Abstract
High mountain environments are often characterised by low temperatures and short growing seasons, yet support high plant endemism and biodiversity. While such ecosystems are considered among the most vulnerable to climatic warming, the impacts of climate change on diversity and composition can be complex. To develop a deeper understanding of these dynamics, changes in vegetation over time along five alpine summits that are part of the Global Observation Research Initiative in Alpine Environment (GLORIA), were assessed including species richness, α-diversity, β-diversity, vegetation and growth form cover as well as composition. The five summits of Mount Clarke in the Australian Alps were surveyed in 2004, 2011 and 2019. Despite increases in species richness over time, there was an overall decline in diversity through biotic homogenisation across the summits. Near complete vegetation cover was recorded in 2004 but increased over the 15 years via in-filling and densification, driven by increasing cover of graminoids and shrubs. Consequently, there was also a shift in species composition which was greatest at higher elevations. The results indicate that there has been a shift towards more competitive and thermophilic composition, which may have implications for flammability in a warming and drying climate. Further assessments will be required to more fully explore the effect of climate variation from climate change, and implications for conservation of this and other alpine floras globally.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High mountain ecosystems are primarily determined by abiotic stressors such as low temperatures and often have short growing seasons (Körner 2003; Nagy and Grabherr 2009). They also often have complex topography that creates environmental heterogeneity and microhabitat differentiation, resulting in high endemism and biodiversity (Körner 2003, 2004; Elsen and Tingley 2015). Above the bioclimatic treeline, most alpine plants are highly specialised, slow growing and long-lived perennials that are resilient to short-term climatic oscillations, but respond to sustained climatic changes (Körner 2003; Nagy and Grabherr 2009; de Witte and Stöcklin 2010). Comparatively, the chemical and biological processes of alpine ecosystems are particularly sensitive to temperature (Körner 2003; Davidson and Janssens 2006; Lütz 2013). With faster rates of warming at higher elevations (Pepin et al. 2015; Wang et al. 2016), alpine ecosystems are considered amongst the most vulnerable and responsive to climate change (Walther et al. 2005; Seddon et al. 2016; Guisan et al. 2019). As a result, there are changes in vegetation due to overall warming but also increasing extreme climatic events such as heatwaves, droughts and fire (Gottfried et al. 1998; Grabherr et al. 2010; Dirnböck et al. 2011; De Boeck et al. 2016; Hock et al. 2019).
With increasing temperatures and longer growing seasons alpine plants can experience direct abiotic stress affecting phenology and physiology (Inouye 2008; Ernakovich et al. 2014; Fu et al. 2015). They are also experiencing increased competition as a result of the expansion of thermophilic species previously restricted to lower elevations (Lamprecht et al. 2018; Steinbauer et al. 2018). In response, there has been an increase in species richness on many alpine summits (Gottfried et al. 2012; Pauli et al. 2012). However, there is a growing ‘extinction debt’ among long-lived alpine plants as climatic thresholds are eclipsed and suitable habitat disappears, particularly for cryophilic species (Dirnböck et al. 2011; Dullinger et al. 2012; Rumpf et al. 2018, 2019). Dispersal lags and fluctuations in biotic interactions are beginning to influence the distribution, diversity and composition of alpine plant communities. Therefore, transient dynamics are likely to dominate the range of responses of alpine plants to climate change in coming decades (Gilman et al. 2010; Anthelme et al. 2014; Alexander et al. 2015, 2018).
Alpine ecosystems are ideal for monitoring changes in vegetation in response to a warming climate. With increasing elevation, the relative importance of biotic interactions such as competition and impacts from anthropogenic land use often decrease (Choler et al. 2001; Körner and Ohsawa 2005; Mark et al. 2015). They also have a global distribution while providing ‘space-for-time natural experiments’ along environmental gradients, and are comparatively simple in their biotic components (Körner 2003; Körner and Paulsen 2004; Grabherr et al. 2010; Körner et al. 2011; Pauli et al. 2015). However, short-term dynamics and natural ecological variability are often difficult to discern from longer-term trends, such as those induced by climate change (Müller et al. 2010). Therefore, multidecadal ecological monitoring using a consistent approach can assist in discerning natural variability from ecosystem dynamics due to a warming climate (Stöckli et al. 2011; Gitzen et al. 2012). Global long-term monitoring initiatives, such as the Global Observation Research Initiative in Alpine Environments (GLORIA—see https://www.gloria.ac.at), provide a standardised and comparable approach to assess vegetation dynamics in response to climate change by monitoring permanent vegetation plots on alpine summits (Vittoz and Guisan 2007; Pauli et al. 2015).
With over 100 study areas and 20 years of monitoring using the same protocols (Pauli et al. 2015), changes in alpine summit vegetation have been reported in Europe (Erschbamer et al. 2011; Michelsen et al. 2011; Gottfried et al. 2012; Pauli et al. 2012; Fernández Calzado and Molero Mesa 2013; Stanisci et al. 2016; Artemov 2018; Lamprecht et al. 2018; Steinbauer et al. 2018; Porro et al. 2019), Asia (Gigauri et al. 2016; Chandra et al. 2018; Salick et al. 2019; Hamid et al. 2020), North America (Malanson and Fagre 2013; Smithers et al. 2020), South America (Carilla et al. 2018) and Oceania (Venn et al. 2012, 2014). With the majority of published GLORIA research from Europe, regionally specific dynamics in diversity and edge expansions or contractions are emerging among those summits. In temperate and boreal parts of Europe, upslope migration has resulted in species enrichment and thermophilisation of assemblages (Gottfried et al. 2012; Pauli et al. 2012; Lamprecht et al. 2018), while in the drier alpine areas of Europe species richness on summits is stagnating or declining (Pauli et al. 2012; Fernández Calzado and Molero Mesa 2013).
Results from GLORIA summits outside Europe are becoming available and have provided new geographical and ecological insights about the processes driving alpine vegetation dynamics (Pauli et al. 2012; Rumpf et al. 2019). In Asia, species enrichment and increasing vegetation cover through time was more pronounced on solar aspects and higher elevation summits (Salick et al. 2019), and there is also evidence of thermophilisation (Gigauri et al. 2016). In North America, there has been increasing dissimilarity in assemblages through time, suggesting considerable species turnover on summits (Malanson and Fagre 2013). However, research assessing climate change impacts on alpine vegetation is sparser for the Southern Hemisphere whether using the GLORIA protocol or other methods (Verrall and Pickering 2020). Using the GLORIA protocols, species enrichment and increasing shrub and graminoid cover have been recorded in South America (Carilla et al. 2018) and Australia (Venn et al. 2012, 2014).
Unlike other alpine environments, many alpine summits in the Australian Alps are characterised by aeolian, deep and well-formed humus soils supporting near complete vegetation cover. The alpine climate is relatively mild with long growing seasons and warm summer temperatures (Costin 1967; Costin et al. 2000; Körner and Paulsen 2004; Green and Osborne 2012). Thus, assessing vegetation dynamics in the Australian Alps provides additional perspectives when understanding climate change impacts on alpine summits. Similar to many alpine environments globally, in the Australian Alps temperatures are increasing, there is declining snow cover, increasing variability in precipitation and changing fire regimes (Williams et al. 2008, 2014; Davis 2013; Sánchez-Bayo and Green 2013; Bradstock et al. 2014; McGowan et al. 2018; Holgate et al. 2020).
Monitoring using the GLORIA protocol across five summits in the Australian Alps, found that elevation and climatic factors, such as soil temperatures and growing season, accounted for some differences in vegetation (Pickering et al. 2008; Pickering and Green 2009). A re-survey in 2011 found that mean species richness at the whole summit scale had increased by approximately 12%, and this was largely driven by new graminoid and shrub species (Venn et al. 2012). Additionally, there were increases in overlapping vegetation cover, but the changes were most pronounced at the lowest elevation summit where graminoids and forbs more than doubled in cover. These compositional and growth form dynamics drove shifts in community trait weighted means, favouring species with more competitive traits, with the most pronounced differences at the lowest elevation summit. Across all summits, there was an increase in the cover of species with higher leaf areas (mm2) and specific leaf area (mm2 mg−1) but decreases in height (mm) and leaf dry matter content (mg g−1) (Venn et al. 2014).
Re-surveying in 2019 provides the opportunity to assess the response of vegetation to further changes in climate by assessing diversity and composition dynamics over more time, building upon a multicomponent approach proposed by Porro et al. (2019). Previous assessments primarily focused on species richness (Venn et al. 2012), but by also assessing changes in α-diversity and β-diversity it may be possible to identify other dynamics such as biotic homogenisation through time (Britton et al. 2009; Liberati et al. 2019). Following on from the previous analysis, assessing dynamics in vegetation and growth form cover on summits with near complete vegetation cover can also provide insights into processes such as densification and gap in-filling (Cannone and Pignatti 2014), and hence potential increases in competitive interactions as well as influential species and growth forms (Pickering and Green 2009; Venn et al. 2014).
The aim of this study incorporating data from the 2019 survey, with those from 2004 and 2011, is to extend the assessment of vegetation dynamics in response to climatic warming for the five alpine mountain summits in the Australian Alps. Specifically, the following questions will be addressed: (1) Has species richness continued to increase in line with previous assessments? (2) Has α-diversity and β-diversity fluctuated across surveys, and do they indicate vegetation homogenisation and overall biodiversity loss? (3) Are there increases in the overlapping cover of vegetation and growth forms via in-filling and densification? (4) Has composition shifted over time, and if so, what species or growth forms are influencing these changes? (5) What are the regional implications of climate drivers on changes in vegetation?
