Abstract
In response to climate warming, high altitude alpine vegetation may be replaced by typically lower altitude species, as species re-assemble and migrate to new areas. However, empirical evidence showing vegetation change in response to climate warming is largely unavailable for Australian alpine areas. Here, we examine changes in species richness with respect to climate and altitude over a 7 year period at a range of spatial scales in a re-survey of five alpine summits that are part of the Global Observation Research Initiative in Alpine Environments monitoring network. Eighty species were recorded in 2011 across all summits, an increase of 6 species since 2004. Mean species richness increased at the whole-of-summit scale from 45 to 50 species (about 12 %). At this scale, the rate of species richness increase was almost one new species per year, with 15 new species recorded at one summit. Here, shrub and graminoid species showed the largest increases. At the smaller spatial scales, changes in species richness were less pronounced. Turnover at the species and community level was typically moderate at all spatial scales and on all summits. The strength and direction of species richness change (the difference in species richness between the two sample periods, +/−) was not related to altitude nor variation in climate. Future re-surveys of the summits will confirm whether these short-term variations in species richness, particularly increases in shrubs, are indeed signals of longer-term trends and interactions with a changing climate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The distribution of alpine plants is strongly controlled by low temperatures and steep gradients in the local climate (Körner and Larcher 1988; Körner 2003). In particular, mountain top vegetation is expected to be highly sensitive to changes in climate (Lesica and Steele 1996), where species may be at their upper altitudinal range limits and topographically featureless summits provide little relief from ambient conditions (Grabherr et al. 1994; Theurillat and Guisan 2001). The general expectation worldwide is for the migration and subsequent replacement of species from the lower altitude alpine areas into the higher alpine and nival areas as species respond to climatic changes, rising temperatures and longer growing seasons (Grabherr et al. 1995; Guisan and Theurillat 2000; Theurillat and Guisan 2001). Indeed, re-surveys of mountain summits from the European Alps, using data from as far back as 1835, have revealed upward shifts of alpine plants and an associated increase in species richness primarily related to recent (late twentieth century) climate warming (Grabherr et al. 1994; Böhm et al. 2001; Holzinger et al. 2008). There are many other examples of shifting species’ ranges in the alpine zone across the European mountains (Kullman 2002; Bahn and Körner 2003; Klanderud and Birks 2003; Walther et al. 2005; Holzinger et al. 2008; Lenoir et al. 2008) and in the upper forest belt (Moiseev and Shiyatov 2003). Recent data from high altitudes showed a consistent temporal increase in species richness in the European Alps (Pauli et al. 2003a; Erschbamer et al. 2011; Pauli et al. 2012). However, continual and rapid climate warming over long periods is eventually expected to reduce species richness and diversity through generalist species out-competing specialised, cold-adapted species in (previously) marginal habitats (Grabherr et al. 1995; Theurillat and Guisan 2001; Lesica and McCune 2004; Venn et al. 2011). These processes therefore threaten rare and endemic high altitude species with narrow distribution ranges via range expansion by lower altitude alpine species (Grabherr et al. 1995; Pauli et al. 2003b; Pauli et al. 2007; Engler et al. 2011; Dullinger et al. 2012).
