Abstract
Green spaces within urban areas provide services and benefits to human populations and habitat for a variety of species. Freshwater, in the form of rivers, canals, lakes, reservoirs and ponds, is an important component of urban greenspaces. This paper focuses on ponds; and specifically ponds within urban areas. This work is timely as during 2008 ponds were designated, in the UK, as habitats of national conservation importance. Yet, while farmland ponds have received considerable attention, there has been little work on the ecology and landscape ecology of urban ponds. Ecological data was collected from 37 ponds in the Borough of Halton (northwest England) over a period of 2 years (2005–2006). The median species richness in these ponds was 28 invertebrate species and 10 macrophyte species. A highly significant correlation was observed between pond density and species richness. The relationship between the richness of different taxa varied according to scale; becoming more significant within pond clusters than within a single pond. These findings have significance for those involved in planning and managing urban environments, further strengthening the need for functional ecological connectivity in urban areas. With pressure to increase infill development, and thus raise housing density, a greater understanding of the affect of urban design on pond ecology will be of importance to urban planners and ecologists alike.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Collectively the mosaics and networks of greenspace (public parks, private gardens, roadside verges and open freshwater bodies) within urban areas provide benefits to human populations (Lees and Evans 2003) and habitat for a variety of species (English Nature 1997). The amount of greenspace within cities in the same country varies greatly: e.g. 12.0% of the City of London and 68.0% of the City of Portsmouth. An important element within urban greenspace is freshwater, both flowing and standing. Almost half (5.8% of the total area) of the greenspace in the City of London is associated with freshwater elements and in the City of Portsmouth 44.0% of the greenspace was accounted for by freshwater (ODPM 2005). The freshwater elements include large features such as rivers, canals, lakes and reservoirs along with the often much smaller ponds. A pond, as defined by Pond Conservation (2002) (a UK charity dedicated to creating and protecting ponds and the wildlife they support) is an area of between 2 m2 and 2 ha that holds water for at least four consecutive months of the year. Ponds are habitats of European significance (Habitats Directive: EC 1992) and during 2008 became part of the UK Biodiversity Action Plan (UK BAP 2008). The UK Biodiversity Action Plan process is a national response to The Convention on Biodiversity (1992) and provides a structure for the protection of habitats and species deemed to be of national importance. Designation as a Biodiversity Action Plan habitat places the management and conservation of ponds on the political agenda, promotes public awareness and requires local authorities to make consideration for the protection and management of ponds within their own jurisdictions.
In some areas ponds have been found to hold a greater diversity of species than nearby lakes or river systems; many of which have been heavily polluted, canalised or over managed (Biggs et al. 2005). Taxa such as amphibians and dragonflies move between several ponds (Gee et al. 1998). Hence, the ecological value of a single pond may be less important than that of the network or “pondscape” as a whole (Boothby 2000).
In the 1950s increasing human population brought over-crowding and associated social problems to many of Britain’s inner cities (Clapton 1998). The New Towns were conceived to address these issues. Central to the vision and design of New Towns, which are, in effect extended suburbs (Clapton 1998) were large areas of open access public greenspace (English Partnerships 2005). These greenspaces were planned as an integrating structure, in the form of extended verges and networks of small greens, rides, shrubberies and small woodlands, within which residential and commercial properties were built. When constructing New Towns extant field ponds, of which there are as many as 4.7 per km2 in England’s northwest (Boothby 2000), were often retained and, additionally, new ponds were created: collectively these contribute to sustainable urban drainage systems, nature conservation and ornamental features (CIRIA 2000; Sutherland and Hill 1995). New Town developments have landscapes which are well connected ecologically and, therefore, provide an opportunity for experimentation regarding urban planning design. This is especially the case when New Town developments are located near, or even adjacent to, older urban developments with different planning designs and, hence, different urban morphologies in which greenspace is both less abundant and less well connected.
Current UK government policy is to increase urban density with the emphasis placed on increasing infill development (Communities and Local Government 2006). With a need for future urban development (Blackman 2007) and a drive to increase urban density a greater understanding of the effect of urban planning regimes on urban ecology is required.
In this paper the authors examine the effect of urban pond density on species richness and present data from a study of 37 ponds in Halton, northwest England. The relationship between pond density and species richness is assessed within two urban design regimes present within Halton: a New Town medium density development and an older, denser development.