Methods
Study area
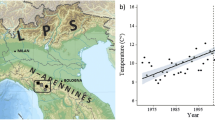
The only GLORIA study area in Australia (AU-KNP) was established in 2004 in the highest and most diverse alpine environment in Australia centred on Mt Kosciuszko (2228 m a.s.l.), the highest peak in the Australian Alps, and continental Australia (Costin 1967; Costin et al. 2000). Five ‘summits’ along a continuous ridgeline from the highest summit of Mount Clarke (2114 m a.s.l.) to near the bottom of the valley by the Snowy River (see Pickering et al. 2008; Pickering and Green 2009) (Fig. 1a). Although all five summits are above the bioclimatic treeline, which occurs around 1800 m a.s.l. (Costin 1967; Costin et al. 2000; Körner and Paulsen 2004), they vary in biotic and abiotic conditions as they range over 301 m of elevation and a horizontal distance of 2240 m (Table 1). Notably, they differ in climatic conditions including the length of the growing season (Pickering and Green 2009; Venn et al. 2014). As for vegetation structure, the two lowest summits (CL4, CL5) are dominated by shrubs while the three highest summits (CL1, CL2, CL3) are dominated by graminoids. The highest summit (CL1) also includes small tracts of windswept feldmark on the exposed western aspect (CSIRO 1972; Pickering and Green 2009).
a Location of the study area within Kosciuszko National Park in Australia and GLORIA summits on Mount Clarke 1–5 (CL1, CL2, CL3, CL4, CL5). b Mean summer temperatures (December to February—December from preceding year) recorded at the Bureau of Meteorology Station 071032 Thredbo AWS (1999–2019, black line; slope = 0.0699, R2 = 0.157, t = 1.879, F1–19 = 3.530, p = 0.076) and soil temperature from Mount Clarke summits (2004–2018, grey line; slope = 0.0555, R2 = 0.272, t = 2.206, F1–13 = 4.865; p = 0.046). Air and soil temperature are positively correlated (Pearson correlation = 0.571, p = 0.026). First year of vegetation monitoring highlighted by vertical dotted line
Summits in the Australian Alps generally have little topographical relief, with many summits more dome-shaped rather than conical peaks. There is also near complete vegetation cover and high plant biomass compared to many other alpine ecosystems (Costin 1967; Costin et al. 2000; Pickering and Green 2009). Disturbance from summer cattle grazing in the area from the late 1830s to 1958 was limited as stock routes at the time avoided Mount Clarke (Costin et al. 2000; Slattery and Worboys 2020). Mount Clarke is also away from any walking tracks and there is very limited off-track walking in this area (Fig. 1a), and very limited evidence of disturbance from the small native mammals found in the region (Green and Osborne 2012; Good and Johnston 2019). Although the population of feral ungulates, such as deer and horses, has been increasing in the area (Driscoll et al. 2019; Ward-Jones et al. 2019), their impacts on these summits have been marginal with little evidence of grazing and the occasional ungulate scat (Pickering and Green 2009; Venn et al. 2012).
While there are no reliable and consistent long-term climatic data for this alpine area, or the Australian Alps in general, air and soil temperature data has more recently become available. For example, hourly air temperature data from 1999 to present are available from an all-weather station maintained by the Australian Bureau of Meteorology approximately 6.3 km to the south of the highest summit (CL1). Soil temperatures have been recorded at 2-h intervals using data loggers (Tinytag Plus—Gemini Data Loggers, Chichester England) at a depth of 10 cm on each aspect of each summit (20 data loggers) from 2004 following the GLORIA protocol (Pauli et al. 2015). These climatic data indicate an overall increase in mean summer air temperatures (R2 = 0.157, t = 1.879, F1–19 = 3.530, p = 0.076) and soil temperatures (R2 = 0.272, t = 2.206, F1–13 = 4.865; p = 0.046) by 0.070° C and 0.056° C per year respectively, beyond the annual variability (Fig. 1b).
Vegetation sampling design
Sampling followed the GLORIA protocol outlined in Pauli et al. (2015) and previous surveys of the five summits (Pickering et al. 2008; Pickering and Green 2009; Venn et al. 2012, 2014). Each summit was split into eight Summit Area Sections (hereafter referred to as “SAS”) by establishing the upper (5 m isoline) and lower (10 m isoline) summit area sections (hereafter referred to as “upper sections” and “lower sections”, respectively) from the highest point, divided by the four cardinal bearings (Fig. S1). For summits with minimal topographical relief, the terminus of the SAS extended 50 m for each section, resulting in variations in the area covered by each SAS (Fig. 1a; Table 1).
Vegetation surveys were conducted in January/February in 2004, 2011 and 2019. This involved surveying vascular plant species composition, bryophyte and lichen (non-vascular) cover, and substrate (solid rock fixed in the ground, loose rock scree, bare soil and organic matter/litter) cover in each SAS. Since this study area has near complete vegetation cover (Costin 1967; Pickering et al. 2008; Pickering and Green 2009), randomised point sampling was used to record the presence and cover of plant species in each SAS supplementing the GLORIA protocol. This involved recording all species intersected by a 1 m length of metal wire (1 mm diameter) lowered vertically toward the ground at 200 randomly located points in each SAS (Evans and Love 1957). After sampling each SAS, a thorough search for rare species that were not recorded during random point sampling was conducted, and any additional species marked as present with a nominal low cover value of 0.1%. From the random point sampling, a species list was generated for all vascular and non-vascular plants for each of the eight SAS at each of the five summits (40 SAS per survey) (Table S1). Vascular plants were characterised into growth forms following Costin et al. (2000) as graminoids (including tussock and non-tussock grasses, sedges and rushes), forbs (including erect, prostrate, creeping and rosette forbs) and shrubs (including subshrubs). Sampling in the 2011 and 2019 re-surveys were conducted ‘blind’, without referring to previous data and species lists (Vittoz et al. 2010a). Species names recorded in the field followed Costin et al. (2000) to be consistent across surveys and the main flora for the region, but have been updated here to match current taxonomy, as outlined by New South Wales Flora Online (https://plantnet.rbgsyd.nsw.gov.au/). Species lists were meticulously checked following surveys and where species could not be accurately and repeatedly identified in the field, genus level data were recorded.
Data processing and analysis
Prior to analysis, five species that that were only recorded in one SAS and one year were removed from the species list (Table S2), Vittoz et al. (2010a), Pauli et al. (2012) and Porro et al. (2019). Then, the randomised point sampling data were used to calculate overlapping cover for all taxa (referred to as species here after, but includes genera for some as indicated above) (hits per species/200 points × 100). The overlapping cover of growth forms (graminoids, forbs and shrubs) was calculated as the sum of all species with that growth form. To account for the varying size of SAS areas, overlapping cover of species was standardised by a corrective coefficient determined by the ratio between the SAS area where the species was recorded and the cumulative SAS area (Porro et al. 2019). Prior to analysis, the standardised overlapping vegetation and growth form cover data were log-transformed to satisfy the assumptions of parametric statistical modelling.
Before assessing the dynamics of species richness, the influence of the species-area relationship was corrected by calculating the standardised residuals of a linear mixed model (LMM) fitted on the 40 SAS species richness values in 2004, 2011 and 2019, with the area of each SAS included as a fixed effect. Due to the repeated measures and spatial structure of the sampling design, each SAS was assigned a unique identifying code (‘SAS Code’—e.g. CL1N05) which was nested within the respective summits (‘Summit Code’—e.g. CL1), with these nested factors included as a random effect in the model. Then, to remove the effect of SAS area on species richness, a linear model (LM) was fitted to the obtained LMM standardised residuals, following the protocol proposed in Vittoz et al. (2010b) and repeated in Porro et al. (2019). To assess species richness dynamics through time for the study area, and determine if summit or section were predictors for species richness, a LMM was conducted with ‘Year’ (2004, 2011 and 2019), ‘Summit’ (CL1, CL2, CL3, CL4 and CL5) and ‘Section’ (upper and lower section) as fixed effects. To test if species richness dynamics were consistent over the study area, interactions of Year, Summit and Section were also included in the model. Then, p-values were attained for fixed effects using the package “lmerTest” v3.1.3 (Kuznetsova et al. 2017) and differences between significant levels of fixed effects and interactions were obtained via the package “emmeans” v1.5.2-1 (Lenth et al. 2020) using Tukey’s HSD adjustment for multiple comparisons.
To further investigate dynamics in α-diversity, diversity profiles for 2004, 2011 and 2019 were calculated and plotted. These profiles were generated from the standardised vegetation cover for the study area and each summit by calculating the exponential of the Rényi index (Rényi 1961; Hill 1973), which provides a series of linked diversity indices, which depend upon the parameter α and the frequencies of each species, defined as:
where pi is the relative frequency of the ith species and S is the total number of species (Tóthmérész 1995). For α = 0, this function produces the total species richness, while α = 1 and α = 2 represent the Shannon index (Shannon 1948) and Simpson index (Simpson 1949), respectively. Generalised diversity profiles have seen a recent revival in ecological analyses (Porro et al. 2019), as different diversity indices have varying sensitivity to the presence of rare and abundant species, and lone indices cannot effectively assess biodiversity and community structure (Magurran 1988). However, diversity profiles have the ability to demonstrate varying measures of diversity simultaneously and permit ranking of the overall diversity of different groupings (in this case, different years) from high to low. If a groups profile runs entirely above another, it is unequivocally more diverse. However, groups cannot be unequivocally ranked if the profiles intersect, as one group contains greater diversity of rare species whereas the other contains greater diversity of common species (Tóthmérész 1995, 1998). Thus, to rank such groupings where profiles intersect, the underlying area of the diversity profile function (hereafter referred to as “diversity area”) was calculated using the trapezoid method (Rohde et al. 2012), as proposed by Di Battista et al. (2017). Diversity area is calculated considering the entire domain and thus, does not preference either species richness nor evenness, with a higher value denoting greater biodiversity. Secondly, to understand the dynamics of varying diversity metrics through time, the following indices were calculated for the study area and each summit: Shannon index (Shannon 1948), Sheldon evenness (Sheldon 1969) and 1—Simpsons Index (Simpson 1949), which represents a Dominance index. All calculations and plotting of α-diversity were executed with the software PAST 4.03 (Hammer et al. 2001).
