Abstract
Cerambycidae have an important ecological role in initiating the degradation process of dead wood, but few studies have evaluated Cerambycidae community attributes in relation to ecosystem phenology. We surveyed the cerambicid fauna of the tropical dry forest in Huatulco, Oaxaca, Mexico, and explored the relationship of Cerambycidae species richness and abundance with phenological changes in vegetation. We applied three collecting methods of light traps, direct collection, and Malaise traps to survey Cerambycidae throughout 2005. To determine seasonal variations, we collected samples in the dry season month of February in the rainy season of May–July and August–September, and in the transition months of October and November through. We collected and identified 145 species, 88 genera, 37 tribes, and four subfamilies. The subfamily with the highest number of species was Cerambycinae (100 species), and the tribe with the highest number of genera and species was Elaphidiini with 13 genera and 33 species. The ICE non-parametric estimator determined an overall expected richness of 373 species, while the overall Shannon Diversity Index was 4.1. Both species richness and abundance varied seasonally, with the highest values recorded in the rainy season and the lowest in the dry season. Overall species abundance was not significantly correlated to monthly rainfall or EVI neither, only for “direct collecting” the EVI vs Richness and EVI vs Shannon Diversity Index were significantly correlated. We propose that the seemingly contradictory relationships between seasonal richness patterns of Cerambycidae and the greening/senescence of vegetation (EVI) may be explained by the seasonal availability of dead organic matter, flowers, or leafy vegetation that may be synchronized with the behavior of different cerambycid species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Cerambycidae family is one of the most diverse groups of the Coleoptera order, accounting for 35,000 known species worldwide (Svacha & Lawrence 2014). Individuals inhabit temperate and tropical forests where they have an ecological role in initiating the degradation process of dead wood, providing an important contribution to the ecosystem’s nutrient cycle (Linsley 1961). Despite such an important ecological role, most of the published research on Cerambycidae deals with taxonomic and natural history aspects (Handley et al 2015). Various studies have described the phenology of Cerambycidae in temperate and tropical communities (Holdefer et al 2015, Handley et al 2015, Hanks et al 2014, Huang et al 2015, Noguera et al 2002, 2007, 2009, 2012), but as far as we know, few studies have explicitly evaluated Cerambycidae community attributes of species richness and diversity in relation to ecosystem phenology.
MODIS (moderate-resolution imaging spectroradiometer) vegetation indices provide spatial and temporal data on the condition of vegetation worldwide and can be used to monitor regional photosynthetic activity and support the interpretation of changes in phenological, biophysical, and land cover conditions (Huete et al 1999). Vegetation indices obtained from remote sensing data have been linked to estimates of biodiversity and land cover heterogeneity (Gould 2000, Kerr et al 2001, Negandra 2001, Oindo & Skidmore 2002, Debinski et al 2006). In particular, several studies have shown how the greening/senescence of forests can be identified by using satellite data, relating vegetation conditions to the presence and activity of insect species such as bettles and moths (Jepsen et al 2009). In a review of how remote sensing has been used to assess faunal diversity, Leyequien et al (2007) conclude that synergy between remote sensing and ecological biodiversity models seems to be a promising approach for assessment, monitoring, prediction, and conservation of faunal diversity. Multiple-date images containing information on structural and phenological properties of vegetation have also significantly improved accuracy for mapping tropical dry forest (Tottrup 2004).
The tropical dry forest is one of the most diverse ecosystems in the Americas and is also one of the most threatened (Janzen 1988). In Mexico, tropical dry forest occurs southwards from the 29° parallel (northern latitude) to the Mexico-Guatemala border (Trejo 2010) and has the largest geographic extent among tropical forests (Dirzo & Ceballos 2010), covering an area equivalent to 8% of the country’s total area (Trejo & Dirzo 2000). Currently, only about 30% of the original extent of tropical dry forest is considered to be in a conserved condition with structurally recognized tree composition and sizes (Trejo 2010), while the rest has been significantly modified by human activities, mainly agriculture and cattle-grazing (Toledo 1992, Maass 1995). Although the deforestation rate for tropical dry forest in Mexico is still under assessment, regional statistics provide estimates that reveal the scale of deforestation. For instance, in the state of Morelos, the deforestation rate is estimated to be 1.4% annually (Trejo & Dirzo 2000), suggesting the need to implement measures to protect and conserve the remaining areas of tropical dry forest.
In the present study, we aim to obtain a better understanding of the local diversity of the Cerambycidae family by exploring the relationship of community attributes with ecosystem phenology, in a site where dry forest cerambicids have been surveyed for more than 20 years (Chemsak & Noguera 1993; Noguera et al 2002, 2007, 2009, 2012; Toledo et al 2002; González-Soriano et al 2008, 2009; Zaragoza-Caballero et al 2010). In particular, we evaluate the relationship between seasonal variation in Cerambycid abundance and species richness with variation in estimates of the amount of green vegetation.
Materials and Methods
Study area
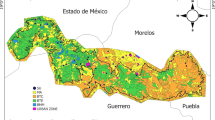
The Huatulco region belongs to the physiographic province known as Costas del Sur on the Pacific Mexican coast (Cervantes-Zamora et al 1990; see Fig 1). This system consists of medium-sized mountains, complex hills and fluvial plains (CONANP 2013). The area is located in the Santa María Huatulco county, next to the Huatulco National Park, a 11,890-ha natural protected area (6374 ha terrestrial and 5516 ha marine). The region comprises part of the southeastern sector of the Río Copalita watershed that is structurally influenced by the slopes of the Cordillera Costera del Sur physiographic province. Some of the streams and rivers included are Cuajinicuil-Xúchilt, Todos Santos, Cacaluta, Tangolunda, Chachacual, and El Arenal (CONANP 2013).
Location of study area. Inset (a) shows in gray the state of Oaxaca within Mexico and the study area located southern the state near by the Pacific Coast. Inset (b) shows the main vegetation types obtained from INEGI (2013) with the rectangle of black border being the inset (c). Inset (c) shows the study area with the location of sampling sites.
According to the Köpen climate classification modified by García (1981), the region’s climate is hot sub-humid, type Awo (w)(i). The rainfall regime is torrential (heavy rain) of short duration with a mean annual rainfall between 1000 and 1500 mm almost 97% of which occurs in the months of June–October. The drought period extends from November to April with occasional aseasonal rain. The mean annual temperature is 28°C with thermic oscillations lower than 5°C.
