Abstract
The aim of this study was to explore the environmental drivers of the aquatic macrophyte assemblage in a large, heterogeneous Spanish region covering a wide altitudinal range. We hypothesized that physicochemical variables affecting assemblages would differ depending on altitude. The study was conducted in 46 plateau ponds and 21 mountain ponds. Our results revealed a shift in hydrophyte assemblage composition and structure along an altitude and water chemistry gradient. However, altitude was not a good predictor of species richness. Conductivity and nutrient concentrations were higher in plateau ponds than in mountain ponds and binary logistic regression showed that conductivity was the best variable for differentiating between both pond types. Canonical correspondence analysis indicated that conductivity was the main factor responsible for the species distribution in both pond types. Generalized linear models showed that in plateau ponds, total phosphorus and mean depth were the strongest predictors of submerged macrophyte coverage, and no model could be created for richness. In the mountain ponds, conductivity and pond area explained coverage of submerged plants, while richness was related to pond area. Our results corroborated the hypothesis to be tested, and the conclusions obtained may be of relevance for making decisions on conservation and restoration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shallow lakes and ponds are the most common lentic continental aquatic ecosystems and the most widely distributed (Downing et al., 2006; Meerhoff & Jeppesen, 2009). Relatively, recent studies suggest that these freshwater ecosystems occupy nearly twice as much area as was previously believed (Downing et al., 2006). Furthermore, several analyses have shown the disproportionately great intensity of many processes in small aquatic ecosystem (Post, 2002) indicating that they play an unexpectedly major role in global cycles (Downing, 2010).
In Europe, ponds are the most widespread aquatic habitat and collectively dominate the total area of continental lentic waters. This assumption is evident especially in Mediterranean countries, such as Spain, where lakes are very scarce (Miracle et al., 2010). There is growing awareness in Europe about the importance of ponds and increasing understanding of the contribution they make to aquatic diversity and catchment functions (Céréghino et al., 2008; Della Bella et al., 2008).
Ponds are considered particularly important in aquatic plant conservation, since they harbour a large number of taxa (Scheffer et al., 2006) including rare and endangered species (Linton & Goulder, 2000; Grillas et al., 2004, Céréghino et al., 2008; Della Bella et al., 2008; Ruiz et al., 2011). Although the importance of aquatic macrophytes is recognized for both ecosystem functioning and conservation purposes, there are important gaps in the knowledge of basic issues concerning this group of plants (Chappuis et al., 2011).
The Mediterranean Basin is one the world’s major biodiversity hot spots (Medail & Quezel, 1999, Myers et al., 2000) and southern and western Europe hold the highest aquatic plant diversity(Chappuis et al., 2012). Among vascular plant, aquatic plants seem to be a special sensitive group with a larger proportion of endangered species than the average (Chappuis et al., 2011).
Macrophytes play an important role in ponds and shallow lakes (Jeppesen et al., 1997; Burks et al., 2006, Declerck et al., 2007). Because of their key ecosystemic functions, aquatic plants are essential for getting a good ecological status of aquatic ecosystems, and it is therefore necessary to preserve such communities in freshwaters. This preservation implies good knowledge on how plant communities are controlled by the principal abiotic parameters that characterize aquatic ecosystems (Bornette & Puijalon, 2011). Existing surveys showed that aquatic plants are synergically influenced by several factors with cross-linked effects (Hrivnák et al., 2013).
Response of aquatic plants to environmental factors has been frequently discussed during the last decades (Lacoul & Freedman, 2006a; Bornette & Puijalon, 2011). In Europe, most publications about aquatic macrophyte abundance and composition and their response to changes in environmental drivers refer to central and northern countries, (e.g. Rørslett, 1991; Vestergaard & Sand–Jensen, 2000; Heegaard et al., 2001; Murphy, 2002; Mäkelä et al., 2004; Penning et al., 2008) where water bodies are rather uniform compared to water bodies in the Mediterranean region. Moreover, these investigations are referred to large aquatic ecosystems in environmentally homogeneous areas. In contrast, the relationships between aquatic macrophytes and environmental factors have been less explored in limnosystems from southern European areas close to the Mediterranean (Alvarez Cobelas et al., 2005; Beklioglu et al., 2007; Chappuis et al., 2011). In these areas, lentic systems often occur over a wide elevation gradient, resulting in high habitat diversity and species richness at a regional scale (Jones et al., 2003; Lacoul & Freedman, 2006b). In Mediterranean countries, most lacustrine ecosystems are small sized. Different European projects (SWALE and ECOFRAME) suggest that Mediterranean lakes operate in a different way compared to the rest of European lakes (Moss et al., 2003, 2004). The high diversity of habitats, in addition to their small area, isolation, strong fluctuations in water level, ecology of submerged macrophytes and varying values for water conductivity are some of the variables explaining why the Mediterranean limnology does not link to many concepts of the temperate limnology (Bécares et al., 2004; Alvarez Cobelas et al., 2005). Furthermore, the implications of climate warming may be even more challenging than in higher latitude lakes, since shallow lakes and ponds in the Mediterranean region are among the most sensitive to extreme climate change (Sánchez et al., 2004).
Species richness has been related to a variety of environmental factors, although sometimes with conflicting results. It has often been found to be correlated with factors such as altitude (Kotze & O’Connor, 2000; Jones et al., 2003; Hrivnák et al., 2013), water body size (Rørslett, 1991; Jones et al., 2003; Mäkelä et al., 2004), trophic state (Toivonen & Huttunen, 1995; Penning et al., 2008; Bornette & Puijalon, 2011), ionic content (Vastergaard & Sand-Jensen, 2000; Mäkelä et al., 2004), pH and alkalinity (Søndergaard et al., 2010; Lauridsen et al., 2015) and human pressures (Sass et al., 2010; Del Pozo et al., 2011). These relations are not always linear and unimodal responses of richness to certain environmental gradients have been described (e.g. trophic gradient; Bornette & Puijalon, 2011).
In the Mediterranean area, only a few studies about macrophyte communities on a regional scale have been carried out. The most relevant ones are those by Gacia et al. (1994), Chappuis et al. (2011), Chappuis et al. (2014) and Pulido et al. (2015), and they are referred to lentic water systems located along altitudinal gradients in the Pyrenees (Catalonia, north-eastern Spain). Considering a latitudinal gradient from north to south Europe, Lauridsen et al. (2015), recently investigated the importance of environmental drivers for aquatic macrophytes in shallow lakes, restricting Spanish lakes to those located in the south (Andalucía and Castilla-La Mancha).
Many factors are known to have an influence on the presence and abundance of aquatic macrophytes, for instance, herbivorism (Lodge, 1991; Pipalová, 2002), substrate characteristics (Lacoul & Freedman, 2006b; Mikulyuk et al., 2011), water-level fluctuations (Fernández-Aláez et al., 1999; Van Geest et al., 2005), land uses in the catchment (Capers et al., 2010; Akasaka et al., 2010) or propagule dispersal (Dahlgren & Ehrlén, 2005). In organisms with high dispersion capacity, local environmental conditions can be expected to explain a large proportion of the assemblage composition (Capers et al., 2010). The distribution of aquatic plants is hardly limited by dispersion because of the ease with which seeds and fruits are carried by waterfowl (Figuerola & Green, 2002). Consequently, the macrophyte assemblage composition is mostly shaped by habitat variables and local characteristics of the pond, particularly chemical composition of the water (Heegaard et al., 2001; Akasaka & Takamura, 2011).