Following on from assessments of α-diversity, β-diversity was also calculated for 2004, 2011 and 2019 to assess if biotic homogenisation had occurred through time. Firstly, the pairwise Simpson dissimilarity (Baselga 2010) was calculated between all the SAS of the study area (40 SAS) as well as the upper and lower section (20 SAS each) for each survey. To assess differences between 2004, 2011 and 2019, the Kruskal–Wallis test with Dunn’s multiple comparison post-hoc test was used to indicate the spatial patterns of diversity without the influence of richness gradients to highlight any differences in β-diversity due to species colonisations and disappearances. Secondly, the multiple-site dissimilarity measures proposed in Baselga (2010) were calculated for the study area, which include βNES (nestedness-resultant multiple-site dissimilarity), βSOR (Sørensen multiple-site dissimilarity) and βSIM (Simpson multiple-site dissimilarity) defined as:
where Si is the total species richness in the SAS i, ST is the total species richness in all the SAS and bij, bji are the number of species exclusive to SAS i and j respectively. These multiple-site measures best represent varying patterns of β-diversity dependent upon the proportion of species shared between different communities (βSOR), species turnover (βSIM) and spatial nestedness (βNES) (Baselga 2010). Analyses of β-diversity were executed in R by means of “betapart” v1.5.2 (Baselga et al. 2020) and the Kruskal–Wallis test was conducted in the baseline package “Stats” v4.0.3 (R CoreTeam 2020).
To assess overlapping cover dynamics through time, the cover of all species was combined for ‘vegetation’ while species were also grouped by ‘growth forms’: graminoids (including rushes, sedges and grasses), forbs and shrubs (including subshrubs and dwarf shrubs) while ferns were excluded due to their rarity. Growth forms represent broad plant functional types and this type of analysis has commonly been used to assess the responses of alpine vegetation to climate change (Pickering and Green 2009; Venn et al. 2012; Porro et al. 2019). First, the standardised sum of overlapping cover was calculated for vegetation and growth forms for each of the 40 SAS for 2004, 2011 and 2019. To assess the dynamics of vegetation and growth form cover through time, LMMs were fitted to log-transformed data via the “lme4” package v1.1-23 (Bates et al. 2015), with the fixed effect ‘Year’ and ‘SAS Code’ nested within ‘Summit Code’ as the random effect to account for the spatiotemporal influence of the sampling design. F-statistics and p-values were calculated in R via the “car” v3.0-10 package (Fox and Weisberg 2019). Where significant variation through time had occurred, subsequent LMMs were executed for each summit (8 SAS) where ‘Year’ was the fixed effect while ‘SAS Code’ was the random effect. Differences in standardised vegetation cover between years were determined via the package “emmeans” v1.5.2-1 (Lenth et al. 2020) using Tukey’s HSD adjustment for multiple comparisons.
To assess species and growth form compositional similarity among years, non-metric dimensional scaling ordinations (NMDS) were used to visualise differences in species and growth form composition for the study area. Firstly, these ordinations were generated from Bray–Curtis distance matrices of square-root transformed standardised species and growth form cover for each SAS in each year (Bray and Curtis 1957; Quinn and Keough 2003; Taguchi and Oono 2005). Secondly, influential species and growth forms driving variances between years were determined by the similarity percentage analysis (SIMPER) using a Pearson correlation co-efficient of 0.75 (Clarke 1993), and were overlaid on these ordinations as proportional vectors. Thirdly, to determine if there had been a compositional shift in species and growth forms through time, one-way analysis of similarity (ANOSIM) with ‘Year’ as the fixed effect was executed using 999 random permutations (Clarke 1993). Pairwise comparisons using the Bonferroni adjustment were then used to determine differences between years. Lastly, ordinations were generated for each summit with subsequent ANOSIM tests to assess temporal changes at the summit level. All calculations and plotting of α-diversity were executed with the software PAST v4.03 (Hammer et al. 2001).
Results
Diversity
A total of 91 plant species from 34 families were recorded across the three surveys in the study area (Table S1). Species richness varied across summits (p < 0.001) (Table 2a), with 51 species recorded cumulatively at the highest summit CL1, 63 species at CL2, CL3 and CL4, and 67 species at the lowest summit, CL5. Species richness also varied through time with 73 species recorded in 2004, 76 in 2011 and 87 in 2019 (p < 0.001) (Table 2a). Specifically, species richness increased from 2004 to 2011 (mean difference 2.7 ± 1.0, p = 0.016) and from 2011 to 2019 (mean difference 5.5 ± 1.0, p < 0.001). Eleven species were lost between 2004 and 2011 and three between 2011 and 2019 (Table S3). There were 14 new species recorded in 2011 and an additional 14 new species recorded between 2011 and 2019. Between 2004 and 2019, there were 16 new species but only two disappearances.
Dynamics in species richness through time were consistent for all summits except the lowest summit, CL5, as demonstrated by the interaction between Year × Summit (p = 0.158) (Table 2a) and Year for each summit individually (CL1, p = 0.002; CL2, p = 0.005; CL3, p = 0.007; CL4, p < 0.001; CL5, p = 0.262) (Table 2b). The number of new species was greater than disappearances between 2004 and 2019 for all summits, but was most pronounced for CL2 and CL4, with a net increase of 19 and 17 species, respectively. Sections also had a significant effect on species richness with the lower sections containing significantly more species than the upper sections (mean difference: 2.3 ± 0.6, p = 0.004) (Table 2a), but variation in species richness over time was not consistent between upper and lower sections (p = 0.004). In particular, species richness was marginally higher in upper sections in 2004 (mean difference: 1.4 ± 1.4, p = 0.322) but in 2011 (mean difference: 3.4 ± 1.4, p = 0.015) and 2019 (mean difference: 5.0 ± 1.4, p < 0.001), species richness was greater in the lower sections. At the summit level, species richness was greater in the lower sections of the two highest summits (CL1, p = 0.023; CL2, p = 0.037).
There was a net decrease in α-diversity through time across the study area with declines in Shannon Index and Sheldon Evenness but an increase in the Dominance Index (Table 3). The Dominance Index (0.19) was highest in 2011 which corresponds to the lowest value for the Shannon index (2.45). However, divergent results were recorded across the summits. There was a net increase in diversity for CL2 as demonstrated by the Shannon Index, while there was a decrease in diversity for all other summits. Considering Sheldon Evenness, there was a net decrease for all summits but there was a net decrease in Dominance Index for CL2 and CL5. These results are supported by the comparable diversity profiles for 2004, 2011 and 2019 for the study area (Fig. 2), where a marked decline in profiles demonstrates the asymmetrical relative abundances of plant species, specifically in 2011. The profiles for the study area could not be unequivocally ranked for α-diversity as they intersected each other, yet the diversity area suggests that diversity was marginally higher in 2019 than 2004, which was largely driven by species enrichment through time. From 2004 to 2019, diversity metrics were variable among summits with values for the Shannon Index decreasing across all summits except CL2, while values for the Dominance Index increased only at CL1, CL2 and CL4. However, there was a consistent decline in Sheldon Evenness across all summits between the first and last survey. Similarly, there were varied patterns in diversity profiles for the five summits (Fig. 2), but a marked increase in species richness for all summits. Since there were no clear differences for any of the profiles at any summit, the diversity area indicates diversity was greatest in 2004 for CL1 and CL3, in 2011 for CL5 and in 2019 for CL2 and CL4.
Considering the dynamics of the ‘pairwise βSIM’, the overall dissimilarity in the study area decreased significantly between 2004 and 2019 (p < 0.001) with the majority of change occurring between 2004 and 2011 (p < 0.001) (Fig. 3). Dynamics at the section level were also significant, with a decrease in dissimilarity between 2004 and 2011 for lower sections (p = 0.026), and between 2004 and 2019 (p = 0.032) (Table S4). However, there was no difference for the upper section between 2004 and 2011 nor 2011 and 2019, but a marked decrease between the first and last survey (p = 0.006). Conversely, dynamics of the ‘multiple-site’ β-diversity metrics were marginal (Table 4). Between 2004 and 2019 for the study area, values for ‘multiple-site ΒSOR’ decreased while ‘multiple-site ΒSIM’ increased, indicating that β-diversity dynamics was influenced less by shared species and more by species turnover through time. The ‘multiple-site ΒSOR’ value was greater for the lower section only in 2004, with the upper sections displaying greater dissimilarity in 2011 and 2019. The ‘multiple-site ΒSIM’ values across all years were higher in the lower sections compared to the upper sections yet the lower sections have increased in dissimilarity between 2004 and 2019 while the upper sections have decreased in dissimilarity in the same period.
