Abstract
Macrophytes are key to the functioning of small shallow lakes (SSLs) because they maintain a clear water state through numerous positive feedbacks. The composition of macrophyte communities changes under anthropogenic pressures; as a result, tools designed to easily and rapidly assess their structure and composition are increasingly requested. We tested three sampling methods (the S3m sampling method, a stratified method, and a mapped inventory) to monitor macrophytes in 26 SSLs. The effect of each method was evaluated on seven descriptors of macrophyte communities, including the median conservation value. The results were comparable for the three methods, but the stratified method failed to accurately monitor the median conservation value and the number of species present at a low frequency, including exotic and patrimonial species, hence serious consequences for management decisions. S3m was applied to 262 SSLs ranging from 1 m2 to 43 ha in surface area. Generalised additive models were used to investigate the environmental factors correlated with four conservation value or ecosystem functioning descriptors. The S3m method showed that surface area, distance from the source, elevation, and bank verticality were determinants of macrophyte richness. Invasive crayfish impacted the macrophyte richness and the coverage of submerged macrophytes, whereas fish presence increased the macrophyte richness and the percentage of exotic macrophytes and reduced patrimonial interest. S3m was successfully applied to a wide diversity of SSLs in France. It proved to be rapid, reproducible, and representative for monitoring macrophytes in SSLs. Therefore, it should be applied for SSL management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Macrophytes strongly influence environmental characteristics in many ways. They modify substrate and water chemistry—e.g., the dissolved oxygen concentration in the rhizosphere (Rehman et al. 2017)—change biogeochemical cycles (Wetzel and Søndergaard 1998), and contribute to primary and secondary productivity (Peters and Lodge 2010). Macrophytes also provide a substrate for epiphytic algae and their grazers (Wolters et al. 2019), act as a food source for fish and birds (Jeppesen et al. 1998), and offer a physical refuge that buffers interactions between fish and zooplankton (Scheffer 2001). They are involved in various feedback mechanisms that tend to maintain a clear-water state (Scheffer and Carpenter 2003). They also provide numerous ecosystem services (Hilt et al. 2017). Macrophyte composition and abundance recently changed in most waterbodies because of various anthropogenic pressures (Körner 2002) such as eutrophication (Rosset et al. 2014), fish stocking (Williams et al. 2002), or biological invasions (Stiers et al. 2011). Furthermore, fish have a positive influence on floristic richness (Hassall et al. 2011), whereas invasive crayfish have a negative influence on macrophyte communities (Lodge and Lorman 1987).
Macrophyte monitoring programs have been widely implemented in the running waters and lakes of European states following the Water Framework Directive (WFD) (Birk et al. 2012), but they have largely failed to include shallow lakes < 50 ha in surface area (Oertli et al. 2005b) and had little influence on their biological conservation (Hassall et al. 2016). Some methods—e.g., the “Biological Macrophyte Index in Lakes” (IBML) in France (Boutry et al. 2013) and the “Ecological State Macrophyte Index” (ESMI) in Poland (Ciecierska and Kolada 2014)—could be applied for monitoring aquatic plant communities in large shallow lakes so as to evaluate their ecological status. However, they were not designed to evaluate the conservation value of the communities. Small shallow lakes and ponds (SSLs) are major contributors to biodiversity conservation in freshwater ecosystems (Lukács et al. 2013; Panzeca et al. 2021). Moreover, the biological monitoring of aquatic plant communities is still in its infancy in European SSLs despite an urgent need to protect them and to preserve ecosystem services (Hill et al. 2018). This implies monitoring the biodiversity of aquatic plant communities with adapted and efficient methodologies. Apart from Great Britain and Switzerland, most European countries still lack ambitious SSL monitoring tools and programs. The British Predictive System for Multimetrics (PSYM) for ponds (Biggs et al. 2000) and the Swiss “Indice Biologique Etangs et Mares” (IBEM) (Indermuehle et al. 2010) provide contemporary examples of SSL monitoring. PSYM is based on the assessment of the richness of invertebrates and macrophytes and associated trophic ranking scores (Palmer 1992). It can be used to assess ponds with surface areas ranging from 1 m2 to 5 ha (Biggs et al. 2000). Macrophyte samplings correspond to a simple qualitative inventory (lists of species present in the lake). The IBEM index is valid in Switzerland and its border regions for waterbodies ranging from 300 to 1000 m above sea level (asl). It can be used to assess the floristic richness of SSLs 50 m2 to 6 ha in surface area and 0.30 m to 9 m mean depth. Neither method can be used to monitor SSLs neglected by the WFD (a few m2 to 50 ha in surface area). As a consequence, a new method called S3m (Sampling of Small Shallow lake macrophytes) derived from the PSYM method was proposed and compared with a stratified method derived from the IBEM method (Indermuehle et al. 2010) and with a mapped inventory method (“mapped inventories” hereafter) (Simpson 1991).
Our goal was to test the efficiency of the S3m method for conducting surveys of plant species composition and abundance in SSLs and calculating diversity and conservation indices. To be applied by a broad range of users, this method should be cost-effective and (1) representative, (2) rapid, (3) reproducible, and (4) flexible. As the choice of the sampling method influences monitoring results and diversity or conservation indices, we compared the S3m method with two other sampling methods: (1) a sampling strategy inspired by the IBEM method (Indermuehle et al. 2010), (2) mapped inventories with coverages computed with the SIG tool (e.g., Hutorowicz 2020). The comparison was performed across a panel of 26 SSLs. Our first hypothesis was that S3m would provide a representative picture of macrophyte richness and of the conservation value of plant communities, and be less time consuming than the stratified and the mapped methods. We investigated the key biotic and abiotic factors of the macrophyte communities of 262 SSLs using the S3m method. Our second hypothesis was that a lower floristic richness and a higher conservation value in high-altitude SSLs would be observed due to harsh climatic conditions. Our third hypothesis was that a high distance from a source—a proxy of SSL connectivity and watershed size—would decrease the conservation value of aquatic plant communities and floristic richness because a high connectivity facilitates biological dispersal and increases nutrient inputs. Our last hypothesis was that the presence of fish and crayfish would reduce the floristic richness and the conservation value of aquatic plant communities.
