Abstract
Brazil has 22 genera and 75 species of Cetoniidae, with the Cerrado hosting the greatest diversity among Brazilian biomes. However, the diversity of groups among the different phytophysiognomies of the biome is not known. The objectives of this study are to assess Cetoniidae diversity and to verify the seasonality of these beetles in three Cerrado phytophysiognomies (gallery forest, cerrado sensu stricto, and campo sujo) located in three conservation units in Brasilia, Distrito Federal, Brazil. We collected adults monthly (October/2016 to September/2018) using 180 traps baited with bananas fermented with sugarcane juice, totaling 1,574 specimens, 8 genera, and 17 species. Cetoniidae diversity was higher in the phytophysiognomies with lower tree density (campo sujo and cerrado sensu stricto) than in gallery forests (forest formation), confirming our hypothesis that more open areas favor the dispersal of these insects due to their diurnal long-flying behavior. The seasonality of Cetoniidae was directly related to the precipitation, with higher numbers of individuals and species in the rainy season. However, the distribution varies among the phytophysiognomies, with aggregated distribution in campo sujo and gallery forest and dispersed in cerrado sensu stricto. Our results suggest that the Cetoniidae take advantage of the open Cerrado physiognomies to locate resources faster and with less energy expenditure, presenting higher diversity in these environments, despite the more ephemeral and dispersed food resources.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Cetoniidae (Insecta: Coleoptera), known as flower and fruit beetles, are insects that have diurnal feeding habits. They feed on nectar, pollen, plant exudates, and ripe fruits (Ritcher 1958; Krikken 1984a), and are highly attracted to fermented fruits such as banana and pineapple (Pacheco et al. 2006; Jákl 2009; Orozco 2012; Evangelista Neto et al. 2017). Some species are recorded as agricultural, horticultural, and ornamental pests, while others are considered pollinators in various parts of the world (Molina 2001; Vuts et al. 2008, 2009; Aydin 2011; Subchev et al. 2011).
They form a diverse group of approximately 4,000 species, 300 of which occur on the American continent (Krikken 1984a; Orozco 2012). In Brazil, 75 species and 22 genera have been reported (Vaz-de-Mello and Puker 2021): three species are recorded from the Cerrado-Pantanal transition (Garcia et al. 2013), 10 in the Atlantic Forest (Gonçalves and Louzada 2005; Puker et al. 2014; Correa et al. 2021a), 16 in the Amazon (Valois and Silva 2015; Alves-Oliveira et al. 2016; Puker et al. 2020a, b), and 21 in the Cerrado (Rodrigues et al. 2013; Evangelista Neto et al. 2017; Correa et al. 2018). The Cerrado biome has the highest richness, accounting for 28% of the species and 36.4% of the genera of Cetoniidae known in the country (Garcia et al. 2013; Rodrigues et al. 2013; Evangelista Neto et al. 2017). This reinforces the importance of preserving the biome for the conservation of this important group of organisms.
In diverse groups of organisms, the spatio-temporal availability of food resources shapes the diversity (species richness and abundance) and behavior of communities of individuals, generating morphological adaptations and preferences for certain habitats (Arruda Almeida et al. 2018; Ribeiro et al. 2022). Representatives of Cetoniidae have a pronounced posthumeral marginalization that enables fast, long (in search of food and other resources), and sonorous flights with closed elytra (Le Gall 2010; Cherman and Morón 2014). For beetles, the elytra protect against dehydration, allowing adaptation to various habitats and as an aid to flight (Le et al. 2010; Johansson et al. 2012). Unlike other Coleoptera, Cetoniidae have better flight control, can hover, and have a quick response to predator threats (Krikken 1984b). The closed elytra flight may be related to diurnal life and sparse food resources, creating the need for long flights (Šípek et al. 2016). The diversity of this group seems to be influenced by microhabitats; for example, areas of cerrado sensu stricto show high species diversity (Evangelista Neto et al. 2017) in contrast to pasture areas and eucalyptus plantations, where fewer species are found (Correa et al. 2021b). Comparing urban parks and the Cerrado nature reserve area, abundance was 1.85 times higher in the nature reserve area even though the species richness was similar (nine species) (Correa et al. 2018).
The Cerrado presents high heterogeneity and strong seasonal variation, with respect to biodiversity and phytophysiognomies with two well-defined seasons: the dry (April to September) and the rainy season (October to March) (Silva et al. 2008). Three main types of vegetation formations are registered in this biome: forest, savanna, and grassland, which have different levels of tree density, humidity, and availability of resources. Among the forest formations, the gallery forest generates a dense cover and maintains high humidity even in the dry season because it accompanies streams and has tall trees (20–30 m tall) (Ribeiro and Walter 2008). In the savannah formations, the cerrado sensu stricto is the characteristic physiognomy of the biome representing 70% of the area (Felfili and Silva Júnior 2005) and presents low, twisted plants, with thick barked trunks and rigid leaves that can be 1.5–5 m tall, demonstrating adaptations to the dry season. In the grassland formations, campo sujo (common in Central Brazil) constitutes an open area with sparse shrubs and subshrubs (Sano et al. 2010).
Climatic seasonality in the Cerrado is reflected in seasonality in insect diversity by increasing or decreasing food resources (Oliveira and Frizzas 2008; Silva et al. 2011; Oliveira et al. 2021) and influencing vegetation (Becerra et al. 2009). Generally, insect diversity tends to be lower in dry months in regions with a well-defined dry season (Wolda 1978; Pinheiro et al. 2002). Studies conducted in the Cerrado have shown seasonality in Coleoptera diversity, with population dynamics being directly influenced by abiotic factors like precipitation and temperature (Evangelista Neto et al. 2017; Frizzas et al. 2020; Evangelista et al. 2021; Oliveira et al. 2021).
The high diversity of plant species recorded in the Cerrado, with 12,356 cataloged species (Mendonça et al. 2008), suggests that a wide range of food resources may contribute to the high diversity of Cetoniidae. However, to date, no study has evaluated the effect of physiognomies, which present variation in food resources and microclimate, on the diversity of these organisms. Thus, the objectives of this study were (i) to evaluate the diversity of Cetoniidae among the physiognomies: gallery forest, cerrado sensu stricto, and campo sujo in the Cerrado biome in Central Brazil, and (ii) to assess the seasonality of this group in these physiognomies.
Since the Cerrado physiognomic forms have different densities of trees, leading to variations in food resources and microclimate, we hypothesized that there will be significant differences in the diversity and seasonality of Cetoniidae among these environments. We expected that the campo sujo will present the highest diversity because it is an open area, which facilitates dispersal, foraging, and the perception of fermented fruit odors characterized by fast and long flights. We also hypothesized that this group of insects would present a seasonal distribution in the phytophysiognomies of cerrado sensu stricto and campo sujo because of the lower humidity in the dry season in this region. We expected that the highest diversity would be synchronized with the rainy season when the availability of food resources is greater. In the gallery forest, we expected a dispersed and more homogeneous distribution of species throughout the year because these areas retain moisture even in the dry season and may have greater availability of food resources throughout the year.