The dominant vegetation type is tropical dry or deciduous forest (Salas-Morales et al 2007, Corona et al 2016). This vegetation type is dominated by short trees with a continuous canopy. The annual rainfall is <1600 mm per year with an evident seasonality, where at least 5 months of the year have <100 mm of rainfall causing the vegetation to drop leaf-cover (Trejo 2010). Other types of vegetation include riparian, costal dunes, manzanillar (a vegetation type dominated by Hippomane mancinella L., a tree growing on the flood plains, in soils where ground water is shallow), savanna, mangrove, and wetlands (CONANP 2013, Salas-Morales et al 2007).
The region contains 736 species of vascular plants of 391 genera and 91 families. Leguminosae is one of the best represented families with 146 especies, Euphorbiaceae has 48 species, Asteraceae 42, and Convolvulaceae 37 species (Salas-Morales et al 2007). Common tree species of tropical dry forest are Amphipterypgium adstringens (Schlecht.) (Julianiaceae), Bursera simaruba (L.) Sarg. (Burseraceae), Apoplanesia paniculata Presl., Caesalpinia eriostachys Benth., Lonchocarpus constrictus Pitt., Lysiloma microphyllum Benth. (Leguminosae), Ceiba aesculifolia (H. B. K.) Britt. and Baker (Bombacaceae), Cochlospermum vitifolium (Willd.) Spreng (Cochlospermaceae), Spondias purpurea L., Comocladia engleriana Loes (Anacardiaceae), Gyrocarpus jatrophifolius Domin (Hernandiaceae), and Guetarda elliptica Sw. (Rubiaceae). In more humid environments of stream valleys, taller trees stand out, such as Calycophyllum candidissimum (Vahl) DC. (Rubiaceae), Ceiba pentandra Gaertn. (Bombacaceae), Sapium sp. (Euphorbiacae), and Ficus cotinifolia H. B. K. (Moraceae). Characteristic species in the coastal dunes are Prosopis juliflora (Sw.) DC. in DC. (Leguminosae), Genipa sp. (Rubiaceae), Guaiacum coulteri A. Gray (Zygophyllaceae), Bursera excelsa (H. B. K.) Engl., (Burseraceae), Karwinskia humboldtiana (Roem. and Schult) Zucc., Ziziphus amole (Sessé and Moc.) M. C. Johnst. (Rhamnaceae), Ficus goldmanii Standl. (Moraceae), and Stenocerus standleyi (González-Ortega) Buxbaum (Cactaceae). Within cliff environments, there are such species as Bursera excelsa (H. B. K.) Engl. (Burseraceae), Amphipterypgium adstringens (Schlecht.) (Julianaceae), and Jatropha ortegae Standl. (Euphorbiaceae) (CONANP 2013).
Collecting techniques and regimes
The geographic coordinates of collection sites is shown in Supplementary Material Appendix 1 (see Fig 1). Fieldwork was completed between February and November 2005. The collections were taken during six time periods: (1) February 6–10, (2) May 29 to June 3, (3) July 6–10, (4) August 31 to September 5, (5) October 3–4, and (6) November 4–8. Collections were carried out over 5 days in each period, beginning 2 days before the new moon, considering that light traps are most effective when the moon sets early (Janzen 1983). However, in October, it was only possible to collect on 1 day due to the presence of a hurricane in the region.
Collecting methods included light trapping, Malaise trapping, and direct collecting. Light trapping employed a combination of two light sources, one mercury vapor lamp and one Minnesota type light trap (Southwood 1966). The latter had two 20 W UV bulbs (one unfiltered) over a single 20-cm-diameter collection jar filled with 70% ethyl alcohol. The light trap and the mercury vapor lamp were placed against a vertical white sheet measuring 1.8 by 1.5 m. These trap systems were located in five different sites and continuously operated during each collecting session. The traps were operated for 4 h each day (from 1900 to 2300 h in the winter and from 2000 to 2400 h in the summer). Six Malaise traps, based on the Townes model (Townes 1972), were placed in different locations inside the forest and remained in position throughout the year (see descriptions of the collecting sites). Each trap was operated for 5 days in each month. Finally, direct collecting was also undertaken during 5 days each month, concentrated between 0900 and 1500 h (1000–1600 h in the summer). Netting, sweeping, and beating were employed, with efforts focused on flowers and woody vegetation in general.
Abundance, richness, and diversity calculations
The composition and structure of the insect community were analyzed by calculating species richness, total and species abundance, along with indices of diversity and evenness. The values of richness and abundance correspond to the number of species and individuals recorded. Diversity and evenness were calculated using the Shannon Index with the natural logarithm. The Evenness Index was calculated because it is a simpler measure of how abundance is distributed among species, with a value of 1 representing a situation in which all species are equally abundant (Magurran 1988).
where
J′ = Evenness Index
H′ = Shannon diversity Index recorded in this study
H max = is the maximum diversity that could possibly occur (situation where all species had equal abundances)
S = number of species or species richness.
These indices were calculated using the program BioDiversity Pro (McAleece et al 1999).
Considering that an observed number of any sample of individuals from a community rich in species underestimates the true number of species present (Chazdon et al 1998), we used a non-parametric estimator of species richness. Additionally, the estimated richness was plotted as a function of the cumulative number of months sampled to graphically evaluate the results on the estimator. The estimator used was ICE (incidence coverage-based estimator), an incidence-based estimator that better satisfies the requirements for an ideal species richness estimator, which is to say, independence of sample size, insensitiveness to patchiness of species distribution and to the order of samples (Chazdon et al 1998). This is based on those species found in ≤10 sampling units (Colwell 2013). The estimates were calculated using EstimateS 9.1 (Colwell 2013). The species collected within each month were considered one sample unit (six in total).
For phenology analysis of these data, it was considered that the rainy season included the period from May to November and the dry season occurred from December to April.
Plant and cerambycid richness relationships
Based on the working hypothesis that cerambycid species richness and plant richness would have a meaningful relationship, we evaluated the plant-cerambycid richness relationship for sites where cerambycid ensembles have previously been described (Chemsak & Noguera 1993; Noguera et al 2002; Toledo et al 2002; Noguera et al 2009; Noguera et al 2012). We compiled data on plant richness for areas neighboring Cerambycidae survey sites: Alamos, Sonora (neighboring San Javier, Sonora); Trinitaria, Chiapas (neighboring El Aguacero, Chiapas); El Limón, Morelos (neighboring Huautla, Morelos); and Cuicatlán, Oaxaca (neighboring Santiago Dominguillo, Oaxaca). For each of these sites, we used the data provided by Trejo (1998) on plant richness per unit area, while Cerambycid richness was calculated based on previous cerambycid surveys (Chemsak & Noguera 1993; Noguera et al 2002; Toledo et al 2002; Noguera et al 2009; Noguera et al 2012). We applied the ICE estimator to determine cerambycid richness for each site, which was then correlated with plant richness per unit area using the non-parametric Spearman rank correlation.