Materials and methods
Site selection and macrophyte sampling methods
Twenty-six SSLs were selected in southwest France. They represented a large panel of morphometric conditions and considerable variations in water quality (Table 1): surface area from 258 to 95,000 m2, mean depth from 0.1 to 5 m, shoreline index D (Hutchinson 1975) from 1.04 to 1.88, and various bank slopes ranging from sites with less than 5% of the shoreline with vertical banks (15 sites) to sites with more than 75% of the shoreline with vertical banks (one site). All field data were collected in the summers of 2013–2014.
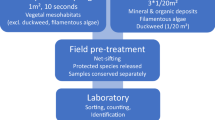
The S3m method was applied using a meander method, which is very efficient in detecting rare species (Huebner 2007). Macrophytes were surveyed in a zigzag pattern, regardless of the depth. Deeper water zones were point-sampled. The IBML littoral five-scale from the French norm XPT90-328 classes (AFNOR 2010) was chosen to estimate plant abundance (class 1: a few individuals; 2: isolated small patches; 3: numerous small patches; 4: large discontinuous patches; 5: large continuous patches).
The stratified sampling method used quadrats and transects. The number of quadrats increased with the surface area of the SSL. A grid pattern was fixed with regularly spaced transects, perpendicular or parallel to the longest axis of the SSL. For each transect, two quadrats were located at each end of the transect, directly against the shoreline, at the usual SSL limit. The SSL shore is usually richer in plant species than the profundal zone (Oertli et al. 2005a), and a higher sampling pressure in this zone determines the stratified strategy. Other quadrats were positioned at each transect intersection. A formula adapted from the IBEM method was used to evaluate the number of quadrats:
Square-metre quadrats, best fitted for sampling macrophyte communities in lakes, were selected (Ling and Jacobs 2010). In the field, transects were identified with pegs. Only the macrophyte species observed in the quadrats were recorded.
For the mapped inventory, coverages of patches and isolated plants were estimated visually as accurately as possible and drawn directly using a recent aerial photograph of each SSL as a guide (Simpson 1991). Drawings were converted into SIG shape files using QGIS software (QGIS Development Team 2021).
All surveys were conducted by walking on the littoral and shallow zones. Vegetation in the deeper zones was surveyed with a grapnel or a rake from a small boat with an electric motor when the depth was beyond 1.5 m or by free diving. Species such as Characeae, Ranunculus subg. Batrachium, and mosses were kept in alcohol or dried for identification in the laboratory. All macrophytes (spermatophytes, bryophytes, ferns, and Characeae) were identified to the species level, when possible.
Comparison of the sampling designs
All data were converted into percent coverages. The relative coverage of each taxon from the mapped inventory, expressed as a percentage of the total area, was considered further as the reference data (the most complete and representative one). It was computed with R software (R Core Team 2020) and the sf R package (Pebesma 2018). For the stratified method, the percentage of quadrats occupied by each species was treated as relative coverage. For the S3m method, a conversion scale (1 = 0.0001, 2 = 0.001, 3 = 0.02, 4 = 0.2, 5 = 0.6) was used. To obtain this scale, each S3m value was converted into corresponding median values obtained with the mapped inventory from 9 randomly chosen SSLs out of the 26 SSLs. The values obtained with the other 17 SSLs were used for data validation tests. Then, 7 descriptors were selected to identify the effects of the sampling method on macrophyte communities: (1) total floristic richness—a synthetic but quantitative picture of biodiversity –, (2) the Shannon diversity index, (3) the median conservation value according to the national red list, (4) the M-NIP trophic index (Sager and Lachavanne 2009), (5) the percent coverage of submerged species (spermatophytes + Characeae + bryophytes + ferns), (6) the percentage of exotic species, and (7) the richness in ‘infrequent species’ (difficult to observe because their coverage is < 1% in mapped inventories). The median conservation value was calculated using the species rarity index (SRI) as follows: (1) all species were assigned a score according to their status on the French national red list, with 32 = CR (Critically Endangered), 16 = EN (Endangered), 8 = VU (Vulnerable), 4 = NT (Near Threatened), 2 = LC (Least Concern), 1 = other, 0 = neophyte; (2) the scores of all species in each sample were summed to provide a species rarity score; and (3) the species rarity score was divided by the number of species recorded in the sample to provide the SRI score (Foster et al. 1989; Rosset et al. 2013).
The sampling methods were compared using Wilcoxon’s test and Spearman’s correlation between the index values from the mapped inventories and the values from the other two methods, and illustrated with linear regression plots.
Finally, the duration of assessment time is key to the success of a monitoring method. The times needed to implement each of the three methods were compared by measuring the time spent monitoring each SSL (sampling + data entry).
Application of the S 3 m method to 262 SSLs: influence of environmental factors on aquatic plant communities
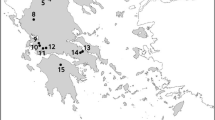
In total, 262 SSLs were selected, differing by their geographical situation and physical features (Fig. 1, Table 2). They were located in various geological bedrocks (acid, calcareous, mixed), under different climatic or altitudinal conditions, and in varied environments (forest, urban, agricultural). They were natural or man-made SSLs, with different hydrological regimes. Twenty-two percent of them dried up in summer. They underwent different biotic pressures (fish, invasive crayfish). Thirteen percent were invaded by exotic crayfish, and 40% harboured fish. Each SSL was sampled once in the summertime. Macrophyte taxa were monitored using S3m from 2013 to 2020.
The influence of environmental factors (detailed in Table 2) was studied on 4 descriptors: (1) total richness, (2) the SRI score, (3) the percent coverage of submerged species, and (4) the percentage of exotic species.