Australian alpine and high mountain areas are considered to be highly vulnerable to climate change (Hughes 2003; Laurance et al. 2011). However, empirical evidence of vegetation shifts in response to recent climate change is largely unavailable, with many studies instead reporting the indirect links of climate change and climate variability with vegetation change; such as investigating the inter-annual variability of snow cover on vegetation patterns (Edmonds et al. 2006) and encroaching sub-alpine treelines into grassy plains (Wearne and Morgan 2001), using space-for-time studies over altitudinal gradients to infer future vegetation change (Venn and Morgan 2005) and using artificial warming experiments in sub-alpine areas to predict climate change effects (Hoffmann et al. 2010). Here, we present the recent results from a comprehensive, on-going, empirical study that has the specific aim of detecting alpine vegetation change on summits in relation to climate change. The study is in its infancy, having been established in 2004, but it is part of a global attempt to detect long-term alpine vegetation change, particularly changes in species richness with respect to local temperatures and altitude, on high mountain summits. An important theoretical basis of this study and the Global Observation Research Initiative in Alpine Environments (GLORIA) monitoring program more generally, is the assumption that patterns in species richness are related to climate. Evidence for this includes patterns of decreasing species richness with increasing altitude at the scale of whole floras and at smaller spatial scales (Körner 1992), as well as the association between vegetation composition and climatic variables (Pickering and Green 2009). Because minimum and maximum temperatures in high mountain areas in Australia have risen (Nicholls 2005) and snow cover has declined over past decades (Green and Pickering 2009), long term monitoring using the GLORIA protocol is therefore timely and essential for detecting and predicting Australian alpine species’ responses to local climate change. The climate predictions for the Australian alpine areas include increases in temperature, particularly minima, and further reductions in snowpack; potentially down from the present mean of 183 days with least 1 cm of snow cover at the highest summit in the region, Mt Kosciuszko 2,228 m, to 87 days by 2050 (Hennessey et al. 2003).
Here, we focus our attention on the level of species, species richness and turnover measures to detect change across five summits in the Snowy Mountains first surveyed using the GLORIA protocol in 2004 and re-surveyed in 2011. At this level, changes in species, particularly those endemic to the region, could highlight more general vegetation changes occurring within the alpine area. Specifically we ask, has the overall species richness across the summits changed since 2004? Are changes in species richness related to the scale of sampling? Are changes in species richness related to altitude? Do different life forms or endemic species show strong changes over time? Is there any evidence of migration of species from lower altitudes into these summits?
Methods
Study sites
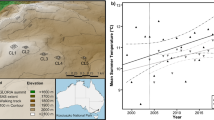
In January 2011 we re-surveyed the five ‘summits’ that were originally surveyed in January 2004 along a continuous ridge from close to the valley floor to the summit of Mt Clarke (Pickering et al. 2008; Pickering and Green 2009) (Fig. 1). The summits cover an altitudinal range of 301 m from the lowest at 1,813 m (Clarke 5) through to the highest at 2,114 m (Clarke 1) (Table 1) and cover a horizontal distance of 1,600 m. The sites were selected for long-term monitoring under the GLORIA sampling protocols (Pauli et al. 2004) (Fig. 2), as they experience similar effects of exposure and differences in climate are most likely due to the altitudinal gradient. They are all relatively flat, rather than cone-shaped peaks, and the vegetation is characteristic of nearby summits in the vicinity. The soils are around 350 ± 110 mm in depth (K. Green, unpublished data), well-formed alpine humus soils (Costin 1954). The highest summits are dominated by tall alpine herbfield, whereas the lower summits are dominated by shrubs. As a result of the continuous, mostly perennial vegetation cover, biomass is high compared to some other alpine regions (Costin 1954). There are some rock outcrops, but these are not a defining feature of the summits. Disturbance is minimal as cattle grazing ceased >60 years ago, the historical stock travelling route avoided these summits and there are few native and no exotic burrowing mammals at these altitudes. No walking tracks cross the summits resulting in low visitation rates.
The layout of sampling methodology of the GLORIA summits showing a the positioning of the upper and lower SASs and clusters of 1 m2 quadrats, and b as viewed from above on a hypothetical summit. Source Pauli et al. (2004)
Vegetation sampling
The top section of each summit was divided into eight summit area sections (SAS), four covering the area down to 5 m below the summit, the 5 m isoline, for each of the four cardinal compass bearings (hereafter referred to as the upper or 5 m SAS), and another four covering the four compass bearings down to the 10 m isoline (hereafter referred to as the lower or 10 m SAS) (Pauli et al. 2004) (Fig. 2). Where the summit was exceptionally flat, the upper area extended 50 m from the summit and lower extended 100 m. At each of the four cardinal bearings at the 5 m isoline, a cluster of four 1 m2 quadrats was established (Fig. 2). Due to the density of tall (>1 m) shrubs on Clarke 5, permanent 1 m2 quadrats were not established. For the other four summits, species composition was recorded in each of the four corner 1 m2 quadrats. In each of the eight SAS a complete species list was compiled of all vascular plant species (Pauli et al. 2004). Species richness data were therefore compiled at four spatial scales: the 1 m2 quadrats, on each aspect of the SAS at the 5 and 10 m contour line and through combining the data from the 5 and 10 m SAS to produce a ‘whole of summit’ species list (5 + 10 m SAS) (Table 1).