In this study, we examined macrophyte communities in relation to some environmental variables (mostly hydrochemical) in a pond set occupying a large region characterized by high geographical heterogeneity, with contrasting altitudinal, topographical, lithological and climatic characteristics. Several criteria have been proposed to differentiate ponds from lakes. They are mostly based on morphological features, particularly pond area (Pond Conservation Group, 1993; Biggs et al., 2005). However, we have used in our study a criterion more linked to water body function, previously proposed by Oertli et al. (2000), who defined pond as “a waterbody with a maximum depth no more than 8 m, offering water plants the potential to colonise almost the entire area of the pond”. Given the important role of macrophytes in keeping good ecological status of lentic sytems, the results obtained by this research will be relevant for management and conservation purposes. If the drivers controlling abundance and richness of macrophytes were different depending on the altitude, management programmes should be altitude-specific. We firstly aimed to identify differences in hydrochemical characteristics along the elevation gradient or between plateau and mountain ponds. Furthermore, we intended to check if the effect of several key environmental factors driving macrophyte assemblages changes with altitude and so the composition and abundance of aquatic plants are determined by different variables in mountain and in plateau ponds. Finally, we aimed to find out how aquatic plant richness is affected by altitude and which chemical and morphometric variables can explain differences in richness within each type of pond. Given the differences in climate, topography and land use along the altitude gradient, we expect the hydrochemical characteristics to differ between lowland and mountain ponds. Besides, macrophyte assemblage composition and abundance could be expected to depend mostly on natural factors in mountain ponds and on variables related to land use in the plateau. We also hypothesize that richness will decrease with increasing altitude, with highest values in plateau ponds.
Study area
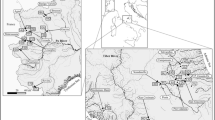
The study was made on 67 ponds scattered over a vast Spanish region, Castilla y León (94,233 km2), mostly drained by the Duero River and its tributaries (Fig. 1). This is an heterogeneous region encompassing a wide altitude range and two distinct landscape regions: a central, flat, relatively low (700–1000 m.a.s.l.) land (the plateau), and a succession of peripheral mountain ranges (elevations up to 2600 m.a.s.l.) surrounding the central area. Plateau and mountain areas differ not only in orographic features but also in climate and land uses. The climate in the central plateau is characterized by hot, dry summers and cold, rainy winters. The average annual rainfall is between 400 and 600 mm. In mountain areas, winters are cold and long, whereas summers are cool and short, and annual precipitation is higher than in the plateau (annual rainfall between 800 and 1500 mm, mostly as snow in winter). Most of the land in the plateau has been cleared and transformed into croplands, only dotted with pine plantations or small oak or holm-oak woods in some zones. In contrast, mountain areas are covered with shrublands, grasslands and, less frequently, forests. The criteria for the site selection were to get ponds from a huge wide altitude gradient as possible, and widespread throughout the region so that the main landscape types in the territory were well represented. Out of the 67 ponds, 46 were plateau ponds (altitudes between 700 and 1000 m.a.s.l.) and 21 were mountain, glacial ponds (altitudes between 1400 and 2120 m.a.s.l.) (Table 1). Most of them are small, shallow and permanent or semi-permanent (they keep water all through the year but may occasionally dry up). Hydrological regime differs between plateau and mountain ponds. Plateau ponds are mostly fed by groundwater and rainfall, whereas mountain ponds depend on surface runoff and precipitation (as snow or rain). Therefore, the whole dataset included two distinct pond types (plateau and mountain), subjected to different land uses and covering a wide elevation range.
Methods
Ponds were sampled once in July 2004–2005 (plateau) or in 2007–2008 (mountain). In order to rule out potential differences caused by the different sampling dates for plateau and mountain ponds, we analysed mean monthly temperature and total monthly precipitation obtained from four weather stations located close to the mountain ponds in the north, west, east and south of the study area. Temperature and precipitation have been considered as the main factors influencing lake mountain functioning (Sovari & Korhola, 1998). Repeated measures ANOVA showed that there were no significant differences in the values of these variables between 2004, 2005, 2007 and 2008. In each pond, several water samples were randomly collected with a tube (6 cm diameter and 1-m long) along a shore-centre transect and were integrated into a single composite sample. The number of samples varied depending on the pond area. Conductivity, pH, temperature and dissolved oxygen were measured on the integrated water sampled using WTW field probes (Model LF 323, Model 330i and Model OXI 320, respectively). The integrated sample was analysed in laboratory to determine total phosphorus (TP), soluble reactive phosphorus (SRP), total nitrogen (TN), nitrate and chlorophyll a concentrations. Nutrient samples were fixed with mercuric chloride and preserved at 4°C until they were analysed. Samples were analysed according to standard methods 1 week after collection (APHA, 1989). Secchi depth was also recorded, but most of the ponds were shallow enough to keep the disc visible up to the bottom. Therefore, the ratio Secchi depth:pond depth (Armengol et al., 2008) was used instead as a variable.
Hydrophyte vegetation (submerged, floating-leaved and free-floating macrophytes) was sampled by placing square sampling units along profiles, which are defined as a line from one shore to the opposite shore at a right angle to the longest length. When the pond could not be crossed because of the depth, transects were used. The number of profiles varied according to the area of the pond and the development of the shore (Jensén, 1977); however, in situ corrections accounted for the heterogeneity of the macrophyte communities and the accessibility to the pond. Square sampling units (0.5× 0.5 m2) were placed along the profiles at varying intervals of 0–5 m, depending on the homogeneity of the vegetation (for example, in the littoral zone, where community changes with increasing depth are quicker, sampling units were placed closer to each other, whereas in the central part of the pond, with more uniform depth, lower richness and less spatial changes in the community, gaps between consecutive samples were increased to 5 m). The total number of units varied according to the width of the pond. Percentage coverage of each species was recorded at each sampling unit. In the deepest areas of the ponds, where direct observation was not possible, the quantification of submerged vegetation was performed by collecting samples with a hook from a boat. For this purpose, four samples were taken in each of 5–20 points, depending on the pond size. Frequency values derived from this hook-sampling were used to estimate plant coverage. For statistical analyses, the coverage values were transformed into a semiquantitative scale ranging from 1 to 7 (1, <1%; 2, 1–5%; 3, 5–10%; 4, 10–25%; 5, 25–20%; 6, 50–75%; 7, >75%). We calculated the mean cover of each species in a pond as the sum of coverages of the species in all the sampling units divided by the total number of sampling units used in the pond.
The nomenclature followed Flora Ibérica (Castroviejo et al., 1986–2010), Flora Europaea (Tutin et al., 1980), Cirujano et al. (2008) and Fernández-Aláez et al. (2012).
Pond areas were measured on images available in SIGPAC (the Spanish Geographical Information System for Agricultural Parcels,http://www.sigpac.jcyl.es/visor/). Mean pond depth was determined by measuring depth at each vegetation sampling unit.
Statistical analyses
Physical and chemical characteristics of the water
Our first interest was to assess the differences between the two pond types (plateau vs. mountain). In the case of water physical and chemical variables, this was firstly addressed by means of Mann–Whitney tests. The values of these variables were represented by box-plots in order to visually highlight differences. Furthermore, we used backward stepwise logistic regression to create a model to discriminate between the two pond types (plateau vs. mountain) on the basis of the physical and chemical characteristics. Prior to the analysis, variables highly intercorrelated (Spearman correlation coefficient >0.6) were removed to avoid problems of collinearity. Logistic regression was chosen because the variables did not satisfy the assumptions demanded by discriminant analysis. The dependent variable (pond type) was treated as a binary variable and several predictor variables were removed from the models in an iterative process using the likelihood ratio statistic based on the maximum partial likelihood estimates. The correct classification rate (percentage of all ponds correctly classified) was used to assess the accuracy of the model. Wald statistic was used to test the significance of individual predictor variables.