A generalised additive model (GAM; Hastie and Tibshirani 1999) between floristic richness and 11 environmental factors was used to identify which factors were the best predictors. The best predictors were identified according to the REML method combined with null space penalisation (Marra and Wood 2011). All GAMs were computed using R (R Core Team 2020) and the mgcv package (Wood 2017). Factors were transformed when appropriate, with assessment for normality and homoscedasticity using Shapiro–Wilk tests and histograms. As suggested by Hassall et al. (2011), the GAM was expected to disentangle non-linear relationships between macrophyte richness and biotic or abiotic factors.
The factors were (1) elevation; (2) surface area; (3) distance from the source (DIS) as proposed in Labat et al. (2021); (4) shoreline development index (Hutchinson 1975); (5) shoreline influence estimated with a 5-scale estimation according to the percentage of perimeters with a slope > 50% or instable banks (0 = 0–5%; 1 = 5–25%; 2 = 26–49%; 3 = 50–75%, 4 > 75%); (6) mean depth; (7) ‘shade’; (8) percentage of woodland in the SSL surroundings (in a 50-m buffer zone); (9) drying (0 = not known to dry up, 1 = known to dry up); (10) presence of fish; and (11) presence of invasive crayfish (0 = unknown presence of fish or crayfish, 1 = known presence of fish or crayfish). The factors were estimated using at least three field observations during various seasons at the time of macrophyte sampling or according to landowners’ observations.
Results
Comparison of the three sampling methods
A total of 148 species—97 helophytes, 33 submerged spermatophytes, 10 bryophytes, and 8 Characeae—were observed in the 26 SSLs used for the sampling comparison. Eight species were exotic, including six infrequent species. According to the French national red list, one species (Utricularia intermedia) was vulnerable and three (Hippuris vulgaris, Potamogeton acutifolius, and Utricularia minor) were near threatened. One species (Caropsis verticillato-inundata) was restricted to the southwest of France, and it is nationally protected and considered vulnerable on the IUCN red lists. Among these five species, three were infrequent (Table S1). The effects of each sampling method on the macrophyte community relevés are illustrated in Table 3 and Fig. 2.
Linear regression plots obtained for each method compared to mapped inventory results for seven descriptors: total floristic richness, Shannon diversity index, species rarity index according to the French national red list (SRI), trophic index for Swiss ponds (M-NIP), relative coverage of submerged vegetation, percentage of exotic species in relation to total richness, and number of infrequent species. The corresponding Spearman correlation (r) with mapped inventories is mentioned in each plot legend
The coefficient of correlation of each descriptor (richness, Shannon diversity index, SRI, M-NIP, percentage of submerged vegetation, and percentage of exotic species) was higher in the mapped inventory and S3m results than in the stratified method results (Table 4). The very high correlations (r = 1) between S3m and the mapped inventory based on qualitative data (floristic richness, SRI, M-NIP, percentage of exotic species, richness in species present at low frequencies) confirmed that all species were inventoried by S3m. Other correlations between S3m and the mapped inventory remained high, as regards descriptors based on quantitative data such as coverage of submerged species (r = 0.95) and the Shannon diversity index (r = 0.85). S3m was well correlated with the stratified method (r ≥ 0.6, except SRI, r = 0.45, and exotic species r = 0.58). The correlation between the stratified method and the mapped inventory was weakest for qualitative descriptors such as SRI and the percentage of exotic species (r = 0.45 and 0.58, respectively), and was high for M-NIP (r = 0.84) and floristic richness (r = 0.81). No significant difference between the three methods was obtained for any of the descriptors, except for the Shannon diversity index and the number of species present at a low frequency (Wilcoxon test, P < 0.005). The stratified method significantly overestimated the Shannon diversity index compared with the mapped inventory and S3m (Table 3). The total number of species present at a low frequency was underestimated by the stratified method compared with the mapped inventory and S3m (Table 3). Consequently, three of the eight exotic species and two of the patrimonial species including vulnerable Utricularia intermedia were not found by the stratified method. Most of the other exotic or patrimonial species were missed in at least one site (Table S1). The monitoring time was lower with S3m (mean time = 80 ± 53 min) than with the stratified method (mean time = 180 ± 134 min). The mapped inventory was the most time-consuming method (243 ± 162 min).
Influence of environmental factors on macrophyte communities in French SSLs
A list of 238 taxa—40 bryophytes, 20 Characeae, 107 helophytes, and 71 submerged or floating spermatophytes or fern species—was established in all 262 SSLs. According to the national red list, 3 species were considered vulnerable, 9 near threatened, 152 least concerned, 1 data deficient, and 13 exotic.
The mean floristic richness per site was 20 ± 14 taxa, with a minimum of 1 species and a maximum of 73 taxa. Sampling time was usually 30 min to 4 h, with a maximum of 8 h in a very unfavourable context (a 7.4 ha SSL, with most of the shoreline colonised by dense brambles, and the whole waterbody occupied by dense Ceratophyllum stands that made it impossible to sail with an electric motor).
GAMs identified 7 key factors of total richness, 6 key factors of SRI, 3 key factors of the coverage of submerged species, and 5 key factors of the percentage of exotic species (Table 5). Fitted relationships are shown in Fig. 3. Surface area was the strongest predictor of total richness, with a logarithmic linear influence. The influence of elevation on floristic richness was not clear from 0 to 1000 m, whereas a clear decrease in floristic richness with increasing elevation was established above 1000 m asl (Fig. 3). DIS—a proxy of SSL connectivity and watershed size—had a positive influence on floristic richness when it was less than 5 km, and a negative effect when it was more than 5 km. The presence of fish favoured floristic richness, whereas the presence of invasive crayfish, a woodland context and bank verticality and instability had negative effects on floristic richness (Fig. 3).