Methods
Study areas
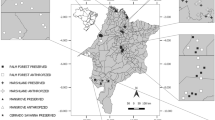
The study was conducted in three conservation units located in Brasilia, Distrito Federal, Brazil (Fig. 1), which have characteristic vegetation of the Cerrado biome. The list of sites is as follows: (i) Parque Nacional de Brasília (PNB) has an area of 42,389 ha and represents the typical ecosystem of the Cerrado of the Central Plateau, including several types of vegetation and abundant diverse fauna. Moreover, it also facilitates the connection with fragments of native Cerrado that are essential for the formation of ecological corridors and the genetic exchange of fauna and flora species (ICMBio 2018). (ii) Fazenda Água Limpa (FAL), belonging to the University of Brasília (UnB), has an area of 4,500 ha including the area of ecological interest (ARIE Capetinga/Taquara) and falls under the Environmental Protection Area (APA of the Basins of Gama and Cabeça do Veado). Environmental heterogeneity provides diverse phytophysiognomies in the Cerrado biome. Both, floristic and faunistic diversities are very high and include species representing endemic plants and animals (PELD 2018). (iii) Reserva Ecológica do IBGE (RECOR) is part of the Environmental Protection Area (APA Gama-Cabeça de Veado District) with an area of 10,000 ha of continuous protected area. It is one of the core areas of the Cerrado Biosphere Reserve, featuring several types of Cerrado biome phytophysiognomies, springs of important watercourses that form the Paranoá Basin, and diverse wildlife (RECOR 2016; PELD 2018).
Conservation units selected for the collection of Cetoniidae in Brasília, Distrito Federal, Brazil (October/2016 to September/2018). PNB - Parque Nacional de Brasília, FAL - Fazenda Água Limpa (FAL), and RECOR - Reserva Ecológica do IBGE, location of the phytophysiognomies in each conservation unit and trap used for the collection of Cetoniidae. Green dots: gallery forest; blue dots: cerrado sensu stricto and yellow dots: campo sujo.
Three phytophysiognomies were selected in each conservation unit: gallery forest, cerrado sensu stricto, and campo sujo, representing forest, savanna, and grassland vegetation formations, respectively. These physiognomies were chosen to select areas with a gradient in tree density, from physiognomy with the lowest tree density (campo sujo) to the physiognomy with the highest tree density (gallery forest) (campo sujo > cerrado sensu stricto > gallery forest).
Insect collection
We collected adults monthly for 24 months (October 2016 to September 2018) using baited traps. The trap consisted of a bait-holder pot (500 g) coupled with a larger plastic pot (1 kg). The larger pot had three 8 × 8 cm side windows and the bottom was removed. The lid of the bait-holder was glued to the bottom of the larger pot (with a hole in the center for the insects to pass through), allowing the bait-holder to be screwed on (Fig. 1). This trap is an adaptation of the pet bottle trap usually used for such collections (Orozco and Pardo-Locarno 2004; Orozco 2012; Rodrigues et al. 2013; Puker et al. 2014). Adaptations were made to facilitate the transport, installation, and removal of traps in the field. The bait-holders could be filled with the bait in the laboratory. In the field, the bait-holders with the collected specimens can be unscrewed and removed to replace with new bait-holders. The bait-holder contained 200 mL of bait, consisting of approximately 150 mL of Nanica banana (Dwarf Cavendish) and 50 mL of sugarcane juice that was fermented for 48 h (Rodrigues et al. 2013; Puker et al. 2014). All traps were installed at approximately 1.50 m from the ground level and remained in the field for seven days. In the gallery forest and cerrado sensu stricto, the traps were tied directly to the trees. In campo sujo, they were set on iron bars because the trees were sparse and low in this phytophysiognomy.
In each phytophysiognomy, two 500 m transects were demarcated, where 10 traps were set, spaced 50 m apart, and 50 m between the transect. Thus, we had 20 traps for each phytophysiognomy. The conservation units were considered repetitions with a total of 180 traps (3 conservation units × 3 phytophysiognomies × 20 traps). A 20 m margin was established from the entry point to the installation of the first trap.
Taxonomic identification
The Cetoniidae were pinned, labeled, separated by species, and counted. Taxonomic identification was performed by comparison with specimens from the Entomological Collection of the Zoology Department of the University of Brasília (DZUB, Brasília, DF, Brazil), and with the assistance of Dr. Fernando Zagury Vaz-de-Mello (UFMT). Vouchers of the collected material were deposited in the DZUB and in the collection of the Universidade Federal do Mato Grosso (CEMT, Cuiabá, MT, Brazil). Climatic data on temperature, relative humidity, and precipitation were obtained from the National Institute of Meteorology (INMET) throughout the collection period.
Data analysis
All data analyses were performed with R Program (R Core Team 2019). The data obtained (abundance and species richness) did not meet normality assumptions relating to the residues and the homogeneity of the variance. A generalized linear model (GLM) was used to verify if there were significant differences in the abundance and species richness (response variables) of Cetoniidae for the three phytophysiognomies (campo sujo, cerrado sensu stricto, and gallery forest). For these comparisons, a GLM was used in a logistic regression model using a negative binomial distribution. GLM selection was previously performed to choose the best model to fit count data using the “hnp” package (Moral et al. 2017). Mean values were statistically separated by Tukey’s HSD test at p < 0.05 using the function “glht” in “multcomp” package (Hothorn et al. 2016).
Similarly, we used GLM in a logistic regression model using a negative binomial distribution to verify the influence of climate variables (monthly average air temperature, monthly average relative air humidity, and monthly cumulative total precipitation) on Cetoniidae abundance and species richness. To identify indicator species among phytophysiognomies types, an indicator value (IndVal) approach was employed to characterize the habitats (Dufrêne and Legendre 1997). The ‘indicspecies’ package was used for the IndVal analysis in R (Caceres et al. 2016). The IndVal indicator attains the maximum value (1.00) when all of the individuals of a species are found in a single treatment (high specificity) and when the species occurs in all replicates of treatment (high fidelity).
The number of the individuals-based protocol was employed, and the accumulation curve was constructed in the R environment, using the “iNEXT” package (Hsieh et al. 2016). In the iNEXT analysis, individual-based abundance data and diversity order based on species richness (q = 0) were used. iNEXT can interpolate and extrapolate species richness by taking into account a measure of sample coverage (Chao and Jost 2012; Chao et al. 2014). Shannon diversity (q = 1) and Pielou evenness indices were calculated using the “BiodiversityR” package (Kindt 2018).
The diversity was analyzed by β-diversity, which expresses species turnover in terms of spatial and/or temporal scales. The β-diversity index expresses the faunistic change (turnover) between sampling (months) for each phytophysiognomy (intra-site heterogeneity) and the species turnover between adjacent phytophysiognomies (inter-site heterogeneity). This index was estimated using the following two formulas. The temporal β-diversity was first measured and calculated using Whittaker’s index: βw = (S/α) – 1, where α is the average of the α-diversity for all sampling periods (months) and S is the total number of species recorded in all sampling periods (Whittaker 1960). The βw value varied between 0 and n-1 (n = 12). The second formula measured the inter-site (spatial) β-diversity and was calculated using Cody’s index: βc = 100 * (Sg + Sl)/2α, where Sg is the increase in the number of species between two phytophysiognomies, Sl is the number of species lost, and α is the average of the α-diversity for the two phytophysiognomies (Cody 1975).
Circular analysis was used to examine the abundance of species in the different months of the year and among all three phytophysiognomies (Agostinelli and Lund 2013). The Rayleigh uniformity test was used to analyze temporal variation data, which analyzes the functional relationship of variables under the concept of dimensional homogeneity (Mendoza 1994).
Results
A total of 1,574 specimens, 8 genera, and 17 species of Cetoniidae were collected in 24 months (Supplementary Material S1). The species with the highest abundance were Gymnetis hebraica, comprising 22.7% of the total collection followed by Hoplopyga brasiliensis (19.8%), and Allorrhina menetriesii (16.3%). For the first time, we recorded the species Gymnetis hieroglyphica, Gymnetis pantherina, Hoplopyga miliaris, and Macrocranius similis.