Taxonomic composition of the aquatic macrophyte assemblages
We first tried to check whether plateau ponds differed from mountain ponds in their taxonomic composition. This issue was addressed in two steps. Firstly, a Detrended Correspondence Analysis (DCA) on semiquantitative coverage data and with rare-species downweighting was performed to make sure that the two types considered, better than other groupings, differed in their assemblage composition. The factor scores of the ponds on the first four DCA axes were correlated with altitude and water physical and chemical parameters using the Spearman correlation coefficient (r s). We selected this coefficient because the variables were not normally distributed. Secondly, we conducted a SIMilarity of PERcentages Analysis (SIMPER) to check whether the mountain and plateau ponds were different in terms of their macrophyte communities and to identify the species that better contributed to the dissimilarities between the two types of ponds.
Next, we intended to identify the environmental drivers influencing the macrophyte assemblage within each of both pond types (plateau and mountain, separately). For this purpose, two partial Canonical Correspondence Analyses (CCA) were carried out, one for plateau and another for mountain ponds, including the physical and chemical characteristics of water as variables and pond area and mean depth as co-variables. Before applying CCA, we verified by means of DCA that variables showed a strong unimodal response (DCA gradient lengths >2 standard units). All the variables, excepting pH, were log (x + 1) transformed prior to analysis. Forward selection with Monte Carlo permutation test (499 permutations, P < 0.05) was applied to select a minimal set of explanatory variables.
Structure of the aquatic macrophyte assemblages
We described the assemblage structure by measuring two parameters: hydrophyte richness and submerged macrophyte coverage. Spearman correlation coefficient was used to explore relationships between altitude and variables describing the assemblage structure in the whole dataset. Next, we used Mann–Whitney tests to check for differences in both structural parameters between the two types of pond.
Finally, the responses of these parameters to environmental variables (physical, chemical, pond area and mean depth) were separately modelled for each pond type using generalized linear models (GLM). Aiming at a robust analysis, several candidate predictors were excluded from GLM analysis due to high or moderate correlation with other predictors, namely SRP (r sp = 0.65 with TP), chlorophyll a (r sp = 0.48 with TN), and pH (r sp = −0.50 with TP) in mountain ponds; and SRP (r sp = 0.83 with TP), and chlorophyll a (r sp = 0.47 with TN), in plateau ponds. Consequently, the variables used in the GLM analyses were conductivity, pH, nitrate, TN, TP, Secchi depth:pond depth, pond area and mean depth (for plateau ponds); and conductivity, nitrate, TN, TP, Secchi depth:pond depth, pond area and mean depth (for mountain ponds). Different analysis options were undertaken depending on the nature of each assemblage parameter. In the case of richness, we used Poisson error distribution and logarithmic link function to model species richness, and statistical significances of the explanatory variables were tested by χ 2 test (P < 0.05). In a full model, we included all explanatory variables uncorrelated and the minimal most parsimonious model was determined by both backward and forward stepwise variable selection based on Akaike’s information criterion. For cover of submerged macrophytes, Quasi-Poisson GLMs (logarithmic link function) were conducted in order to compensate for overdispersion. We construct a full model with all uncorrelalated variables and manually and sequentially we removed the variables that were not significant (P > 0.05). The final model was that showing the greatest amount of explanatory power and with all of the included variables being significant. The statistical significance of each variable was tested using χ 2 tests.
Mann–Whitney test and Spearman correlations were calculated with STATISTICA v.8., while DCA and CCA were performed with CANOCO for Windows 4.5. Binary logistic regression and SIMPER analysis were carried out with SPSS v.21 and PRIMER v.5., respectively, and we used R statistical package (R Core Team, 2014) to elaborate GLMs.
Results
Taxonomic composition of the aquatic macrophyte assemblages
The DCA applied to the whole dataset (67 ponds) revealed altitude and pond type (plateau vs mountain) as the main drivers of the macrophyte assemblage (Fig. 2A) over factors such as geographical position or pond size. In fact, the scores of the samples on the first axis were closely related to altitude (Spearman rank correlation, 0.650, P < 0.001), as well as with environmental variables related to altitude in the study area, specifically conductivity (r sp = −0.705, P < 0.001), pH (r sp = −0.625, P < 0.001), TP (r sp = −0.684, P < 0.001) and TN (r sp = −0.528, P < 0.001). The high eigenvalue (0.81) showed that a substantial part of the total variation in species composition among the ponds was expressed along this axis. Sites on the farthest right end of axis 1 corresponded to mountain ponds above 2000 m.a.s.l (Fig. 2B) and were characterized by the presence of Subularia aquatica L., Sparganium angustifolium Michx, Luronium natans (L.) Raf. and Isoetes echinospora Durieu. Plateau ponds were plotted towards the left end of the first DCA axis (Fig. 2B), with Tolypella hispanica Nordst. ex T.F. Allen, Chara canescens Desv. & Loisel, Chara connivens Salzm. Ex A. Braun and Ranunculus peltatus subsp. saniculifolius (Viv.) C.D.K. as most characteristic species, typically growing in ponds at altitudes only slightly above 700 m a.s.l. (Fig. 2B). These patterns revealed an evident shift in macrophyte assemblage composition along an altitude and a water chemistry gradient.
Detrended correspondence analysis (DCA) performed on semiquantitative coverage data of hydrophyte species. A Ordination of the ponds. B Ordination of the hydrophyte species. Open circles, species shared by plateau and mountain ponds; black circles, species unique to plateau ponds; black squares, species unique to mountain ponds. Taxon codes as in Table 3
Thirty-four and twenty-one taxa (including submerged, floating-leaved and free-floating taxa) were recorded in plateau and mountain ponds, respectively. Of the 34 taxa in the plateau ponds, 7 were charophyte species, and 1 was a bryophyte. In mountain ponds, 2 of the taxa were charophyte species and 5 were bryophytes. The number of taxa per pond ranged from 1 to 12 in the plateau ponds and from 1 to 10 in the mountain ponds. SIMPER revealed a high taxonomical dissimilarity (94.5%) between the two types of ponds. The taxa contributing most to this dissimilarity were Myriophyllum alterniflorum Dc., Ranunculus peltatus subsp. peltatus Schrank, Potamogeton trichoides Cham. & Schltdl and Sphagnum sp. (Table 2). The average similarity among plateau ponds and among mountain ponds was 16.50 and 21.10%, respectively. P. trichoides, M. alterniflorum and Polygonum amphibium L. were the most frequent and abundant species in plateau ponds and 23 species were exclusive of them. R. peltatus subsp. peltatus, Sphagnum sp., and Callitriche brutia Petagna were the most frequent and abundant species in mountain ponds and 9 species were only recorded in these ponds (Table 3).
Physical and chemical characteristics of the water
Values of all the environmental variables but ratio Secchi depth:pond depth were significantly higher in plateau ponds than in mountain ponds (Mann–Whitney U test, P < 0.05). Plateau ponds were characterized by a high variability in conductivity (median 241 μS cm−1, minimum of 50 μS cm−1 and maximum of 1025 μS cm−1). In contrast, conductivity values in mountain ponds were low and with low variability (median 17 μS cm−1, minimum of 7.4 μS cm−1 and maximum of 188 μS cm−1). Median values of pH in plateau and mountain ponds were 8.1 and 6.6, respectively, with higher variability in mountain ponds than in plateau ponds. The highest phosphorus concentrations and highest among-site variability were measured in plateau ponds, with median values of TP and SRP of 116.04 and 24.55 μg l−1, respectively. In mountain ponds, median values of TP and SRP were 29.05 and 8.72 μg l−1, respectively. Similar patterns were recorded for nitrogen, with median values of TN and nitrate in plateau ponds of 1.5 and 0.036 mg l−1, respectively, while those values for mountain ponds were 0.81 and 0.018 mg l−1. Median values of chlorophyll a in the plateau and the mountain ponds were 11.8 and 7.3 μg l−1, respectively (Fig. 3).