Sampling in 2011 was conducted ‘blind’ without referring to the 2004 data. Sampling in each of the SAS and quadrats was performed by the same people to ensure consistency in the data (Vittoz et al. 2010). Post sampling, a rigorous species identification checking procedure was used to ensure changes in species between 2004 and 2011 were not pseudo-changes, arising from difficulties in species identification. All species names follow Costin et al. (2000) to be consistent with the initial 2004 survey.
Climatic variation
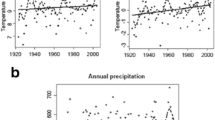
Temperature loggers (Tinytag Plus—Gemini Data Loggers, Chichester England) were buried 10 cm below the ground surface from January 2004 through to January 2011, in the centre of the cluster of four quadrats on each aspect of each summit. Temperatures were recorded every 2 h. Temperature data from across this time period were used to calculate annual values of absolute minimum soil temperature, annual daily mean soil temperature, absolute maximum soil temperature, temperature sums (>5 °C), growing degree days and the length of the growing season across the years sampled. Several climate parameters were derived from these data and used in the subsequent analyses. Precipitation data were collected between 2003 and 2011 from an automated weather station about 8 km to the south at Thredbo (1,957 m) (Bureau of Meteorology), and from Pengilley Bog 13 km to the north-east (1,730 m), during the growing season only.
Data analysis
Variations with climate
Climate parameters derived from the temperature data included mean, minimum and maximum temperatures for each year, mean season days and mean thaw date for each site. We used ANCOVA to determine separately whether these climate parameters explained change in species richness (the difference between the 2004 and the 2011 samples) for both the 5 and 10 m SAS (dependent variables), using altitude as a covariate (see Appendix Table 5). Data from each aspect from each SAS were used in these analyses. To determine the strength of relationships between species richness and climatic parameters, simple linear regression was used with species richness values from the 5 and 10 m SAS from each aspect of each site. In addition, linear regression was used to investigate any relationships between the change (+/−) in species richness, using data from each aspect of each summit as separate data points, with the climatic parameters in both the 5 and the 10 m SAS (see Appendix Table 5).
Changes in species richness
Significant differences in total species richness between 2004 and 2011 were determined within summits and in the 5 and 10 m SAS and the 1 m2 quadrats using t tests and data from each aspect (see Appendix Table 5). The change in species richness, interpreted as the difference (+/−) between the 2004 and 2011 sample times, was analysed with simple linear regression against altitude for all species and for four groups of species: Kosciuszko alpine area endemic species (KNP endemics, Costin et al. 2000), forbs, graminoids and shrubs, in both the 5 and the 5 + 10 m SAS.
Species and vegetation turnover
Frequency data (presence/absence) from 2004 and 2011 were used to calculate turnover of individual species and the vegetation overall. We calculated the individual species turnover (Tsp) from the quadrat data (presence/absence in 1 m2 quadrats) at Clarke 1–4 according to the equation:
where A is the frequency of quadrats where the species appeared in 2011; D is the frequency of quadrats where the species disappeared; U is the frequency of quadrats where the species’ frequency was unchanged (Milberg and Hansson 1993). In addition, a vegetation turnover index (Tveg) was calculated for overall changes in species richness at different spatial scales (SAS), life forms and groups according to the equation:
where A is the number of new species in 2011; D is the number of disappearing species; B is the number of species present in both years of comparison. For both indices, low turnover values are close to 0.01 and complete turnover is 1.0.