We collected 106 individuals and 9 species from the gallery forest, 423 individuals and 15 species from the cerrado sensu stricto, and 1,045 individuals and 15 species from campo sujo (Fig. 2). The abundance and species richness of Cetoniidae were significantly lower (p < 0.01) for the gallery forest and did not differ between the phytophysiognomies cerrado sensu stricto and campo sujo (Fig. 2). Eight species were common to the three types of physiognomy while G. hieroglyphica and M. similis were collected only from campo sujo (Fig. 3b). No exclusive species were found in cerrado sensu stricto and gallery forest areas (Fig. 3c and d).
Boxplot graph of abundance and species richness of Cetoniidae species collected in traps baited with fermented fruit in campo sujo, cerrado sensu stricto, and gallery forest, in three conservation units (Fazenda Água Limpa, Parque Nacional de Brasília, and Reserva Ecológica do IBGE) in Brasília/DF, Brazil (October 2016 to September 2018). Values followed by the same letter in the columns do not differ significantly (p < 0.01) by Tukey’s HSD test
Cetoniidae species collected in traps baited with fermented fruit in campo sujo, cerrado sensu stricto, and gallery forest, in three conservation units (Fazenda Água Limpa, Parque Nacional de Brasília, and IBGE Ecological Reserve) in Brasília/DF, Brazil (October 2016 to September 2018). (A) Total, (B) campo sujo, (C) cerrado sensu stricto and (D) gallery forest
The seven species considered as indicators of campo sujo physiognomy were Hoplopyga albiventris (IndVal = 0.749; p < 0.001), E. lurida (IndVal = 0.726; p < 0.001), A. menetriesii (IndVal = 0.599; p < 0. 001), Hologymnetis undulata (IndVal = 0.581; p < 0.001), Hoplopyga singularis (IndVal = 0.580; p < 0.001), G. hieroglyphica (IndVal = 0.535; p < 0.001), and Hoplopyga miliaris (IndVal = 0.498; p < 0.001). For the gallery forest, the two indicator species were Inca bonplandi (IndVal = 0.665; p < 0.001) and Hoplopyga liturata (IndVal = 0.602; p < 0.001). For campo sujo and cerrado sensu stricto, the four indicator species were: G. hebraica (IndVal = 0.833, p < 0.001), H. brasiliensis (IndVal = 0.691; p < 0.001), Gymnetis rufilatris (Indval = 0.667, p < 0.001), and Allorrhina cincta (IndVal = 0.386; p < 0.001). No species were indicated as exclusive indicators of cerrado sensu stricto physiognomy.
The species accumulation curve indicated that sampling effort was satisfactory for campo sujo and cerrado sensu stricto, whereas, for the gallery forest, a greater sampling effort is necessary to reach the asymptote (Fig. 4). The campo sujo physiognomy showed the highest Shannon diversity index (H’ = 2.12) and highest Pielou’s equitability (J = 0.78), followed by the gallery forest (H’ = 1.61 and J = 0.73) and the cerrado sensu stricto (H’= 1.37 and J = 0.50).
Individuals-based accumulation curve of Cetoniidae species [based on species richness (q = 0)] collected in traps baited with fermented fruit in campo sujo, cerrado sensu stricto, and gallery forest in three conservation units (Fazenda Água Limpa, Parque Nacional de Brasília, and Reserva Ecológica do IBGE) in Brasília/DF, Brazil (October 2016 to September 2018)
The β-diversity index expressing the faunistic change (turnover) between sampling months was higher in the gallery forest (βw = 3.50), followed by cerrado sensu stricto (βw = 2.40) and campo sujo (βw = 1.90). In terms of species turnover between adjacent phytophysiognomies (inter-site heterogeneity-β-spatial diversity), the largest difference occurred between campo sujo and gallery forest with βc = 41.7%, followed by cerrado sensu stricto and gallery forest with βc = 29.2%, and the smallest difference was found between campo sujo and cerrado sensu stricto (βc = 13.3%).
Cetoniidae were collected in all months of the year, with the highest abundance (n = 811) and richness (n = 15) observed in November, while the lowest abundance (n = 6) was observed in September and the lowest species richness (n = 1) in July and August (Fig. 5). In the rainy season (October to March), 1,424 individuals (90.5% of the total collected) and 17 species were collected. In the dry season (April to September), 150 individuals (9.5% of the total) and 7 species (42% of the species) were collected (Figs. 3a and 5). Except for G. hebraica, which was found throughout the year, most species were collected during the rainy season. Although April falls in the dry season, it represents a transition period, with 37 individuals and 7 species collected during this period. Gymnetis flava was collected only during the transition period and in the dry season. Precipitation showed a significant effect on abundance (z = 3.346 and p = 0.00082) and species richness (z = 3.059 and p = 0.00222) of Cetoniidae.
Species richness and abundance of Cetoniidae collected in traps baited with fermented fruit in campo sujo, cerrado sensu stricto, and gallery forest in three conservation units (Fazenda Água Limpa, Parque Nacional de Brasília, and Reserva Ecológica do IBGE) in Brasília/DF, Brazil (October 2016 to September 2018) during dry and rainy seasons in the Cerrado
The circular analysis showed that the abundance of Cetoniidae presents a well-defined peak in November for all physiognomies (Fig. 6); however, the distribution throughout the rainy season varied. The aggregate distribution of abundance was concentrated in campo sujo during November and in the gallery forest between November and January. Cerrado sensu stricto showed a dispersed distribution with a wider distribution throughout the rainy season (October to January) (Fig. 6), with small peaks during the dry season due to the presence of G. hebraica (Table 1). Rayleigh’s uniformity test showed that there was significant temporal variation in Cetoniidae abundance (p < 0.0001) in the three phytophysiognomies (Table 1).
Circular analysis for total abundance of Cetoniidae (A) collected in traps baited with fermented fruit in campo sujo (B), cerrado sensu stricto (C) and gallery forest (D) phytophysiognomies in three conservation units (Fazenda Água Limpa, Parque Nacional de Brasília, and Reserva Ecológica do IBGE) in Brasília/DF, Brazil, (October 2016 to September 2018)
Discussion
Our results confirm our hypothesis that more open Cerrado physiognomies (campo sujo and cerrado sensu stricto) support the highest diversity of Cetoniidae when compared to forested areas (gallery forest). We also confirmed that although the Cetoniidae occur throughout the year in the Cerrado, in general, abundance and species richness were concentrated in the rainy season. However, the seasonality of the group varies among the three phytophysiognomies, being aggregated in campo sujo and gallery forest and dispersed in cerrado sensu stricto. Thus, the hypothesis that the community in the gallery forest would not be affected by the seasonality of the climate was not confirmed, possibly because the species are linked to the seasonality of the Cerrado biome and not to the specific microclimate of the physiognomy. Further studies are required to address the biology of the species, interactions with plants, natural history, and ecosystem services.