Pairwise Simpson dissimilarity indices (‘pairwise βSIM’ as proposed by Baselga (2010)) for the study area upper and lower sections in 2004, 2011 and 2019. Asterisks indicate significant changes between years (*p < 0.05; **p < 0.01; ***p < 0.001)
Cover and composition
In 2004 nearly all of the summit areas were covered by vegetation. However, overlapping cover of vegetation, graminoids and shrubs increased in the study area from 2004 to 2019 (p < 0.001) (Table 5a), although there were differential responses among summits (Table 5b), particularly when changes occurred. Graminoid cover increased markedly over the 15 years (p < 0.001) but most of the change occurred between 2004 and 2011 (mean difference: 7.8, p < 0.001) rather than between 2011 and 2019 (mean difference: 3.5, p = 0.002) (Table S5). Shrub cover also increased over the 15 years (p < 0.001) but unlike graminoid cover, it was mostly between 2011 and 2019 (mean difference: 3.3, p < 0.001) with less variation between 2004 and 2011 (mean difference: 1.9, p = 0.025) (Table S5). In contrast to graminoids and shrubs, forb cover was low with no consistent change over time (Fig. 4).
There were clear patterns in cover among individual summits over time (Fig. 5). Overlapping vegetation cover increased across all summits between 2004 and 2019, but for the three lower elevation summits (CL3, p < 0.001; CL4, p < 0.001; CL5, p < 0.001), most of the change occurred between 2004 and 2011, while the majority of change for the two higher summits (CL1, p = 0.005; CL2, p < 0.001) occurred between 2011 and 2019. There was a similar pattern in graminoid cover, with increases across all summits over the 15 years, but no increases for lowest three summits between 2011 and 2019, while for the two higher summits there were increases between 2011 and 2019. Shrub cover also increased across all summits over the 15 years, but it was only significant between 2011 and 2019 for some summits (CL1, p = 0.049; CL3, p < 0.001; CL4, p = 0.012).
There was a significant shift in species (R = 0.1134, p < 0.001) and growth form composition (R = 0.0422, p = 0.004) through time for the study area (Fig. 6). Species composition differed between 2004 and 2011 (R = 0.1451, p < 0.001) but not between 2011 and 2019 (Table S6). As for growth forms, composition was similar between 2004 and 2011 and between 2011 and 2019, but there were differences over the 15 years (R = 0.0100, p < 0.001). There was a shift in species composition for the four higher summits (CL1, p < 0.001; CL2, p = 0.029; CL3, p = 0.005; CL4, p = 0.011), but changes in growth form composition was only significant for the highest summit (CL1, p = 0.047). The majority of the variation in species composition occurred between 2004 and 2011, with shifts for CL1 (p = 0.003), CL3 (p = 0.002) and CL4 (p = 0.032). However, for growth form composition, the only difference was for CL1 between 2011 and 2019 (p = 0.012).
Non-metric dimensional scaling ordinations using Bray–Curtis similarity index on square-root transformed standardized overlapping cover data with 95% confidence ellipses of a species (R = 0.1134, p < 0.001), b growth form (R = 0.0422, p = 0.004) composition shifts across surveys for the study area. Vectors demonstrate Pearson correlations (> 0.75) of the relative influence of species (1a—Trisetum spicatum subsp. australiense, 2a—Poa Sp., 3a—Empodisma minus, 4a—Nematolepis ovatifolum, 5a—Kunzea muelleri) and growth forms (1b—Shrubs, 2b—Graminoids, 3b—Forbs) of compositional shift through time. Shifts in c species and growth form composition with maximum extent convex hulls for each summit
Discussion
Discerning diversity dynamics
Species enrichment has continued on the summits of Mount Clarke in the Australian Alps consistent with previous assessments (Venn et al. 2012), and with summits in the Central European Alps also using the GLORIA protocol (Erschbamer et al. 2011; Lamprecht et al. 2018; Porro et al. 2019). This contrasts with results from drier alpine environments, such as in the Mediterranean (Pauli et al. 2012) and the Andes (Carilla et al. 2018), where there were declines in species richness. At a summit scale, the maximum increase in species richness for Mount Clarke in the Australian Alps was on the second highest summit (47 to 59 species recorded on CL2 from 2011 to 2019) but was less than the prior assessments (41 to 56 species on CL4 from 2004 to 2011) (Venn et al. 2012). Species richness increased in all but the lowest summit (CL5) over the three surveys, suggesting that species enrichment may be stagnating at lower elevations, which appears to be also occurring in the Altai mountains of Russia (Artemov 2018). Furthermore, the increase in mean species richness for Mount Clarke summits suggests a longer-term trend rather than a short-term fluctuation (Erschbamer et al. 2011; Venn et al. 2012). Between the first and last survey, there were 16 new species and only two of these, Epilobium gunnianum and Oreomyrrhis brevipes, are restricted to this alpine zone (Wright et al. 2010). Therefore, the increases in species richness were predominately driven by species with broader environmental tolerance including subalpine and montane zones. This is consistent with monitoring at a continental scale from Europe, where species richness and abundance increases were driven by thermophilic species from lower elevations responding to climate warming (Gottfried et al. 2012). Climate induced changes in summit vegetation have also been suggested by Lamprecht et al. (2018), Porro et al. (2019) and Steinbauer et al. (2018), among others. These changes on Mount Clarke summits may also be climate-induced, as conditions in the area have warmed, growing seasons lengthened, and snow cover reduced, while the area has experienced limited other types of disturbance such as from trampling or grazing for nearly 60 years (Slattery and Worboys 2020). Furthermore, the disappearance and re-appearance of species between surveys may be explained by observer error including misidentifications (Vittoz and Guisan 2007; Vittoz et al. 2010a; Futschik et al. 2020), as well as drought legacy effects where certain species may have experience drought-induced mortality but following an increase in precipitation in 2010 (Holgate et al. 2020), competitive species may have established themselves more readily (Venn and Morgan 2009).
There were differing patterns in α-diversity for the Mount Clarke study area. From 2004 to 2019, evenness decreased but there were fluctuations in dominance and diversity (Shannon index). However, disentangling dynamics of species richness and α-diversity has proven difficult and led to mixed conclusions (Britton et al. 2009; Ross et al. 2012). In response, diversity profiles were used here as they embody various α-diversity metrics and dynamics through time. These profiles more effectively encapsulate multifaceted ecological processes driving vegetation dynamics in response to climate change, such as upslope migration, leading or trailing edge range dynamics, localised extinctions, competition and densification (Rumpf et al. 2018, 2019; Porro et al. 2019). Across all summits, diversity was greatest in 2019, but this can largely be attributed to increasing species richness, as denoted by the larger area under the diversity profiles at low α values. Conversely, α-diversity was lowest in 2011, which may due to the dominance of competitive species following a pulse of recruitment at the end of the millennial drought (Venn and Morgan 2009). The second highest summit (CL2) displayed the greatest increase in α-diversity, which was largely driven by 19 new species from 2004 to 2019. Excluding this summit, diversity dynamics seem to follow a trend with elevation, similar to the findings of Porro et al. (2019). The greatest loss of α-diversity over the 15 years occurred at the highest summit (CL1), followed by the third highest summit (CL3), while the two lower summits became more diverse. The loss of diversity over time at higher elevations may be explained by the combination of upslope migration of more thermally-tolerant plants (Artemov 2018; Steinbauer et al. 2018) and the in-filling and expansion of competitive species resulting in densification (Cannone and Pignatti 2014; Rumpf et al. 2018).
Considering β-diversity, the decline in the values of ‘pairwise βSIM’ through time indicates biotic homogenisation across the study area. These results are similar to those for northern Apennines summits in Italy (Porro et al. 2019), but β-diversity is yet to be investigated for other GLORIA summits. Biotic homogenisation is ongoing for the Mount Clarke study area and was first detected in the lower sections of summits in 2011, but appears to have now progressed upslope to the upper sections potentially demonstrating the influence of elevation on climate-induced vegetation dynamics (Artemov 2018; Rumpf et al. 2018; Smithers et al. 2020). The upper sections were less affected by upslope migration and densification, but over the 15 years there was a strong homogenisation signal, demonstrating the importance of longer-term monitoring (Walther et al. 2005; Alexander et al. 2018; Futschik et al. 2020). The dynamics in β-diversity are mostly attributed to species turnover rather than shared species or nestedness, as indicated by the greater values of ‘multiple-site βSIM’ (Baselga 2010). Several species were replaced but few were lost from the study area, which is similar to changes documented on Italian summits (Porro et al. 2019) and is consistent with previous assessments of the same summits (Venn et al. 2012).
Densification of vegetation driving compositional dynamics
Unlike many other alpine areas globally, there is near complete vegetation cover across the Mount Clarke summits, as is the case for many other alpine summits in the Australian Alps (Costin 1967; Costin et al. 2000; Pickering and Green 2009; Venn et al. 2017). Therefore, dynamics in vegetation cover were the result of in-filling or densification with more species recorded in areas where vegetation cover is complete but less dense, such as inter-tussock spaces and under shrub canopies rather than colonisation of bare ground (Cannone and Pignatti 2014). There was a considerable increase in graminoid and shrub cover through time, as found in the previous assessment of the summits (Venn et al. 2014) as well as for other alpine and tundra environments (Björk and Molau 2007; Cannone et al. 2007; Wilson and Nilsson 2009; Ababneh and Woolfenden 2010; Myers-Smith et al. 2011; Elmendorf et al. 2012; Gao et al. 2016; Amagai et al. 2018). More specifically, the increasing presence of thermophilic graminoids and shrubs is well documented throughout the GLORIA network (Erschbamer et al. 2009, 2011; Grabherr et al. 2010; Gottfried et al. 2012; Fernández Calzado and Molero Mesa 2013; Gigauri et al. 2016; Stanisci et al. 2016; Lamprecht et al. 2018; Hamid et al. 2020). The process of ‘grassification’ of alpine environments can alter biotic interactions and increase intraspecific and interspecific competition (Choler et al. 2001; Callaway et al. 2002; Alexander et al. 2015). The expansion of graminoids on alpine summits in the Australian Alps may reflect their capacity to out-compete forbs and recruit within the canopy of senescing shrubs following considerable precipitation (Williams and Ashton 1988; Jarrad et al. 2008). Similarly, shrubification has implications for ecosystem processes such as localised snow cover and associated hydrological dynamics (Körner 2003; Myers-Smith et al. 2011). With an increase in shrub cover and height, they can outcompete graminoids and forbs by forming dense thickets with closed canopies that transform microclimatic conditions and biotic interactions in tundra and alpine environments (Myers-Smith et al. 2011; Ballantyne and Pickering 2015; Cáceres et al. 2015). Shrub cover also affects the duration of snow cover and hence affects hydrological dynamics (Costin et al. 2000; Körner 2003; Myers-Smith et al. 2011; Venn and Green 2018), as well as the size and extent of subnivean spaces that provide critical overwintering habitat for many alpine animals (Green and Osborne 2012).