Fitted relationships between residuals of the generalised additive models (GAMs) and each significant predictor factor for floristic richness, coverage of submerged species, and species rarity index according to the French national red list (SRI), and percentage of exotic species in relation to the total richness of the 262 SSLs. Solid lines are fitted splines in the continuous factors and fitted values for each of the categorical factors. Dashed lines are standard errors
SRI was well explained by environmental factors (R2 = 0.23). Surface area and elevation tended to have a positive influence, whereas the presence of fish, DIS and woodland had a negative one. Mean depth had a positive influence on SRI from 0 to 1.8 m, and a negative one when it was more than 1.8 m. Three factors—invasive crayfish, shade, and mean depth—had a weak impact (R2 = 0.12) on submerged species coverage (Fig. 3). A decrease was observed in the submerged vegetation cover along an increasing shade gradient. The impact of mean depth on the submerged vegetation cover was measured when depth was > 1.8 m. At less than 1.8 m depth, the influence of mean depth was positive (Fig. 3). The presence of invasive crayfish reduced the cover of submerged plants.
The percentage of exotic species was favoured by the distance from the source, the presence of fish, and mean depth (Table 5). Elevation and the presence of invasive crayfish tended to limit the percentage of exotic plant species (Fig. 3, Table 5).
Discussion
Comparison of the efficiency of the three sampling methods
SSLs are a matter of concern due to conservation issues, hence the importance of using a sampling method able to detect floristic biodiversity. Moreover, the accuracy and reproducibility of macrophyte coverage estimations are a prerequisite to effectively monitor biological integrity (Karr and Chu 1997). The S3m method is a rapid assessment method that provides a representative picture of macrophyte richness and structure within the aquatic plant communities of SSLs. These results validate our first hypothesis.
Comparable results were obtained with S3m and the mapped method to compute species coverage, and they were more accurate and complete than those obtained with the stratified method. Moreover, sampling with the S3m method required less than half the time required by the other two methods. Species aggregation in patches can strongly affect the accuracy of stratified methods (May et al. 2018). Consequently, the stratified method failed to provide an accurate picture of the conservation value because the sampled richness was incomplete. Moreover, the stratified method failed to consider numerous low-frequency species, so that it underestimated the floristic richness and SRI and overestimated the percentage of exotic species. However, it was accurate for monitoring trophic levels, confirming the relevance of stratified methods for monitoring trophic alterations in various standing water bodies.
Our findings do not allow us to conclude that any one method was best for estimating species coverage. Stratified sampling failed to identify small, isolated vegetation patches and rare species which may represent almost half of the species. However, it was effective in estimating the coverage of dominant species (Pante and Dustan 2012). The mapped and S3m methods did not include plant density in patches and were poorly fitted to report the coverage of dispersed or low-coverage species. A simple visual estimation can be more representative (Dethier et al. 1993; Bråkenhielm and Qinghong 1995) and less time-consuming (Lillie 2000) than quadrats when the distribution is patchy, at least in small lakes. The accuracy of visual estimation can decrease with increasing surface area (Traxler 1997). The accuracy of the IBML 5-scale can limit this bias. All three methods were valuable for estimating species coverage or at least the coverage of functional groups and dominant species. However, the Shannon index results obtained with the stratified method suggested that stratified methods are less efficient for monitoring community diversity.
A comparison of our S3m data with literature data showed that S3m recorded a higher number of plant taxa than other sampling methods did in other studies (Table 6). A comparison of the number of plant species found in literature data with those found using S3m showed its efficiency for monitoring the macrophyte richness of SSLs. However, these differences are only indicative, and could be explained by different local conditions or geographical isolation (Scheffer et al. 2006; Gledhill et al. 2008).
The accuracy of S3m is particularly useful to evaluate the conservation value or monitor low-frequency patrimonial taxa or exotic species. However, the inclusion or exclusion of infrequent species in biological monitoring is an old debate (Van Sickle et al. 2007). Deleting infrequent species can alter the sensitivity of community-based methods when it comes to detecting ecological changes (Cao et al. 1998). These species can also play a key role in management decisions when they have a patrimonial value (Thompson 2013).
S3m is also useful for detecting exotic species early, which is essential for implementing a rapid response and minimising the impact of potential invasive species (Reaser et al. 2020). For instance, two small stands of Myriophyllum aquaticum were observed, corresponding to 0.2% of the SSL surface area in one of the 26 SSLs used for comparison. Four years later, its coverage represented approximately half of the surface area. This dramatically increased the cost of its control or eradication and reduced the coverage of other macrophytes such as Luronium natans—a species protected at the national scale. Our results from the 26 SSLs highlight that numerous exotic or patrimonial species can be missed when a stratified method is used. Moreover, the exotic or patrimonial species inventoried in the 262 SSLs were mostly infrequent according to their mean coverage (Table S2). Consequently, the use of stratified methods induces serious consequences in management decisions. The S3m method is cost effective, rapid (less time consuming than the other methods), and efficient for assessing aquatic plant communities in SSLs.
Influence of environmental factors on aquatic plant communities
The higher richness found among the 262 sites vs. the 26 sites (238 vs. 148 taxa) can be explained by the diversity of climatic, elevational, and geological conditions (e.g., Fig. 1).
Our results, from lowly to highly impacted SSLs, confirmed the key role of surface area, elevation, and distance from the source previously established on French lowimpacted SSLs (Labat et al. 2021). The decrease in floristic richness above 1,000 m asl was probably due to an indirect effect of the short duration of the growing season, cold temperatures (Jones et al. 2003), and maybe lower nutrient availability (Lacoul and Freedman 2005). Despite a lower floristic richness, altitude ponds are often occupied by transition mire and quaking bogs, usually combined with oligotrophic to mesotrophic vegetation in standing waters. This vegetation includes rare or specific species like Sparganium angustifolium or Callitriche palustris at the highest elevation. Consequently, the conservation value increases with elevation despite lower floristic richness. The influence of anthropogenic pressure cannot be excluded, even if it is usually lower with elevation (Fernández-Aláez et al. 2018). Thus, our second hypothesis was validated.