Methods
Study area
The study is focused in the administrative district of Halton which lies between Manchester and Liverpool in the northwest of England. Halton straddles the River Mersey and comprises two towns, Runcorn and Widnes, several villages and some agricultural land. The total area of the Borough is in the region of 91 km2 (ODPM 2005) and the population was 118,000 in 2004 (Halton Borough Council 2007). The relatively homogeneous solid geology of the Halton area comprises Triassic strata of sandstone and pebble beds north of the River Mersey with sandstone and mudstones predominating to the south. The solid geology is overlain by marine and estuarine alluvium associated with the River Mersey, and glacial deposits comprising glacial till (boulder clay, marl clay) and glacial sands and gravels (Cross 2005). This geology contributes to a landscape rich in naturally mesotrophic, neutral to alkaline waterbodies that form readily in low-lying areas or within excavations in the clay.
The low-lying areas of Halton either side of the river have been extensively developed for both residential and commercial use. This has required large-scale drainage of flood areas in the Mersey flood plain. The two towns of Runcorn and Widnes developed separately in the 19th century: Widnes around the pioneering early chemical industry and Runcorn as a canal port with associated shipbuilding and chemical industries such as tanneries and soap works that spilled across the river from Widnes. This varied history has resulted in a mixture of urban designs with both older traditional densely built urban areas and the medium density Runcorn New Town developments of the 1960s and 1970s (English Partnerships 2005). It is this juxtaposition of urban morphologies and the concentration of ponds (the Ponds Research Unit’s Pond Audit estimates 371 ± 10% extant ponds outside of gardens, within Halton i.e. a pond density of approximately 4.1 ponds per km2) that make Halton an ideal study location in which to study urban ponds within different urban designs and to evaluate the relevance of landscape ecological concepts such as connectivity and metapopulation theory.
Ecological and chemical assessment
Between May 2004 and September 2006, ecological data was collected from 37 ponds located in areas of public access (i.e. a 10% sample of ponds outside of domestic gardens). Selection of ponds to survey was stratified in order to ensure representation of the two contrasting urban morphologies associated with the New Town development and the adjacent older, more densely developed areas, and a range of pond successional stages. Hydrophyte and aquatic macro-invertebrate species richness within the ponds was assessed by Andrew Harmer Consultants and Bill Bellemy Ecological Consultants under contract to the local administration’s Landscape Services Department.
Invertebrates were sampled using a standard Freshwater Biological Association (FBA) pattern long-handled pond net with a mesh size of 0.5 mm until no new species were recorded (Guest and Bentley 1998). Invertebrates were collected from all microhabitats (e.g. floating vegetation, submerged vegetation, open water etc.). Samples were taken twice per year: in late spring/summer and autumn, and then aggregated for each pond. All aquatic macrophytes rooted below the winter high water mark were identified. The presence and breeding of amphibians was assessed by visual counting and netting of adults and larvae, and egg searches among submerged vegetation (English Nature 2001) expending 15 min of survey effort per method per 50 m of the ponds perimeter (Gent and Gibson 2003).
Jaccard’s index of similarity was used to measure the similarity of invertebrate and plant communities between ponds in the old and New town areas (Eq. 1)
where j = the number of species found in both ponds and r = the number of species found in only one or the other pond.
In addition, physical variables (altitude, shade, cover of aquatic plants, depth and surface area), chemical characteristics (pH, conductivity, dissolved oxygen and hardness) and data concerning the nature of the surrounding landscape at a radius of 250 m (proportion of soft surfaces and housing density) were collated for each pond by the authors. The altitude (metres above sea level) and six figure grid reference for each pond were derived from data supplied by Halton Borough Council, based on Ordnance Survey MasterMaps® (1:1,250 scale). The shade (percentage cover of pond surface at midday, in summer), percentage cover of vegetation (in autumn) and maximum depth in spring (following winter rains) were measured on site.
Pond area was estimated from aerial photographs (Google Earth™) with reference to known distances measured on the ground. Density of housing (within 250 m) and the percentage of soft surfaces (soil, vegetation, water within 250 m) where measured from the same images.
Water chemistry was analysed in both the field and the laboratory. Electronic meters were used to measure dissolved oxygen (Dryden, Aqua Traul© Water Proof Series-DO 300), pH (Hanna Champ® HI98106) and conductivity (Hanna Dist 4 HI98304) at monthly intervals from April to the following March in each of 3 years (2005, 2006 and 2007). Total hardness (mg of CaCO3) was assessed by titration (Rump and Krist 1999; Stoianov et al. 2000) under laboratory conditions from samples collected in April, May and June 2005, 2006 and 2007.
Spatial analysis
Differences between the species richness, physical and chemical parameters of ponds in the New Town and old town areas were explored using the non-parametric Mann–Whitney Test as data was found to be of a non-normal distribution (using Minitab 13 statistical software).