The graminoid and shrub driven densification of vegetation across the Mount Clarke summits also resulted in shifts in composition. Although many new species were forbs, they remained scarce and their contribution to assemblages was minimal. Earlier assessments of this study area demonstrated a similar pattern of graminoid and shrub driven compositional dynamics (Venn et al. 2012, 2014). Across a wide range of alpine summits monitored using the GLORIA protocol, shifts in composition have also been detected in Europe (Erschbamer et al. 2009; Fernández Calzado and Molero Mesa 2013; Malanson and Fagre 2013; Lamprecht et al. 2018), Asia (Gigauri et al. 2013; Hamid et al. 2020) and South America (Carilla et al. 2018). The majority of changes across the Mount Clarke summits occurred between the first two surveys. Such changes may have been due to lagged responses to climate change and increased competitive interactions as suggested for other alpine summits (Erschbamer et al. 2009; Alexander et al. 2018), but also may have occurred in response to end of a major drought in the region (Venn and Morgan 2009; Holgate et al. 2020) and/or as a consequence of observer error compounded through time (Vittoz and Guisan 2007; Vittoz et al. 2010a; Futschik et al. 2020). There were shifts in species composition in all but the lowest elevation summit, indicating potential stabilisation of assemblages. Since this summit (CL5) was already dominated by dense thickets of thermophilic shrubs with closed canopies found at lower elevations in 2004 (i.e. Kunzea mullerei and Prosanthera cuneata) (Wright et al. 2010), colonisation by other species and growth forms may have been restricted. Similarly, the divergent responses in diversity, cover and composition of each summit may be explained by varying thresholds for change as determined by the underlying alpine plant communities and differing abiotic factors (Pickering and Green 2009). The exclusion of other species amidst increasing and densifying shrub cover has been established in other alpine ecosystems in Australia, with a positive feedback loop between warming and shrub proliferation (Scherrer and Pickering 2005; Wahren et al. 2013; Camac et al. 2017). Although there are many benefits from assessing growth form or functional type dynamics, Australian alpine plants have shown species-specific varied responses to warming (Hoffmann et al. 2010). Therefore, continued long-term monitoring and more rigorous phenological and experimental warming research is required to more fully understand species-specific responses to climate change in these environments.
Influence of climatic drivers and regional implications
Long-term dynamics in vegetation are often masked by short-term responses and variability to cyclical climate influences (Greenland 1999; Müller et al. 2010). The main climate drivers that cause variability in the study area are the El-Niño-Southern Oscillation (ENSO), Indian Ocean Dipole (IOD) and Southern Annular Mode (SAM). The interaction of these largely independent drivers leads to considerable climatic variability, where warmer temperatures and drought conditions are often the result of a negative IOD, positive SAM during summer and El-Niño (Holgate et al. 2020). Thus, interpreting long-term changes in vegetation requires consideration of the influence of shorter-term climatic variation. Specifically, the baseline data for the Mount Clarke summits in 2004 was collected during relatively cool conditions, but also in the middle of the ‘Millennial Drought’ (Van Dijk et al. 2013), which persisted in the area until 2010 (Holgate et al. 2020), while the second survey occurred a year after drought-breaking precipitation. This shorter-term climatic variation may explain some of the vegetation dynamics seen, such as an increase in dominance and species richness, as herbaceous vegetation can rapidly respond to an influx of precipitation after the end of drought conditions (Scherrer and Pickering 2005; Venn et al. 2014). The third survey was during a period of below average precipitation but higher temperatures. Thus, difficulties arise from attempting to deduce the influence of climate drivers verses climate change on ecological processes but continued long-term monitoring aims to address this ambiguity (Walther et al. 2002; Müller et al. 2010).
The results for Mount Clarke summits and other studies of Australian alpine areas (e.g. Gallagher et al. 2009; Pickering et al. 2014; Annandale and Kirkpatrick 2017; Kirchoff 2020) indicate that the Australian alpine vegetation is changing, and that it is likely that at least some of the change is associated with warming conditions. Such changes are of particular concern as the area is considered to be one of the most at risk from climate change in Australia (Hughes 2003; Laurance et al. 2011; Hoffmann et al. 2019), and contains many endemic animal and plant species (Costin et al. 2000; Green and Osborne 2012). Australian alpine environments occur as isolated fragments with restricted elevation gradients as treelines are close to summits (Costin 1967; Costin et al. 2000). Thus, there is limited potential for upslope migration, particularly for cryophilic and endemic species as conditions warm, increased variation in precipitation and declining snow cover (Hennessy et al. 2003; Sánchez-Bayo and Green 2013; Holgate et al. 2020).
Of particular issue for Australian alpine regions, is increasing fire severity and frequency with climate change (Hennessy et al. 2005; Zylstra 2018), as these regions have historically experienced few fires (Dodson et al. 1994; Williams et al. 2008; Kirkpatrick and Bridle 2013). While some alpine plant communities have recovered from fire in the past, specialist communities and cryophilic species had limited capacity to recover post fire (Kirkpatrick et al. 2010; Venn et al. 2016). In the past decades, fires in the Australian Alps are becoming hotter, more frequent and covering larger areas with fires burning into alpine areas previously thought to have low flammability. In some cases, there has already been repeat burning of alpine vegetation within a few years (Williams et al. 2008; Camac et al. 2013, 2017; Verrall 2018; Zylstra 2018). It is possible that the densification and in-filling of vegetation driven by graminoids and shrubs across the Mount Clarke summits over 15 years, if more widespread, may also increase connectivity, landscape flammability and increased fuel loads as conditions become warmer and drier (Williams et al. 2006; Fraser et al. 2016).
Conclusion
This study found dynamics in alpine vegetation diversity, cover and composition over a 15-year period using a global, standardised monitoring protocol. Across the Mount Clarke summits, air and soil temperatures have increased steadily since the beginning of the millennium. In the same period, α-diversity decreased at higher elevations despite considerable species enrichment. There were no observable patterns in species enrichment along the elevation gradient summits, yet due to upslope migration, lower summit area sections displayed greater shifts in diversity in comparison to upper summit area sections. More broadly, there was evidence of ongoing biodiversity loss via biotic homogenisation with reductions in β-diversity. There were also marked increases in vegetation cover via in-filling and densification, driven primarily by graminoids and shrubs. Although new species contributed little to overall vegetation cover and composition, there were shifts in species composition. These compositional shifts were more pronounced at higher elevations but the majority of changes occurred between the first and second survey (2004–2011). While this study provides some support for climate-induced dynamics in alpine vegetation, further monitoring is required to disentangle responses to climatic variability verses climate change. However, with clear differences across elevation gradients in Australia’s highest and most diverse alpine environment, there is cause for concern. Consequently, as the climate continues to warm, it is likely that there will be further transitions in vegetation assemblages towards more thermophilic species currently characteristic of lower elevations with significant implications for conservation and ecological process in the region including fires.
Data availability
Data is available at https://dataverse.harvard.edu/dataset.xhtml?persistentId=doi:10.7910/DVN/VWBZHN.