The distance from the source had an influence on the macrophyte community composition (Labat et al. 2021). Moreover, higher DIS was often characterised by communities typical of natural eutrophic lakes including Magnopotamion or Hydrocharition alliances, whereas lower DIS was characterised by communities of oligotrophic to mesotrophic standing waters including Littorelletea uniflorae and/or the Isoeto-Nanojuncetea. When DIS = 0 (isolated ponds), vegetation was usually typical of natural dystrophic lakes and ponds, or hard oligo-mesotrophic standing waters with benthic vegetation composed of Charophytes. DIS also had a positive influence on floristic richness up to 5 km, and a negative one above 5 km. Our third hypothesis stipulating that distance from the source would influence floristic richness was validated. Connectivity between SSLs and rivers facilitates the dispersal of plant propagules (Vogt et al. 2006), particularly from exotic species (Parendes and Jones 2000). Above 5 km, the effect of DIS on total floristic richness became negative, perhaps because (1) colonisation by invasive species was higher with higher DIS (Fig. 3), and (2) eutrophication was higher because of larger watersheds and increasing anthropogenic pressure. Vertical or unstable banks also impacted floristic richness by reducing the expression of the pool of species (cf. the colonisation gradient based on the littoral depth gradient (e.g., Pokorný and Björk 2010)).
Our hypothesis on the positive effect of fish on floristic richness was validated. This effect had already been found (Linton and Goulder 2000; Hassall et al. 2011). However, the presence of fish could have an impact on the conservation value of the communities, as suggested by our SRI index results. The influence of fish, especially on submerged species, could depend on fish communities. Large cyprinids reduce hydrophyte coverage and composition (Pı́palová 2002), whereas their impact on macrophyte communities can be limited by piscivorous fish (Tátrai et al. 2009). Goulder (2001) suggested that higher floristic richness in fishponds can be explained by moderate trampling by anglers. Many anglers or other users can also carry native and exotic plant fragments or seeds on their footwear (Ware et al. 2012) or on their boats. Higher depths motivate boating or sailing for fish or sport, which is a major vector of native and exotic species dispersal (Rothlisberger et al. 2010; Sytsma and Pennington 2015) and can partly explain the positive influence of depth on the percentage of exotic species.
As hypothesised, exotic crayfish had a strong impact on floristic richness, especially on submerged macrophytes and could limit the abundance of exotic macrophyte species. Exotic crayfish can consume and fragment macrophytes, drastically reduce plant biomass and remove some plant species (Carreira et al. 2014). However, no impact of crayfish on the patrimonial value of SSLs was found by the SRI score, probably because the contribution of submerged species to SRI was generally weak (low richness, and usually a low conservation score). An indirect impact of exotic crayfish on the conservation value is not excluded because submerged species usually shelter numerous invertebrate and fish species (Engel 1988) and strongly influence zooplankton and algal communities (Celewicz-Gołdyn and Kuczyńska-Kippen 2017).
Bank verticality and woodland were also key for plant richness, whereas shade impacted submerged plants. These findings are congruent with literature data (Cowardin et al. 2005; Joye et al. 2006; Angélibert et al. 2010; Hassall et al. 2011; Sender 2016).
The key factors of conservation interest identified from the SRI score should be viewed cautiously. Furthermore, the reliability of red lists in conservation evaluation is a matter of discussion because the red list status is based on expert opinion or political or empirical decision (Le Berre et al. 2019). It might be useful to define rarity as including functional rarity (Loiseau et al. 2020) to better understand the implications of human and natural pressures in SSL functioning or to simply define species sensitivity to alterations, as suggested in numerous integrity indices in North American lakes (Beck et al. 2010) and for depressional wetlands (e.g., Reiss and Brown 2005).
The plants listed in red lists can strongly differ between neighbour countries or regions or according to the scale (regional, national, European). Differences can also be noticed as regards taxa. The French national red list used for SRI computing excludes mosses and Characeae and can miss key factors for these groups or overestimate the influence of factors key to groups included in this red list. Moreover, most of the species in our database were considered LC in the national red list, which tends to have less influence than exotic species in SRI trends. This can be observed through opposite trends for elevation, DIS, mean depth, and fish presence between the SRI and the percentage of exotic species. The SRI score was also influenced by surface area and woodland.
Conclusion
This study proposes and validates a new sampling method (S3m) adapted to SSLs. S3m provided similar or even better results than the stratified method and was less time consuming. Therefore, it constitutes an efficient tool for assessing the plant communities of permanent or temporary SSLs. The conservation value of SSLs still remains neglected because of the lack of adapted sampling methods. We tested S3m on a wide range of French SSLs differing by their locations, physical features, and human uses, and confirmed (1) the importance of biodiversity, including patrimonial species, in SSLs, and (2) that macrophyte richness and submerged macrophyte coverage can be predicted using physical or biological factors.
Large lowland SSLs defined by a short distance from the source, a low coverage of nearby woodlands and the presence of fish harboured potentially higher floristic richness than small mountainous SSLs. Therefore, heterogeneity of the key factors is a prerequisite for the conservation of various communities harboured by SSLs at the landscape level.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to private funding but are available from the corresponding author upon reasonable request.
