as well as anammox activity (Meyer et al. 2005; Rich et al. 2008; Risgaard-Petersen et al. 2004; Trimmer et al. 2003) in estuarine sediments, both processes increase in activity in response to increased nitrate availability. To evaluate the impact of increased nitrogen loading on the relative importance of anaerobic ammonium oxidation in salt marsh sediments, denitrification and anammox were examined in experimentally fertilized marshes of the Plum Island Estuary and in marshes with a high input of groundwater-borne nitrate in Great Sippewissett marsh, Cape Cod.
Methods and Materials
Study Areas and Sampling Strategy
The Plum Island Sound Estuary is located in northeastern Massachusetts and has one of the largest tidal marsh areas on the northeastern coast of the USA. Plum Island Sound is a 25-km long, macro-tidal estuary with a mean tidal range of 2.9 m. It contains a highly productive, riverine, tidal marsh system with a salinity gradient ranging from 0‰ to 32‰. The marsh vegetation is typical of New England marshes. S. alterniflora and Spartina patens dominates the vegetation in brackish and saline regions, whereas T. angustifolia dominates the vegetation in freshwater areas. Phragmites australis is common and can be found in clusters along the landward edges of the marsh.
The relative importance of anammox as a nitrogen-removing pathway was surveyed in the marshes of the Plum Island Sound Estuary in June 2006. Anammox was investigated in the marsh sediment over a salinity gradient expanding over four vegetations zones: S. alterniflora, S. patens, P. australis, and T. angustifolia (Table 1). The impact of increased nitrogen loading on the role of anammox in tidal marshes was investigated in marsh sediments in a long-term marsh fertilization experiment in the Plum Island Sound Estuary and over a natural nitrogen-loading gradient in Great Sippewissett marsh, Cape Cod, MA.
The marsh fertilization in the Plum Island Sound Estuary was begun in 2004 as part of the Trophic cascades and Interacting control processes in a Detritus-based aquatic Ecosystem (TIDE) Project, fertilizing ∼60,000 m2 of pristine salt marsh. An area of similar size, with similar hydrology, vegetation, and water chemistry as the fertilized marsh, was designated as a reference site. The fertilizer addition mimicked the natural route of exposure for anthropogenic nitrogen to salt marsh environments by adding nitrate and phosphate directly to the flooding water in the tidal creeks on every incoming tide. Each year, the marsh was fertilized throughout the growing season (May–September). The fertilizer was added continuously during rising tide, automatically adjusting the fertilizer addition to the increasing water mass resulting in a constant nitrate and phosphate concentration in the water column throughout the duration of the tide. The nitrogen loading was increased by more than an order of magnitude compared to the reference site aiming for an approximate nutrient concentration in the creek water of 70 μM nitrate and 4 μM phosphate in the fertilized creek (Deegan et al. 2007). In 2006, when this research was carried out, the seasonal average in nitrate concentration was higher than anticipated. In the fertilized tidal creek, the average nitrate concentration was 132 ± 22 μM, whereas the unfertilized reference creek had an average concentration of 7.4 ± 0.7 μM.
Anammox was investigated in the sediment on two locations in the experimentally fertilized tidal creek (Sweeney Creek) and the unfertilized reference creek (West Creek). In July 2006, cores were collected in the permanently inundated bare sediment at the bottom of the tidal creeks and in the vegetated marsh platform at a distance of 15–20 m from the creek banks in an area dominated by S. patens (Table 1). The latter location was only exposed to the added fertilizer during inundation at high tide.
Anammox was also measured in two locations in Great Sippewissett marsh, Cape Cod, MA, over a natural nitrogen-loading gradient. This marsh receives nitrate from residential areas with septic tanks located at the fringe of the marsh. The nitrate is transported directly into the marsh via a ground water flow, which is also the main freshwater input to this marsh. The groundwater enters the marsh through seepage and via springs, in which the nitrate concentrations can exceed 200 μM.
In November 2005, sediment cores were collected on two locations in Great Sippewissett marsh, at variable distance from a nitrate-rich groundwater spring (spring water nitrate concentration 220 μM). Sediment cores were collected at the mouth of the groundwater spring, where the sediment pore water was highly affected by the nearby spring ([NO3 −]pw = 25.6 ± 7.2 μM) and at location 10 m from spring, where the sediment was less exposed to the groundwater-borne nitrate ([NO3 −]pw = 1.5 ± 0.2 μM; Table 1)
Pore water was extracted from surface sediment (3–4 cm) and nitrate concentrations were measured using standard spectrophotometric methods (Crompton 2005).