Binary logistic regression performed on water physical and chemical variables (explanatory variables) and pond type (binary response variable) allowed us to construct models to discriminate between plateau and mountain ponds. SRP (highly correlated with TP, r sp = 0.788 P < 0.001) and pH (correlated with conductivity r sp = 0.624 P < 0.001) were removed prior to the statistical analysis, so binary logistic regression was performed on the following variables: conductivity, nitrate, TN, chlorophyll a and Secchi depth:pond depth. Conductivity (P < 0.05) and TN (P < 0.1) were the only variables significantly differentiating the two types of pond. The results of the analysis are shown in Table 4. The model made it possible to reliably discriminate between mountain and plateau ponds, since 92.3% of the cases were correctly classified (93.5% of the plateau ponds and 90.5% of the mountain ponds).
Relationship between environmental variables and assemblage composition
Canonical correspondence analyses (CCA) were independently made for plateau and mountain ponds in order to identify driving factors within each pond type. The first two axis of the CCA for plateau ponds explained 75.2% of the variance of the species–environment relationships. The forward selection process kept four environmental variables (conductivity, TP, chlorophyll a and pH) significantly contributing to the variance in the taxonomic composition (Fig. 4). These four variables explained 72.3% of the total variance. Axis 1 was strongly correlated with conductivity, TP and pH, and therefore, differentiated the ponds on the basis of their ionic content and trophic status. Callitriche hamulata Kütz ex W.D.K. Koch, Nitella translucens Ag, Isoetes sp. and Utricularia australis R. Br. were most frequent in ponds with low conductivity, pH and phosphorus concentration. In contrast, Lemna minor L., Nymphaea alba L., Potamogeton pectinatus L., Lemna gibba L. and Myriophyllum spicatum L. were typical of ponds with high ionic content, whereas L. minor, Ceratophyllum submersum L. and N. alba were associated to eutrophic ponds. Axis 2 was related to chlorophylla a concentration, with Chara hispida var. major (Harm.) R.D. Wood, Chara aspera var. aspera Dethard. ex Willd., Groenlandia densa (L.) Fourr., Chara vulgaris var. vulgaris L. and Zannichellia pedunculata Rchb. in Mossler, Handb placed towards the negative end of this axis.
Canonical correspondence analysis (CCA) diagram performed on physical and chemical variables and semiquantitative coverage data of hydrophyte species in plateau ponds. Taxon codes as in Table 4
In mountain ponds, the first two CCA axes explained 100% of the variance of the species–environment relationship. Forward selection showed that conductivity, and TP (altogether explaining 58.9% of the total variance) were the main drivers of macrophyte assemblage composition (Fig. 5). The axis 1 could be identified as a gradient of ionic content. I. echinospora, S. angustifolium, S. aquatica and Warnstorfia exannulata (Schimp.) Loeske were associated with low-conductivity ponds, while Chara fragilis Desv. and G. densa were found in the ponds with highest conductivity values. P. amphibium, M. spicatum, Drepanocladus aduncus (Hedw.) Warnstand, Ranunculus trichophyllus Chaix were related to both high conductivity and phosphorus concentration values. The second axis represented a phosphorus gradient, although concentrations were below 60 μg l−1 in all the ponds. Nevertheless, four species, G. densa, Ch. fragilis, M. alterniflorum and I. echinospora, could be identified as typical of the most oligotrophic water bodies.
Canonical correspondence analysis (CCA) diagram performed on physical and chemical variables and semiquantitative coverage data of hydrophyte species in mountain ponds. Taxon codes as in Table 4
Relationship between environmental variables and assemblage structure
Submerged macrophyte coverage significantly declined as altitude increased (r S = −0.3621, P < 0.01) and, in addition, the values of this variable were significantly higher in plateau ponds than in mountain ponds. In contrast, richness did not significantly differ between pond types or along the elevation gradient (Fig. 6).
The model constructed for submerged macrophyte coverage in plateau ponds included TP, mean depth (both negatively related) and, to a lesser extent, pH (positively related) as explanatory variables. In mountain ponds, submerged macrophyte coverage was negatively correlated with pond area and positively with conductivity (Table 5). Species richness in plateau ponds was only positively related to pond area (Table 6). However, no significant variables allowed to build a model for mountain ponds, that is, hydrophyte richness patterns could not be explained by any of the variables included in the study.
Discussion
Influence of altitude on macrophyte communities is well known (Lacoul & Freedman, 2006a; Chappuis et al., 2011; Hrivnák et al., 2013). It is less known which physical and chemical variables are responsible for macrophyte assemblage composition and structure at different altitudes. To address this issue, we have selected for the present study a heterogeneous pond set occupying a wide elevation gradient and with varying physical and chemical characteristics. It was distinctively divided into two groups: mountain ponds located at relatively high altitude and plateau ponds at lower elevation. These two groups also differed in variables other than altitude, particularly in conductivity which was the variable (among those measured in this study) contributing most to the distinctness of mountain and plateau ponds, as suggested by the logistic regression analysis. Conductivity values were usually higher in lowland ponds due to comparatively high summer temperatures (and increased ionic content induced by evaporation), but also due to higher nutrient content, for which conductivity can be considered as a good surrogate (Toivonen & Huttunen, 1995). Furthermore, the two types of ponds significantly differed in the values of nutrient concentrations, chlorophyll a and ionic content. Likewise, plateau ponds encompassed wide environmental gradients, specially of conductivity and nutrient concentrations.
Relationship between environmental variables and assemblage composition
Consistently with these environmental divergences, a marked difference in macrophyte assemblage composition between flatland and mountain ponds was observed. Only 10 species occurred in both types of ponds, 6 of them just poorly represented in mountain ponds (present only in a few of them and with low abundances). M. alterniflorum and P. trichoides, both typical of mesotrophic water bodies (Nurminen, 2003; Steffen et al., 2014), were the most frequent and abundant species in the plateau. Two taxa typical of acidic and oligotrophic waters, Sphagnum sp. and W. exannulata (Karttunen & Toivonen, 1995; Murphy, 2002), and one species usually growing in soft, poor-nutrient waters, C. brutia (García-Baquero & Crujeiras, 2015), were very frequent in mountain areas and were exclusively recorded here. These differences were well captured by the first DCA axis, which was closely related to elevation. This was an expected outcome since altitude is an indirect factor summarizing a set of essential climate drivers such as light, precipitation and temperature, with a known influence on macrophyte communities (Lacoul & Freedman, 2006a, b). However, species distribution was related to ionic content (indicated by conductivity) and pond trophy (indicated by nitrogen and phosphorus concentrations) as well as with altitude. In fact, disentangling the effects of these three variables in the study area is a trying task, since decreasing altitudes were related to increasing eutrophication and ionic content, as often reported by studies on areas with altitude gradients of varying amplitudes (Heegaard et al., 2001; Chappuis et al., 2014). Such relationship can be easily explained by the intense agriculture activity in the plateau. Therefore, the ordination of species along the elevation gradient was likely due to climate factors, but it was also a response to local conditions.
Consistent with our hypothesis, the macrophyte assemblages seemed to be governed by different driving factors in plateau and mountain areas. From among the variables included in the study, the ionic content, measured by conductivity, was the main predictor of the assemblage composition in both cases, as shown by a number of studies (Gacia et al., 1994; Heegaard et al., 2001; Chappuis et al., 2014). However, the meaning of this variable was not the same in the two pond types. In mountain areas, it reflected the catchment lithological composition, whereas in the plateau it was closely related to pH and phosphorus concentrations.