The gallery forest showed the lowest diversity compared to the grasslands and savannah vegetation formations (Fig. 2). Higher diversity observed in campo sujo and cerrado sensu stricto can be explained by the drier microclimate in these areas, which may favor a faster loss of moisture from the diet and the intensification of volatiles from fermentation. As open areas are more, the volatiles can spread easily and quickly via air. The lower presence of physical barriers (lower density of woody plants) compared to forest formations may also facilitate the displacement of specimens (Fuentes et al. 2016). Cetoniidae are diurnal beetles with long flights (Le Gall 2010; Cherman and Morón 2014) and probably take advantage of more open Cerrado areas. This is because despite sparser food resources, locating is faster and less energetically expensive; the time spent foraging is reduced thereby reducing the probability of predation (Goodell 2003). Another important fact is that grassland (8,848 plant species) and savanna (7,618 plant species) formations have a higher vegetation diversity than forest formations (6,998 plant species) (Mendonça et al. 2008), which may also have contributed to the higher diversity of Cetoniidae in these types of vegetation formation by representing a greater source of food resources.
Insects have flight properties such as height, speed, and maneuvering ability. These may vary among species and are directly linked to ecological factors such as feeding habits, habitat, and predation (Šípek et al. 2016; Kojima and Kato 2017; Le Roy et al. 2019; Farisenkov et al. 2020). Most beetles open the elytra during flight; however, the families Cetoniidae, Scarabaeidae, Cerambycidae, and Buprestidae have species that are known to fly with the elytra closed or partially raised (Krikken 1984a; Šípek et al. 2016; Kojima and Kato 2017). Rapid flight has the benefit of dodging predators, and the closed elytra may reduce water loss during diurnal flight (Chown et al. 2011; Šípek et al. 2016). This type of flight is associated with diurnal life, allowing insects to utilize dispersed and unpredictably distributed food resources represented by uneven flowering of fruit trees (Šípek et al. 2016), as occurs in the Cerrado.
It is also worth noting that the larvae and adults of Cetoniidae have distinct nutritional biology. Larvae are usually saprophytes or saproxylophages, and can often be found in soil, decaying vegetables, rotting wood, tree cavities, feces (Luederwaldt 1911; Morón and Arce 2002; Puker et al. 2014), and may also live in association with social insects (Krikken 1984a; Micó et al. 2000; Peter and Johnson 2009; Puker et al. 2012). Adults typically seek out flowers or inflorescences that are large and open, yellow, white, purple, or pink, as well as fermented fruits (Peter and Johnson 2009; Puker et al. 2014, 2015), foods with high sugar concentration, and fermenting yeasts. After emergence, males search for trunks, branches, flowers/inflorescences, or fruits to find mating partners (Arce-Pérez and Morón 1999). The search for mates and food, therefore, has an important relationship with the dispersal ability of these insects, especially in males. After mating, males disperse widely in search of food in flowers. Despite the distinct biology of immatures and adults, most developmental requirements of the Cetoniidae are met in the Cerrado explaining the high richness of the group in this biome. The Brazilian Cerrado contains 12,356 plant species (Mendonça et al. 2008) and has approximately 44% of endemic flora making it the most diverse savannah on the planet (Klink and Machado 2005). It contains several endemic fruiting species that are rich in nutrients and diverse flowers.
We recorded 13 species that are considered indicators for the three physiognomies. We found seven species for campo sujo, two for gallery forest, and no exclusive indicator species for cerrado sensu stricto, indicating that this may be a transition area between closed and open formations. Among the indicator species of campo sujo, immatures of H. albiventris and H. singularis were found associated with termites and the adults feeding on the sap of Asteraceae Baccharis and Vernonia (Shaughney and Ratcliffe 2015). Adults of E. lurida can be considered generalists because they are reported pests of maize, apple, persimmon, grapes, peach, safflower (Bertels and Baucke 1966; Garcia and Corseuil 1999; Cunha et al. 2007; Androcioli et al. 2017). They also act as floral visitors of sunflower (Torretta et al. 2009) and cotton (Dutra et al. 2012) and as pollinators of orchids (Singer and Cocucci 1997). Adults of H. miliaris are described as floral visitors of the Apocynaceae (Koschnitzke 2015). Cetoniidae tend to be regarded as resource exploiters rather than pollinators (Di Iorio 2013; Iorio 2014) because they may pollinate flowers accidentally while searching for resources (Peter and Johnson 2009).
In the gallery forest, the indicator species I. bonplandi and H. liturata were more abundant in August (Fig. 3D), which is characteristic of the dry season in the Cerrado. Despite having smaller areas and being more isolated, gallery forests have a vegetational structure characterized by the dominance of tree species and canopy formation that are associated with watercourses (Ribeiro and Walter 2008). This probably maintains humidity in dry months and ensures the maintenance of resident species during this period. These species may also be favored by less competition from other species because the diversity of insects and the occurrence of natural enemies are lower in the Cerrado during the dry season (Ramos and Diniz 1993; Morais and Diniz 1999; Silva et al. 2011). Adults of I. bonplandi feed on plant exudates and flowers (Boos and Ratcliffe 1985) and immatures on decaying wood (Morón 1983; Costa et al. 1988) and the organic material derived from palm trees (Sousa et al. 2018). Hoplopyga liturata adults feed on exudates from plants of the families Boraginaceae, Fabaceae, Zingiberaceae, and Rhamnaceae, whereas, immatures are found in decomposing organic material of the families Myrtaceae, Burseraceae, Solanaceae, and Moraceae (Shaughney and Ratcliffe 2015).
The species H. brasiliensis, G. hebraica, G. rufilatris, and A. cincta were recorded as indicator species of open Cerrado vegetation formations (cerrado sensu stricto and campo sujo). Immatures of H. brasiliensis are found in association with the termite species Cornitermes cumulans, which is considered a key species because of its abundance and the consequent impact on the environment. Moreover, the nests of this species are capable of harboring several groups of arthropods (Redford 1984). The abundance of H. brasiliensis adults, mostly collected in campo sujo, may be linked to the presence of C. cumulans nests. Gymnetis hebraica is one of the most abundant species and is the only species collected year-round in the open Cerrado, mainly in cerrado sensu stricto. It is a generalist species well adapted to the Cerrado climate conditions and this behavior of G. hebraica was observed in another study in the Cerrado (Evangelista Neto et al. 2017). For most other collected species, no information on their biology or natural history was found.
Regarding the sampling effort, we observed that the species accumulation curves for campo sujo and cerrado sensu stricto reached the asymptote, but not for the gallery forest (Fig. 4). The gallery forest showed a large number of rare species (J = 0.73), which reinforces the need for greater sampling effort for this type of physiognomy in future studies.
The ability to fly allows organisms to colonize different environments, and the species that constitute these communities may share similarities or differences based on the specific requirements of the organisms and the differences and biotic and abiotic similarities of the environments. In the present study, temporal β-diversity was higher in the gallery forest, suggesting that a greater variation (qualitative and/or quantitative) in the availability of resources throughout the year possibly promotes changes in the species composition of Cetoniidae in this environment. Regarding the βc diversity index, which measures the changes in composition among the phytophysiognomies, it was observed that the communities of open areas were more similar to each other with a change in species composition of 13.3% throughout the study. This change in composition was maximum between campo sujo and gallery forest (41.7%), showing a gradient of similarity from the more open areas to the more closed areas. For the Cetoniidae, it was observed that the gallery forest has a specific community and that this community is the one that most varies over time, indicating that conservation efforts in this phytophysiognomy need more attention.