Sampling Technique
At all locations, replicate sediment cores (n = 4 in Plum Island Estuary, n = 3 in Great Sippewissett marsh) were collected using acrylic cylinders (7.5 cm ID). Sediment cores were brought to the laboratory within 24 h of collection and processed.
Sediment Slurry Incubations
Measurement of denitrification and anammox followed procedures for sediment slurry incubations as described by Thamdrup and Dalsgaard (2002) with some modifications. In the laboratory, the sediment cores were sliced, and the upper 3–4 cm of each sediment core was selected for analysis in order to get a representative sample of the surface sediment, which may be very uneven in vegetated tidal marshes. At some locations, the sediment cores were not waterlogged, and the top sediment was oxidized. In these cases, the sediment cores were sliced (size, 3–4 cm) at a depth corresponding with the water level.
The selected core sections were immediately placed in a glove bag in an oxygen-free, 100% N2 atmosphere. In the glove bag, sediment was homogenized, and larger pieces of root material were removed. Approximately 14 subsamples of 2–3 g (wet weight) were weighted and placed in 25 ml glass culture tubes. One hundred percent N2-saturated artificial seawater (oxygen-free), with a salinity corresponding to the pore water salinity, was added filling the culture tubes, and the tubes were sealed with silicon/polytetrafluoroethylene septa in open-top phenolic screw caps. The samples were pre-incubated overnight to allow for consumption of any ambient nitrate and to insure completely anoxic conditions in the tubes; all incubations occurred at temperatures similar to the temperature in the surface sediment on the sampling day. All slurries from Plum Island Estuary were incubated between 18°C and 22°C. Slurries from Great Sippewissett, which were collected later in the year, were incubated at approximately 10°C. The following day, 15N-nitrogen tracers were added through the septum using a syringe. For each location studied, the sediment from each replicate core was split into two batches, each making up five to seven sediment slurries; one batch was added labeled 15N–NH4 + and unlabeled 14N–NO3 − for anammox measurements, whereas the other batch was added labeled 15N–NO3 − only for denitrification measurements. Ammonium and nitrate was added to a concentration of 300 μM. The purity of the labeled ammonium and nitrate added to the sediment slurries were, as stated by the manufacturer, isotopic ratios of 98% and 99%, respectively, and the r 29 and r 30 were corrected for the unlabeled impurities accordingly. 30N2 (denitrification) and 29N2 (anammox) production was quantified measuring the concentration of [29N2] and [30N2] in the sediment slurries over time, using membrane inlet mass spectrometry (Kana et al. 1994). One sediment slurry from each batch was sacrificed at every time point. N2 concentrations were measured directly in the sediment slurry immediately as each culture tube holding the slurry was opened. In this way, preservation of samples using biocides such as mercury were avoided as the gaseous nitrogen production was measured in real time, at intervals of 8–24 h, for a total of up to 120 h.
Measuring N2 production from anaerobic ammonium oxidation using only one treatment adding 15N–NH4 + and 14N–NO3 − does not distinguish anaerobic ammonium oxidation via the anammox pathway, which uses nitrite as electron acceptor, from other thermodynamically feasible ammonium-oxidation pathways using other electron acceptors. However, since Planctomyces bacteria carrying out anammox are found widespread in marine as well as freshwater environments (Penton et al. 2006; Schmid et al. 2007), it is highly likely that anammox is the anaerobic ammonium-oxidation pathway observed in these sediments. Hence, the N2 production from anaerobic ammonium oxidation observed in these studied will be referred to as anammox.
Denitrification and anammox were measured under elevated ammonium and nitrate conditions, ensuring unlimited nitrogen availability for both denitrifiers and anammox bacteria. This approach is based on the assumption that the relative importance of the two processes is unaffected by the elevated concentrations of inorganic nitrogen in the slurries. In this case, the relative importance measured in the laboratory would also reflect the relative importance of the two processes in situ. This assumption was tested by Thamdrup and Dalsgaard (2002), who found the relative importance of the two processes in slurry incubations to be independent of ammonium as well as nitrate concentrations.