The ANCOVA procedure was performed with SPSS version 18. All other statistical analyses were performed with SYSTAT version 10.
Results
General patterns
There was a change in species richness across all spatial scales, with an overall pattern of increasing richness. Across all summits at the whole-of-summit scale (5 + 10 m SAS), 80 species were recorded compared to 74 in 2004 (Table 2). Overall, mean species richness increased at the scale of whole summits (5 + 10 m SAS) from 45 to 50 species (about 12 %) between 2004 and 2011. At the level of individual summits, changes in richness ranged from −1 at Clarke 3, to +15 at Clarke 4. The largest changes in species richness occurred at Clarke 4 (1,948 m) where 17 new species were recorded in the 5 + 10 m SAS (a total increase of +15 species). Mean species richness within the SAS (using the data from each aspect as four replicates) revealed changes between −1.3 and +11.5 mean species (Tables 1, 3), with the largest increases in mean species richness occurring at Clarke 4. The range in mean species richness in 2011 at the quadrat level across sites was less pronounced (between 6.6 and 9.1 species), and the change in mean species richness between 2004 and 2011 resulted in either a gain or loss of ~1 species (Table 1). Among the life forms considered (forbs, graminoids and shrubs), there was variation in species richness between the two time periods with a tendency for increases in shrub and graminoid richness and a decrease in herb richness.
Variation with climate
There were no consistent trends (increases or decreases) in climatic variables between 2004 and 2011 (Fig. 3), although relationships between site altitude and climate between 2004 and 2011 remained consistent; minimum temperatures (R 2 = 0.28, P = 0.016), mean temperatures (R 2 = 0.44, P = 0.005) and number of growing season days (snow free days) (R 2 = 0.23, P = 0.032) all significantly decreased with increasing altitude but not maximum temperatures (using the data from four loggers at each site as replicates).
The climate parameters appeared to have little influence on the changes in species richness between 2004 and 2011, in any of the SAS tested, as the ANCOVA analyses revealed no significant effects (see Appendix Table 6). Linear regressions between climate parameters and the mean species richness from the 5 and 10 m SAS across the altitudinal gradient of sites revealed few significant, strengthened, weakened or predictive relationships (see Appendix Table 6). Only relationships between species richness and the mean soil temperatures from each aspect at each site were significant with any reasonable predictive power (5 m SAS: 2004 R 2 = 0.26, P = 0.02, 2011 R 2 = 0.25, P = 0.03. 10 m SAS: 2004 R 2 = 0.06, P = 0.26, 2011 R 2 = 0.41, P = 0.002). Linear regressions between the change (+/−) in species richness at each altitude and the climatic parameters revealed no significant relationships with any predictive power for either the 5 or the 10 m SAS (see Appendix Table 6).
Precipitation data from the nearby rain gauges revealed substantial increases in annual and growing season precipitation over the 2010/2011 growing season (start of October to the end of April) in comparison with previous years in which the region experienced low rainfall conditions for almost a decade. At Thredbo, there was a 30 % increase in annual precipitation in 2011 compared with 2004 (mean precipitation between 2004 and 2009 (2010 data missing) was 1,136 mm, whereas in 2011 it was 1,647 mm). Growing season precipitation (October to the end of April) at Pengilley Bog was about 50 % higher in 2011 compared with previous years (mean growing season precipitation between 2003/2004 and 2009/2010 was 575 mm, whereas in 2010/2011 it was 1,182 mm).
Changes in species richness
The t tests comparing the total species richness between 2004 and 2011 revealed no significant differences in the 5 m SAS at any sites. In the 10 m SAS, however, there were significant differences between 2004 and 2011 at the two lowest summits, Clarke 4 and Clarke 5 (P = 0.03 and P = 0.04 respectively) (see Appendix Table 6). The overall total species richness across all sites in the 10 m SAS was higher in 2011 than in 2004 (n = 20, mean ± 1 SE: 2004 = 25.8 ± 1.1, 2011 = 31 ± 1.2, P < 0.001). There were no overall significant differences in the 5 m SAS between years (see Appendix Table 6).