The seasonality in the abundance of insects is synchronized with the availability of food resources, and these in turn vary seasonally depending on the climate (Wolda 1978). The Cerrado has two well-defined seasons, and the majority of adult insect populations can be found in the rainy season from October to March (Silva et al. 2011; Oliveira et al. 2021). The seasonal variation in abundance and richness of Cetoniidae is directly related to the climatic characteristics of the Cerrado, with 90.5% of individuals and 100% of species occurring in the rainy season and 9.5% of individuals and 41.2% of species in the dry season. Furthermore, we recorded a direct relationship between precipitation and the diversity of Cetoniidae. The onset of rainfall is considered to trigger increased population density (Wolda 1978; Silva et al. 2011), and other studies conducted with Cetoniidae in the Cerrado (Evangelista Neto et al. 2017; Correa et al. 2018), Cerrado-Pantanal transition (Garcia et al. 2013), Atlantic Forest (Puker et al. 2014), and Amazon (Puker et al. 2020a) also recorded this behavior. The circular analysis confirmed this pattern, showing that the distribution of abundance was concentrated in the first quarter of the rainy season with November presenting the highest number of individuals. However, there was a variation in the distribution of abundance throughout the rainy season among the three phytophysiognomies. The overabundance of some species such as A. menetriesii in November in campo sujo or the presence of abundant species that are distributed throughout the year as G. hebraica in cerrado sensu stricto can generate differences in the patterns observed in different physiognomies.
The Cerrado offers favorable conditions for agriculture. Consequently, this region has experienced a constant reduction in natural vegetation. The Cerrado in the central-western region of Brazil has the highest degree of deforestation (61.6%) (Tisott and Schmidt 2021). The increase and strengthening of agribusiness in the biome trigger negative environmental effects, such as deforestation, biodiversity loss, groundwater pollution, and soil erosion (Brussaard et al. 2010). The fragmentation of natural habitats due to the advancement of agricultural frontiers modifies niches and the microclimate of these habitats, reducing the availability of food resources (quality and/or quantity), and shelter for numerous species, including the insects and plants that shelter these species (Turner 1997). The increase in agricultural areas in the Cerrado may also increase the incorporation of organic matter into the soil through no-till farming and generate a population growth of some species. For example, in Peru, an increase in the population of Gymnetis species was observed in apple plantations due to environmental factors (incorporation of organic matter into the soil and constant humidity) and the high number of adults searching for food resulted in the invasion of beehives by insects attracted by concentrated sugar substances (Tejada and Morón 2015).
Knowledge about the ecological requirements of species is crucial for their conservation, especially if they live in threatened habitat types such as old-growth forests. Studies on the factors that affect the abundance and distribution of insects and the ecological requirements that influence habitat selection are vital to ensure proper species and habitat management (Thomas et al. 1998, 2001; Krämer et al. 2012) because they elucidate the complex trade-off between various biotic and abiotic factors. The information gathered here is important for the assessment and preservation of habitats by assisting in public measures for the maintenance and creation of conservation units. The Cerrado presents 11 main types of physiognomies (Ribeiro and Walter 2008), and we evaluated only three of these physiognomies. Moreover, four species of Cetoniidae were recorded for the first time in the Federal District, suggesting the need for more studies in other physiognomies to uncover information about the real diversity of Cetoniidae in the Cerrado biome.
Data Availability
Data supporting the results and conclusions of this study are available upon request from the corresponding author.
References
Agostinelli C, Lund U (2013) R Package ’Circular’: Circular Statistics (Version 0.47). Available from URL: https://rforge.r-project.org/projects/circular/
Alves-Oliveira JR, Mendes DMM, Campos D, Silva-Neto AM, Morais JW, Grossi PC (2016) First report of two species of scarab beetles (Coleoptera, Scarabaeidae) inside nests of Azteca cf. chartifex Forel (Hymenoptera, Formicidae) in Brazilian Amazonian Rainforest. Rev Bras Entomol 60:359–361. https://doi.org/10.1016/j.rbe.2016.07.003
Androcioli HG, Hoshino AT, Pastório MA, Cardoso PC, Araújo PM, Fernandes TAP, Menezes AO Jr (2017) First record of Euphoria lurida Fabricius (Coleoptera: Scarabaeidae) injurious to safflower (Carthamus tinctorius L.) (Asterales: Asteraceae) in Brazil. Neotrop Entomol 46:130–132. https://doi.org/10.1007/s13744-016-0434-6
Arce-Pérez R, Morón MA (1999) El ciclo de vida de Paragymnetis flavomarginata sallei Schaum, 1849 (Coleoptera: Melolonthidae: Cetoniinae), con observaciones sobre su biologia. Folia Entomol Mex 105:37–54
Arruda Almeida B, Green AJ, Sebastián-González E, Dos Anjos L (2018) Comparing species richness, functional diversity and functional composition of waterbird communities along environmental gradients in the neotropics. PLoS ONE 13(7):e0200959. doi: https://doi.org/10.1371/journal.pone.0200959
Aydin G (2011) Plant phenology-related shifts in color preferences of Epicometis (Tropinota) hirta (Coleoptera: Scarabaeidae: Cetoniinae) adults - key to effective population monitoring and suppression. Fla Entomol 94(4):832–838. https://doi.org/10.1653/024.094.0415
Becerra JAB, Shimabukuro YE, Alvalá RCS (2009) Relação do padrão sazonal da vegetação com precipitação na região de Cerrado da Amazônia legal, usando índices espectrais de vegetação. Rev Bras Meteorol 24(2):125–134. https://doi.org/10.1590/S0102-77862009000200002
Bertels A, Baucke O (1966) Segunda relação das pragas das plantas cultivadas no Rio Grande do Sul. Pesq Agropec Bras 1:17–46
Boos J, Ratcliffe BC (1985) A new subspecies of Inca clathrata (Olivier) from Trinidad, West Indies, and range extensions for Inca clathrata sommeri Westwood (Coleoptera: Scarabaeidae: Trichiinae). Coleopt Bull 39:381–389
Brussaard L, Caron P, Campbell B, Lipper L, Mainka S, Rabbinge R, Babin D, Pulleman M (2010) Reconciling biodiversity conservation and food security: scientific challenges for a new agriculture. Curr Opin Sust 2:34–42. https://doi.org/10.1016/j.cosust.2010.03.007