Rates of anammox and denitrification were measured as the production of 29N2 and 30N2 in excess of the concentration present at time zero, normalized for the weight of the sediment samples in the culture tubes (Trimmer et al. 2003). The rates were calculated using a linear regression and are referred to as r 29 (nmol 29N2 (g ww sed)−1 h−1) and r 30 (nmol 30N2 (g ww sed)−1 h−1). However, not all regressions from these time series were statistically significant. Generally, average rates of anammox and denitrification from each location were calculated only based on the time series that showed statistically significant regressions (p < 0.05; Table 1). An exception to this rule was the denitrification rates measured in the S. patens zone. In this case, some of the highest 30N2 concentration in this study was found in some of these samples, but the time series was too scattered to calculate significant rates for denitrification, at the p < 0.05 level. Because the high concentrations of 30N2 observed here could only originate from denitrification, rates were estimated based on the best possible fit of the data available and included in the study. Anammox rates were nondetectable at this location.
Anammox and denitrification rates were corrected for the unlabeled 14N impurities in the 15N spike material. Denitrification rates were measured as 30N2 production in sediment samples added 15N–NO3 − only. The fraction of 15N in the nitrate added was 0.990 (f N(E) = 0.990; “N(E)” refers to enriched nitrate). Total denitrification (D total) was calculated according to Eq. 1:
Anammox rates were measured as 29N2 production (r 29) in sediment samples added 15N–NH4 + with a 15N fraction of 0.98 (f A = 0.98; “A” refers to ammonium) and 14N–NO3 − with a 15N fraction of 0.0036 (f N(N) = 0.0036; “N(N)” refers to natural abundance nitrate). In the anammox measurements, denitrification of the added nitrate produced a small amount of 29N2 from the small amount of 15N–NO3 − naturally present in the nitrate. The contribution of 29N2 production from denitrification was calculated based on the denitrification rate from Eq. 1 and subtracted from the 29N2 production measured in the samples for anammox analyses. Subsequently, the remaining 29N2 production, originating from anaerobic ammonium oxidation, was corrected for the 14N impurities in the added 15N–NH4 + and the 15N impurities in the 14N–NO3 − added, yielding the rates of anaerobic ammonium oxidation. Eq. 2:
These calculations are modified after Thamdrup and Dalsgaard (2002).
In the anammox measurements, the slurries may contain a small amount of unlabeled ammonium from the sediment pore water, and unlabeled ammonium may be produced through ammonification and dissimilatory nitrate reduction to ammonium during incubation. Both processes have been shown to be important in marsh sediments (Hopkinson and Giblin 2008). However, since labeled ammonium was added in concentrations as high as 300 μM for unlabeled ammonium were considered negligible.
Results and Discussion
Anammox Vegetation Survey
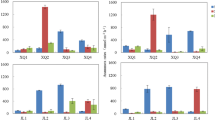
The importance of the anammox process was studied across a salinity transect expanding over four vegetation zones. On the day of sampling, pore water salinity varied from 0 to 15 psu (Table 1). Anammox was detected in most vegetation types, except for S. patens. The rates were low, ranging from 0 to 0.2 nmol N (g ww sed)−1 h−1. Denitrification rates were significantly higher than the anammox rates ranging from 9 to 31 nmol N (g ww sed)−1 h−1 over the entire marsh. Consequently, N2 production from anammox was trivial compared to the overall N2 production (Fig. 1). Over the salinity gradient, the relative importance of anammox increased from contributing less than 1% of the total N2 production in the S. alterniflora-dominated, high-salinity zone to approximately 3% in the freshwater-dominated, T. angustifolia zone (Fig. 2). This increase in anammox’s relative importance was primarily driven by denitrification decreasing with decreasing salinity. However, the anammox rates also increased slightly with decreasing salinity (Fig. 1).