The change in species richness between the two sample times for particular groups of species (life forms etc.) in both the 5 and the 5 + 10 m SAS showed no significant trends with altitude. The species richness of KNP endemics however, showed weak non-significant trends with altitude for both the 5 and the 5 + 10 m SAS (R 2 = 0.68, P = 0.061, R 2 = 0.74, P = 0.08 respectively), with the higher altitude sites losing one or two species and the lower sites gaining one or two species between sample times. Overall, most sites experienced an increase in species richness between the 2004 and the 2011 sample times, with several new records of graminoid and shrub species contributing to the increases in both the 5 and the 5 + 10 m SAS. The most dramatic decreases in species richness across the study were among forb species at Clarke 3 and 5 (−4 and −6 species respectively) within the top 5 m SAS (Table 3).
Species and vegetation turnover
At the quadrat level, mean individual species turnover (Tsp) was highest at Clarke 1 and 2, and lower at Clark 3 and 4 (0.67, 0.72, 0.59, 0.25 Clarke 1–4 respectively). Mean Tsp over these four sites was relatively high, 0.65. Most forb species had relatively low turnover (Tsp between 0 and 0.5), but there were a few exceptions where new occurrences or absences in some quadrats produced complete turnover (Tsp = 1.0) at one or more sites (Table 4). For example, Euphrasia alsa, Scleranthus biflorus and S. singuliflorus had either very high or very low Tsp, where less than three occurrences in either 2004 or 2011 produced very high or complete turnover. Geranium potentilloides, a common forb species from lower altitudes in the region, was a new occurrence in the quadrats, appearing in 2011 once at Clarke 4 (Table 4). The prominent snowgrass, Poa sp., was present in every quadrat at each site and this did not change between 2004 and 2011 (Table 4). The inconspicuous hook sedge, Uncinia sp. was not present in the quadrats in 2011, but this species was recorded within the SAS at the two highest sites. The larger shrub species recorded at the lower altitude sites (Prostanthera cuneata and Phebalium ovatifolium) showed no turnover whatsoever. Epacris microphylla, a prominent shrub present at all sites, also showed no change or any turnover at the three highest sites (Clarke 1–3), and only a minor change at Clarke 4. Kunzea muelleri, a common larger alpine shrub, also showed no change between 2004 and 2011 (Table 4). Other shrub species had low turnover values, mostly as a result of fewer records in quadrats in 2011.
Vegetation turnover (Tveg) across the five sites within the 5 m SAS were between 0.31 and 0.40 (Table 3) with no clear trends with site altitude. Within the 10 m SAS (not including the 5 m SAS) Tveg values were between 0.32 and 0.45 across the five sites (Table 3). Overall vegetation turnover in the quadrats was similar to that of the SAS, Tveg between 0.25 and 0.47. Kosciuszko endemic species had higher turnover, especially within the 5 + 10 m SAS (between 0.20 and 0.60). Forb turnover was fairly similar across summits and SAS of different areas (between 0.27 and 0.5) as was graminoid turnover, although the Tveg values were overall slightly higher (between 0.38 and 0.62). Shrub species at the 5 m and the 5 + 10 m SAS showed considerably lower Tveg values (between 0.08 and 0.40). Exploratory analyses of the data revealed no clear relationships with the Tveg values of any life form and site altitude.