The assemblage composition in mountain regions was primarily related to the ionic content (conductivity) of the water and secondarily to trophic state (phosphorus concentration). In the further north of the study area, carbonated rocks (limestone and dolomite) are dominant, and the resulting higher conductivity values favoured elodeids (P. natans, P. amphibium, and G. densa) and charophytes (Ch. fragilis). The remaining mountain areas are dominated by siliceous rocks and the ponds were frequently colonized by isoetids and bryophytes (I. echinospora, S. aquatica, W. exannulata), well adapted to live in soft waters (Murphy, 2002; Pulido et al., 2015). The apparently relevant role of phosphorus concentration should be rather interpreted as a relationship between pond depth and assemblage composition, since the highest phosphorus concentrations were recorded in the shallowest water bodies. These ponds held dense populations of helophytes that accumulate high amounts of nutrients (Abdo & Da Silva, 2002), which are released to the water column through decomposition after death. Therefore, macrophytes might be playing an essential role as nutrient source influencing the pond trophy. Nevertheless, we could hardly identify a set of species typical of shallow waters as many of them were present in shallow ponds as well as in the littoral zone of deeper ones.
The chemical gradient underlying species compositional variation in the plateau ponds was also the ionic content; but, unlike mountain ponds, this gradient was related to pH and phosphorus concentration. Certainly, high ionic content in these lowland ponds is caused to a large extent by nutrient enrichment originated from anthropogenic land use, mainly agriculture. Thus, conductivity was indicative of trophic state (Crowder et al., 1977).
Relationship between environmental variables and assemblage structure
Submerged macrophyte coverage
Not only assemblage composition but submerged macrophyte coverage differed between plateau and mountain ponds as a response to the altitude gradient. Furthermore, submerged macrophyte coverage was inversely related to altitude over the whole dataset. Lower temperatures (Barko et al., 1986) or coarser sediments (often sandy to stony) at higher altitudes could negatively affect the growing of macrophytes. It has been suggested that the physical properties, particularly texture of sediments, can influence growing by hindering root penetration (Deny, 1980). Moreover, coarse-textured sediments can be considered nutritionally poor for macrophyte growth (Barko et al., 1986).
Community structure also seemed to be shaped by different factors in plateau and mountain areas. In plateau ponds, phosphorus concentrations and depth had a negative effect on the coverage of submerged macrophytes. It is widely accepted that phosphorus is the nutrient controlling productivity in aquatic ecosystems and, when in high concentrations, the growth of macrophytes declines even in the littoral zone, mostly due to shading by phytoplankton and filamentous algae (Blindow, 1992; Morris et al., 2003). This principle is consistent with our results, where ponds with highest phosphorus and chlorophyll concentrations were associated to low coverage values. The GLM models created for submerged macrophyte coverage showed a negative relationship between coverage and pond depth. This suggests that the deepest areas of the ponds were unfavourable for macrophytes, probably because of reduced light intensity or quality (Scheffer, 1998). This effect can hardly be due to depth itself since mean depth was seldom above 2.5 m, but to a combination of depth and turbidity. In this respect, it should be pointed out that in deep ponds the ratio Secchi depth:mean depth was often below 0.5 due to high turbidity caused by increased eutrophication. In the model, pH was selected as a significant variable together with depth and phosphorus concentration. However, it is very likely that high pH values were a consequence of high macrophyte productivity and not the opposite. A potential relationship between pH and alkalinity, which might be an alternative explanation, can almost certainly be rejected because previous studies on similar plateau ponds of the region failed to find a significant relationship between those variables (Fernández-Aláez et al., 2006; Fernández-Alaez & Fernández-Aláez, 2010).
Of all the variables tested, conductivity, but not pH or phosphorus concentration, was the best predictor of submerged macrophyte coverage in mountain ponds. A likely explanation can be found in the type of vegetation present. Low-conductivity water bodies are considered to be more appropriate for isoetids, which hardly take up bicarbonates (Gacia & Peñuelas, 1991). In fact, isoetids, together with bryophytes, were dominant in ponds with low ionic content. They are both small-sized plants with low growth rate. In contrast, moderate conductivity levels are more favourable for myriophylloides, which can hamper the development of isoetids and bryophytes through increased coverage and shading.
On the other hand, pond area was inversely related to submerged plant coverage. Although it has been found that the cover of hydrophytes is relatively smaller in larger waterbodies (Duarte et al., 1986; Van Geest et al., 2003), the effects of area on aquatic plant abundance have hardly received any attention. Van Geest et al. (2003) suggested several mechanisms to explain this negative relationship, e.g. the effect of wind stress on macrophytes (Hudon et al., 2000), the effect of the turbidity as result of resuspension in large lakes, the persistence of the submerged macrophytes throughout the winter in sheltered small water bodies or the effects of the trophic cascade leading to clear water. However, in our study, these hypotheses were not checked and further research is required.
Hydrophyte richness
In contrast to submerged macrophyte coverage, hydrophyte richness did not significantly differ between plateau and mountain ponds nor was it related to altitude in spite of the wide elevation range (1420 m), well above that in some previous studies (Bagella et al., 2010). Our results seem to challenge the widespread opinion that altitude is a major environmental driver of macrophyte richness (e.g. Heegaard et al., 2001; Jones et al., 2003, Rolon & Maltchik, 2006). Probably, we failed to find the expected negative relationship between altitude and richness because a set of interacting factors caused a complex response of the assemblage. For instance, we expected richness to be higher in lowland, plateau sites, and it was often so. However, a number of plateau ponds, those on the extreme of the conductivity (>800 μS cm−1) and TP (>1000 μg l−1) gradients, supported very poor assemblages composed by some of the few species tolerating these conditions, such as C. demersum, P. pectinatus, M. spicatum and Ch. connivens. These species might have become dominant in such harsh environments as a result of a process of competitive exclusion (Sand-Jensen, 1997).
Neither in mountain or plateau ponds was it possible to create a GLM model explaining differences in richness on the basis of trophic state or ionic content. In mountain ponds, this was an expected result because the available range of conductivity and nutrient concentrations was narrow. In water bodies with low or moderate nutrient levels, like our mountain ponds, plant communities are usually most influenced by sediment texture, pH or alkalinity (Frink & Norwell, 1984). Pond area was the only explanatory variable significantly related to richness of hydrophytes in mountain ponds. This result is in accordance with the positive species–area relationship (MacArthur & Wilson, 1967; Cam et al., 2002), which is one of the most robust generalizations in ecology (He & Legendre, 2002). The lack of relationship between richness and physical or chemical variables was more puzzling in plateau ponds, where the ranges of nutrients, conductivity and area were much wider. Such variables have been identified as reliable predictors of plant richness in aquatic environments (Mäkelä et al., 2004; Della Bella et al., 2008; Capers et al., 2009; Søndergaard et al., 2010). However, none of these variables had a significant effect on hydrophyte richness changes in lowland ponds, indicating that hydrophyte richness might depend on factors not measured, including interspecific relationships, disturbance or stress (e.g. intra-annual water-level fluctuations). Nevertheless, we suspect that the large amount of unexplained variation in richness indicates the importance of stochastic processes (Capers et al., 2010). This is just another example of the difficulty to quantify the relative contributions of different processes to observed richness patterns (van Groenendael et al., 2000). Ponds represent dynamic ecosystems in which species richness do not only reflect the current environmental conditions but also historical pond-specific events (Edvardsen & Økland, 2006).