Rates of anammox and denitrification in four vegetation zones: S. alterniflora, S. patens *, P. australis, and T. angustifolia (mean ± SE). Asterisk Rates representing denitrification in the S. patens zone in the Plum Island Estuary marshes were not statistically significant, but high dinitrogen production was detected in several samples indicating high denitrification activity. N.D., not detectable
The relative importance of anammox, expressed as the percentage of the total N2 production accounted for by anammox in four vegetation zones: S. alterniflora, S. patens *, P. australis, and T. angustifolia. Asterisk Rates representing denitrification in the S. patens zone in the Plum Island Estuary marshes were not statistically significant, but high dinitrogen production was detected in several samples indicating high denitrification activity. N.D., not detectable
All the anammox rates were low compared to those observed in most other coastal sediments (Dalsgaard et al. 2005). Denitrification was the major pathway for conversion of bioavailable inorganic nitrogen to gaseous nitrogen in natural marshes, and anammox only played a minor role in the nitrogen removal capacity.
These findings are consistent with anammox being of minor importance in shallow coastal sediment (<10 m) and in wetland sediments (Dalsgaard et al. 2005; Matheson et al. 2003; Meyer et al. 2005; Rich et al. 2008; Risgaard-Petersen et al. 2004; Trimmer et al. 2003). Even though anammox bacteria are widespread in many marine environments (Penton et al. 2006), the heterotrophic nature of most shallow coastal marine sediment are favorable to denitrifiers. Denitrifying bacteria are also widespread in the marine environment and belong to a very diverse group of heterotrophic marine organisms, including representative from Bacteria, Archaea, and even Eukarya (Foraminifera) (Francis et al. 2007), whereas anammox bacteria are found only among Planctomyces (Strous et al. 1999). The growth rate of the denitrifiers is significantly higher than the Planctomyces bacteria responsible for anammox, which are slow-growing chemolithoautotrophic organisms (Strous et al. 1999; van de Vossenberg et al. 2008), and consequently, denitrification is the major N2-producing process in most shallow organic-rich coastal sediments.
The Impact of Increased Nitrogen Loading on the Relative Importance of Anammox in Salt Marshes
In the Plum Island Estuary marsh fertilization experiment, anammox was detectable in both the tidal creek and marsh platform sediments, in fertilized marsh, as well as in the unfertilized reference marsh. However, at all sites, nitrogen removal through anammox was insignificant compared to denitrification and accounted for less than 1% of the total N2 production (Figs. 3 and 4). Anammox activity was also low in sediments near a nitrate-rich groundwater spring entering Great Sippewissett Marsh. No significant differences (t tests, p > 0.05) could be observed for the anammox rates, or the relative importance of anammox, between the fertilized and reference marsh in the Plum Island Estuary or between locations with variable distance from the nitrate-rich ground water spring in Great Sippewissett marsh.
In the Plum Island Sound Estuary, denitrification rates in the marsh platform sediment were not affected by fertilization (t test, p = 0.82). In the tidal creek sediment, however, denitrification was 47% higher in the fertilized creek compared to the reference creek (t test, p = 0.013), showing that the denitrification capacity in the creek sediment was significantly increased by increased nitrogen loading. The different response observed for the platform and creek sediment is ascribed to a difference in exposure to the added fertilizer. The bottom of the tidal creek is permanently inundated, experiencing full exposure to the added fertilizer, whereas the platform only is flooded during high tide, 12% of the day on average. In general, these studies showed that increasing nitrogen loading entering the surface of the vegetated marsh, or the tidal creek sediment, did not affect the absolute rates of anammox or change the overall contribution from anammox to the total N2 production, which was low at all times, but may affect denitrification in the tidal creek sediment. In Great Sippewissett marsh, denitrification was not affected by the difference in nitrogen loading from the groundwater spring. In these marsh sites, denitrification appears to be more sensitive to increased nitrogen loading than anammox.
Salinity as a Controlling Factor of N2-Producing Pathways
The highest anammox rates were found in the freshwater-dominated T. angustifolia and P. australis vegetation zones. Investigations of the anammox rates from all locations with low nitrogen levels (including unfertilized locations in Plum Island and the location in Great Sippewissett marsh, furthest away from the spring) found no significant correlation with salinity. However, the relative importance of anammox (the percentage of the total N2 production accounted for by anammox) decreased linearly as a function of salinity (p = 0.03; Fig. 5). This decrease was almost exclusively driven by denitrification rates being lower in freshwater-dominated areas.