References
AFNOR (2010) XP T90–328 - Échantillonnage des communautés de macrophytes en plans d’eau. AFNOR, Paris
Angélibert S, Rosset V, Indermuehle N, Oertli B (2010) The pond biodiversity index “IBEM”: a new tool for the rapid assessment of biodiversity in ponds from Switzerland. Part 1. Index development. Limnetica 29:93–104
Beck MW, Hatch LK, Vondracek B, Valley RD (2010) Development of a macrophyte-based index of biotic integrity for Minnesota lakes. Ecol Indic 10:968–979. https://doi.org/10.1016/j.ecolind.2010.02.006
Biggs J, Williams P, Whitfield M et al (2000) Biological techniques of still water quality assessment phase 3. Method development. Environment Agency, Bristol
Birk S, Bonne W, Borja A et al (2012) Three hundred ways to assess Europe’s surface waters: an almost complete overview of biological methods to implement the water framework directive. Ecol Indic 18:31–41. https://doi.org/10.1016/j.ecolind.2011.10.009
Boutry S, Bertrin V, Dutartre A (2013) Méthode d’évaluation de la qualité écologique des plans d’eau basée sur les communautés de macrophytes Indice Biologique Macrophytique en Lac (IBML) - Rapport d’avancement. IRSTEA
Bråkenhielm S, Qinghong L (1995) Comparison of field methods in vegetation monitoring. Water Air Soil Pollut 79:75–87
Cao Y, Williams DD, Williams NE (1998) How important are rare species in aquatic community ecology and bioassessment? Limnol Oceanogr 43:1403–1409. https://doi.org/10.4319/lo.1998.43.7.1403
Carreira B, Dias M, Rebelo R (2014) How consumption and fragmentation of macrophytes by the invasive crayfish Procambarus clarkii shape the macrophyte communities of temporary ponds. Hydrobiologia. https://doi.org/10.1007/s10750-013-1651-1
Celewicz-Gołdyn S, Kuczyńska-Kippen N (2017) Ecological value of macrophyte cover in creating habitat for microalgae (diatoms) and zooplankton (rotifers and crustaceans) in small field and forest water bodies. PLoS ONE 12:e0177317. https://doi.org/10.1371/journal.pone.0177317
Ciecierska H, Kolada A (2014) ESMI: a macrophyte index for assessing the ecological status of lakes. Environ Monit Assess 186:5501–5517. https://doi.org/10.1007/s10661-014-3799-1
Cowardin LM, Carter V, Golet FC, Laroe ET (2005) Classification of wetlands and deepwater habitats of the United States. In: Lehr JH, Keeley J (eds) Water encyclopedia. Wiley, Hoboken
Dethier M, Graham E, Cohen S, Tear L (1993) Visual versus random-point percent cover estimations: “objective” is not always better. Mar Ecol Prog Ser 96:93–100. https://doi.org/10.3354/meps096093
Engel S (1988) The role and interactions of submersed macrophytes in a Shallow Wisconsin Lake. J Freshw Ecol 4:329–341. https://doi.org/10.1080/02705060.1988.9665182
Fernández-Aláez C, Fernández-Aláez M, García-Criado F, García-Girón J (2018) Environmental drivers of aquatic macrophyte assemblages in ponds along an altitudinal gradient. Hydrobiologia 812:79–98. https://doi.org/10.1007/s10750-016-2832-5
Foster GN, Foster AP, Eyre MD, Bilton DT (1989) Classification of water beetle assemblages in arable fenland and ranking of sites in relation to conservation value. Freshw Biol 22:343–354. https://doi.org/10.1111/j.1365-2427.1989.tb01109.x
Gledhill DG, James P, Davies DH (2008) Pond density as a determinant of aquatic species richness in an urban landscape. Landsc Ecol 23:1219–1230. https://doi.org/10.1007/s10980-008-9292-x
Goulder R (2001) Angling and species richness of aquatic macrophytes in ponds. Freshw Forum 15:71–76
Hassall C, Hollinshead J, Hull A (2011) Environmental correlates of plant and invertebrate species richness in ponds. Biodivers Conserv 20:3189–3222. https://doi.org/10.1007/s10531-011-0142-9
Hassall C, Hill M, Gledhill D, Biggs J (2016) The ecology and management of urban pondscapes. In: Francis RA, Millington JDA, Chadwick MA (eds) Urban landscape ecology: science, policy and practice. Routledge, Abingdon, pp 129–147
Hastie T, Tibshirani R (1999) Generalized additive models. Chapman & Hall/CRC, Boca Raton
Hill MJ, Hassall C, Oertli B et al (2018) New policy directions for global pond conservation. Conserv Lett 11:e12447. https://doi.org/10.1111/conl.12447
Hilt S, Brothers S, Jeppesen E et al (2017) Translating regime shifts in shallow lakes into changes in ecosystem functions and services. Bioscience 67:928–936. https://doi.org/10.1093/biosci/bix106
Huebner CD (2007) Detection and monitoring of invasive exotic plants: a comparison of four sampling methods. Northeast Nat 14:183–206
Hutchinson GE (1975) A treatise on limnology: Vol-1 Part-1: geography and physics of lakes. Wiley, New York
Hutorowicz A (2020) A retrospective ecological status assessment of the lakes based on historical and current maps of submerged vegetation—a case study from five stratified lakes in Poland. Water 12:2607. https://doi.org/10.3390/w12092607
Indermuehle N, Angélibert S, Rosset V, Oertli B (2010) The pond biodiversity index “IBEM”: a new tool for the rapid assessment of biodiversity in ponds from Switzerland. Part 2. Method description and examples of application. Limnetica 29:105–120. https://doi.org/10.23818/limn.29.08
Jeppesen E, Søndergaard M, Søndergaard M, Christoffersen K (1998) The structuring role of submerged macrophytes in lakes. Springer, New York
Jones JI, Li W, Maberly SC (2003) Area, altitude and aquatic plant diversity. Ecography 26:411–420
Joye DA, Oertli B, Lehmann A et al (2006) The prediction of macrophyte species occurrence in Swiss ponds. Hydrobiologia 570:175–182. https://doi.org/10.1007/s10750-006-0178-0
Karr JR, Chu EW (1997) Biological monitoring and assessment: using multimetric indexes effectively. University of Washington, Seattle
Körner S (2002) Loss of submerged macrophytes in shallow lakes in north-eastern Germany. Int Rev Hydrobiol 87:375–384
Labat F, Thiébaut G, Piscart C (2021) Principal determinants of aquatic macrophyte communities in least-impacted small shallow lakes in France. Water 13:609. https://doi.org/10.3390/w13050609