Discussion
Short-term changes in species richness and variation through sampling scale
The short-term increases in species richness across the summits on the Mt Clarke ridge between 2004 and 2011 are consistent with the trends from similar monitoring studies in the European Alps (Grabherr et al. 1994; Walther et al. 2005; Pauli et al. 2007; Holzinger et al. 2008; Erschbamer et al. 2011). The magnitude of the species richness increase on a single summit (up to 17 new species not previously recorded in 2004) is within the range of increases reported from Europe (Holzinger et al. 2008), however, future surveys of the summits at Mt Clarke will reveal whether these are merely short-term fluctuations or part of longer-term trends (Erschbamer et al. 2011). The largest changes (mostly increases) in species richness were at the lower mountain sections (10 m SAS), and moderate changes occurred at the whole of summit scale (5 + 10 m SAS), where the observed changes across summits equate to an increase of almost one extra species per year, taking into account species absences. This is in line with recent findings from Europe where, at the continent scale, Gottfried et al. (2012) report an increase in species numbers and abundance of species that predominantly grow at lower elevation mountain summits, in response to climate warming. In contrast, the observed change in species richness at the very top of the Australian summits (the 5 m SAS) was approximately 0.2 species per year. At the whole summit scale, including data across all summits, total mean species richness in 2011 had increased by about 12 % since 2004; whereas at the very top of each summit, changes were more subtle and total mean species richness across all summits had only increased by 4.4 %. Climate variables measured on site did not change significantly over this time and so these observed changes in species richness are more likely a result of species responses to longer-term increases in temperatures, declines in snowpack (Green and Pickering 2009), or possibly positive biotic interactions (Callaway et al. 2002; Venn et al. 2009) facilitating the co-existence of many species on the summits, whereby lower temperatures on summits (compared to lower mountain environments) are negated by individuals sheltering next to one another. Continued warming trends may, however, alter or reverse such plant–plant interactions leading to an increase of negative (competitive) interactions (Venn et al. 2009) and stagnating species richness. The recent, large increases in regional precipitation may have resulted in a pulse of recruitment (Venn and Morgan 2009), but are unlikely to result in large changes in species migration and subsequent species richness at the summits within the same year/growing season. At the 1 m2 quadrat scale, changes in total species richness between 2004 and 2011 were subtle, and with no appreciable mean net increase or decrease in species numbers across summits Clarke 1–4. Over future sampling intervals of 5–7 years, appreciable changes to species richness are likely to be at the larger spatial scales at these summits, not just because of the larger area and therefore a greater chance of encountering more species, but because many high elevation species in Australia are large themselves (broad leaved herbs with leaves up 20 cm, and shrubs with diameters up to 1.5 m). Whereas at smaller scales, the 1 m2 quadrat level, total species richness may be limited by the physical size of individual plants, and smaller changes over time are expected through species losses and gains countering each other.
Turnover dynamics between 2004 and 2011
The high proportion of perennial species among the Australian alpine flora (Costin et al. 2000) and the relatively tall and closed vegetation structure, which may act to reduce the availability of micro-habitats for colonisers, are the most likely explanations for the lack of pattern and the overall moderate values of vegetation turnover within the larger SAS areas. Slow growing perennials may also integrate climatic changes that occur over longer periods better than short-lived species. Although there has been much variation in the annual snow amount (metre-days), the general trend is an overall decline with a 30 % reduction over the past five decades (Green and Pickering, 2009; Green, 2010) with the date of snow melt also having advanced by 2.75 days per decade (Green 2010). The downward trend in depth of snow is associated with increasing temperature rather than reduced precipitation (Nicholls 2005). Therefore longer-term increases in temperature and snowpack declines (Green and Pickering 2009) may be key determinants of turnover dynamics in these systems. Recent turnover in European mountain summits has also been moderate in magnitude with, on average, increasing species richness in temperate and boreal regions, but strikingly in stagnation or decreases on the Mediterranean mountains; the latter consisting of very open vegetation where water availability in summer is an important factor (Pauli et al. 2012). Climatic water balance also appears to be the key driver of species’ (often downhill) niche-tracking on Californian summits, despite twentieth century warming (Crimmins et al. 2011).
In the Snowy Mountains, the tenacity of the grass tussocks, larger forbs and shrubs may prevent many dispersing species from entering the community through the effects of competition (Venn et al. 2009; Venn et al. 2011). Additionally, attached leaf litter surrounding Poa tussocks and many Asteraceae forbs and ground litter from shrubs effectively insulate soils and meristematic regions close to the ground, to the benefit of the individual, whilst preventing new seedlings from establishing (Dullinger et al. 2003; Körner 2003; Buckeridge et al. 2010; Myers-Smith et al. 2011). However, at the higher summits, facilitative (positive) effects of the standing vegetation are more likely to play a role in sheltering and protecting new species from the harsher climatic conditions at high altitudes (Callaway 1995; Callaway et al. 2002), and potentially creating establishment opportunities for typically lower altitude species.