These findings are consistent with studies of estuarine sediments with a salinity gradient, where anammox was also found to have the highest relative importance in the freshwater-dominated areas, even though the overall importance was low (Rich et al. 2008; Trimmer et al. 2003). Since both anammox bacteria and denitrifying bacteria tolerate changes in salinity (Boran et al. 2006; Magalhaes et al. 2005), the observed correlation between the importance of anammox and salinity is most likely to be linked to other environmental factors controlled by salinity, such as ammonium retention and vegetation zones.
Conclusion
Anammox activity was low at all locations studied in tidal marsh sediments and accounted for less than 3% of the total N2 production at all times. Over a natural marsh salinity gradient from the S. alterniflora-dominated salt marsh to the T. angustifolia-dominated oligohaline marsh, anammox had the highest relative importance (∼3%) in the freshwater marsh, where denitrification rates were lower and anammox rates a little higher than in the salt marshes. These findings are consistent with anammox being of minor importance in shallow coastal sediment, which has been the outcome of most near-shore anammox studies. The relative importance of anammox in marshes was unaffected by increased nitrogen loading but decreased linearly with salinity.
References
Bodelier, P.L.E., J.A. Libochant, C. Blom, and H.J. Laanbroek. 1996. Dynamics of nitrification and denitrification in root-oxygenated sediments and adaptation of ammonia-oxidizing bacteria to low-oxygen or anoxic habitats. Applied and Environmental Microbiology 62: 4100–4107.
Boran, K., M. Kolevab, R. Arsovb, W. van der Starc, M.S.M. Jetten, and M. Strous. 2006. Adaptation of a freshwater anammox population to high salinity wastewater. Journal of Biotechnology 126: 546–553. doi:10.1016/j.jbiotec.2006.05.012.
Brandes, J.A., A.H. Devol, and C. Deutsch. 2007. New developments in the marine nitrogen cycle. Chemical Reviews 107: 577–589. doi:10.1021/cr050377t.
Burgin, A., and S. Hamilton. 2007. Nitrate removal in aquatic ecosystems. Frontiers in Ecology and the Environment 5: 89–96. doi:10.1890/1540-9295(2007)5[89:HWOTRO]2.0.CO;2.
Crompton, T.R. 2005. Analysis of seawater—a guide for the analytical and environmental chemist. Berlin: Springer.
Dalsgaard, T., D.E. Canfield, J. Petersen, B. Thamdrup, and J. Acuna-Gonzalez. 2003. N-2 production by the anammox reaction in the anoxic water column of Golfo Dulce, Costa Rica. Nature 422: 606–608. doi:10.1038/nature01526.
Dalsgaard, T., B. Thamdrup, and D.E. Canfield. 2005. Anaerobic ammonium oxidation (anammox) in the marine environment. Research in Microbiology 156: 457–464. doi:10.1016/j.resmic.2005.01.011.
Deegan, L.A., J.L. Bowen, D. Drake, J.W. Fleeger, C.T. Friedrichs, K.A. Galvan, J.E. Hobble et al. 2007. Susceptibility of salt marshes to nutrient enrichment and predator removal. Ecological Applications 17: S42–S63. doi:10.1890/06-0452.1.
Dollhopf, S.L., J.H. Hyun, A.C. Smith, H.J. Adams, S. O’Brien, and J.E. Kostka. 2005. Quantification of ammonia-oxidizing bacteria and factors controlling nitrification in salt marsh sediments. Applied and Environmental Microbiology 71: 240–246. doi:10.1128/AEM.71.1.240-246.2005.
Dong, Z.Q., and T.H. Sun. 2007. A potential new process for improving nitrogen removal in constructed wetlands—promoting coexistence of partial-nitrification and ANAMMOX. Ecological Engineering 31: 69–78. doi:10.1016/j.ecoleng.2007.04.009.
Engstrom, P., T. Dalsgaard, S. Hulth, and R.C. Aller. 2005. Anaerobic ammonium oxidation by nitrite (anammox): Implications for N-2 production in coastal marine sediments. Geochimica Et Cosmochimica Acta 69: 2057–2065. doi:10.1016/j.gca.2004.09.032.
Francis, C.A., J.M. Beman, and M.M.M. Kuypers. 2007. New processes and players in the nitrogen cycle: the microbial ecology of anaerobic and archaeal ammonia oxidation. Isme Journal 1: 19–27. doi:10.1038/ismej.2007.8.