Remote sensing vegetation indices
The phenological behavior of vegetation was estimated by assembling time series of the MODIS Vegetation Indices dataset (MYD13Q1), which is a 16-day global dataset at 250-m spatial resolution and distributed as a gridded level-3 Aqua/Terra product. These data were obtained from the Data Pool Land Processes Distributed Active Archive Center (USGS; http://e4ftl01.cr.usgs.gov/MOLA/MYD13Q1.005/).
This study used the two MODIS vegetation indexes (VI) products: the standard Normalized Vegetation Index (NDVI) and the Enhanced Vegetation Index (EVI). The NDVI provides continuity to historic datasets while the EVI provides improved sensitivity over high biomass regions and improved vegetation capability through a de-coupling of the canopy background signal and reduction in atmosphere influence (Solano et al 2010). The NDVI is a normalized transform of the near infrared (NIR) to red reflectance ratio (\( \raisebox{1ex}{${\rho}_{\mathrm{NIR}}$}\!\left/ \!\raisebox{-1ex}{${\uprho}_{\mathrm{RED}}$}\right. \)):
As a ratio, the NDVI has the advantage of minimizing certain types of band-correlated noise (positively correlated) and influences attributed to variations in direct/diffuse irradiance, clouds and cloud shadows, sun and view angles, topography, and atmospheric attenuation (Solano et al 2010). On the other hand, the EVI incorporates the Atmospheric Resistant Index (ARVI), along with the removal of soil-brightness-induced variations in VI as in the Soil Adjusted Vegetation Index (SAVI). Additionally, EVI decouples the soil and atmospheric influences from the vegetation signal by including a feedback term for simultaneous correction (Solano et al 2010).
where
ρ x = full or partially atmospheric-corrected (for Rayleigh scattering and ozone absorption) surface reflectances
L = canopy background ajustment for correcting non-linear differerential NIR and red radiant transfer through a canopy
C 1 and C 2 are the coefficients of the aerosol resistence term (which uses the blue band to correct for aerosol influences in the red band)
G = a gain or scaling factor.
The MODIS Vegetation Indexes were correlated and plotted against both species richness and species abundance for three sampling datasets, described below. Initially, the seasonal behavior of vegetation indexes was analyzed on a monthly basis. However, vegetation phenology and species richness/diversity were related at five time periods (February, May/June, July, August/September, and November) because of the necessity for differentiating seasonal climate at the same time of making sure the MODIS data were clear of clouds. Therefore, 15-day MODIS vegetation index images were combined by selecting the highest value and then related to average species richness/diversity. After revising our results, it was apparent that the NDVI data reached higher values than EVI data and how EVI data differentiated values more clearly along the time axis. We believe these circumstances made the EVI more useful for our comparative purposes.
Relationship of species richness and abundance to phenology of tropical dry forest
To relate species richness and diversity with the vegetation indexes, non-parametric correlation calculations were explored along with visual analysis of plotted results. Mean monthly precipitation data (CONGUA 2014) was included to compare the significance of the relationship between diversity/richness and vegetation phenological conditions. The results shown correspond to data obtained from the five sampling localities of site group #1 described above, in which light traps were located. In these sites, collecting efforts were constant to adequately reflect the variation in richness and diversity of the study area.
The analysis was conducted with richness and diversity calculations on three data sets obtained by applying different collection methods: (1) light trap, (2) direct collecting, and (3) the combined values of 1 and 2 (“all samples”). The Malaise trap method was applied but excluded because did not produce records for any species. Previous studies in tropical deciduous forest (Noguera et al 2002, 2007, 2009, 2012) have shown that many species of Cerambycidae respond immediately to the first rainfall events, but other species begin their activity later on as their adults use food resources (flowers or herbaceous plants) not present at the start of the rainy season. In this sense, the light trap sampling registers many of the species of the first group, but not of the second; whereas, the direct collecting method records principally species that visit flowers or use herbaceous plants, and therefore, species of the second group are recorded. Thus, analyzing the three sets of data allowed for more detail in measuring variations of species richness and diversity and their possible relationship with the vegetation indexes. Rainfall data was also used for comparing the significance of such a relationship. Correlation analyses were performed applying the non-parametric Spearman Rho (ρs) using the PASW software version 18.0.0.
Backup samples
All material collected was deposited in the Chamela Research Station’s Entomological Collection which is part of the Intituto de Biologia, Universidad Nacional Autónoma de México.
Results
Species richness
We recorded a total of 145 cerambicid species in Huatulco, of which only 122 could be assigned to a described species, the remainder (24 species) belonged to either problematic taxonomic groups or were undescribed species. Supplementary Material Appendix 2 contains the complete species list. The described species included 32 registered for the first time within the state of Oaxaca (22% of total). The species found belong to 88 genera, 37 tribes, and four subfamilies (Table 1). Cerambycinae was the subfamily with the highest number of species (100 species, 68% of total), followed by Lamiinae with 33 species (23% of total). The same pattern was found for the number of genera/tribes: 62/20 for Cerambycinae and 24/13 for Lamiinae, respectively (Table 1). Elaphidiini and Trachyderini were the tribes with highest numbers of genera/species (13/33 and 13/18, respectively), while 20 tribes consisted of only one species (Supplementary Material Appendix 2).
Genera with highest species richness were Phaea Newman with 7 species, Eburia Lepeletier and Strangalia Audinet-Serville each with 6 species, and Anelaphus Linsley, Psyrassa Pascoe, Obrium Dejean and Ameriphoderes Clarke, each with 5 species. Supplementary Material Appendix 2 contains the species that belong to the complete list of genera. In summary, 85% of genera were represented by only one or two species, with the exception of the seven genera referred to above which included 27% of total registered species.
Estimated richness
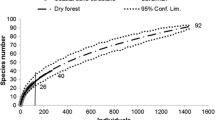
The species richness value obtained from the non-parametric ICE estimate was higher than the observed richness value (373 vs 145, respectively), meaning that only 39% of the local species richness was registered by the field sampling. The species accumulation curve had not yet reached an asymptote, but the observed value approached the ICE estimate (Fig 2).