This study revealed clear patterns of macrophyte assemblage composition and structure over an altitudinal gradient. Such gradient was unquestionably associated to environmental variables such as conductivity, pH and nutrient content. The most outstanding result was that assemblages in lowland, plateau ponds and mountain ponds were governed by different factors. Mountain assemblages proved to be driven mostly by natural factors (lithological characteristics of the catchment in particular), whereas assemblages in plateau ponds were clearly influenced by anthropic factors (specially nutrient enrichment). It is evident from these results that plateau and mountain ponds are two different types of water bodies which must be separately taken into consideration when applying management programmes. These differences should also be considered for typology design.
References
Abdo, M. S. A. & C. J. Da Silva, 2002. Nutrient stock in the aquatic macrophytes Eichornia crassipes and Pistia stratiotes in the Pantanal, Brazil. In Lieberei, R., H. K. Bianchi, V. Boehm & C. Reisdorff (eds), Neotropical Ecosystems. Proceedings of the German-Brazilian Workshop. Hamburg 2000, GKSS-Geesthacht: 875–880.
Akasaka, M. & N. Takamura, 2011. The relative importance of dispersal and the local environment for species richness in two aquatic plant growth forms. Oikos 120(1): 38–46.
Akasaka, M., N. Takamura, H. Mitsuhashi & Y. Kadono, 2010. Effects of land use on aquatic macrophyte diversity and water quality of ponds. Freshwater Biology 55: 902–922.
Alvarez Cobelas, M., C. Rojo & D. G. Angeler, 2005. Mediterranean limnology: current status, gaps and the future. Journal of Limnology 64(1): 13–29.
APHA, 1989. Standard Methods for the Examination of Water and Wastewater. 17th edition. American Public Health Association, Washington, DC.
Armengol, X., M. Antón-Pardo, F. Atiénzar, J. L. Echevarrías & E. Barba, 2008. Limnological variables relevant to the presence of the endangered white-headed duck in southestern Spanish wetlands during a dry period. Acta Zoologica Academia Scintiarum Hungaricae 54(Suppl. 1): 54–60.
Bagella, S., S. Gascón, M. C. Caria, J. Sala, M. A. Mariani & D. Boix, 2010. Identifying key environmental factors related to plant and crustacean assemblages in Mediterranean temporary ponds. Biodiversity and Conservation 19: 1749–1768.
Barko, J. W., M. S. Adams & N. L. Clesceri, 1986. Environmental factors and their consideration in the management of submerged aquatic vegetation: a review. Journal of Aquatic Plant Management 24: 1–10.
Bécares, E., A. Conty, C. Rodríguez & S. Blanco, 2004. Funcionamiento de los lagos someros mediterráneos. Ecosistemas 13(2): 2–16.
Beklioglu, M., S. Romo, I. Kagalou, X. Quintana & E. Bécares, 2007. State of the art in the functioning of shallow Mediterranean Lakes: workshop conclusions. Hydrobiologia 584: 317–326.
Biggs, J., P. Williams, P. Whitfield, P. Nicolet & A. Weatherby, 2005. 15 years of pond assessment in Britain: results and lessons learned from the work of Pond Conservation. Aquatic Conservation: Marine and Freshwater Ecosystems 15: 693–714.
Blindow, I., 1992. Decline of charophytes during eutrophication: comparison with angiosperms. Freshwater Biology 28: 9–14.
Bornette, G. & S. Puijalon, 2011. Response of aquatic plants to abiotic factors: a review. Aquatic Sciences 73: 1–14.
Burks, R. L., G. Mulderij, E. Gross, I. Jones, L. Jacobsen, E. Jeppesen & E. Van Donk, 2006. Center stage: the crucial role of macrophytes in regulating trophic interactions in shallow lake wetlands. In Bobbink, R., B. Beltman, J. T. A. Verhoeven & D. F. Whigham (eds.), Wetlands: Functioning, Biodiversity Conservation, and Restoration. Ecological Studies, Vol. 191. Springer, Berlin: 537–559.
Cam, E., J. D. Nichols, J. E. Hines, J. R. Sauer, R. Alpizar-Jara & C. H. Flather, 2002. Disentangling sampling and ecological explanations underlying species–area relationships. Ecology 83: 1118–1130.
Capers, R. S., R. Selsky, G. J. Bugbee & C. White, 2009. Species richness of both native and invasive aquatic plants influenced by environmental conditions and human activity. Botany 87: 306–314.
Capers, R. S., R. Selsky & G. J. Bugbee, 2010. The relative importance of local conditions and regional processes in structuring aquatic plant communities. Freshwater Biology 55: 952–966.
Castroviejo, S., M. Lainz, G. López González, P. Montserrat & F. Múñoz Garmendia et al., 1986, 1990, 1993, 1997, 2001, 2007–2010. Flora Ibérica: Plantas vasculares de la Península Ibérica e Islas Baleares,Vols. I–III, VIII, X, XIV–XV, XVII–XVIII. Real Jardín Botánico, C.S.I.C. Madrid.
Céréghino, R., J. Biggs, B. Oertli & S. Declerck, 2008. The ecology of European ponds: defining the characteristics of a neglected freshwater habitat. Hydrobiologia 597: 1–6.
Chappuis, E., E. Ballesteros & E. Gacia, 2011. Aquatic macrophytes and vegetation in the Mediterranean area of Catalonia: patterns across an altitudinal gradient. Phytocoenologia 41(1): 35–44.
Chappuis, E., E. Ballesteros & E. Gacia, 2012. Distribution and richness of aquatic plants across Europe and Mediterranean countries: patterns, environmental driving factors and comparison with total plant richness. Journal of Vegetation Science 23: 985–997.
Chappuis, E., E. Gacia & E. Ballesteros, 2014. Environmental factors explaining the distribution and diversity of vascular aquatic macrophytes in a highly heterogeneous Mediterranean region. Aquatic Botany 113: 72–82.
Cirujano, S., J. Cambra, P. M. Sánchez Castillo, A. Meco & N. Flor Arnau, 2008. Flora Ibérica, Algas Continentales: Carófitos (Characeae). Real Jardín Botánico, C.S.I.C. Madrid.
Crowder, A. A., J. M. Bristow, M. R. King & S. Vanderkloet, 1977. The aquatic macrophytes of some lakes in southeastern Ontario. Naturaliste Canadien 104: 457–464.
Dahlgren, J. P. & J. Ehrlén, 2005. Distribution patterns of vascular plants in lakes – the role of metapopulation dynamics. Ecography 28: 49–58.
Declerck, S., M. Vanderstukken, A. Pals, K. Muylaert & L. de Meester, 2007. Plankton biodiversity along a gradient of productivity and its mediation by macrophytes. Ecology 88: 2199–2210.
Del Pozo, R., C. Fernández-Aláez & M. Fernández-Aláez, 2011. The relative importance of natural and anthropogenic effects on community composition of aquatic macrophytes in Mediterranean ponds. Marine and Freshwater Research 62: 101–109.
Della Bella, V., M. Bazzanti, M. G. Dowgiallo & M. Iberit, 2008. Macrophyte diversity and physico-chemical characteristics of Tyrrhenian coast ponds in central Italy: implications for conservation. Hydrobiologia 597: 85–89.
Deny, P., 1980. Solute movement in submerged angiosperms. Biological Reviews 55: 65–92.
Downing, J. A., 2010. Emerging global role of small lakes and ponds: little things mean a lot. Limnetica 29(1): 9–24.
Downing, J. A., Y. T. Prairie, J. J. Cole, C. M. Duarte, L. J. Tranvik, R. G. Striegl, W. H. McDowell, P. Kortelainen, N. F. Caraco, J. Melack & J. Middelburg, 2006. The global abundance and size distribution of lakes, ponds, and impoundments. Limnology and Oceanography 51: 2388–2397.