Hamersley, M.R. 2002. The role of denitrification in the nitrogen cycle of new england salt marshes. Ph.D. thesis, Woods Hole Oceanographic Institution/Massachusetts Institute of Technology, Woods Hole, MA, USA.
Hammersley, M.R., G. Lavik, D. Woebken, J.E. Rattray, P. Lam, H. Hopmans, J.S. Sinninghe Damsté et al. 2007. Ammonium oxidation contributes significantly to nitrogen loss from the Peruvian oxygen minimum zone. Limnology and Oceanography 52: 923–933.
Hopkinson, C., and A. Giblin. 2008. Nitrogen dynamics in salt marsh ecosystems. In Nitrogen in the marine environment, eds. D. Capone, D. Bronk, D. Mulholland, and E. CarpenterNew York: Academic.
Kana, T.M., C. Darkangelo, M.D. Hunt, J.B. Oldham, G.E. Bennett, and J.C. Cornwell. 1994. Membrane inlet mass-spectrometer for rapid high-precision determination of N-2, O-2, and Ar in Environmental Water Samples. Analytical Chemistry 66: 4166–4170. doi:10.1021/ac00095a009.
Kuypers, M.M.M., G. Lavik, D. Woebken, M. Schmid, B.M. Fuchs, R. Amann, B.B. Jorgensen et al. 2005. Massive nitrogen loss from the Benguela upwelling system through anaerobic ammonium oxidation. Proceedings of the National Academy of Sciences of the United States of America 102: 6478–6483. doi:10.1073/pnas.0502088102.
Magalhaes, C.M., S.B. Joye, R.M. Moreira, W.J. Wiebe, and A.A. Borlado. 2005. Effect of salinity and inorganic nitrogen concentrations on nitrification and denitrification rates in intertidal sediments and rocky biofilms of the Douro River estuary, Portugal. Water Research 39: 1783–1794. doi:10.1016/j.watres.2005.03.008.
Matheson, F.E., M.L. Nguyen, A.B. Cooper, and T.P. Burt. 2003. Short-term nitrogen transformation rates in riparian wetland soil determined with nitrogen-15. Biology and Fertility of Soils 38: 129–136. doi:10.1007/s00374-003-0640-3.
Meyer, R.L., N. Risgaard-Petersen, and D.E. Allen. 2005. Correlation between anammox activity and microscale distribution of nitrite in a subtropical mangrove sediment. Applied and Environmental Microbiology 71: 6142–6149. doi:10.1128/AEM.71.10.6142-6149.2005.
Mulder, A., A.A. Vandegraaf, L.A. Robertson, and J.G. Kuenen. 1995. Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor. FEMS Microbiology Ecology 16: 177–183. doi:10.1111/j.1574-6941.1995.tb00281.x.
Paredes, D., P. Kuschk, and H. Koser. 2007. Influence of plants and organic matter on the nitrogen removal in laboratory-scale model subsurface flow constructed wetlands inoculated with anaerobic ammonium oxidizing bacteria. Engineering in Life Sciences 7: 565–576. doi:10.1002/elsc.200700030.
Penton, C.R., A.H. Devol, and J.M. Tiedje. 2006. Molecular evidence for the broad distribution of anaerobic ammonium-oxidizing bacteria in freshwater and marine sediments. Applied and Environmental Microbiology 72: 6829–6832. doi:10.1128/AEM.01254-06.
Reddy, K.R., W.H. Patrick, and C.W. Lindau. 1989. Nitrification–denitrification at the plant root–sediment interface in wetlands. Limnology and Oceanography 34: 1004–1013.
Rich, J.J., O.R. Dale, B. Song, and B.B. Ward. 2008. Anaerobic ammonium oxidation (Anammox) in Chesapeake Bay sediments. Microbial Ecology 55: 311–320. doi:10.1007/s00248-007-9277-3.
Risgaard-Petersen, N., R.L. Meyer, M. Schmid, M.S.M. Jetten, A. Enrich-Prast, S. Rysgaard, and N.P. Revsbech. 2004. Anaerobic ammonium oxidation in an estuarine sediment. Aquatic Microbial Ecology 36: 293–304. doi:10.3354/ame036293.