Lacoul P, Freedman B (2005) Physical and chemical limnology of 34 lentic waterbodies along a tropical-to-alpine altitudinal gradient in Nepal. Int Rev Hydrobiol 90:254–276. https://doi.org/10.1002/iroh.200410766
Le Berre M, Noble V, Pires M et al (2019) How to hierarchise species to determine priorities for conservation action? A critical analysis. Biodivers Conserv 28:3051–3071. https://doi.org/10.1007/s10531-019-01820-w
Léquivard L, Millouet J-C (2013) Flore des mares de l’Orléanais. Symbioses 30:17–26
Lillie RA (2000) Development of a biological index and classification system for wisconsin wetlands using macroinvertebrates and plants. Wisconsin Department of Natural Resources, Monona, Wisconsin
Ling JE, Jacobs SWJ (2010) Biological assessment of wetlands: testing techniques—preliminary results. Wetl Aust 21:36–55. https://doi.org/10.31646/wa.250
Linton S, Goulder R (2000) Botanical conservation value related to origin and management of ponds. Aquat Conserv Mar Freshw Ecosyst 10:77–91. https://doi.org/10.1002/(SICI)1099-0755(200003/04)10:2%3c77::AID-AQC391%3e3.0.CO;2-Y
Lodge DM, Lorman JG (1987) Reductions in submersed macrophyte biomass and species richness by the crayfish Orconectes rusticus. Can J Fish Aquat Sci 44:591–597. https://doi.org/10.1139/f87-072
Loiseau N, Mouquet N, Casajus N et al (2020) Global distribution and conservation status of ecologically rare mammal and bird species. Nat Commun 11:5071. https://doi.org/10.1038/s41467-020-18779-w
Lukács BA, Sramkó G, Molnár VA (2013) Plant diversity and conservation value of continental temporary pools. Biol Conserv 158:393–400. https://doi.org/10.1016/j.biocon.2012.08.024
Marra G, Wood SN (2011) Practical variable selection for generalized additive models. Comput Stat Data Anal 55:2372–2387. https://doi.org/10.1016/j.csda.2011.02.004
May F, Gerstner K, McGlinn DJ et al (2018) mobsim: an r package for the simulation and measurement of biodiversity across spatial scales. Methods Ecol Evol 9:1401–1408. https://doi.org/10.1111/2041-210X.12986
Oertli B, Auderset Joye D, Castella E et al (2005a) PLOCH: a standardized method for sampling and assessing the biodiversity in ponds. Aquat Conserv Mar Freshw Ecosyst 15:665–679. https://doi.org/10.1002/aqc.744
Oertli B, Biggs J, Céréghino R et al (2005b) Conservation and monitoring of pond biodiversity: introduction. Aquat Conserv Mar Freshw Ecosyst 15:535–540. https://doi.org/10.1002/aqc.752
Oertli B, Auderset Joye D, Castella E, et al (2000) Diversité biologique et typologie écologique des étangs et petits lacs de Suisse
Palmer M (1992) A botanical classification of standing waters in Great Britain and a method for the use of macrophyte flora in assessing changes in water quality. incorporing a reworking data, 1992. Nature Conservancy Council
Pante E, Dustan P (2012) Getting to the point: accuracy of point count in monitoring ecosystem change. J Mar Biol 2012:7. https://doi.org/10.1155/2012/802875
Panzeca P, Troia A, Madonia P (2021) Aquatic macrophytes occurrence in Mediterranean farm ponds: preliminary investigations in north-western Sicily (Italy). Plants 10:1292. https://doi.org/10.3390/plants10071292
Parendes L, Jones J (2000) Role of light availability and dispersal in exotic plant invasion along roads and streams in the HJ Andrews experimental forest, Oregon. Conserv Biol 14:64–75
Pebesma E (2018) Simple features for R: standardized support for spatial vector data. R J 10:439. https://doi.org/10.32614/RJ-2018-009
Peters JA, Lodge DM (2010) Littoral zone. In: Likens GE (ed) Lake ecosystem ecology: a global perspective: a derivative of encyclopedia of inland waters. Elsevier/Academic Press, Amsterdam, Boston, pp 18–25
Pı́palová I (2002) Initial impact of low stocking density of grass carp on aquatic macrophytes. Aquat Bot 73:9–18. https://doi.org/10.1016/S0304-3770(01)00222-4
Pokorný J, Björk S (2010) Development of aquatic macrophytes in shallow lakes and ponds. In: Eiseltová M (ed) Restoration of lakes, streams, floodplains, and bogs in Europe. Springer, Netherlands, Dordrecht, pp 37–43
QGIS Development Team (2021) QGIS geographic information system. Open source geospatial foundation project. https://qgis.org/fr/site/. Accessed 6 Mar 2021
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 7 Dec 2020
Reaser JK, Burgiel SW, Kirkey J et al (2020) The early detection of and rapid response (EDRR) to invasive species: a conceptual framework and federal capacities assessment. Biol Invasions 22:1–19. https://doi.org/10.1007/s10530-019-02156-w
Rehman F, Pervez A, Khattak BN, Ahmad R (2017) Constructed wetlands: perspectives of the oxygen released in the rhizosphere of macrophytes: water. Clean: Soil, Air, Water. https://doi.org/10.1002/clen.201600054
Reiss KC, Brown MT (2005) Developing biological indicators for isolated depressional forested wetlands. University of Florida, Gainesville
Rosset V, Simaika JP, Arthaud F et al (2013) Comparative assessment of scoring methods to evaluate the conservation value of pond and small lake biodiversity: assessment of conservation value of pond and small lake biodiversity. Aquat Conserv Mar Freshw Ecosyst 23:23–36. https://doi.org/10.1002/aqc.2287
Rosset V, Angélibert S, Arthaud F et al (2014) Is eutrophication really a major impairment for small waterbody biodiversity? J Appl Ecol 51:415–425. https://doi.org/10.1111/1365-2664.12201
Rothlisberger J, Chadderton W, McNulty J, Lodge D (2010) Aquatic invasive species transport via trailered boats: what is being moved, who is moving it, and what can be done. Fishereis 35:121–132. https://doi.org/10.1577/1548-8446-35.3.121
Sager L, Lachavanne J-B (2009) The M-NIP: a macrophyte-based Nutrient Index for ponds. Hydrobiologia 634:43–63. https://doi.org/10.1007/s10750-009-9899-1