The pondscape of the Borough was established using data from the Ponds Research Unit’s Pond Audit (based in Liverpool John Moores University). The audit includes pond locations drawn from Ordnance Survey map data and aerial photography, and holds data on pond distribution across the northwest region of England. In order to take account of the wider landscape the total number of ponds in each adjoining grid square i.e. the value assigned to each 1 × 1 km grid square was the sum of the eight adjoining squares plus the number of ponds in that square. In this way landscape scale variation in pond numbers could be visualised. At smaller scales the number of ponds within radii of 0–250 m, 0–500 m, 0–750 m and 0–1,000 m of each sample pond were counted. Pond densities at these varying scales where then used as part of multiple regression analysis. With each regression all other variables remained constant, only the scale at which pond density was measured was altered. Spearman’s coefficient of rank correlation (Minitab statistical software version 13) was used to explore relationships between the pondscape density in which a sampled pond was located and the invertebrate and floral richness of the sampled pond.
Clusters of sampled ponds were identified by mapping functional connection between ponds based on the dispersal of the great or northern crested newt (Triturus cristatus), a species of conservation priority in Britain (English Nature 2001), which according to Arntzen and Wallis (1991) and Swan and Oldham (1993) can be taken at a maximum of 1 km. It can be assumed that if the pond landscape is suitable in its spatial arrangement for this species of conservation importance then it will meet the needs of the many invertebrate species that have equal or greater powers of dispersal (Rundle et al. 2002; Delettre and Morvan 2000; Conrad et al. 1999).
Backward stepwise regression analysis (Minitab statistical software version 13) was used to identify relationships between species richness of the taxa studied and variables recorded for each pond. This method includes all variables in the regression model and then excludes those with a calculated α value below the set levels, in this instance 0.01 and 0.05. This allowed for those factors with the greatest influence on species richness to be identified. Multicollinearity within the dataset was tested for by calculating the variation inflation factor (VIF) for each independent variable, retaining only variables with a VIF of less than 5 (Montgomery and Peck 1982). This allowed for the determination of the unique effect of each predictor variable on the richness of species and removed the effect of those variables that are strongly related to each other e.g. as conductivity and hardness are correlated only one or the other variable would be retained. Data was normalised by transformation to z values (Subtract mean and divide by standard deviation) to facilitate regression analysis.
Results
During the ecological survey of 37 ponds, conducted between 2004 and 2006, a total of 119 species of aquatic macro-invertebrates, 57 species of aquatic macrophytes and four species of amphibian were found. The amphibian species were the common frog (Rana temporaria), the common toad (Bufo bufo), the smooth or common newt (Lissotriton vulgaris syn. Triturus vulgaris) and the great or northern crested newt (Triturus cristatus).
Lissotriton vulgaris was recorded in 45% of ponds, while Rana temporaria was recorded in 67% of ponds. T. cristatus was found to be breeding in four ponds (10%). Two of the breeding sites where located within a large area of urban greenspace consisting of open parkland, meadow and woodland, known as Phoenix Park that occupies 304 hectares in the centre of Runcorn New Town. The other two ponds in which T. cristatus was found were located on a golf course and had been designed in conjunction with the local authority and created by the green staff for nature conservation. Of the 37 ponds surveyed amphibian species were absent from only five.
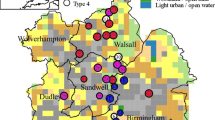
There were significant differences in the richness of both aquatic invertebrates and aquatic macrophytes found in the older established urban areas that constitute the original settlements of Widnes and Runcorn (located on both sides of the River Mersey) and the purpose built New Town developments on the southern side of the River. The median numbers of aquatic plant species was 11.5 in ponds located in the New Town area and 9.0 in ponds located within the old town (W = 369.5, P = 0.045). The difference for aquatic invertebrates was even higher with the median number of species per New Town pond being 37.0, compared to only 18.0 for the old town ponds (W = 409, P < 0.001). The ponds with the highest species richness were located in the New Town area, this distribution is especially apparent with aquatic invertebrates: in general, ponds with the highest species richness were located in close proximity to each other (Fig. 1).
Schematic distribution of ponds in the Halton study. Numbers next to each point indicates the richness of aquatic invertebrates species found at each pond (figure in brackets indicates number of plant species). Diagonal line indicates position of the River Mersey in relation to ponds, shaded area indicates New Town
Backward stepwise regression analysis indicated that, for invertebrate, pond density (in adjacent 1 km squares), vegetation cover and minimum recorded dissolved oxygen (% saturation) produced α values above 0.05 and thus remained in the regression model. These were the predictors with the greatest impact on determining species richness. Only pond density remained in the regression model when α = 0.01. The variable with the greatest influence on plant species richness was pond density (in adjacent 1 km square) at both α levels.