References
Ababneh L, Woolfenden W (2010) Monitoring for potential effects of climate change on the vegetation of two alpine meadows in the White Mountains of California, USA. Quat Int 215:3–14. https://doi.org/10.1016/j.quaint.2009.05.013
Alexander JM, Diez JM, Levine JM (2015) Novel competitors shape species’ responses to climate change. Nature 525:515–518. https://doi.org/10.1038/nature14952
Alexander JM et al (2018) Lags in the response of mountain plant communities to climate change. Glob Change Biol 24:563–579. https://doi.org/10.1111/gcb.13976
Amagai Y, Kudo G, Sato K (2018) Changes in alpine plant communities under climate change: dynamics of snow-meadow vegetation in northern Japan over the last 40 years. Appl Veg Sci 21:561–571. https://doi.org/10.1111/avsc.12387
Annandale B, Kirkpatrick JB (2017) Diurnal to decadal changes in the balance between vegetation and bare ground in Tasmanian fjaeldmark. Arct Antarct Alp Res 49:473–486. https://doi.org/10.1657/aaar0017-001
Anthelme F, Cavieres LA, Dangles O (2014) Facilitation among plants in alpine environments in the face of climate change. Front Plant Sci. https://doi.org/10.3389/fpls.2014.00387
Artemov IA (2018) Changes in the altitudinal distribution of alpine plants in Katunskiy Biosphere Reserve (Central Altai) revealed on the basis of multiyear monitoring data. Contemp Probl Ecol. https://doi.org/10.1134/S1995425518010018
Ballantyne M, Pickering CM (2015) Shrub facilitation is an important driver of alpine plant community diversity and functional composition. Biodivers Conserv 24:1859–1875. https://doi.org/10.1007/s10531-015-0910-z
Baselga A (2010) Partitioning the turnover and nestedness components of beta diversity. Global Ecol Biogeogr 19:134–143. https://doi.org/10.1111/j.1466-8238.2009.00490.x
Baselga A, Orme D, Villeger S, De Bortoli J, Leprieur F, Logez M, Henriques-Silva R (2020) betapart: partitioning beta diversity into turnover and nestedness components. R package version 1.5.2, https://CRAN.R-project.org/package=betapart
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw. https://doi.org/10.18637/jss.v067.i01
Björk RG, Molau U (2007) Ecology of alpine snowbeds and the impact of global change. Arct Antarct Alp Res 39:34–43. https://doi.org/10.1657/1523-0430(2007)39[34:EOASAT]2.0.CO;2
Bradstock R, Penman T, Boer M, Price O, Clarke H (2014) Divergent responses of fire to recent warming and drying across south-eastern Australia. Glob Change Biol 20:1412–1428. https://doi.org/10.1111/gcb.12449
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of Southern Wisconsin. Ecol Monogr 27:326–349
Britton AJ, Beale CM, Towers W, Hewison RL (2009) Biodiversity gains and losses: evidence for homogenisation of Scottish alpine vegetation. Biol Conserv 142:1728–1739. https://doi.org/10.1016/j.biocon.2009.03.010
Cáceres Y, Llambí LD, Rada F (2015) Shrubs as foundation species in a high tropical alpine ecosystem: a multi-scale analysis of plant spatial interactions. Plant Ecol Divers 8:147–161. https://doi.org/10.1080/17550874.2014.960173
Callaway RM et al (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848. https://doi.org/10.1038/nature00812
Camac JS, Williams RJ, Wahren CH, Morris WK, Morgan JW (2013) Post-fire regeneration in alpine heathland: Does fire severity matter? Austral Ecol 38:199–207. https://doi.org/10.1111/j.1442-9993.2012.02392.x
Camac JS, Williams RJ, Wahren CH, Hoffmann AA, Vesk PA (2017) Climatic warming strengthens a positive feedback between alpine shrubs and fire. Glob Change Biol 23:3249–3258. https://doi.org/10.1111/gcb.13614
Cannone N, Pignatti S (2014) Ecological responses of plant species and communities to climate warming: upward shift or range filling processes? Clim Change 123:201–214. https://doi.org/10.1007/s10584-014-1065-8
Cannone N, Sgorbati S, Guglielmin M (2007) Unexpected impacts of climate change on alpine vegetation. Front Ecol Envir 5:360–364. https://doi.org/10.1890/1540-9295(2007)5[360:UIOCCO]2.0.CO;2
Carilla J, Halloy S, Cuello S, Grau A, Malizia A, Cuesta F (2018) Vegetation trends over eleven years on mountain summits in NW Argentina. Ecol Evol 8:11554–11567. https://doi.org/10.1002/ece3.4602
Chandra S, Singh A, Singh CP, Nautiyal MC, Rawat LS (2018) Vascular plants distribution in relation to topography and environmental variables in alpine zone of Kedarnath Wild Life Sanctuary, West Himalaya. J Mt Sci 15:1936–1949. https://doi.org/10.1007/s11629-017-4738-8
Choler P, Michalet R, Callaway RM (2001) Facilitation and competition on gradients in alpine plant communities. Ecology 82:3295–3308. https://doi.org/10.1890/0012-9658
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Costin A (1967) Alpine ecosystems of the Australasian region. In: Wright HE, Osburn WH (eds) Arctic and alpine environments. Indiana University Press, Bloomington, pp 55–87
Costin A, Gray M, Totterdell C, Wimbush D (2000) Kosciuszko alpine flora. CSIRO Publishing, Clayton South
CSIRO DoPI (1972) Kosciuszko National Park Alpine Vegetation 1966. CSIRO Publishing, Clayton South
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173. https://doi.org/10.1038/nature04514
Davis CJ (2013) Towards the development of long-term winter records for the Snowy Mountains. Aust Meteorol Oceanogr J 63:303–313. https://doi.org/10.22499/2.6302.003
De Boeck HJ, Bassin S, Verlinden M, Zeiter M, Hiltbrunner E (2016) Simulated heat waves affected alpine grassland only in combination with drought. New Phytol 209:531–541. https://doi.org/10.1111/nph.13601
de Witte LC, Stöcklin J (2010) Longevity of clonal plants: why it matters and how to measure it. Ann Bot 106:859–870. https://doi.org/10.1093/aob/mcq191
Di Battista T, Fortuna F, Maturo F (2017) BioFTF: an R package for biodiversity assessment with the functional data analysis approach. Ecol Ind 73:726–732. https://doi.org/10.1016/j.ecolind.2016.10.032
Dirnböck T, Essl F, Rabitsch W (2011) Disproportional risk for habitat loss of high-altitude endemic species under climate change. Global Change Biol 17:990–996. https://doi.org/10.1111/j.1365-2486.2010.02266.x
Dodson JR, SaliS TD, Myers CA, Sharp AJ (1994) A thousand years of environmental change and human impact in the alpine zone at Mt. Kosciusko, New South Wales. Aust Geogr 25:77–87. https://doi.org/10.1080/00049189408703100
Driscoll DA et al (2019) Impacts of feral horses in the Australian Alps and evidence-based solutions. Ecol Manag Restor 20:63–72. https://doi.org/10.1111/emr.12357
Dullinger S et al (2012) Extinction debt of high-mountain plants under twenty-first-century climate change. Nat Clim Change 2:619–622. https://doi.org/10.1038/nclimate1514
Elmendorf SC et al (2012) Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol Lett 15:164–175. https://doi.org/10.1111/j.1461-0248.2011.01716.x
Elsen PR, Tingley MW (2015) Global mountain topography and the fate of montane species under climate change. Nat Clim Change 5:772–776. https://doi.org/10.1038/nclimate2656
Ernakovich JG, Hopping KA, Berdanier AB, Simpson RT, Kachergis EJ, Steltzer H, Wallenstein MD (2014) Predicted responses of arctic and alpine ecosystems to altered seasonality under climate change. Glob Change Biol 20:3256–3269. https://doi.org/10.1111/gcb.12568
Erschbamer B, Kiebacher T, Mallaun M, Unterluggauer P (2009) Short-term signals of climate change along an altitudinal gradient in the South Alps. Plant Ecol 202:79–89. https://doi.org/10.1007/s11258-008-9556-1
Erschbamer B, Unterluggauer P, Winkler E, Mallaun M (2011) Changes in plant species diversity revealed by long-term monitoring on mountain summits in the Dolomites (northern Italy). Preslia 83:387–401
Evans RA, Love RM (1957) The step-point method of sampling: a practical tool in range research. Rangel Ecol Manag 10:208–212
Fernández Calzado MR, Molero Mesa J (2013) Changes in the summit flora of a Mediterranean mountain (Sierra Nevada, Spain) as a possible effect of climate change. Lazaroa 34:65–75. https://doi.org/10.5209/rev_LAZA.2013.v34.n1.41523
Fox J, Weisberg S (2019) An R companion to applied regression, 3rd edn. Sage Publications Ltd., Thousand Oaks
Fraser IP, Williams RJ, Murphy BP, Camac JS, Vesk PA (2016) Fuels and landscape flammability in an Australian alpine environment. Austral Ecol 41:657–670. https://doi.org/10.1111/aec.12355
Fu G, Shen ZX, Sun W, Zhong ZM, Zhang XZ, Zhou YT (2015) A meta-analysis of the effects of experimental warming on plant physiology and growth on the Tibetan Plateau. J Plant Growth Regul 34:57–65. https://doi.org/10.1007/s00344-014-9442-0
Futschik A et al (2020) Disentangling observer error and climate change effects in long-term monitoring of alpine plant species composition and cover. J Veg Sci 31:14–25. https://doi.org/10.1111/jvs.12822
Gallagher RV, Hughes L, Leishman MR (2009) Phenological trends among Australian alpine species: using herbarium records to identify climate-change indicators. Aust J Bot 57:1–9. https://doi.org/10.1071/BT08051
Gao Q et al (2016) Climate change and its impacts on vegetation distribution and net primary productivity of the alpine ecosystem in the Qinghai-Tibetan Plateau. Sci Total Environ 554–555:34–41. https://doi.org/10.1016/j.scitotenv.2016.02.131
Gigauri K, Akhalkatsi M, Nakhutsrishvili G, Abdaladze O (2013) Monitoring of vascular plant diversity in a changing climate in the alpine zone of the Central Greater Caucasus. Turk J Bot 37:1104–1114. https://doi.org/10.3906/bot-1301-38