Abundance
A total of 1093 individuals were collected in this study. The distribution of individuals by species was heterogeneous; a limited number of species were very abundant, with most of them represented by a few individuals (Fig 3a). The most abundant species was Ameriphoderes cribricollis (Bates) with 66 individuals, followed by Stizocera submetallica (Chemsak & Linsley) with 64, Coscinedes gracilis Bates with 62, Stenobatyle eburata (Chevrolat) with 60, S. miniaticollis (Chevrolat) with 57, Pachymerola ruficollis ruficollis Giesbert with 56 and Sternidius naeviicornis (Bates) with 47. On the other hand, there were 51 species with just one individual and 116 species represented by ≤10 individuals.
Species abundance: a relative to overall abundance and b by species according to two data samples, “direct collect samples” (dark gray boxplots) and “light trap” (light gray boxplots). Boxplots show median (darker line) and 25 (box’s bottom) and 75 (box’s top) percentiles of abundance values at the different time periods; the star outliers mean extreme values located >1.5 times than the boxes’ height, while the empty circle outliers are extreme values <1.5 times than the boxes’ height.
Species abundance also varied at the different time periods (Fig 3b), a relationship that enables to compare “direct collection” versus “light trap” collecting methods. Both groups of samples had outlier values with the exception of November, reflecting that a few species contained most of the individuals. Median abundance values were slightly higher for “light trap” during May/June and Aug/Sep, and the opposite occurred for July and November. If the species with highest abundance are excluded (outliers in Fig 3b), it is apparent that for both sampling groups (“direct collection” and “light traps”), there were significant differences in species abundances according to the time period: In May/June, there were 55 species (319 indivuduals) collected by the “light trap” method versus 7 species (16 individuals) obtained by “direct collection.” In the following time period of July, the situation was reversed according to sampling method: 35 species (253 individuals) were obtained by “direct collection” versus 21 species (112 individuals) by “light traps.” This trend remained for the periods Aug/Sep and November, there were higher number of species and abundance collected by the “direct collect” method.
Diversity
Based on the overall number of species throughout the year, we obtained a Shannon Diversity Index of 4.1, with an evenness value of 0.83. Diversity and evenness values varied during the year (Fig 4a). November showed the lowest diversity value (1.7) and May/June and July the highest (3.5 and 3.2, respectively). The highest evenness values were reached in February (0.89) due to the occurrence of a few species with low abundances, while the lowest evenness value of 0.75 occurred in November.
Phenology
Overall, the number of active species varied seasonally, being the highest during the rainy season with 68 species in July at the start of the rainy season (Fig 4b). By comparison, the lowest number of species occurred during the dry season with 12 species in February. Although there were only 14 species in November, considered the end of the rainy season, an overall assessment shows that 118 species (81.3%) were recorded during the rainy season, whereas 19 species (13%) were exclusive of the dry season and 8 species (5.4%) occurred in both seasons. Therefore, the rainy season comprised 86.7% of overall species richness of Cerambycidae. Species abundances also varied seasonally, with 994 individuals (91%) registered during the rainy season, and 99 individuals (9%) registered during the dry season. By survey period, the highest number of individuals (496) occurred in July, while the lowest (21 individuals) occurred in February (Fig 4b).
Plant and cerambycid richness relationships
Linear regression analysis determined a significant positive relationship between plant richness and cerambycid species richness (ρs = 0.829; Sig = 0.042; Fig 5). One site, Cuicatlán, Oaxaca, showed an atypical low value for cerambycid species richness (Fig 5), which if removed, increases the significance of the relationship based on the other 5 sites (ρs = 1.000; Sig = 0.00).
Correlation between plant species richness and cerambycid species at six study sites in Mexico. Chamela, Jalisco; Alamos, Sonora (neighbor of San Javier, Sonora); Trinitaria, Chiapas (neighbor of El Aguacero, Chiapas); El Limón, Morelos (neighbor of Huautla, Morelos); and Cuicatlán, Oaxaca (neighbor of Santiago Dominguillo, Oaxaca).
Species richness and vegetation phenology
As expected, both vegetation indexes (NDVI and EVI) exhibited the seasonal pattern of tropical deciduous forest at the different sampling sites, and were closely correlated to rainfall (ρs = 0.900; Sig = 0.037; for both indexes). For EVI, the drying out and greening-up time periods and rates are clearly differentiated (Fig 6). However, while the EVI shows similar low values from February to May, the NDVI showed greater differentiation for the dry season months of February to May, with the driest month of April having the lowest NDVI (Fig 6). The greening process starts gradually in May and occurs at a faster rate from June to July with onset of the rainy season. The highest EVI occurred in the rainy season months of August and September, with September also having the highest NDVI. Both indexes decreased in October when the drying out trend becomes evident. However, the EVI index decreased faster than the NDVI, where the EVI drops under 0.40 in November, while the NDVI shows a relatively high value >0.75. Both indexes consistently showed the drying out process that continues until January of the following year; however, the EVI more clearly stretches out the drying process.
Non-parametric Spearman rank correlations for the three types of sampling data (all samples, light trap and direct collecting) against both the EVI and the average monthly rainfall regime (CONAGUA 2014) showed that overall species abundance was not correlated with either monthly rainfall or EVI. This is true even though it would seem there is a close relationship between species abundance and monthly rainfall and EVI, particularly during the first three time periods (Fig 7a, b). Only for “direct collecting,” the EVI vs Richness and EVI vs Shannon Diversity Index were significantly correlated (ρs = 0.900; Sig = 0.037), while both “all samples” and “light traps” datasets showed very poor correlations between EVI or precipitation against species diversity and species richness.
Examining the relationship between EVI values and species richness and diversity showed two types of relationship, one for the “all samples” and “light traps” data sets and another for the “direct collection” data set (Fig 7c, e). For the former two methods, there was a slow increase in EVI values from February to May–June coinciding with a rapid and single increase in both species richness and species diversity (Fig 7c, e). From May–June to July, there was a rapid increase in EVI, while richness and diversity clearly dropped. EVI then increased slightly over the rainy season until Aug/Sep, and then fell rapidly, while richness and diversity continuously dropped until reaching minimum values in November (Fig 7c, e).