Naturally long-lived species will show little turnover over short time periods, as demonstrated by many of the alpine shrubs in this study. Shrub population dynamics may also offer additional insights into future vegetation change, given the differential growth rates between species and recognised expansion with respect to land-use legacies and climate warming in this region (McDougall 2003; Scherrer 2003; Scherrer and Pickering 2005). Surprisingly however, the total number of shrub species increased over the study period at the whole summit scale, with marked increases occurring at Clarke 4 (Fig. 2). Additionally, three individuals of Podocarpus lawrencei (Podocarpaceae), a distinctive, slow growing and extremely long-lived shrub species (up to 400 year) (McDougall et al. 2012), were recorded for the first time in 2011 within the 5 + 10 m SAS at Clarke 3 and 5, thereby challenging the idea that shrub species are mostly static in the landscape and regenerate clonally, whereas some species have the potential to migrate to suitable habitats faster than previously thought. At the quadrat scale, turnover in most shrub species was small, although a new occurrence of Melicytus sp. was recorded at Clarke 4. Over time, new shrub occurrences combined with the obvious decaying branches of senescing shrubs, will act as indicators of directional and/or cyclical vegetation change. Shrubs may therefore demonstrate the longer-term effects of climatic change, whereas many graminoid and forb species may be too short-lived or decay too quickly to leave evidence of their establishment/decline within a seven-year sampling interval.
The largest declines in species richness were among forb species within the top 5 m SAS at some of the lower sites, Clarke 3 and Clarke 5 (declines of 18 and 25 % respectively). None of the species which contributed to these particular declines were locally endemic and they were all present in either the lower SAS on those summits or at other sites. Until species are repeatedly absent from future re-surveys, perhaps as a result of climatic changes affecting the summits differentially according to altitude, local extinctions of the summit flora are unlikely in the short-term. Longer-term predictions however, should include measures of population dynamics and identification of species ranges that are still occupied through species resilience, but which in future may become climatically unsuitable (Dullinger et al. 2012) and disappear once certain thresholds are exceeded, creating an extinction debt.
Individual species losses from quadrats did not contribute to the species absence from any of the summits. Species turnover and apparent losses at small spatial scales may also indicate identification issues surrounding certain genera. Whilst the data indicate substantial declines and losses of several species, substantial increases of closely related species occurred concurrently (Table 2). This was particularly evident in genera such as Carex, Luzula and Prasophyllum where accurately identifying individuals to species was at times impossible in the field because flowering material was not available. Additionally, on several occasions in 2011 the closely related and difficult to distinguish species did occur together in the same plot. The values presented here are therefore conservative in terms of changes in species numbers; if several individuals of the difficult to distinguish species were together in the same plot and could not be identified further, they were classed as one taxonomic unit. Every effort was made accurately to identify, record and match species data between the sampling years. Subsequent re-surveys will determine whether complete species turnover in 2011 were genuine losses or gains, or indeed misidentifications.