Gigauri K, Akhalkatsi M, Abdaladze O, Nakhutsrishvili G (2016) Alpine plant distribution and thermic vegetation indicator on Gloria summits in the Central Greater Caucasus. Pak J Bot 48:1893–1902
Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD (2010) A framework for community interactions under climate change. Trends Ecol Evol 25:325–331. https://doi.org/10.1016/j.tree.2010.03.002
Gitzen RA, Millspaugh JJ, Cooper AB, Licht DS (2012) Design and analysis of long-term ecological monitoring studies. Cambridge University Press, Cambridge. https://doi.org/10.1017/CBO9781139022422
Good R, Johnston S (2019) Rehabilitation and revegetation of the Kosciuszko summit area, following the removal of grazing: an historic review. Ecol Manage Restor 20:13–20. https://doi.org/10.1111/emr.12363
Gottfried M, Pauli H, Grabherr G (1998) Prediction of vegetation patterns at the limits of plant life: a new view of the alpine-nival ecotone. Arct Alp Res 30:207–221
Gottfried M et al (2012) Continent-wide response of mountain vegetation to climate change. Nat Clim Change 2:111–115. https://doi.org/10.1038/nclimate1329
Grabherr G, Gottfried M, Pauli H (2010) Climate change impacts in alpine environments. Geogr Compass 4:1133–1153. https://doi.org/10.1111/j.1749-8198.2010.00356.x
Green K, Osborne WS (2012) Field guide to wildlife of the Australian snow-country, 2nd edn. New Holland Publishers, Chatswood
Greenland D (1999) Enso-related phenomena at long-term ecological research sites. Phys Geogr 20:491–507. https://doi.org/10.1080/02723646.1999.10642692
Guisan AA et al (2019) Climate change impacts on mountain biodiversity. In: Lovejoy TE, Hannah L (eds) Biodiversity and climate change: transforming the biosphere. Yale University Press, New Haven, pp 221–233
Hamid M, Khuroo AA, Malik AH, Ahmad R, Singh CP, Dolezal J, Haq SM (2020) Early evidence of shifts in alpine summit vegetation: a case study from Kashmir Himalaya. Front Plant Sci 11:16. https://doi.org/10.3389/fpls.2020.00421
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:19–20
Hennessy K, Whetton P, Smith I, Bathols J, Hutchinson M, Sharples J (2003) The impact of climate change on snow conditions in mainland Australia. CSIRO Atmospheric Research, Melbourne, Australia
Hennessy K, Lucas C, Nicholls N, Bathols J, Suppiah R, Ricketts J (2005) Climate change impacts on fire-weather in south-east Australia. CSIRO Marine and Atmospheric Research, Melbourne
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432. https://doi.org/10.2307/1934352
Hock R et al (2019) High Mountain Areas. In: Pörtner HO et al (eds) IPCC Special Report on the ocean and cryosphere in a changing climate. IPCC, Geneva, p 72
Hoffmann AA, Camac JS, Williams RJ, Papst W, Jarrad FC, Wahren C (2010) Phenological changes in six Australian subalpine plants in response to experimental warming and year-to-year variation. J Ecol 98:927–937. https://doi.org/10.1111/j.1365-2745.2010.01667.x
Hoffmann AA et al (2019) Impacts of recent climate change on terrestrial flora and fauna: some emerging Australian examples. Austral Ecol 44:3–27. https://doi.org/10.1111/aec.12674
Holgate CM, Van Dijk AIJM, Evans JP, Pitman AJ (2020) Local and remote drivers of southeast australian drought. Geophys Res Lett. https://doi.org/10.1029/2020GL090238
Hughes L (2003) Climate change and Australia: trends, projections and impacts. Austral Ecol. https://doi.org/10.1046/j.1442-9993.2003.01300.x
Inouye DW (2008) Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89:353–362. https://doi.org/10.1890/06-2128.1
Jarrad FC, Wahren C-H, Williams RJ, Burgman MA (2008) Impacts of experimental warming and fire on phenology of subalpine open-heath species. Aust J Bot 56:617–629. https://doi.org/10.1071/BT08018
Kirchoff CM (2020) Rapid assessment of future habitat suitability: a case-study of the snowy mountains endemic alpine flora using the Biodiversity and Climate Change Virtual Library (BCCVL). Austral Plant Conserv 28:5
Kirkpatrick JB, Bridle KL (2013) Natural and cultural histories of fire differ between Tasmanian and mainland Australian alpine vegetation. Aust J Bot 61:465–474. https://doi.org/10.1071/BT13128
Kirkpatrick JB, Bridle KL, Dickinson KJM (2010) Decades-scale vegetation change in burned and unburned alpine coniferous heath. Aust J Bot 58:453–462. https://doi.org/10.1071/BT10138
Körner C (2003) Alpine plant life: functional plant ecology of high mountain ecosystems; with 47 tables, 2nd edn. Springer, Berlin
Körner C (2004) Mountain biodiversity, its causes and function. Ambio 33:11–17
Körner C, Ohsawa M (2005) Mountain Systems. In: Hassan R, Scholes R, Ash N (eds) Ecosystems and human well-being: current state and trends, vol 1. Island Press, Washington, pp 683–716
Körner C, Paulsen J (2004) A world-wide study of high altitude treeline temperatures. J Biogeogr 31:713–732. https://doi.org/10.1111/j.1365-2699.2003.01043.x
Körner C, Paulsen J, Spehn EM (2011) A definition of mountains and their bioclimatic belts for global comparisons of biodiversity data. Alpine Bot 121:73–78. https://doi.org/10.1007/s00035-011-0094-4
Kuznetsova A, Brockhoff PB, Christensen RHB (2017) lmerTest Package: tests in linear mixed effects models. J Stat Softw 82:1–26. https://doi.org/10.18637/jss.v082.i13
Lamprecht A, Semenchuk PR, Steinbauer K, Winkler M, Pauli H (2018) Climate change leads to accelerated transformation of high-elevation vegetation in the central Alps. New Phytol 220:447–459. https://doi.org/10.1111/nph.15290
Laurance WF et al (2011) The 10 Australian ecosystems most vulnerable to tipping points. Biol Conserv 144:1472–1480. https://doi.org/10.1016/j.biocon.2011.01.016
Lenth R, Buerkner P, Herve M, Love J, Riebl H, Singmann H (2020) emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.5.2-1, https://CRAN.R-project.org/package=emmeans
Liberati L, Messerli S, Matteodo M, Vittoz P (2019) Contrasting impacts of climate change on the vegetation of windy ridges and snowbeds in the Swiss Alps. Alpine Bot 129:95–105. https://doi.org/10.1007/s00035-019-00223-5
Lütz C (2013) Plants in Alpine regions: cell physiology of adaption and survival strategies. Plants in alpine regions: cell physiology of Adaption and Survival Strategies. Springer, Wien. https://doi.org/10.1007/978-3-7091-0136-0
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press, Princeton
Malanson GP, Fagre DB (2013) Spatial contexts for temporal variability in alpine vegetation under ongoing climate change. Plant Ecol 214:1309–1319. https://doi.org/10.1007/s11258-013-0253-3
Mark AF et al (2015) Ecological responses to 52 years of experimental snow manipulation in high-alpine cushionfield, Old Man Range, South-Central New Zealand. Arct Antarct Alp Res 47:751–772. https://doi.org/10.1657/AAAR0014-098
McGowan H, Callow JN, Soderholm J, McGrath G, Campbell M, Zhao JX (2018) Global warming in the context of 2000 years of Australian alpine temperature and snow cover. Sci Rep 8:8. https://doi.org/10.1038/s41598-018-22766-z
Michelsen O, Syverhuset AO, Pedersen B, Holten JI (2011) The impact of climate change on recent vegetation changes on Dovrefjell, Norway. Diversity 3:91–111. https://doi.org/10.3390/d3010091
Müller F, Baessler C, Schubert H, Klotz S (2010) Long-term ecological research: between theory and application. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-8782-9
Myers-Smith IH et al (2011) Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ Res Lett. https://doi.org/10.1088/1748-9326/6/4/045509
Nagy L, Grabherr G (2009) The biology of alpine habitats. Oxford University Press, Oxford
Pauli H et al (2012) Recent plant diversity changes on Europe’s mountain summits. Science 336:353–355. https://doi.org/10.1126/science.1219033
Pauli H et al (eds) (2015) The GLORIA field manual:standard multi-summit approach, supplementary methods and extra approaches, 5th edn. Austrian Academy of Sciences and University of Natural Resources and Life Sciences, Vienna
Pepin N et al (2015) Elevation-dependent warming in mountain regions of the world. Nat Clim Change 5:424–430. https://doi.org/10.1038/nclimate2563
Pickering CM, Green K (2009) Vascular plant distribution in relation to topography, soils and micro-climate at five GLORIA sites in the snowy mountains, Australia. Aust J Bot 57:189–199. https://doi.org/10.1071/BT08133
Pickering CM, Hill W, Green K (2008) Vascular plant diversity and climate change in the alpine zone of the Snowy Mountains, Australia. Biodivers Conserv 17:1627–1644. https://doi.org/10.1007/s10531-008-9371-y
Pickering CM, Green K, Barros AA, Venn S (2014) A resurvey of late-lying snowpatches reveals changes in both species and functional composition across snowmelt zones. Alpine Bot 124:93–103. https://doi.org/10.1007/s00035-014-0140-0
Porro F et al (2019) Could plant diversity metrics explain climate-driven vegetation changes on mountain summits of the GLORIA network? Biodivers Conserv 28:3575–3596. https://doi.org/10.1007/s10531-019-01837-1
Quinn GP, Keough MJ (2003) Experimental design and data analysis for biologist. Cambridge University Press, Port Melbourne
Rényi A (1961) On measures of entropy and information. In: Neyman J (ed) Proceedings of the 4th Berkeley symposium on mathematical statistics and probability, vol I. University of California Press, Berkeley, California, pp 547–561
Rohde UL, Jain GC, Poddar AK, Ghosh AK (2012) Introduction to integral calculus. Wiley, Hoboken
Ross LC, Woodin SJ, Hester AJ, Thompson DBA, Birks HJB (2012) Biotic homogenization of upland vegetation: patterns and drivers at multiple spatial scales over five decades. J Veg Sci 23:755–770. https://doi.org/10.1111/j.1654-1103.2012.01390.x