Schmid, M.C., N. Risgaard-Petersen, J. van de Vossenberg, M.M.M. Kuypers, G. Lavik, J. Petersen, S. Hulth et al. 2007. Anaerobic ammonium-oxidizing bacteria in marine environments: widespread occurrence but low diversity. Environmental Microbiology 9: 1476–1484. doi:10.1111/j.1462-2920.2007.01266.x.
Seitzinger, S.P. 1988. Denitrification in freshwater and coastal marine ecosystems: ecological and geochemical significance. Limnology and Oceanography 33: 702–724.
Seitzinger, S.P., W.S. Gardner, and A.K. Spratt. 1991. The effect of salinity on ammonium sorption in aquatic sediments: Implications for benthic nutrient recycling. Estuaries 14: 167–174. doi:10.2307/1351690.
Sherr, B.F., and W.J. Payne. 1978. Effect of the Spartina alterniflora root–rhizome system on salt marsh soil denitrifying bacteria. Applied and Environmental Microbiology 35: 724–729.
Strous, M., J.A. Fuerst, E.H.M. Kramer, S. Logemann, G. Muyzer, K.T. van de Pas-Schoonen, R. Webb et al. 1999. Missing lithotroph identified as new planctomycete. Nature 400: 446–449. doi:10.1038/22749.
Teal, J.M., and B.L. Howes. 2000. Salt marsh values: Retrospection from the end of the century. In Concepts and controversies in tidal marsh ecology, eds. M.P. Weinstein, and D.A. Kreeger, 9–19. Dordrecht: Kluwer Academic.
Thamdrup, B., and T. Dalsgaard. 2002. Production of N-2 through anaerobic ammonium oxidation coupled to nitrate reduction in marine sediments. Applied and Environmental Microbiology 68: 1312–1318. doi:10.1128/AEM.68.3.1312-1318.2002.
Thompson, S.P., H.W. Paerl, and M.C. Go. 1995. Seasonal patterns of nitrification and denitrification in a natural and a restored salt-marsh. Estuaries 18: 399–408. doi:10.2307/1352322.
Trimmer, M., J.C. Nicholls, and B. Deflandre. 2003. Anaerobic ammonium oxidation measured in sediments along the Thames estuary, United Kingdom. Applied and Environmental Microbiology 69: 6447–6454. doi:10.1128/AEM.69.11.6447-6454.2003.
Valiela, I., and M.L. Cole. 2002. Comparative evidence that salt marshes and mangroves may protect seagrass meadows from land-derived nitrogen loads. Ecosystems 5: 92–102. doi:10.1007/s10021-001-0058-4.
Van de Graaf, A.A., A. Mulder, P. Debruijn, M.S.M. Jetten, L.A. Robertson, and J.G. Kuenen. 1995. Anaerobic oxidation of ammonium is a biologically mediated process. Applied and Environmental Microbiology 61: 1246–1251.
Van de Graaf, A.A., P. deBruijn, L.A. Robertson, M.S.M. Jetten, and J.G. Kuenen. 1996. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 142: 2187–2196.
van de Vossenberg, J., J.E. Rattray, W. Geerts, B. Kartal, L. van Niftrik, E. van Donselaar, J.S. Sinninghe Damste et al. 2008. Enrichment and characterization of marine anammox bacteria associated with global nitrogen gas production. Functional diversity of fresh water and marine anammox bacteria. Environmental Microbiology 10: 3120–3129. doi:10.1111/j.1462-2920.2008.01643.x.
Acknowledgments
The research was supported by the National Science Foundation PIE-LTER (NSF-OCE-9726921; NSF-OCE-0423565); NSF DEB 0213767 and the NOAA, Department of Commerce under Grant number NA16RG2273, Woods Hole Oceanographic Institutions Sea Grant project R/M-50 and R/M-53. The views expressed here are those of the authors and so do not necessarily reflect the views of NOAA or any of its sub-agencies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koop-Jakobsen, K., Giblin, A.E. Anammox in Tidal Marsh Sediments: The Role of Salinity, Nitrogen Loading, and Marsh Vegetation. Estuaries and Coasts 32, 238–245 (2009). https://doi.org/10.1007/s12237-008-9131-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-008-9131-y