Duarte, C. M., J. Kalff & R. H. Peters, 1986. Patterns in biomass and cover of aquatic macrophytes in lakes. Canadian Journal of Fisheries and Aquatic Sciences 43: 1900–1908.
Edvardsen, A. & R. H. Okland, 2006. Variation in plant species richness in and adjacent to 64 ponds in SE Norwegian agricultural landscapes. Aquatic Botany 85: 79–91.
Fernández-Aláez, C., M. Fernández-Aláez & E. Bécares, 1999. Influence of water level fluctuation on the structure and composition of the macrophyte vegetation in two small temporary lakes in the northwest of Spain. Hydrobiologia 415: 155–162.
Fernández-Aláez, C., M. Fernández-Aláez, C. Trigal & B. Luis, 2006. Hydrochemistry of northwest Spain ponds and its relationships to groundwaters. Limnetica 25(1–2): 433–452.
Fernández-Aláez, M. & C. Fernández-Aláez, 2010. Effects of the intense summer dessication and the autumn filling on the water chemistry in some Mediterranean ponds. Limnetica 29(1): 59–74.
Fernández-Aláez, C., M. Fernández-Aláez, N. F. Santiago & M. Aboal, 2012. ID-tax. Catálogo y claves de identificación de organismos del grupo macrófitos utilizados como elementos de calidad en las redes de control del estado ecológico. Ministerio de Agricultura, Alimentación y Medio Ambiente.
Figuerola, J. & A. J. Green, 2002. Dispersal of aquatic organisms by waterbirds: a review of past research and priorities for future studies. Freshwater Biology 47: 483–494.
Frink, C. R. & W. A. Norvell, 1984. Chemical and physical properties of Connecticut Lakes. Connecticut Agricultural Experiment Station Bulletin No. 817, New Haven, Connecticut.
Gacia, E. & J. Peñuelas, 1991. Carbon assimilation of Isoetes 1acustris L. from Pyrenean lakes. Photosynthetica 25: 97–104.
Gacia, E., E. Ballesteros, L. Camarero, O. Delgado, A. Palau, J. L. Riera & J. Catalan, 1994. Macrophytes from lakes in the eastern Pyrenees: community composition and ordination in relation to environmental factors. Freshwater Biology 32: 73–81.
García-Baquero, G. & R. M. Crujeiras, 2015. Can environmental constraints determine random patterns of plant species co-occurrence? Ecology and evolution 5(5): 1088–1099.
Grillas, P., P. Gauthier, N. Yavercovski & C. Perennou, 2004. Mediterranean Temporary Pools, Issues Relating to Conservation, Functioning and Management, Vol. 1. Station Biologique de la Tour du Valat, Arlek.
He, F. & P. Legendre, 2002. Species diversity patterns derived from species-area models. Ecology 85: 1185–1198.
Heegaard, E., H. H. Birks, C. E. Gibson, S. J. Smith & S. Wolfe-Murphy, 2001. Species–environmental relationship of aquatic macrophytes in Northern Ireland. Aquatic Botany 70: 175–223.
Hrivnák, R., H. Ot’ahel’ová, J. Kochjarová & P. Pal’ove-Balang., 2013. Effect of environmental conditions on species composition of macrophytes – study from two distinct biogeographical regions of Central Europe. Knowledge and Management of Aquatic Ecosystems 411, 09.
Hudon, C., S. Lalonde & P. Gagnon, 2000. Ranking the effects of site exposure, plant growth form, water depth, and transparency on aquatic plant biomass. Canadian Journal of Fisheries and Aquatic Sciences 57: 31–42.
Jensén, S., 1977. An objective method for sampling the macrophyte vegetation in lakes. Vegetatio 33: 107–118.
Jeppesen, E., J. P. Jensen, M. Søndergaard, T. Lauridsen, L. J. Pedersen & L. Jensen, 1997. Top-down control in freshwater lakes: the role of nutrient state, submerged macrophytes and water depth. Hydrobiologia 342(343): 151–164.
Jones, J. I., W. Li & S. C. Maberly, 2003. Area, altitude aquatic plant diversity. Ecography 26: 411–420.
Karttunen, K. & H. Toivonen, 1995. Ecology of aquatic bryophyte assemblage in 54 small Finnish lakes, and their changes in 30 years. Annales Botanici Fennici 32: 75–90.
Kotze, D. C. & T. G. O’Connor, 2000. Vegetation variation within and among palustrine wetlands along an altitudinal gradient in KwaZulu-Natal, South Africa. Plant Ecology 146: 77–96.
Lacoul, P. & B. Freedman, 2006a. Environmental influences on aquatic plants in freshwater ecosystems. Environmental Reviews. 14: 89–136.
Lacoul, P. & B. Freedman, 2006b. Relationships between aquatic plants and environmental factors along a steep Himalayan altitudinal gradient. Aquatic Botany 84: 3–16.
Lauridsen, T. L., E. Jeppesen, S. A. J. Declerck, L. De Meester, J. M. Conde-Porcuna, W. Rommens & S. Brucet, 2015. The importance of environmental variables for submerged macrophyte community assemblage and coverage in shallow lakes: differences between northern and southern Europe. Hydrobiologia 744: 49–61.
Linton, S. & R. Goulder, 2000. Botanical conservation value related to origin and management of ponds. Aquatic Conservation: Marine and Freshwater Ecosystems 10: 77–91.
Lodge, D. M., 1991. Herbivory on freshwater macrophytes. Aquatic Botany 41: 195–224.
MacArthur, R. H. & E. O. Wilson, 1967. The Theory of Island Biogeography. Princeton University Press, Princeton.
Mäkelä, S., E. Huitu & L. Arvola, 2004. Spatial patterns in aquatic vegetation composition and environmental covariates along chains of lakes in the Kokemäenjoki watershed (S. Finland). Aquatic Botany 80: 253–269.
Medail, F. & P. Quezel, 1999. Biodiversity hotspots in the Mediterranean Basin: setting global conservation priorities. Conservation Biology 13: 1510–1513.
Meerhoff, M. & E. Jeppesen, 2009. Shallow Lakes and Ponds. In G. E. Likens (ed), Encyclopedia of Inlands Waters, Vol. 2. Elsevier, Oxford: 645–655.
Mikulyuk, A., S. Sharma, S. Van Egeren, E. Erdmann, M. E. Nault & J. Hauxwell, 2011. The relative role of environmental spatial and land-use patterns in explaining aquatic macrophyte community composition. Canadian Journal of Fisheries and Aquatic Sciences 68: 1778–1789.
Miracle, M. R., B. Oertli, R. Céréghino & A. Hull, 2010. Preface: conservation of european ponds-current knowledge and future needs. Limnetica 29(1): 1–8.
Morris, K., P. C. Bailey, P. I. Boon & L. Hughes, 2003. Alternative stable states in the aquatic vegetation of shallow urban lakes. Catastrophic loss of aquatic plants consequent to nutrient enrichment. Marine and Freshwater Research 54: 201–215.