Rumpf SB et al (2018) Range dynamics of mountain plants decrease with elevation. Proc Natl Acad Sci USA 115:1848–1853. https://doi.org/10.1073/pnas.1713936115
Rumpf SB, Hulber K, Zimmermann NE, Dullinger S (2019) Elevational rear edges shifted at least as much as leading edges over the last century. Global Ecol Biogeogr 28:533–543. https://doi.org/10.1111/geb.12865
Salick J, Fang ZD, Hart R (2019) Rapid changes in eastern Himalayan alpine flora with climate change. Am J Bot 106:520–530. https://doi.org/10.1002/ajb2.1263
Sánchez-Bayo F, Green K (2013) Australian snowpack disappearing under the influence of global warming and solar activity. Arct Antarct Alp Res 45:107–118. https://doi.org/10.1657/1938-4246-45.1.107
Scherrer P, Pickering CM (2005) Recovery of alpine vegetation from grazing and drought: data from long-term photoquadrats in Kosciuszko National Park, Australia. Arct Antarct Alp Res 37:574–584. https://doi.org/10.1657/1523-0430
Seddon AWR, Macias-Fauria M, Long PR, Benz D, Willis KJ (2016) Sensitivity of global terrestrial ecosystems to climate variability. Nature 531:229–232. https://doi.org/10.1038/nature16986
Shannon CE (1948) A mathematical theory of communication. Bell Labs Tech J 27:623–656. https://doi.org/10.1002/j.1538-7305.1948.tb00917.x
Sheldon AL (1969) Equitability indices: dependence on the species count. Ecology 50:466–467. https://doi.org/10.2307/1933900
Simpson EH (1949) Measurement of diversity. Nature 163:688. https://doi.org/10.1038/163688a0
Slattery D, Worboys GL (2020) Kosciuszko. A Great National Park. Envirobook Publishing, Sussex Inlet
Smithers BV, Oldfather MF, Koontz MJ, Bishop J, Bishop C, Nachlinger J, Sheth SN (2020) Community turnover by composition and climatic affinity across scales in an alpine system. Am J Bot 107:239–249. https://doi.org/10.1002/ajb2.1376
Stanisci A, Frate L, Di Cella UM, Pelino G, Petey M, Siniscalco C, Carranza ML (2016) Short-term signals of climate change in Italian summit vegetation: observations at two GLORIA sites. Plant Biosyst 150:227–235. https://doi.org/10.1080/11263504.2014.968232
Steinbauer MJ et al (2018) Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 556:231. https://doi.org/10.1038/s41586-018-0005-6
Stöckli V, Wipf S, Nilsson C, Rixen C (2011) Using historical plant surveys to track biodiversity on mountain summits. Plant Ecol Divers 4:415–425. https://doi.org/10.1080/17550874.2011.651504
Taguchi YH, Oono Y (2005) Relational patterns of gene expression via non-metric multidimensional scaling analysis. Bioinformatics 21:730–740. https://doi.org/10.1093/bioinformatics/bti067
Team RC (2020) R: a language and environment for statistical computing. Version 4.0.3. R Foundation for Statistical Computing, Vienna, Austria, https://www.R-project.org/
Tóthmérész B (1995) Comparison of different methods for diversity ordering. J Veg Sci 6:283–290. https://doi.org/10.2307/3236223
Tóthmérész B (1998) On the characterization of scale-dependent diversity. Abstr Bot 22:149–156
Van Dijk AIJM et al (2013) The millennium drought in southeast Australia (2001–2009): natural and human causes and implications for water resources, ecosystems, economy, and society. Water Resour Res 49:1040–1057. https://doi.org/10.1002/wrcr.20123
Venn SE, Green K (2018) Evergreen alpine shrubs have high freezing resistance in spring, irrespective of snowmelt timing and exposure to frost: an investigation from the Snowy Mountains, Australia. Plant Ecol 219:209–216. https://doi.org/10.1007/s11258-017-0789-8
Venn SE, Morgan JW (2009) Patterns in alpine seedling emergence and establishment across a stress gradient of mountain summits in south-eastern Australia. Plant Ecol Divers 2:5–16. https://doi.org/10.1080/17550870802691356
Venn S, Pickering CM, Green K (2012) Short-term variation in species richness across an altitudinal gradient of alpine summits. Biodivers Conserv 21:3157–3186. https://doi.org/10.1007/s10531-012-0359-2
Venn SE, Pickering CM, Green K (2014) Spatial and temporal functional changes in alpine summit vegetation are driven by increases in shrubs and graminoids. AoB Plants. https://doi.org/10.1093/aobpla/plu008
Venn SE, Pickering CM, Butler SA, Letten AD (2016) Using a model based fourth-corner analysis to explain vegetation change following an extraordinary fire disturbance. Oecologia 182:855–863. https://doi.org/10.1007/s00442-016-3700-8
Venn S, Kirkpatrick J, McDougall K, Walsh N, Whinam J, Williams RJ, Keith DA (2017) Alpine, sub-alpine and sub-antarctic vegetation of Australia. Australian Vegetation, 3rd edn. Cambridge University Press, Cambridge
Verrall B (2018) Recovery of snow dependent plant communities in Australia from fire. Honours thesis for Griffith University School of Environment and Science, Gold Coast.
Verrall B, Pickering CM (2020) Alpine vegetation in the context of climate change: a global review of past research and future directions. Sci Total Environ 748:141344. https://doi.org/10.1016/j.scitotenv.2020.141344
Vittoz P, Guisan A (2007) How reliable is the monitoring of permanent vegetation plots? A test with multiple observers. J Veg Sci 18:413–422. https://doi.org/10.1658/1100-9233(2007)18[413:HRITMO]2.0.CO;2
Vittoz P, Bayfield N, Brooker R, Elston DA, Duff EI, Theurillat JP, Guisan A (2010a) Reproducibility of species lists, visual cover estimates and frequency methods for recording high-mountain vegetation. J Veg Sci 21:1035–1047. https://doi.org/10.1111/j.1654-1103.2010.01216.x
Vittoz P, Camenisch M, Mayor R, Miserere L, Vust M, Theurillat JP (2010b) Subalpine-nival gradient of species richness for vascular plants, bryophytes and lichens in the Swiss Inner Alps. Bot Helv 120:139–149. https://doi.org/10.1007/s00035-010-0079-8
Wahren CH, Camac JS, Jarrad FC, Williams RJ, Papst WA, Hoffmann AA (2013) Experimental warming and long-term vegetation dynamics in an alpine heathland. Aust J Bot 61:36–51. https://doi.org/10.1071/BT12234
Walther GR et al (2002) Ecological responses to recent climate change. Nature 416:389–395. https://doi.org/10.1038/416389a
Walther GR, Beißner S, Burga CA (2005) Trends in the upward shift of alpine plants. J Veg Sci 16:541–548. https://doi.org/10.1658/1100-9233
Wang Q, Fan X, Wang M (2016) Evidence of high-elevation amplification versus Arctic amplification. Sci Rep. https://doi.org/10.1038/srep19219
Ward-Jones J, Pulsford I, Thackway R, Bishwokarma D, Freudenberger D (2019) Impacts of feral horses and deer on an endangered woodland of Kosciuszko National Park. Ecol Manag Restor 20:37–46. https://doi.org/10.1111/emr.12353
Williams R, Ashton D (1988) Cyclical Patterns of regeneration in subalpine heathland communities on the Bogong high-plains, Victoria. Aust J Bot 36:605–619. https://doi.org/10.1071/BT9880605
Williams RJ, Wahren CH, Bradstock RA, Müller WJ (2006) Does alpine grazing reduce blazing? A landscape test of a widely-held hypothesis. Austral Ecol 31:925–936. https://doi.org/10.1111/j.1442-9993.2006.01655.x
Williams RJ et al (2008) Large fires in Australian alpine landscapes: their part in the historical fire regime and their impacts on alpine biodiversity. Int J Wildl Fire 17:793–808. https://doi.org/10.1071/WF07154
Williams RJ, Papst WA, McDougall KL (2014) Alpine ecosystems. In: Lindenmayer DB, Burns E, Thurgate N, Lowe A (eds) Biodiversity and environmental change: monitoring, challenges and directions. CSIRO Publishing, Melbourne, pp 167–212
Wilson SD, Nilsson C (2009) Arctic alpine vegetation change over 20 years. Glob Change Biol 15:1676–1684. https://doi.org/10.1111/j.1365-2486.2009.01896.x
Wright G, McDougall KL, Duncan A (2010) Guide to the native ferns, conifers and flowering plants in Kosciuszko National Park. New South Wales National Parks and Wildlife Service, Sydney
Zylstra PJ (2018) Flammability dynamics in the Australian Alps. Austral Ecol 43:578–591. https://doi.org/10.1111/aec.12594
Acknowledgements
We acknowledge the ngarigo and kombumerri as First Nations and Traditional Custodians of the land on which the fieldwork and desktop research for this study was conducted. We pay our respect to their Elders past and present, recognise that sovereignty was never ceded and acknowledge their continued connection to country and culture. Thank you to P. Norman, R. Stewart, G. Wright, M. Schroder, G. Cobb and J. Dodd for assisting with fieldwork, and we extend our thanks to those whom were involved in previous assessments. We would also like to thank C. Morrison for proofing the manuscript. This study was supported by funding from New South Wales National Parks and Wildlife as well as funding from Griffith University by a Research Degree scholarship for one of the authors (BV).
Funding
This manuscript is to be used in Biodiversity and Conservation and was funded by Griffith University.
Author information
Authors and Affiliations
Contributions
BV contributed to data collection in 2019, data analysis and writing. KG contributed to project design and data collection in 2004, 2011 and 2019. CMP contributed to project design, data collection in 2004, 2011 and 2019 and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Communicated by Daniel Sanchez Mata.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Verrall, B., Green, K. & Pickering, C.M. Dynamics in plant diversity and composition on Australian alpine summits over time. Biodivers Conserv 30, 1855–1880 (2021). https://doi.org/10.1007/s10531-021-02171-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-021-02171-1