Scheffer M (2001) Alternative attractors of shallow lakes. Sci World J 1:254–263. https://doi.org/10.1100/tsw.2001.62
Scheffer M, Carpenter SR (2003) Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol Evol 18:648–656. https://doi.org/10.1016/j.tree.2003.09.002
Scheffer M, van Geest GJ, Zimmer K et al (2006) Small habitat size and isolation can promote species richness: second-order effects on biodiversity in shallow lakes and ponds. Oikos 112:227–231. https://doi.org/10.1111/j.0030-1299.2006.14145.x
Sender J (2016) The effect of riparian forest shade on the structural characteristics of macrophytes in a mid-forest lake. Appl Ecol Environ Res 14:249–261. https://doi.org/10.15666/aeer/1403_249261
Simpson JT (1991) Volunteer lake monitoring: a methods manual. U.S., Washington
Stiers I, Crohain N, Josens G, Triest L (2011) Impact of three aquatic invasive species on native plants and macroinvertebrates in temperate ponds. Biol Invasions 13:2715–2726. https://doi.org/10.1007/s10530-011-9942-9
Sytsma MD, Pennington T (2015) 3. Vectors for spread of invasive freshwater vascular plants with a North American analysis. In: Canning-Clode J (ed) Biological invasions in changing ecosystems. De Gruyter Open Poland, Berlin, pp 55–74
Tátrai I, Mátyás K, Korponai J et al (2009) Changes in water clarity during fish manipulation and post-manipulation periods in a shallow eutrophic lake. Fundam Appl Limnol Arch Für Hydrobiol 174:135–145. https://doi.org/10.1127/1863-9135/2009/0174-0135
Thompson W (2013) Sampling rare or elusive species concepts, designs, and techniques for estimating population parameters. William L. Thompson, Island, Washington
Traxler A (1997) Handbuch des vegetationsökologischen Monitorings. Methoden, Praxis, angewandte Projekte. Teil A: Methoden. Umweltbundesamt, Wien
Van Sickle J, Larsen DP, Hawkins CP (2007) Exclusion of rare taxa affects performance of the O/E index in bioassessments. J N Am Benthol Soc 26:319–331. https://doi.org/10.1899/0887-3593(2007)26[319:EORTAP]2.0.CO;2
Vogt K, Rasran L, Jensen K (2006) Seed deposition in drift lines during an extreme flooding event—evidence for hydrochorous dispersal? Basic Appl Ecol 7:422–432. https://doi.org/10.1016/j.baae.2006.05.007
Ware C, Bergstrom D, Müller E, Alsos I (2012) Humans introduce viable seeds to the Arctic on footwear. Biol Invasions 14:567–577. https://doi.org/10.1007/s10530-011-0098-4
Wetzel RG, Søndergaard M (1998) Role of submerged macrophytes for the microbial community and dynamics of dissolved organic carbon in aquatic ecosystems. In: Jeppesen E, Søndergaard M, Søndergaard M, Christoffersen K (eds) The structuring role of submerged macrophytes in lakes. Springer, New York, pp 133–148
Williams AE, Moss B, Eaton J (2002) Fish induced macrophyte loss in shallow lakes: top-down and bottom-up processes in mesocosm experiments. Freshw Biol 47:2216–2232. https://doi.org/10.1046/j.1365-2427.2002.00963.x
Wolters J, Reitsema RE, Verdonschot RCM et al (2019) Macrophyte-specific effects on epiphyton quality and quantity and resulting effects on grazing macroinvertebrates. Freshw Biol 64:1131–1142. https://doi.org/10.1111/fwb.13290
Wood SN (2017) Generalized additive models: an introduction with R, 2nd edn. CRC Press/Taylor & Francis Group, Boca Raton
Acknowledgements
We thank the Aquabio team, especially Joyce Lambert, David Serrette, and Nicolas Tarozzi, who assisted the first author in macrophyte sampling and identification. We also thank the Pyrenees National Park, the Regional Natural Parks of Bauges, Ballon des Vosges, Causses du Quercy, Perigord-Limousin, Plateau des Millevaches, Prealps Cotes d’Azur, Volcans d’Auvergne, and Vosges du Nord; the EPTB Seine Grands Lacs; the Regional Conservatories of Natural Spaces of Aquitaine, Bourgogne, Lorraine and Limousin; the French National Forestry Office; the French National Office of Hunting and Wildlife; the Chartered Fisheries and Aquatic Environment Protection Departmental Federations of Dordogne, Gironde, Puy de Dome and Vosges; the Gironde Hunting Federation; Pinail and Glomel Reserve Associations, and the communities of the cities of Andernos, Sage-Blavet, and Tregor-Lannion. Special thanks to the managers or site owners who have granted permission to carry out sampling. The BIOME project was labelled by the scientific council of DREAM Competitiveness Cluster.
Funding
This research followed the BIOME (BIOindication des Mares et Etangs) project, funded by Aquabio, and the call for proposals “IPME Biodiversité” launched by ADEME, Grant number 1682C0129.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Additional information
Communicated by Daniel Sanchez Mata.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10531_2022_2416_MOESM1_ESM.csv
Supplementary file1 (CSV 5 kb) Table S1 French national status (“National red list” or “exotic”), frequency, mean coverage, and standard deviation of each macrophyte species from the 26 SSLs sampled with the mapped inventory method. Species without a status were omitted. DD = data deficient; LC = least concern; NT = near-threatened; VU = vulnerable
10531_2022_2416_MOESM2_ESM.csv
Supplementary file2 (CSV 8 kb) Table S2 French national status (“National red list” or “exotic”), frequency, mean coverage, and standard deviation of each macrophyte species from the 262 SSLs sampled with the S3m method. Total mean coverages and corresponding deviations for all plants for each status are also indicated. Species without a status were omitted. DD = data deficient; LC = least concern; NT = near threatened; VU = vulnerable
Rights and permissions
About this article
Cite this article
Labat, F., Thiébaut, G. & Piscart, C. A new method for monitoring macrophyte communities in small shallow lakes and ponds. Biodivers Conserv 31, 1627–1645 (2022). https://doi.org/10.1007/s10531-022-02416-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-022-02416-7