Caceres M, Jansen F, de Caceres MM(2016) Package ‘indicspecies’. Relationship between species and groups of sites.R package version, 1(6)
Chao A, Jost L (2012) Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93:2533–2547. https://doi.org/10.1890/11-1952.1
Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK, Ellison AM (2014) Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Monogr 84:45–67. https://doi.org/10.1890/13-0133.1
Cherman MA, Morón MA (2014) Validación de la família Melolonthidae Leach, 1819 (Coleoptera: Scarabaeoidea). Acta Zool Mex 30(1):201–220
Chown SL, Sorensen JG, Terblanche JS (2011) Water loss in insects: An environmental change perspective. J Insect Physiol 57:1070–1084. https://doi.org/10.1016/j.jinsphys.2011.05.004
Cody ML (1975) Towards a theory of continental species diversity bird distribution over Mediterranean habitat gradients. In: Cody ML, Diamond JM (eds) Ecology and evolution of communities. Harvard University Press. Cambridge, pp 214-257
Costa C, Vanin AS, Casari-Chen SA (1988) Larvas de Coleoptera do Brasil. Museu de Zoologia, Universidade de São Paulo, São Paulo
Correa CMA, Puker A, Lara MA, Rosa CS, Korasaki V (2018) Importance of urban parks in conserving biodiversity of flower chafer beetles (Coleoptera: Scarabaeoidea: Cetoniinae) in brazilian Cerrado. Environ Entomol 48(1):97–104. https://doi.org/10.1093/ee/nvy176
Correa CMA, da Silva PG, Puker A, Ad’vincula HL (2021a) Pastureland is better than eucalyptus monoculture: β-diversity responses of flower chafer beetles to Brazilian Atlantic Forest conversion. Int J Trop Insect Sci 41:137–144. https://doi.org/10.1007/s42690-020-00186-9
Correa CMA, Silva PG, Lara MA, Puker A (2021b) Spatiotemporal patterns of β-diversity of flower chafer beetles in urban park and natural reserve sites in Brazilian Cerrado. Int J Trop Insect Sci 41(1):681–691. https://doi.org/10.1007/s42690-020-00257-x
Cunha US, Grützmacher AD, Martins JFS, Stefanello GJ, Jardim EO (2007) Ocorrência de Euphoria lurida (Fabricius) (Coleoptera: Scarabaeidae) em milho cultivado em Várzea no Rio Grande do Sul. Neotrop Entomol 36(6):976–979. https://doi.org/10.1590/S1519-566X2007000600023
Di Iorio O (2013) A review of the Cetoniinae (Coleoptera: Scarabaeidae) from Argentina and adjacent countries: systematics and geographic distributions. Zootaxa 3668:1–87. https://doi.org/10.11646/zootaxa.3668.1.1
Di Iorio O (2014) A review of the natural history of adult Cetoniinae (Coleoptera: Scarabaeidae) from Argentina and adjacent countries. Zootaxa 3790:281–318. https://doi.org/10.11646/zootaxa.3790.2.3
Dufrêne M, Legendre P (1997) Species assemblages and indicator species: the need for a flexible asymmetrical approach. Ecol Monogr 67:345–366. https://doi.org/10.1890/0012-9615(1997)067[0345:SAAIST]2.0.CO;2
Dutra CC, Meotti C, Fernandes MG, Raizer J (2012) Riqueza e composição de espécies de insetos visitantes florais de algodoeiro Bt e não-Bt. Arq Inst Biol 79:353–361
Evangelista Neto J, Oliveira CM, Vaz-De-Mello FZ, Frizzas MR (2017) Diversity of Cetoniidae (Insecta: Coleoptera) in the Cerrado of Central Brazil. Entomol Sci 20:1–9. https://doi.org/10.1111/ens.12284
Evangelista J, Rocha MVC, Monné ML, Monné MA, Frizzas MR (2021) Diversity of Cerambycidae (Insecta: Coleoptera) in the Cerrado of Central Brazil using a new type of bait. Biota Neotrop 21(1):e20201103. https://doi.org/10.1590/1676-0611-BN-2020-1103
Farisenkov SE, Lapina NA, Petrov PN, Polilov AA (2020) Extraordinary flight performance of the smallest beetles. PNAS 117:24643–24645. https://doi.org/10.1073/pnas.2012404117
Felfili JM, Silva Júnior MC (2005) Diversidade alfa e beta no cerrado sensu strictu, Distrito Federal, Goiás, Minas Gerias e Bahia. In: Scariot A, Sousa-Silva JC, Felfili JM (eds) Cerrado: ecologia, biodiversidade e conservação. Ministério do Meio Ambiente (MMA), Brasília
Frizzas MR, Batista JLFL, Rocha MVC, Oliveira CM (2020) Diversity of Scarabaeinae (Coleoptera: Scarabaeidae) in an urban fragment of Cerrado in Central Brazil. Eur J Entomol 117:273–281. https://doi.org/10.14411/eje.2020.031
Fuentes JD, Chamecki M, Roulston T, Chen B, Pratt KR (2016) Air pollutants degrade floral scents and increase insect foraging times. Atmos Environ 141:361–374. https://doi.org/10.1016/j.atmosenv.2016.07.002
Garcia FRM, Corseuil E (1999) Flutuação populacional de cerambicídeos e escarabeídeos (Coleoptera) em pomares de pessegueiro no município de Porto Alegre, Rio Grande do Sul. Revista da FZVA 5/6(1):69–81
Garcia FP, Rodrigues SR, Bagnara CAC, Oliveira DS (2013) Survey of saproxylophagous Melolonthidae (Coleoptera) and some biological aspects in Aquidauana. MS Biota Neotrop 13(3). https://doi.org/10.1590/S1676-06032013000300004
Gonçalves TT, Louzada JNC (2005) Estratificação vertical de coleópteros carpófilos (Insecta: Coleoptera) em fragmentos florestais do sul do Estado de Minas Gerais, Brasil. Ecol Austral 15:101–110
Goodell K (2003) Food availability affects Osmia pumila (Hymenoptera: Megachilidae) foraging, reproduction, and brood parasitism. Oecologia 134(4):518–527. https://doi.org/10.1007/s00442-002-1159-2
Hothorn T, Bretz F, Westfall P, Heiberger RM, Schuetzenmeister A, Scheibe S, Hothorn MT (2016) Package ‘multcomp’. Simultaneous inference in general parametric models. Project for Statistical Computing, Vienna
Hsieh TC, Ma KH, Chao A (2016) iNEXT: an R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol Evol 7:1451–1456. https://doi.org/10.1111/2041-210X.12613
INSTITUTO CHICO MENDES DE CONSERVAÇÃO DA BIODIVERSIDADE (ICMBIO). Plano de manejo do Parque Nacional de Brasília. Disponível em: http://www.icmbio.gov.br/portal/images/stories/imgs-unidadescoservacao/PARNA%20Brasilia.pdf Acesso em: 26 de junho de 2018
Jákl S (2009) Results of entomological expeditions to Yamdena, Larat, Tandula, Selaru and Molu islands (Indonesia, Moluccas, Tanimbar islands) with the description of new genus, three new species and four new subspecies (Coleoptera: Cetoniinae). Stud Rep Distr Mus Prague-east Taxonomical Ser 5(1–2):139–158
Johansson LC, Engel S, Baird E, Dacke M, Muijres FT, Hedenström A (2012) Elytra boost lift, but reduce aerodynamic efficiency in flying beetles. J R Soc Interface 9:2745–2748. https://doi.org/10.1098/rsif.2012.0053
Klink CA, Machado RB (2005) A conservação do Cerrado brasileiro. Megadiversidade 1(1):147–155
Kindt R(2018) Package “BiodiversityR”. Package for community ecology and suitability analysis. Version 1.10-1. The comprehensive R archive network. URL: https://cran.r-project.org/web/packages/BiodiversityR/index.html
Kojima W, Kato T (2017) Correlated evolution between flight habit and diel activity in Coleoptera. Biol J Linn Soc 121:530–539. https://doi.org/10.1093/biolinnean/blx008