No significant differences could be found with regard to environmental and chemical variables between ponds in the New Town and those in the old town (Table 1). The density of ponds was significantly higher in the Runcorn New Town area than in the older Runcorn and Widnes areas. The mean number of ponds in all adjoining 1 × 1 km grid squares for ponds in the New Town was 49 compared to 32 ponds for those in the old town (W = 455.0, P < 0.001).
The difference in pond density between the New Town and old town areas is reflected in a significant difference in the richness of species within the sample ponds. There was a positive linear relationship between increasing pond density and richness of both invertebrate (Rs = 0.742, P < 0.001) and plant species in the ponds (Rs = 0.528, P < 0.001) (Fig. 2).
The impact of pond density as a predictor for species richness of both plants and invertebrates varies depending on the scale at which that density is measured (see Table 2). Species richness for both taxa increases along with increasing pond density at all scales. However, the influence of density becomes greater at larger scales. If using pond density within 250 m from the surveyed ponds as a component of multiple regression analysis the resulting R 2 value is 0.43 for invertebrates (F = 1.72, P = 0.126) and 0.56 for plants (F = 2.91, P = 0.013). If pond density within 750 m is substituted for density within 250 m (all other variables remain unchanged) then the R 2 value increases marginally to 0.47 for invertebrates (F = 2.08, P = 0.063) and 0.58 for plants (F = 3.16, P = 0.008). If however, the density of ponds at a wider landscape scale i.e. number of ponds within all adjacent 1 × 1 km grid squares, the R 2 increases to 0.71 for invertebrates (F = 5.8, P ≤ 0.001) and 0.68 for plants (F = 4.91, P ≤ 0.0001).
Plant and aquatic invertebrate species richness is also positively correlated to each other, so that those sites with highest number of plant species also have the highest number of aquatic invertebrate species. Regression analysis indicated that plant species richness accounted for 37.1% of the observed variation in aquatic invertebrate species richness (P < 0.001, see Fig. 3 for regression equation). However, the strength of the relationship increases substantially when considering the sum total of species richness within clusters of ponds (where each pond is no more than 1 km from its nearest neighbour). In this case, the richness of plant species within each cluster accounted for 83.4% (P = 0.007) of the observed invertebrate species variation (see Fig. 3 for regression equation).
The median similarity of invertebrate communities (Jaccard’s Index) between all ponds was 21.2 and 16.1% for plant communities. The median similarity for invertebrates between old town ponds was 15.8%, while for New Town ponds it was significantly higher 31.1% (W = 26880.5, P ≤ 0.001). For plant communities the median similarity in old town areas was 17.3%, compared to 38.2% in the New Town (W = 34111.8, P ≤ 0.001).
Discussion
During 2004 and 2005 the ecology of 37 ponds in Halton, northwest England was investigated. The species richness of both aquatic invertebrates and aquatic macrophytes was significantly higher in ponds located in the New Town developments than in those located in the older established urban areas. Backward stepwise regression analysis indicated the pond density to be the major determining variable for aquatic species richness. The highest density of ponds was to be found in the New Town area. As pond density increased so did the richness of both invertebrate and plant species in those ponds.
Of the 37 ponds surveyed in Halton amphibians were present in 32. Of the five ponds in which amphibians were absent one is contaminated with heavy metal, to which amphibians are known to be sensitive (Horne and Dunson 1995). Another pond is periodically inundated during higher than normal tides by brackish water from the River Mersey. And the other three ponds where characterised by a combination of extreme shade and precipitously, inaccessible pond margins: all features which are not conducive to amphibians (Foster 2007). In the survey reported here Triturus cristatus was found in 10% of ponds. The EU funded Pond Life project, which surveyed a 1,000 ponds in northwest England between 1995 and 1998, found this species present in 25% of ponds surveyed (Gledhill 1999). The northwest of England is known to be a strong hold for T. cristatus in mainland Britain (Swan and Oldham 1993). Hence, the population in Halton appears to be below the level of the wider region. Amongst the factors known to adversely affect T. cristatus are excess growth of macrophytes and the presence of fish. The probability of T. cristatus occupying a pond decreases substantially once the total cover of macrophytes exceeds 75% (Swan and Oldham 1993). Only three surveyed ponds in Halton exhibit this degree of macrophyte growth (with T. cristatus absent from all three). The presence of fish is likely to be a much more detrimental factor. Based on the aquatic habitat requirements of the species alone i.e. size, vegetation cover, shade and water quality (Oldham et al. 2000), 21 ponds would appear to be potentially suitable. Of these, four are actually occupied and of the remaining 17 considerable fish populations were present in 11 and judged to be the most likely explanation for the absence of T. cristatus, which is know to be very adversely affected by the presence of fish (Oldham et al. 2000). The nature of the terrestrial matrix may also be a major factor contributing to the absence of T. cristatus from ponds that would otherwise appear suitable (Oldham et al. 2000). The density of ponds observed in Halton would appear to meet the requirements of the species, its relative scarcity further point to issues of pond quality and the nature of the terrestrial matrix as potential factors to explain its distribution in the Borough.