Direct collection showed a different relationship where EVI and Cerambicydae species richness and diversity were positively correlated (ρs = 0.900; Sig = 0.037). The increase shown by the EVI from February to May–June and from May–June to July coincided with increases in Cerambycidae richness and diversity. This resembled the strong correlation of EVI with monthly precipitation (Fig 6a). The lack of correlation between precipitation and Cerambycidae richness and diversity was highlighted by the pattern observed from July to August–September when precipitation increased, while both diversity and richness of Cerambycidae decreased (Fig 7d, f).
Captures by collecting method
For the three collecting methods, 82 taxa were collected by direct collecting, 75 species were collected at light traps, but no species were obtained by Malaise traps. There were 12 species that were collected by both direct collecting and light traps, 70 species obtained only by direct collection, and 63 species obtained only with light traps. Hence, direct collecting was marginally the most productive method, recording 57% of the species, followed by light collecting with 52% of species. Considering only species collected exclusively by one of these methods, direct collecting obtained 48% of species, and light traps obtained 43% of species.
Comparison with other tropical dry forest regions
The number of Cerambycidae species recorded in Huatulco, Oaxaca, was lower than the number of species recorded in other regions of Mexico, with 306 species in Chamela, Jalisco (Chemsak & Noguera 1993), 153 species in Huautla, Morelos (Noguera et al 2002), and 203 species in El Aguacero, Chiapas (Toledo et al 2002). Other regions with fewer Cerambycidae species include San Buenaventura, Jalisco (109 species; Noguera et al 2007), San Javier, Sonora (82 species; Noguera et al 2009), and Santiago Dominguillo, Oaxaca (97 species; Noguera et al 2012).
Of the 122 species identified in the region of Huatulco Oaxaca, 88 (72%) are shared with the region of Chamela (Chemsak & Noguera 1993), 47 (39%) with Huautla (Noguera et al 2002), and 48 (39%) with El Aguacero (Toledo et al 2002). Species exclusively recorded in Huatulco, as compared with those mentioned above, were Lasiogaster costipennis Gahan, Strangalia xanthotela (Bates), Eburia (Eburia) aegrota Bates, Eburia (E.) stigma (Olivier), Anelaphus undulatum (Bates), Mephritus apicatus (Linsley), Micropsyrassa glabrata Martins and Chemsak, Diasporidion duplicatum (Gounelle), Piezocera serraticollis Linell, Lophalia cribricollis (Bates), Tessarecphora aracnoides centralis Monné, Oncideres pallifasciata Noguera, and Phaea marthae Chemsak.
Huatulco shared the following genera with other regions: 81 (92%) with Chamela, 70 (80%) with Huautla, 55 (63%) with El Aguacero, 48 (55%) with San Buenaventura (Noguera et al 2007), 46 (52%) with San Javier (Noguera et al 2009), and 41 (47%) with Dominguillo (Noguera et al 2012). Indeed, only four genera are exclusive from Huatulco, as compared to the other tropical dry forest referred above: Lasiogaster Gahan, Mephritus Pascoe, Tessarecphora Thomson, and Eranina Monné. The last three groups have a neotropical distribution, while Lasiogaster is a monotipic genus, only distributed in México and Central America (Monné 2015a, b, c).
At a continental level, 50 of the species identified in this study have only been recorded from Mexico, 47 species extend their distribution between Mexico and Central America, 5 between Mexico and South America, 6 between United States of America (USA) and Mexico, 8 between USA and Central America, 2 between USA, Central America and The Antilles, 2 between USA to South America and The Antilles, 1 between Canada and Mexico and 1 between Canada and Central America. These values indicate that 41% of the Cerambycidae species collected in Huatulco are endemic to Mexico.
Discussion
These results significantly contribute to the series of studies developed by our team across the country to better understand the composition, distribution, and abundance of cerambycid species, along with the identification of key associated environmental factors. Species richness showed similar local and temporal patterns as previous studies (Noguera et al 2002, 2007, 2009, 2012, Toledo et al 2002).
Estimates of species richness indicate that not all potential species were collected, and we had 62% of species that were collected in just 1 month, with 36% of species represented by only one individual, and 70% had ≤5 individuals. This underestimate of species richness may be due to methodological aspects of sampling design, the role of environmental heterogeneity, and the natural history of Cerambycidae. Our sampling methodology involved just five collecting days per month, which given the short life span of Cerambycidae species means that species with short activity periods may not have been collected because of the mismatch in timing between the period the species is active and the days when sampling was carried out. Alternatively, the sampling design may not have been efficient enough to deal with the high heterogeneity of tropical dry forest (Trejo 1998), and its influence on the local and seasonal rarity of some cerambycid species.
The lower than expected species richness could also be associated with human induced processes such as land use/land cover changes occurring at local and regional scales. Deforestation may have caused local extinction or drastic reduction of some plant species important for cerambycid species, particularly if other sources of food have disappeared or have been drastically diminished, or if cerambycids show very specialized food habits.
Although the estimated species curve was far from reaching an asymptote, meaning that richness values can significantly vary, the estimated richness value of 373 species is higher than the 306 Cerambycidae species recorded at the Chamela tropical dry forest site, which has been to date the site with the highest number of recorded Cerambycidae species (Chemsak & Noguera 1993). In contrast with other sites, data from Chamela were gathered over 10 years of field work, enabling us to set this as a base line for comparison purposes. Huautla, Morelos had an estimated number of species of 251 (Noguera et al 2002), El Aguacero, Chiapas with 228 (Toledo et al 2002), San Buenaventura, Jalisco with 151 (Noguera et al 2007), San Javier, Sonora with 121 (Noguera et al 2009) and Santiago Dominguillo, Oaxaca had 97 species (Noguera et al 2012).
The Huatulco site located within the Huatulco National Park is well preserved, similar to the conservation status of the tropical dry forest at the Chamela site (Lira & Ceballos 2010). In contrast, the other sites sampled in Mexico have different levels of disturbance. It is possible therefore that differences in local disturbance regimes and plant richness are two of the main factors driving cerambycid species richness. Even though such a hypothesis needs to be specifically tested, Huatulco and Chamela are clearly the two sites with the highest estimated and actual recorded cerambycid species richness and the best preserved natural areas. This association of species richness with forest conservation has been documented for cerambycid species in other regions (Makino et al 2007, Meng et al 2013) and for other groups of insects (Hanski et al 2007, Diniz et al 2010, Kambach et al 2013).