Krikken J (1984a) A new key to the suprageneric taxa in the beetle family Cetoniidae, with annotated lists of the known genera. Zool Verh 210:1–175
Krikken J (1984b) A generic reclassification of the afrotropical Bolboceratini (Coleoptera: Geotrupidae). Zool Meded 58(3):23–45
Koschnitzke C(2015) Polinizadores e visitantes florais de três táxons de Asclepiadoideae (Apocynaceae) na restinga de Maricá, Rio de Janeiro, Brasil. Natureza online 13 (4):165–176
Krämer B, Kämpf I, Enderle J, Poniatowski D, Fartmann T (2012) Microhabitat selection in a grassland butterfly: a trade-off between microclimate and food availability. J Insect Conserv 16:857–865. https://doi.org/10.1007/s10841-012-9473-4
Le TQ, Byun D, Saputra Ko JH, Park HC, Kim M (2010) Numerical investigation of the aerodynamic characteristics of a hovering Coleopteran insect. J Theor Biol 266:485–495. https://doi.org/10.1016/j.jtbi.2010.07.013
Le Gall P (2010) Affinités biogéographiques des Insectes du “Dahomey gap” présence d’une popuiation de Goliathus goliatus Linné, 1771, au Bénin (Coleoptera, Scarabaeidae, Cetoniinae). B Soc Entomol Fr 115(1):17–21. https://doi.org/10.3406/bsef.2010.2821
Le Roy C, Debat V, Llaurens V (2019) Adaptive evolution of butterfly wing shape: from morphology to behaviour. Biol Rev 94:1261–1281. https://doi.org/10.1111/brv.12500
Luederwaldt G (1911) Quatro lamellicorneos termitophilos. Revista del Museo Paulista 8:405–413
Mendonça RD, Felfili JM, Walter BMT, Silva Júnior MD, Rezende AV, Filgueiras TDS, Nogueira PE, Fagg CW (2008) Flora vascular do bioma Cerrado: checklist com 12.356 espécies. In: Sano SM, Almeida SP, Ribeiro JF (eds) Cerrado: ecologia e flora. Embrapa Cerrados. Planaltina –DF, pp 422–442
Mendoza C (1994) A theorem for Rayleigh’s method of dimensional analysis and its proof. Mech Res Commun 21:103–107
Micó E, Smith A, Morón MA (2000) New larval descriptions for two species of Euphoria Burmeister (Coleoptera: Scarabaeidae: Cetoniinae: Cetoniini: Euphoriina) with a key to the known larvae and a review of the larval biology. Ann Entomol Soc Am 93:795–801. https://doi.org/10.1603/0013-8746(2000)093[0795:NLDFTS]2.0.CO;2
Molina JM (2001) Incidencia de Tropinota squalida (Scopoli, 1783) (Coleoptera: Scarabaeidae) en el cultivo del arándano en Huelva (España): problemática asociada a su control. Zapateri: Revista Aragonesa de Entomología 9:93–98
Morais HC, Diniz IR (1999) Caterpillar seasonality in a central Brazilian Cerrado. Rev Biol Trop 47:1025–1033
Moral RA, Hinde J, Demétrio CGB (2017) Half-normal plots and overdispersed models in R: The hnp Package. — J. Stat. Softw
Morón MA (1983) Los estados immaduros de Inca clathrata sommeri Westwood (Coleoptera, Melolonthidae, Trichiinae); con observaciones sobre el crecimiento alometrico del imago. Folia Entomol Mex 56:31–51
Morón MA, Arce R (2002) Descriptions of the immature stages of five mexican species of Gymnetini (Coleoptera: Scarabaeidae: Cetoniinae). P Entomol Soc Wash 104:1036–1054
Oliveira CM, Frizzas MR (2008) Insetos de Cerrado: distribuição estacional e abundância. Boletim de pesquisa e desenvolvimento. Embrapa Cerrados, p 26
Oliveira CP, Oliveira CM, Specht A, Frizzas MR (2021) Seasonality and distribution of Coleoptera families (Arthropoda, Insecta) in the Cerrado of Central Brazil. Rev Bras Entomol 65(3). https://doi.org/10.1590/1806-9665-RBENT-2021-0025
Orozco J (2012) Monographic revision of the American genus Euphoria Burmeister, 1842 (Coleoptera: Scarabaeidae: Cetoniinae). Coleop Bull 11:1–182. https://doi.org/10.1649/0010-066X-66.mo4.1
Orozco J, Pardo-Locarno LC (2004) Description of immature stages of three species of American Cetoniinae (Coleoptera: Scarabaeidae: Cetoniinae). Zootaxa 769:1–14. https://doi.org/10.11646/zootaxa.769.1.1
Pacheco FC, Deloya C, Cortes GP (2006) Phytophagous scarab beetles from the Central Region of Guerrero, Mexico (Coleoptera: Scarabaeidae: Melolonthinae, Rutelinae, Dynastinae, Cetoniinae). Rev Colomb Entomol 32(2):191–199
Peter CI, Johnson SD (2009) Pollination by flower chafer beetles in Eulophia ensata and Eulophia welwitschii (Orchidaceae). S Afr J Bot 75:762–770. https://doi.org/10.1016/j.sajb.2009.07.008
Pinheiro F, Diniz IR, Coelho D, Bandeira MPS (2002) Seasonal pattern of insect abundance in the Brazilian Cerrado. Austral Ecol 27(2):132–136. https://doi.org/10.1046/j.1442-9993.2002.01165.x
PROGRAMA ECOLÓGICO DE LONGA DURAÇÃO (PELD). Áreas de estudo. Disponível em: http://www.peld.unb.br/index.php?option=com_content&view=article&id=8&Itemid=10 Acesso em: 26 de junho de 2018
Puker A, Ad’vincula HL, Korasaki V, Ferreira FNF, Orozco J (2014) Biodiversity of Cetoniinae beetles (Coleoptera: Scarabaeidae) in introduced and native habitats in the Brazilian Atlantic Forest. Entomol Sci 17:309–315. https://doi.org/10.1111/ens.12069
Puker A, Correia CMA, Silva AS, Silva JVO, Korasaki V, Grossi PC (2020a) Effects of fruit-baited trap height on flower and leaf chafer scarab beetles sampling in Amazon rainforest. Entomol Sci 23(3):245–255. https://doi.org/10.1111/ens.12418
Puker A, Ferreira KR, Correa CMA (2020b) Sampling flower chafer beetles (Coleoptera: Cetoniidae) in the Amazon Rainforest: the role of bait types and trap installation heights. Environ Entomol 49(5):1096–1104. https://doi.org/10.1093/ee/nvaa097
Puker A, Lopes-Andrade C, Rosa CS, Grossi PC (2012) New records of termite hosts for two species of Hoplopyga, with notes on the life cycle of Hoplopyga brasiliensis (Coleoptera: Scarabaeidae: Cetoniinae). Ann Entomol Soc Am 105:872–878. https://doi.org/10.1603/AN12068
Puker A, Rosa CS, Orozco J, Solar RR, Feitosa RM (2015) Insights on the association of American Cetoniinae beetles with ants: Myrmecophily in beetles. Entomol Sci 18:21–30. https://doi.org/10.1111/ens.12085
Ramos FA, Diniz IR (1993) Seasonal cycles, survivorship and growth of colonies of Polistes versicolor (Hymenoptera: Vespidae) in the urban area of Brasília. Brazil Entomol 112:191–200
R CORE TEAM (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www. R-project.org/
Redford KH (1984) The termitaria of Cornitermes cumulans (Isoptera, Termitidae) and their role in determining a potential keystone species. Biotropica 16(2):112–119
RESERVA ECOLÓGICA DO IBGE (RECOR). Conheça a RECOR. Disponível em: http://www.recor.org.br/cid360/conheca-a-recor.html Acesso em: 25 de outubro de 2016
Ribeiro JF, Walter BMT (2008) As principais fitofisionomias do bioma Cerrado. In: Sano SM, Almeida SP (eds) Cerrado: ecologia e flora. Embrapa Cerrados. Planaltina –DF, pp 89–166
Ribeiro PHO, Togni PHB, Frizzas MR (2022) Spatial and temporal segregation in dung beetles (Coleoptera: Scarabaeinae) in the Cerrado of Central Brazil. https://doi.org/10.1007/s10531-022-02453-2. Biodivers Conserv