Lissotriton vulgaris occurred in 40% of the Pond Life survey ponds and in the study reported in this paper it was present in 45% of ponds. L. vulgaris has much less specific habitats requirements, than T. cristatus (Gledhill 1999; Guest and Harmer 2006) and occurs regularly in urban areas (Wyatt 1998).
The current Halton study found a median richness of 10.0 species of aquatic macrophytes per pond. In order to place the results of the current study in context this data can be compared to that presented by three other surveys. The Lowland Pond Survey (Pond Action 1998) reported a median diversity of 9.6 species of aquatic macrophyte from 156 reference ponds across Britain. However, in a study of 146 ponds in Cardiff, Wales, Rich (2000) reported a median richness of 16.1 species of macrophyte per pond. The Pond Life Project survey, the median richness was 22.0 species of aquatic macrophyte (Guest and Bentley 1998). The botanical species richness of Halton’s ponds is, therefore, comparable with the national survey (Rich 2000), but lower, than that of both the Cardiff survey (which included urban sites) and the Pond Life dataset from mostly rural sites in the same geographic region as Halton.
Aquatic macro-invertebrate richness was similarly lower in the Halton ponds than in the wider northwest. The figure for the Halton ponds was 28.0 species per pond compared to 32.0 found in the Pond Life Project (Guest and Bentley 1998). If pond density is a major determinant of species richness, then this would account for the northwest regions higher species richness compared to that found nationally. The density of ponds is much higher in the northwest of England (4.7 ponds per km2, Boothby 2000) than the national average (1.4 ponds per km2, Swan and Oldham 1997). If this is the case one could expect there to be differences within species richness within Halton related to the pond density within different parts of the Borough.
Significant differences were noted between the number of both plant species and invertebrate species per pond in the New Town ponds (11.5 species of plants and 37.0 species of invertebrates) and those in old town areas (9.6 species of plants and 18.0 species of invertebrates). This could not be explained by variation in either water chemistry or physical parameters such as the size of the ponds, their depth or altitude (Table 1).
The density of ponds in the landscape can clearly been seen to be a major contributing factor to the richness for hydrophytes and aquatic macro-invertebrates. Species richness for both groups was highest in the densest pondscapes. The apparent connection between botanical richness and dissolved oxygen reflects the observation that the ponds with the highest number of plant species were also those with the greatest extent of vegetation cover. The higher oxygen production, therefore, probably results not from species richness but from the quantity of plant material present.
The impact of pond density on the species richness of a pond depends on the scale at which that density is measured. Pond density has its largest effect at the largest landscape scale. This would indicate that the density of ponds within the wider landscape scale is more important in determining the richness of species than the density of ponds within a given ponds immediate surroundings (see Table 2). For aquatic invertebrates the major influence of pond density was at the scale of 1 × 1 km grid squares. For aquatic plants this was still true, however the effect of pond density at smaller scales was also significant. This reflects the varying dispersal abilities of different taxa. From the management perspective the distances between ponds in a network depend on the species of primary conservation concern, with higher plant species richness being favoured by a high density of ponds located closer to the pond in question. Linton and Goulder (2003) observed from a study of botanical diversity of ponds in northeast England that the species in a given pond represent both species present throughout the area and those that have colonised from neighbouring ponds. This study not only found that the number of other ponds within 500 m of a particular pond had an impact on its botanical richness but so did the botanical richness of those neighbouring ponds (Linton and Goulder 2003).
While the species richness of the ponds in the New Town was higher, the assemblages of species for both invertebrates and aquatic macrophytes were more homogenous than those in the old areas. This is potentially due to higher connectivity between ponds in the New Town areas allowing species to spread more easily between ponds than in the old town areas.
In Halton the highest densities of ponds are found in the New Town areas and are associated with the greater provision of open access greenspaces in which the ponds are situated and with lower levels of built density within this urban design style. However, since the proportion of soft space did not emerge as a significant variable in the analysis of species richness it is likely that a simple measure of the amount of green space is inadequate to account for variations in pond biota. Rather it may be the precise nature and composition of that surrounding landscape that has a determining effect on the species present within a pond or pondscape. Data from the Lowland Pond Survey indicated isolation to be a major negative factor with regard to the species richness of ponds for both aquatic invertebrates and macrophytes (Biggs et al. 1998). In addition, pond isolation was also significantly correlated with the rarity of aquatic macrophytes (P = 0.001 for submerged aquatics and P = 0.05 for emergent plants) (Biggs et al. 1998). The most isolated ponds from the Lowland Pond Survey dataset are those with the lowest species richness and also those least likely to contain rare plant species. Indeed, increased fragmentation and thus isolation of pond habitats associated with pond loss is likely to be a contributing factor to the decline of these species.