Regarding plant species richness, the Huatulco region seems to be poorly documented. CONANP (2013) reports only 413 plant species, a low number when compared to the 1149 plant species reported for the Chamela region (Lott 2002). However, in a study of plant richness in Mexico’s tropical dry forests, Trejo (1998) reported a richness of 107 plant species per area unit (0.1 ha) for Copalita, an area adjacent to the Huatulco region, which is very similar to the equivalent parameter of 103 plant species/0.1 ha found in Chamela, Jalisco (Lott et al 1987). The close positive relationship between plant and cerambycid species richness was clearly revealed by our correlation analysis for six study sites in Mexico and corresponds with the correlation of plant-insect richness found in other studies (STRI 2006, Graham et al 2012, Meng et al 2013).
The seasonal pattern of cerambycid species richness and diversity identified in this study is similar to that found in other regions of Mexico where the activity of adult individuals is concentrated during the rainy season. The explanation of this seems to be straightforward; the combination of most of species’ food habits and the higher availability of food and other resources during the rainy season. Vegetation indexes obtained from remote sensing data are demonstrated to be good predictors of ecosystem productivity (Buermann et al 2002, Wang et al 2005). However, in the present study, we found that richness and diversity of cerambycid species were related to the EVI vegetation index in two ostensibly contradictory directions: by separating the data by collecting method and allowing the identification of potentially meaningful relationships. For direct collection, EVI was significantly correlated to Cerambycidae community attributes, where species richness and diversity of cerambycids increased as greenness increased and decreased as vegetation dried out. This may be explained as adult cerambycid obtained by direct collecting feed mainly on flowers, herbs, and trees that blossom in sync with the greening of vegetation (F. A. Noguera, personal observation). Resources used by these adult individuals represent needed nutrients for sexual maturation and increased fertility for some female individuals (e.g., Li & Liu 1997), promoting also the successful location of potential mates (Hanks 1999). Species of adult individuals directly collected also use the death matter for larvae development; such resources are derived from branches falling on the ground because of storm winds and the increase of humidity (weigh) during the rainy season.
On the other hand, the light trap sampling method showed a non-linear relationship between EVI and cerambycid richness and diversity. Two steps can be visualized to explain this relationship. In the first part of the phenological cycle prior to EVI greening of the vegetation, the activity peak of cerambycids collected by light traps occurred in May/June at the end of the dry season and 2 months before the peak in rainfall and greening of vegetation. This is when a maximum amount of recently dead trees and fallen branches occur on the ground (Martínez-Yrízar 1995). This decomposing organic matter may provide resources for the larvae of most cerambycid species (Linsley 1961); hence, it can be hypothesized that although species collected by light traps are as strongly associated with decomposing organic matter as with the abundance of green organic matter, they take advantage of tree and branches which die at the end of the dry season. In the second part of the phenological cycle when high values of EVI indicate vegetation greenness (July to November), richness and diversity of cerambicid species, collected by either direct or light trapping, dropped rapidly. This relationship seems to be related to a combination of conditions such as changes in the quality of resources available (green and death matter) and higher cost for reproduction.
References
Buermann W, Wang Y, Dong J, Zhou L, Zeng X, Dickinson RE, Potter CS, Myneni RB (2002) Analysis of a multiyear global vegetation leaf area index data set. J Geophys Res 107(D22):4646. doi:10.1029/2001JD000975
Cervantes-Zamora Y, Cornejo-Olgín SL, Lucero-Márquez R, Espinoza-Rodríguez JM, Miranda-Viquez E, Pineda-Velázquez A (1990) Provincias Fisiográficas de México. Extracted from Regiones Naturales de México II, IV.10.2. Atlas Nacional de México. Vol. II. Escala 1:4000000. Instituto de Geografía, UNAM, México
Chazdon RL, Colwell RK, Denslow JS, Guariguata MR (1998) Statistical methods for estimating species richness of woody regeneration in primary and secondary rain forests of Northeastern Costa Rica. In: Dallmeier F, Comiskey JA (eds) Forest biodiversity research, monitoring and modeling. Conceptual background and old world case studies. UNESCO and The Parthenon Publishing Group, Paris, pp 285–309
Chemsak JA, Noguera FA (1993) Annotated checklist of the Cerambycidae of the Estación de Biología Chamela, Jalisco, México (Coleoptera), with descriptions of a new genera and species. Folia Entomol Mex 89:55–102
Colwell RK (2013) Estimates: statistical estimation of species richness and shared species from samples. Version 9 and earlier. User’s Guide and application. http://viceroy.eeb.uconn.edu/estimates/. Accessed 11 Jan 2016
CONAGUA (Comisión Nacional del Agua) (2014) Servicio Meteorológico Nacional. Normales Climatológicas para el Estado de Oaxaca 1951–2010. http://smn.cna.gob.mx/climatologia/Normales5110/NORMAL20194.TXT/ Accessed 11 Jan 2016
CONANP (Comisión Nacional de Áreas Protegidas) (2013) Programa de Manejo Parque Nacional Huatulco. Comisión Nacional de Áreas Protegidas, México, p 208
Corona R, Galicia L, Palacio-Prieto JL, Burgi M, Hersperger A (2016) Local deforestation patterns and their driving forces of tropical dry forest in two municipalities in Southern Oaxaca, Mexico (1985–2006). Inv Geog, Bol Ins Geog. doi:10.14350/rig.50918
Debinski DM, VanNimwegen RE, Jakubauskas ME (2006) Quantifying relationships between bird and butterfly community shifts and environmental change. Ecol Appl 16:380–393