Ritcher PO (1958) Biology of Scarabaeidae. Annu Rev Entomol 3:311–334
Rodrigues SR, Oliveira JLN, Bagnara CAC, Puker A (2013) Cetoniinae (Coleoptera: Scarabaeidae) attracted to fruit-baited traps near Aquidauana, Mato Grosso Do Sul, Brazil. Coleopt Bull 67(2):119–122. https://doi.org/10.1649/0010-065X-67.2.119
Sano EE, Rosa R, Brito JLS, Ferreira LG (2010) Land cover mapping of the tropical savanna region in Brazil. Environ Monit Assess 166:113–124
Shaughney JM, Ratcliffe BC (2015) A monographic revision of the genus Hoplopyga Thomson, 1880 (Coleoptera: Scarabaeidae: Cetoniinae: Gymnetini). Coleopt Bull 69:579–638. https://doi.org/10.1649/0010-065X-69.4.579
Silva FAM, Assad ED, Evangelista BA(2008) Caracterização climática do bioma Cerrado. In: Sano SM, Almeida SP, Ribeiro JF (eds). Cerrado: Ecologia e Flora. Embrapa Cerrados e Embrapa Informação Tecnológica. Brasília, pp. 69–88
Silva NAP, Frizzas MR, Oliveira CM (2011) Seasonality in insect abundance in the Cerrado of Goiás State, Brazil. Rev Bras Entomol 55(1):79–87. https://doi.org/10.1590/S0085-56262011000100013
Singer RB, Cocucci AA (1997) Pollination of Pteroglossaspis ruwenzoriensis (Rendle) rolfe (Orchidaceae) by beetles in Argentina. Bot Acta 110:338–342
Šípek P, Fabrizi S, Eberle J, Ahrens D (2016) A molecular phylogeny of rose chafers (Coleoptera: Scarabaeidae: Cetoniinae) reveals a complex and concerted morphological evolution related to their flight mode. Mol Phylogenet Evol 101:163–175. https://doi.org/10.1016/j.ympev.2016.05.012
Sousa R, Fuhrmann J, Kouklík O, Šípek P (2018) Immature stages of three species of Inca LePeletier & Serville, 1828 (Coleoptera: Scarabaeidae: Cetoniinae) and morphology of phytophagous scarab beetle pupa. Zootaxa 4434(1):65–88. https://doi.org/10.11646/zootaxa.4434.1.4
Subchev MA, Toshova TB, Andreev RA, Petrova VD, Maneva VD, Spasova TS, Marinova NT, Minkov PM, Velchev DI (2011) Employing floral baited traps for detection and seasonal monitoring of Tropinota (Epicometis) hirta (Poda) (Coleoptera: Cetoniidae) in Bulgaria. Acta Zool Bulg 63(3):269–276
Tejada G, Morón MA (2015) Exceptional observations on species of Gymnetis (Coleoptera: Cetoniidae) in honey bee hives (Hymenoptera: Apidae) in Peru. Acta Zool Mex 31(1):143–145. https://doi.org/10.21829/azm.2015.311533
Thomas JA, Bourn NAD, Clarke RT, Stewart KE, Simcox DJ, Pearman GS, Curtis R, Goodger B (2001) The quality and isolation of habitat patches both determine where butterflies persist in fragmented landscapes. Proc Biol Sci 268:1791–1796. https://doi.org/10.1098/rspb.2001.1693
Thomas JA, Simcox DJ, Wardlaw JC, Elmes GW, Hochberg ME, Clarke RT (1998) Effects of latitude, altitude and climate on the habitat and conservation of the endangered butterfly Maculinea arion and its Myrmica ant hosts. J Insect Conserv 2:39–46. https://doi.org/10.1023/A:1009640706218
Tisott ST, Schmidt V (2021) Expansion and intensification of agricultural crops in the Cerrado biome in the Center-West Region of Brazil. Braz J of Bus 3(3):2280–2294. https://doi.org/10.34140/bjbv3n3-020
Torretta JP, Navarro F, Medan D (2009) Visitantes florales nocturnos del girasol (Helianthus annuus, Asterales: Asteraceae) en la Argentina. Rev Soc Entomol Argent 68:339–350
Turner AM (1997) Contrasting short-term and long-term effects of predation risk on consumer habitat use and resources. Behav Ecol 8(2):120–125. https://doi.org/10.1093/beheco/8.2.120
Valois M, Silva F (2015) A new species of Golinca Thomson (Coleoptera: Scarabaeidae: Cetoniinae): first record of the genus for Brazil. Zootaxa 3919(1):192–196. https://doi.org/10.11646/zootaxa.3919.1.9
Vaz-de-Mello FZ, Puker A(2021) Cetoniidae in Catálogo Taxonômico da Fauna do Brasil. PNUD. Disponível em: <http://fauna.jbrj.gov.br/fauna/faunadobrasil/115404. Acesso em: 28 Abr. 2021
Vuts J, Zoltán I, Tóth M (2008) Development of an attractant-baited trap for Oxythyrea funesta Poda (Coleoptera: Scarabaeidae, Cetoniinae). Z Naturforsch 63(9–10):761–768. https://doi.org/10.1515/znc-2008-9-1023
Vuts J, Szarukán I, Subchev M, Toshova T, Tóth M (2009) Improving the floral attractant to lure Epicometis hirta Poda (Coleoptera: Scarabaeidae, Cetoniinae). J Pest Sci 83:15–20. https://doi.org/10.1007/s10340-009-0263-z
Whittaker RH (1960) Vegetation of the Siskiyou mountains, Oregon and California. Ecol Monogr 30:279–338
Wolda H (1978) Seasonal fluctuations in rainfall, food and abundance of tropical insects. J Anim Ecol 47(2):369–381
Acknowledgements
The authors thank the University of Brasília and the Graduate Program in Zoology for the infrastructure made available for this study, and the Coordination for the Improvement of Higher Education Personnel (CAPES) for providing scholarships to the first author and PPG/ZOO (04/2018) for financing a part of this project. We thank Marcus Vinícius Celani Rocha, Isabela Silva Oliveira, Maycon Vinícius Laia de Aquino, Túlio Martins Campo e Thales de Castro Silva for their great help in the field and Fernando Zagury Vaz-de-Mello (UFMT) for helping with Cetoniidae identification. The MRF has a CNPq (National Council for Scientific and Technological Development) fellowship (process # 313952/2018-3). The collection was performed with permits from ICMBio (license # 53797-1).
Funding
This work was supported by project 04/2018 of the Programa de Excelência Acadêmica of the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - PROEX/CAPES fornecido pelo Programa de Pós-Graduação em Zoologia (PPG/ZOO). Author J.E. has received scholarships from the Coordination for the Improvement of Higher Education Personnel (CAPES), and the author M.R.F. received research support from Fundação de Apoio a Pesquisa do Distrito Federal (FAPDF; process # 0193.000958/2015) and fellowship from the National Council for Scientific and Technological Development (CNPq - process # 313952/2018-3).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Data collection and the first draft of the manuscript were performed by Juliane Evangelista. The analysis was performed by Charles Martins de Oliveira. Marina Regina Frizzas provided logistic support, guidance, and proofreading of the manuscript. All authors commented on the previous versions of the manuscript and have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Communicated by P. Ponel.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Evangelista, J., Oliveira, C.M. & Frizzas, M.R. Open vegetation formations (grasslands and savannahs) support a higher diversity of Cetoniidae (Insecta: Coleoptera) than forest formations in the brazilian Cerrado. Biodivers Conserv 31, 2875–2892 (2022). https://doi.org/10.1007/s10531-022-02467-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-022-02467-w