The richness of aquatic invertebrates and macrophytes were positively related: invertebrate communities being richest in those ponds with the highest botanical richness. Furthermore this relationship was much stronger at the pondscape scale i.e. when looking at clusters of ponds rather than individual ponds. This would seem to reflect the mobility of invertebrate species within the network and suggest that maximising the ecological status of one pond relies not just on the prevailing conditions in or affecting the individual pond, or even on the immediate terrestrial matrix, but also on managing the condition of other neighbouring ponds and the wider terrestrial landscape. Briers and Briggs (2005) working at large spatial scales (60 × 60 km square) in Oxfordshire, England found evidence for spatial autocorrelation of invertebrate communities over distances of up to 13 km. The study also showed that, within the same sample of ponds, at the local scale similarity of communities was often low (Briers and Briggs 2005).
While factors such as water chemistry, aquatic vegetation structure and shade may determine the precise composition of the invertebrate and plant community within a pond or cluster of ponds, pond density appears as a much greater determinant of the species richness. Ponds are by their nature fragmented habitats i.e. patches of aquatic habitat surrounded by a terrestrial matrix; this is true whether they are urban or rural. Urban habitats in general are frequently highly fragmented (English Nature 1997). Many pond species have, developed strategies to cope with life in such fragmented habitats. The implications for the planning and design of urban areas are that the retention and/or creation of pond networks are vital for the conservation of freshwater species. The National Amphibian Survey (Swan and Oldham 1993) recommended a minimum of 2 ponds per km2 to ensure the persistence of amphibian populations in the medium and long term, with more being preferable. Bullock et al. (1998) concluded that the optimum pond density for T. cristatus was closer to 5 ponds per km2. Boothby (2000) based on the Pond Life dataset combined with a wider audit of the pond resource of northwest England suggested a density of at least 4 ponds per km2 to maintain the connectivity of the pond landscape. Extrapolating from the data in the current study of the relationship between invertebrate species richness and pond density in the Halton survey, on the basis of invertebrates being the most strongly correlated to density (as shown in Fig. 2), it would require a pond density of 4.5 ponds per km2 to achieve species richness in line with national and regional. Finding space for such a number of ponds in ever more densely developed urban areas may be problematic. However, the current paper has taken into account the distribution of ponds in areas of public greenspace and has not considered the benefits and services provided by garden ponds.
In some cases the loss of ponds due to urban expansion can be offset by the creation of new ponds in gardens. Swan and Oldham (1997) reported a net increase in pond numbers following conversion of farmland to housing development. This was explained by number of ponds created within domestic gardens. Pond density in urban areas is variable and can be high e.g. in the City of Brighton (on the south coast of England) the density of garden ponds is 200 per km2 (Beebe 1979), Banks and Laverick (1986) working in the conurbation of Sunderland in northeast England estimated a density of garden ponds of 100 per km2 and the Biodiversity of Urban Gardens in Sheffield project (BUGS) estimated a density of 67 garden ponds per km2 (Beer 2003). Working with the most modest of these estimates (67 garden ponds per km2) results in an estimate of 994 ponds in the Borough. Although garden ponds may vastly out number those in the wider public landscape this does not mean that they provide the same quality of habitat. Intensive management, poor design and the presence of ornamental fish species can all impact negatively on the species richness of garden ponds (Swan and Oldham 1997; Gaston et al. 2005). Garden ponds tend to be much smaller than those in the public realm. In a study of 900 garden ponds in Birmingham (Wyatt 1998) the median size of ponds encountered was 3.14 m2, by comparison the median size of the surveyed ponds in Halton was 302 m2. Although pond size did not emerge as a significant contributing factor to species richness, even the smallest of the ponds in the Halton sample (32 m2) was an order of magnitude larger than those in the Birmingham study. In addition, pond size is known to be a factor in the occurrence of particular species such as T. cristatus (Bullock et al. 1998). As they are under the direct control of individual households rather than the local authority planners and ecologists whatever the relative value of garden ponds to nature conservation they cannot be planned at a landscape scale. However, urban designers and planners are able to consider the implications of the size and juxtaposition of private gardens in making their professional decisions.