Diniz S, Prado PI, Lewinsohn TM (2010) Species richness in natural and disturbed habitats: Asteraceae and flower-head insects (Tephritidae: Diptera). Neotrop Entomol 39:163–171
Dirzo R, Ceballos G (2010) Las selvas secas de México: un reservorio de biodiversidad and laboratorio viviente. In: Ceballos G, Martínez L, García A, Espinoza E, Bezaury-Creel J, Dirzo R (eds) Diversidad, amenazas and áreas prioritarias para la conservación de las selvas secas del Pacífico de México. Fondo de Cultura Económica/CONABIO, México, pp 13–17
García E (1981) Modificaciones al sistema de clasificación climática de Köpen. Instituto de Geografía, Universidad Nacional Autónoma de México, México, D.F. p 90
González-Soriano E, Noguera FA, Zaragoza-Caballero S, Morales-Barrera MA, Ayala-Barajas R, Rodríguez-Palafox A, Ramírez-García E (2008) Odonata diversity in a tropical dry forest of Mexico. I. Sierra de Huautla, Morelos. Odonatologica 37:305–315
González-Soriano E, Noguera FA, Zaragoza-Caballero S, Ramírez-García E (2009) Odonata de un bosque tropical caducifolio: sierra de San Javier, Sonora, México. Rev Mex Biodivers 80:341–348
Gould W (2000) Remote sensing of vegetation, plant species richness, and regional biodiversity hotspots. Ecol Appl 10:1861–1870
Graham EE, Poland TM, McCullough DG, Millar JG (2012) A comparison of trap type and height for capturing cerambycid beetles (Coleoptera). J Econ Entomol 105:837–846
Handley G, Hough-Goldstein J, Hanks LM, Millar JG, D`Amico V (2015) Species richness and phenology of cerambycid beetles in urban forest fragments of northern Delawere. Ann Entomol Soc Am 108(3):251–262
Hanks LM (1999) Influence of the larval host plant on reproductive strategies of cerambycid beetles. Annu Rev Entomol 44:483–505
Hanks LM, Reagel PF, Mitchell RF, Wong JCH, Meier LR, Silliman CA, Graham EE, Striman BL, Robinson KP, Mongold-Diers JA, Millar JG (2014) Seasonal phenology of the cerambycid beetles of east-central Illinois. Ann Entomol Soc Am 107(1):211–226
Hanski I, Koivulehto H, Cameron A, Rahagalala P (2007) Deforestation and apparent extinctions of endemic forest beetles in Madagascar. Biol Lett 3:344–347. doi:10.1098/rsbl.2007.0043
Holdefer DR, Mello-Garcia FR (2015) Análise faunística de cerambicídeos (Coleoptera, Cerambycidae) en floresta subtropical úmida brasileira. Entomotropica 30(13):118–134
Huang J, Zhang J, Li M-J, Xia T-F (2015) Seasonal variations in the incidence of Monochamus alternatus adults (Coleoptera: Cerambycidae) and other major Coleoptera: a two-year monitor in the pine forests of Hangzhou, Eastern China. Scandinavian J F Res 30(6):507–515
Huete A, Justice C, van Leeuwen W (1999) MODIS VEGETATION INDEX (MOD13) algorithm theoretical basis document version 3. University of Virginia, Department of Environmental Sciences, Charlottesville
INEGI (Instituto Nacional de Estadística, Geografía e Informática) (2013) Conjunto de datos vectoriales de Uso de Suelo y Vegetación Escala 1:250 000, Serie V (Capa Unión)
Janzen DH (1983) Insects: introduction. In: Janzen DH (ed.) Costa Rican Natural History. The University of Chicago Press, Chicago, pp 619–645. http://www.inegi.org.mx/gep/contenidos/recnat/usossuelo/Default.aspx/. Accessed 24 June 2015
Janzen DH (1988) Tropical dry forest. The most endangered major tropical ecosystem. In: Wilson EO (ed) Biodiversity. National Academy Press, Washington, DC, pp 130–137
Jepsen JU, Hagen SB, Høgda KA, Ims RA, Karlsen SR, Tømmervik H, Yoccoz NG (2009) Monitoring the spatio-temporal dynamics of geometrid moth outbreaks in birch forest using MODIS-NDVI data. Remote Sens Environ 113:1939–1947
Kambach S, Guerra F, Beck SG, Hensen I, Schleuning M (2013) Human-induced disturbance alters pollinator communities in tropical mountain forests. Diversity 5:1–14. doi:10.3390/d5010001
Kerr JT, Southwood TRE, Cihlar J (2001) Remotely sensed habitat diversity predicts butterfly species richness and community similarity in Canada. Proc Natl Acad Sci U S A 98(20):11365–11370
Leyequien E, Verrelst J, Slot M, Schaepman-Strub G, Heitkönig IMA, Skidmore A (2007) Capturing the fugitive: applying remote sensing to terrestrial animal distribution and diversity. Int J Appl Earth Obs G 9:1–20
Li D, Liu Y (1997) Correlations between sexual development, age, maturation feeding, and mating of adult Anoplophora glabripennis Motsch. (Coleoptera: Cerambycidae). J Northwest Forest Coll 12(4):19–23
Linsley EG (1961) The Cerambycidae of North America. Part I. Introduction. Univ Calif Publ Entomol 18:1–135
Lira I, Ceballos G (2010) Huatulco, Oaxaca. In: Ceballos G, Martínez L, García A, Espinoza E, Bezaury J, Dirzo R (eds) Diversidad, amenazas y áreas prioritarias para la conservación de las selvas secas del Pacífico de México. CONABIO y Fondo de Cultura Económica, México, D. F., pp 520–526
Lott EJ (2002) Lista anotada de las plantas vasculares de Chamela-Cuixmala. In: Noguera FA, Vega-Rivera JH, García-Aldrete AN, Quesada-Avendaño M (eds) Historia Natural de Chamela. Instituto de Biología, México, pp 99–136
Lott EJ, Bullock SH, Solís-Magallanes A (1987) Floristic diversity and structure of upland and arroyo forests of coastal Jalisco. Biotropica 19:228–235
Maass M (1995) Conversion of tropical dry forest to pasture and agriculture. In: Bullock SH, Mooney HA, Medina E (eds) Seasonally dry tropical forests. Cambridge University Press, Cambridge, pp 399–422
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, Princeton, p 192
Makino S, Goto H, Hasegawa M, Okabe K, Tanaka H, Inoue T, Okochi I (2007) Degradation of longicorn beetle (Coleoptera, Cerambycidae, Disteniidae) fauna caused by conversation from broad-leaved to man-made conifer stands of Cryptometris japonica (Taxodiaceae) in central Japan. Ecol Res 22:372–381
Martínez-Yrízar A (1995) Biomass distribution and primary productivity of tropical dry forests. In: Bullock SH, Mooney HA, Medina E (eds) Seasonally dry tropical forests. Cambridge University Press, Cambridge, pp 326–345