Moss, B., D. Stephen, C. Álvarez, E. Becares, W. Van de Bund, S. E. Collings, E. Van Donk, E. De Eyto, T. Feldmann, C. Fernández-Aláez, M. Fernández-Aláez, R. J. Franken, F. García-Criado, E. M. Gross, M. Gyllström, L. A. Hansson, K. Irvine, A. Järvalt, J. P. Jensen, E. Jeppensen, T. Kairesalo, R. Kornijow, T. Krause, H. Künnap, A. Laas, E. Lill, B. Lorens, H. Luup, M. R. Miracle, P. Noges, T. Noges, M. Nykänen, I. Ott, W. Peczula, E. Peeters, G. Phillips, S. Romo, V. Russell, J. Salujoe, M. Scheffer, K. Siewertsen, H. Smal, C. Tesch, H. Timm, L. Tuvikene, I. Tonno, T. Virro, E. Vicente & D. Wilson, 2003. The determination of ecological status in shallow lakes – a tested system (ECOFRAME) for implementation of the European Water Framework Directive. Aquatic Conservation: Marine and Freshwater Ecosystems 13: 507–549.
Moss, B., D. Stephen, D. M. Balayla, E. Bécares, S. E. Collings, C. Fernández-Aláez, M. Fernández-Aláez, C. Ferriol, P. García, J. Gomá, M. Gyllström, L.-A. Hansson, J. Hietala, T. Kairesalo, M. R. Miracle, S. Romo, J. Rueda, V. Russell, A. Stahl-Delbanco, M. Vensson, K. Vakkilainen, M. Valentín, W. J. Van de Bund, E. Van Donk, E. Vicente & M. J. Villena, 2004. Continental-scale patterns of nutrient and fish effects on shallow lakes: synthesis of a pan-European mesocosm experiment. Freshwater Biology 49: 1633–1649.
Murphy, K. J., 2002. Plant communities and plant diversity in softwater lakes of northern Europe. Aquatic Botany 73: 287–324.
Myers, N., R. A. Mittermeier, C. G. Mittermeier, G. A. B. da Fonseca & J. Kent, 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853–858.
Nurminen, L., 2003. Macrophyte species composition reflecting water quality changes in adjacent water bodies of lake Hiidenvesi, SW Finland. Annales Botanici Fennici 40: 199–208.
Oertli B., D. A. Joye, E. Castella, R. Juge & J.-B. Lachavanne, 2000. Diversite´ biologique et typologie e´cologique des e´tangs et petits lacs de Suisse. Swiss Agency for the Environment, Forests and Landscape, Laboratory of Ecology and Aquatic Biology, University of Geneva, Geneva.
Penning, W., M. Mjelde, B. Dudley, S. Hellsten, J. Hanganu, A. Kolada, M. Van Den Berg, S. Poikane, G. Phillips, N. Willby & F. Ecke, 2008. Classifying aquatic macrophytes as indicators of eutrophication in European lakes. Aquatic Ecology 42: 237–251.
Pìpalová, I., 2002. Initial impact of low stocking density of grass carp on aquatic macrophytes. Aquatic Botany 73: 9–18.
Pond Conservation Group, 1993. A future for Britain’s ponds: An agenda for action. Pond Conservation Group, Oxford.
Post, D. M., 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703–718.
Pulido, C., J. Riera, E. Ballesteros, E. Chappuis & E. Gacia, 2015. Predicting aquatic macrophyte occurrence in soft-water oligotrophic lakes (Pyrenees mountain range). Journal of Limnology 74(1): 143–154.
R Core Team, 2014. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/.
Rolon, A. S. & L. Maltchik, 2006. Environmental factors as predictors of aquatic macrophyte richness and composition in wetlands of southern Brazil. Hydrobiologia 556: 221–231.
Rørslett, B., 1991. Principal determinants of aquatic macrophyte richness in northern European lakes. Aquatic Botany 39: 173–193.
Ruiz, C., G. Martinez, M. Toro & A. Camacho, 2011. A Review: macrophytes in the Assessment of Spanish Lakes Ecological Status Under the Water Framework Directive (WFD). Ambientalia: 1–25.
Sánchez, E., C. Gallardo, M. A. Gaertner, A. Arribas & M. Castro, 2004. Future climate extreme events in the Mediterranean simulated by a regional climate model: a first approach. Global and Planetary Change 44: 163–180.
Sand-Jensen, K., 1997. Eutrophication and plant communities in Lake Fure during 100 years. In Sand-Jensen, K. & O. Pedersen (eds), Freshwater Biology – Priorities and Development in Danish Research. G.E.C Gad, Copenhagen: 26–53.
Sass, L. L., M. A. Bozek, J. A. Hauxwell, K. Wagner & S. Wright, 2010. Response of aquatic macrophytes to human land use perturbations in the watersheds of Wisconsin lakes, USA. Aquatic Botany 93: 1–8.
Scheffer, M., 1998. Ecology of shallow lakes. Chapman and Hall, London.
Scheffer, M., G. J. van Geest, K. Zimmer, E. Jeppesen, M. Søndergaard, M. G. Butler, M. A. Hanson, S. Declerck & L. De Meester, 2006. Small habitat size and isolation can promote species richness: second-order effects on biodiversity in Shallow Lakes and ponds. Oikos 112: 227–231.
Søndergaard, M., L. S. Johansson, T. L. Lauridsen, T. B. Jørgensen, L. Liboriussen & E. Jeppesen, 2010. Submerged macrophytes as indicators of the ecological quality of lakes. Freshwater Biology 55: 893–908.
Sovari, S. & A. Korhola, 1998. Recent diatom assemblage changes in subartic Lake Saanajärvi, NW Finnish Lapland, and their palaeoenvironmental implications. Journal of Paleolimnology 20: 205–215.
Steffen, K., C, Leuschner, U. Müller, G. Wiegleb & T. Becker, 2014. Relationships between macrophyte vegetation and physical and chemical conditions in northwest German running waters. Aquatic Botany 113: 46–55.
Toivonen, H. & P. Huttunen, 1995. Aquatic macrophytes and ecological gradients in 57 small lakes in southern Finland. Aquatic Botany 51: 197–221.
Tutin, T. G., V. H. Heywood, N. A. Burges, D. M. Moore, D. H. Valentine, S. M. Walters & D. A. Webbs (eds.), 1980. Flora Europaea. Cambridge University Press, Cambridge.
Van Geest, G. J., F. C. J. M. Roozen, H. Coops, R. M. M. Roijackers, A. D. Buijse, E. T. H. M. Peeters & M. Scheeffer, 2003. Vegetation abundance in lowland flood plan lakes determined by surface area, age and connectivity. Freshwater Biology 48: 440–454.
Van Geest, G. J., H. Wolters, F. C. J. M. Roozen, H. Coops, R. M. M. Roijackers, A. D. Buijse & M. Scheeffer, 2005. Water-level fluctuations affect macrophyte richness in floodplain lakes. Hydrobiologia 539: 239–248.
Van Groenendael, J., J. Ehrlén & B. M. Svensson, 2000. Dispersal and persistence: population processes and community dynamics. Folia Geobotanica 35(2): 107–114.
Vestergaard, O. & K. Sand-Jensen, 2000. Aquatic macrophyte richness in Danish lakes in relation to alkalinity, transparency and lake area. Canadian Journal of Fisheries and Aquatic Sciences 57: 2022–2031.
Acknowledgments
This research was funded by the Spanish Ministry of Science and Technology (project REN2003–03718/HID), by the Spanish Ministry of Education and Science project (project CGL2006-03927) and by the Junta of Castilla and León (projects LE33/03 and UMC1/04).
Author information
Authors and Affiliations
Additional information
Guest editors: M. T. O’Hare, F. C. Aguiar, E. S. Bakker & K. A. Wood / Plants in Aquatic Systems – a 21st Century Perspective
Rights and permissions
About this article
Cite this article
Fernández-Aláez, C., Fernández-Aláez, M., García-Criado, F. et al. Environmental drivers of aquatic macrophyte assemblages in ponds along an altitudinal gradient. Hydrobiologia 812, 79–98 (2018). https://doi.org/10.1007/s10750-016-2832-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2832-5