Despite the potential problems with garden ponds they may still make a substantial contribution to local species richness, especially for mobile taxa such as invertebrates or amphibians by acting as temporary refuges. In such a way garden ponds have the potential to form stepping stones between other ponds in the wider landscape. Low density developments would seem to favour the protection of pond networks, both in public spaces and by providing a greater garden resource. However, growing pressure to increase urban infill development is likely to see a rise in building density and make the retention and management of urban blue/green spaces ever more challenging. Both conservation and planning must move past single site definitions to consider the broader pond landscape and the maintenance of diversity within it. Much insight could be gained from longitudinal studies in these newly developed areas to assess the long term impact of changes in the surrounding landscape. The dataset collected as part of the current study could form part of such long term monitoring. In this study a comparison has been made been two urban designs. Other designs have come forward e.g. New Urbanism and much could be gained through a study which compares a wide variety of urban designs and the associated biodiversity.
References
Arntzen JW, Wallis GP (1991) Restricted gene flow in a moving hybrid zone of the newts Triturus cristatus and T. marmoratus in Western France. Evolution 45:805–826. doi:10.2307/2409691
Banks B, Laverick G (1986) Garden ponds as amphibian breeding sites in a conurbation in northeast England (Sunderland, Tyne and Wear). Herpetol J 1:44–50
Beebe TJC (1979) Habitats of British amphibians (2) suburban parks and gardens. Biol Conserv 15:241–247. doi:10.1016/0006-3207(79)90046-6
Beer AR (2003) Greenstructure and urban planning—case study. Research into Biodiversity in Domestic Gardens, Sheffield, UK. Available at http://www.map21ltd.com/COSTC11/Sheffield/sh-domest-gdns.htm. Accessed 17 March 2008
Biggs J, Whitfield M, Williams P, Fox G, Nicolet P (1998) Factors affecting the nature conservation value of ponds: results of the National Pond Survey. In: Proceedings of the ponds conference 1998, Denman College, Abingdon, Oxon, 10–12th December 1998
Biggs J, Williams P, Whitfield M, Nicolet P, Weatherby A (2005) 15 years of pond assessment in the Britain: results and lessons learned from the work of pond conservation. Aquat Conserv Mar Freshwat Ecosyst 15(6):693–714. doi:10.1002/aqc.745
Blackman D (2007) 30,000 homes needed each year. Society guardian [Online], Wednesday, 27th April 2005. Available online at http://society.guardian.co.uk/housing/story/0,1470568,00.html. Accessed 27 September 2007
Boothby J (ed) (2000) A landscape worth saving: final report of the pond biodiversity survey of northwest England. Pond life project. Liverpool John Moores University, Liverpool
Briers RA, Biggs J (2005) Spatial patterns in pond invertebrate communities: separating environmental and distance effects. Aquat Conserv Mar Freshwat Ecosyst 15:549–557. doi:10.1002/aqc.742
Bullock DJ, Oldham K, Corbett K (1998) Habitats and their management. In: Gent T, Gibson S (eds) Herpetofauna workers manual. JNCC, Peterborough
CIRIA (2000) Sustainable urban drainage systems—design manual for Scotland and Northern Ireland, CIRIA report C521. CIRIA, London
Clapton M (1998) Invincible green suburbs, brave new towns. Manchester University Press, Manchester
Communities and Local Government (2006) Planning policy statement 3: housing. Department of Communities and Local Government, London
Conrad KF, Willson KH, Harvey IF, Sherratt TN (1999) Dispersal characteristics of seven odonate species in an agricultural landscape. Ecography 22:524–531
Convention on Biodiversity (1992) Text of the convention on biodiversity available at http://www.cbd.int/doc/legal/cbd-un-en.pdf. Accessed 18 March 2008
Cross J (2005) Halton Borough Council Provisional Local Transport Plan 2006/07 to 2010/11 appendix 3: provisional strategic environmental assessment environmental report. Report no. 12175/R03, produced by Gifford Ltd on behalf of Halton Borough Council, July 2005
Delettre YR, Morvan N (2000) Dispersal of adult aquatic Chironomidae (Diptera) in agricultural landscapes. Freshw Biol 44(3):399–411. doi:10.1046/j.1365-2427.2000.00578.x
English Nature (1997) A framework for the future: green networks with multiple uses in and around towns and cities. English Nature, Research report no 256, Peterborough
English Nature (2001) Great crested newt mitigation guidelines. English Nature (now Natural England), Peterborough
English Partnerships (2005) English Partnerships, the National Regeneration Agency: New towns. Available at http://www.englishpartnerships.co.uk. Accessed 4 June 2007