McAleece N, Lambshead PJD, Cage JD (1999) BioDiversity Pro. User’s guide and application. http://www.nrmc.demon.co.uk/bdpro/. Accessed 11 Jan 2016
Meng L-Z, Martin K, Weigel A, Yang X-D (2013) Tree diversity mediates the distribution of longhorn beetles (Coleoptera: Cerambycidae) in a changing tropical landscape (Southern Yunnan, SW China). PLoS One 8:e75481. doi:10.1371/journal.pone.0075481
Monné MA (2015a) Catalogue of the Cerambycidae (Coleoptera) of the Neotropical Region. Part I. Subfamily Cerambycinae. http://www.cerambyxcat.com/Parte1_Cerambycinae.pdf/. Accessed 11 Jan 2016
Monné MA (2015b) Catalogue of the Cerambycidae (Coleoptera) of the Neotropical Region. Part II. Subfamily Lamiinae. http://www.cerambyxcat.com/Parte2_Lamiinae.pdf/. Accessed 11 Jan 2016
Monné MA (2015c) Catalogue of the Cerambycidae (Coleoptera) of the Neotropical Region. Part III. Subfamilies Lepturinae, Necydalinae, Parandrinae, Prioninae, Spondylidinae and Families Oxypeltidae, Vesperidae and Disteniidae. http://www.cerambyxcat.com/Parte3_Lepturinae_e_outros.pdf/. Accessed 11 Jan 2016
Negandra H (2001) Using remote sensing to assess biodiversity. Int J Remote Sens 22:237–240
Noguera FA, Zaragoza-Caballero S, Chemsak JA, Rodríguez-Palafox A, Ramírez E, González-Soriano E, Ayala R (2002) Diversity of the Cerambycidae (Coleoptera) of the tropical dry forest of México. I. Sierra de Huautla, Morelos. Ann Entomol Soc Am 95:617–627
Noguera FA, Chemsak JA, Zaragoza-Caballero S, Rodríguez-Palafox A, Ramírez-García E, González-Soriano E, Ayala R (2007) A faunal study of Cerambycidae (Coleoptera) from one region with tropical dry Forest in México: San Buenaventura, Jalisco. Pan-Pac Entomol 83(4):296–314
Noguera FA, Ortega-Huerta MA, Zaragoza-Caballero S, Ramírez-García E, González-Soriano E (2009) A faunal study of Cerambycidae (Coleoptera) from one region with tropical dry forest in México: San Javier, Sonora. Pan-Pac Entomol 85:70–90
Noguera FA, Zaragoza-Caballero S, Rodríguez-Palafox A, González-Soriano E, Ramírez-García E, Ayala R, Ortega-Huerta MA (2012) Cerambícidos (Coleoptera: Cerambycidae) del bosque tropical caducifolio en Santiago Dominguillo, Oaxaca, México. Rev Mex Biodivers 83(3):611–622
Oindo BO, Skidmore AK (2002) Interannual variability of NDVI and species richness in Kenya. Int J Remote Sens 23:85–298
Salas-Morales SH, Schibli L, Nava-Zafra A, Saynes-Vázquez A (2007) Flora de la costa de Oaxaca, Mèxico (2): lista florística comentada del parque nacional Huatulco. Bol Soc Bot Mex 81:101–130
Solano R, Didan K, Jacobson A, Huete A (2010) MODIS vegetation indices (MOD13) C5, user’s guide. Terrestrial Biophisics and Remote Sensing Lab., The University of Arizona. Version 1.0, May 27, 2010. http://www.ctahr.hawaii.edu/grem/modis-ug.pdf/. Accessed 11 Jan 2016
Southwood TRE (1966) Ecological methods with particular reference to the study of insect population. Methuen, London, p 524
STRI (Smithsonian Tropical Research Institute) (2006) Direct link established between tropical tree and insect diversity. ScienceDaily. http://www.sciencedaily.com/releases/2006/07/060721202616.htm
Svacha, P, JF Lawrence (2014) 2.4 Cerambycidae Latreille, 1802. In: Richard AB, Beutel RG (eds) Handbook of Zoology, Arthropoda: Insecta. Coleoptera (Beetles). Morphology and systematic (Phytophaga), vol 3. Walter de Gruyter, Berlin/Boston, pp 16–177
Toledo VM (1992) Bio-economic cost. In: Downing T, Hecht S, Pearson H (eds) Development or destruction? The conversion of tropical forest to pasture in Latin American. Westview, New York, pp 63–71
Toledo VH, Noguera FA, Chemsak JA, Hovore FT, Giesbert EF (2002) The cerambycid fauna of the tropical dry forest of “El Aguacero”, Chiapas, México (Coleoptera: Cerambycidae). Coleopts Bull 56:515–532
Tottrup C (2004) Improving tropical forest mapping using multi-date Landsat TM data and pre-classification image smoothing. Int J Remote Sens 25:717–730
Townes H (1972) A light-weight trampa Malaise. Entomol News 83:239–247
Trejo, I (1998) Distribución y diversidad de selvas bajas de México: relaciones con el clima y el suelo. Ph.D. dissertation, Universidad Nacional Autónoma de México, México, p 210
Trejo I (2010) Las selvas secas del Pacífico mexicano. In: Ceballos G, Martínez L, García A, Espinoza E, Bezaury-Creel J, Dirzo R (eds) Diversidad, amenazas and áreas prioritarias para la conservación de las selvas secas del Pacífico de México. Fondo de Cultura Económica/CONABIO, México, pp 41–51
Trejo I, Dirzo R (2000) Deforestation of seasonally dry tropical forest: a national and local analysis in Mexico. Biol Conserv 94:133–142
Wang Q, Adiku S, Tenhunen J, Granier A (2005) On the relationship of NDVI with leaf area index in a deciduous forest site. Remote Sens Environ 94:244–255
Zaragoza-Caballero S, Noguera FA, González-Soriano E, Ramírez-García E, Rodríguez-Palafox A (2010) Insectos. In: Ceballos G, Martínez L, García A, Espinoza E, Bezaury-Creel J, Dirzo R (eds) Diversidad, amenazas y áreas prioritarias para la conservación de las selvas secas del Pacífico de México. Fondo de Cultura Económica-CONABIO, México, pp 195–214
Acknowledgments
We would like to acknowledge the reviewers’ opinions and suggestions which significantly improved our work. We also thank our colleague Dr. Katherine Renton for helping us to improve the writing. Finally, we thank the Consejo Nacional de Ciencia y Tecnología (CONACyT) for providing funds used for the development of our work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by Fernando B Noll – UNESP
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM
(DOCX 49.4 kb)
Rights and permissions
About this article
Cite this article
Noguera, F.A., Ortega-Huerta, M.A., Zaragoza-Caballero, S. et al. Species Richness and Abundance of Cerambycidae (Coleoptera) in Huatulco, Oaxaca, Mexico; Relationships with Phenological Changes in the Tropical Dry Forest. Neotrop Entomol 47, 457–469 (2018). https://doi.org/10.1007/s13744-017-0534-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13744-017-0534-y