European Commission (1992) Directive/92/43/EC of the European Parliament and the Council, 21st May 1992: the conservation of natural habitats and of wild flora and fauna (Habitats directive). Off J EC L206(50)
Foster J (2007) Amphibians in the garden: your questions answered. Natural England gardening for nature, no: 18. Natural England, Peterborough
Gaston KJ, Smith RM, Thompson K, Warren PH (2005) Urban domestic gardens: experimental tests of methods for increasing biodiversity. Biodivers Conserv 14:395–413. doi:10.1007/s10531-004-6066-x
Gee JHR, Smith BD, Lee KM, Wynn-Griffiths S (1998) The ecological basis of freshwater pond management for biodiversity. Aquat Conserv Mar Freshwat Ecosyst 17(2):91–104
Gent T, Gibson S (2003) The Herpetofauna worker’s manual. Joint Nature Conservation Committee, Peterborough
Gledhill DG (1999) Factors affecting the occurrence and distribution of native amphibians species in northwest England. Undergraduate dissertation, School of Biological and Earth Sciences, Liverpool John Moores University, Liverpool England
Guest JP, Bentley D (1998) Critical pond biodiversity survey. Pond life project. Liverpool John Moores University, Liverpool, England. Unpublished survey report
Guest J, Harmer A (2006) Amphibian atlas of Cheshire and the Wirral. England
Halton Borough Council (2007) Your council: Halton population. Available at http://www2.halton.gov.uk/yourcouncil/population?a=5441. Accessed 24 June 2007
Horne MT, Dunson WA (1995) Effects of low pH, metals, and water hardness on larval amphibians. Arch Environ Contam Toxicol 29(4):500–505. doi:10.1007/BF00208380
Lees S, Evans P (2003) Biodiversity’s contribution to the quality of life. A research report for English Nature, no 510. Environmental impacts team, English Nature, February 2003
Linton S, Goulder R (2003) Species richness of aquatic macrophytes in ponds related to number of species in neighbouring water bodies. Arch Hydrobiol 157(4):555–565
Montgomery DC, Peck EA (1982) Introduction to linear regression analysis. Wiley, New York
ODPM (2005) Generalised land use data (GLUD) statistics for England. Office of the Deputy Prime Minister, London
Oldham RS, Keeble J, Swan MJS, Jeffcote M (2000) Evaluating the suitability of habitat for the great crested newt. Herpetol J 10:143–156
Pond Action (1998) National Lowland Pond Survey 1996. Pond Action Oxford Brookes University, England
Ponds Conservation (2002) A guide to monitoring the ecological quality of ponds and canals using PSYM. Pond Conservation Trust, Oxford Brookes University/the Environment Agency, Oxford/West Midlands
Rich T (2000) A comparison of the ponds in the County of Cardiff with the national statistics from the Lowland Pond Survey. In: Pond Action (2000). Proceedings of the Ponds conference 1998. Pond Action, Oxford
Rump H, Krist H (1999) Laboratory manual for the examination of water, waste water and soil. Wiley, Chichester
Rundle SD, Foggo A, Choiseul V, Bilton DT (2002) Are distribution patterns linked to dispersal mechanism? An investigation using pond invertebrate assemblages. Freshw Biol 47(9):1571–1581. doi:10.1046/j.1365-2427.2002.00886.x
Stoianov I, Charpra S, Maksimovic C (2000) A framework linking urban park land use with pond water quality. Urban Water 2:47–62. doi:10.1016/S1462-0758(00)00039-X
Sutherland WJ, Hill DA (1995) Managing habitats for conservation. Cambridge University Press, Cambridge
Swan MJS, Oldham RS (1993) National Amphibian Survey: final report. English Nature research reports no. 38, Peterborough
Swan MJS, Oldham RS (1997) Pond loss and amphibians: a historical perspective. In: Boothby J (ed) British pond landscape: action for protection and enhancement. UK conference of the Pond Life Project, University College Chester, 7–9 September 1997. Pond Life Project, Liverpool, pp 3–16
UK BAP (2008) UK list of priority habitats available at http://www.ukbap.org.uk/PriorityHabitats.aspx. Accessed 8 March 2008
Wyatt N (1998) Birmingham pond survey. Urban Wildlife Trust and Birmingham City Council, Birmingham
Acknowledgements
The authors would like to thank: Andrew Harmer Consultants and Bill Bellamy Associates for species data collected as part of the Heritage Lottery Funded project “Making the Most of Halton’s Ponds” and Paul Oldfield (Nature Conservation Officer, Halton Borough Council) for granting access to pond sites. Pond Audit data was provided by the Ponds Research Unit, Liverpool John Moores University. Laboratory water analysis was carried out with the assistance of Vanessa Barat, Mathieu Viossanges, Thomas Auger, Angélique Autef and Audrey Arnaud.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gledhill, D.G., James, P. & Davies, D.H. Pond density as a determinant of aquatic species richness in an urban landscape. Landscape Ecol 23, 1219–1230 (2008). https://doi.org/10.1007/s10980-008-9292-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-008-9292-x