Factors governing species richness change at Mt Clarke
The inter-annual variability in climate in the Snowy Mountains over the period of this study does not reflect the longer-term trends in climate warming for the region; increasing temperatures (Nicholls 2005) and around a 30 % reduction in snow (metre days) over the past five decades in combination with snowmelt having advanced by 2.75 days per decade (Green and Pickering 2009; Green 2010).The predicted effects of long term climate change for the region, however, include a lengthening of the growing season, increased end of season variability in snow cover and an increase in mean minimum temperatures (Hennessey et al. 2003), all of which could potentially make higher altitude habitats more suitable for typically lower altitude species (Venn and Morgan 2005). The overall general increase in species richness across the summits recorded during this re-survey and the marked increase in shrub and graminoid species, indicate that the vegetation may be responding to one or more environmental factors present at these sites. However, patterns in species richness and co-existence are determined by a host of interacting factors including rainfall, microsite availability (Zobel et al. 2000; Venn and Morgan 2009), species interactions (positive and negative) (Choler et al. 2001; Cavieres et al. 2006; Venn et al. 2009) and the dispersal and recruitment potential of species in the regional species pool (Dirnböck and Dullinger 2004), a thorough discussion of which is beyond the scope of this study. Bear in mind that species and life forms will respond to environmental stimuli and predicted changes across the altitudinal gradient of sites differently. For example, a large component of the herbaceous vegetation across the study sites at Mt Clarke are Asteraceae forbs, which in general have reproductive and dispersal traits synonymous with successful colonisation (Venn 2007; Venn and Morgan 2010). However, only the taller, mat-forming and more vigorous genera of this family (Craspedia, Celmisia, Podolepis) are predicted to be competitive with environmental change (Venn et al. 2011), although colonisation may be slow. Contrary to expectation, these genera did not show clear patterns of increases at any spatial scale between 2004 and 2011, indicating that perhaps longer intervals between samples may be needed to detect change and that even widespread, common species with relatively well understood biology might not demonstrate directional change or migration predictably. Overall, the specific environmental factors that may determine species enrichment or species decline across the altitudinal gradient of sites at Mt Clarke remain undetermined.
Conclusions
Species richness changes over short time scales may not reflect the longer-term trends or be indicative of longer-term trends in climate. Here, the variation in climate between 2004 and 2011 showed no directional change for any parameters except recent rainfall and the increases/decreases in species richness across the summits were not apparently related to short-term variation in climate. Climatic variation over the past few decades is therefore more likely to explain changes in species richness across the sites. Over the short-term, changes in species richness could not be predicted from site altitude, but increases in species richness, particularly consisting of shrub and graminoid species were more pronounced at the lower altitudes. These results suggest shrub migration onto the lower SAS of the summits is underway, whereas dramatic changes to the uppermost summit vegetation at these sites seems unlikely in the short term. Given that the local lower elevation species pool is dominated by expanding shrubby vegetation, over longer time periods shrubs are expected to continue to increase in abundance at the lower summit sites, potentially causing decreases in overall species richness at those sites. Results from this re-survey do not suggest that Kosciuszko endemic species are any more or less threatened by the short-term variation in climate than the more widespread species, however, future surveys will reveal whether these species become more marginalised or continue to turn over at moderate rates across the gradient of sites at Mt Clarke.
References
Bahn M, Körner C (2003) Recent increases in summit flora caused by warming in the Alps. In: Nagy L, Grabherr G, Körner C, Thompson DBA (eds) Alpine biodiversity in Europe. Springer, Berlin, pp 437–441
Böhm R, Auer I, Brunetti M, Maugeri M, Nanni T, Schöner W (2001) Regional temperature variability in the European Alps: 1760–1998 from homogenized instrumental time series. Int J Climatol 21:1779–1801
Buckeridge KM, Zufelt E, Chu H, Grogan P (2010) Soil nitrogen cycling rates in low arctic shrub tundra are enhanced by litter feedbacks. Plant Soil 330:407–421
Callaway RM (1995) Positive interactions among plants. Bot Rev 61(4):306–349
Callaway RM, Brooker RW, Choler P, Kikvidze Z, Lortie CJ, Newingham B, Aschehoug ET, Armas C, Kikodze D, Cook BJ (2002) Positive interactions among alpine plants increase with stress. Nature 417:844–848
Cavieres LA, Badano EI, Sierra-Almeida A, Gómez-González S, Molina-Montenegro MA (2006) Positive interactions between alpine plant species and the nurse cushion plant Laretia acaulis do not increase with elevation in the Andes of central Chile. New Phytol 169(1):59–69
Choler P, Michalet R, Callaway RM (2001) Facilitation and competition on gradients in alpine plant communities. Ecology 82:3295–3308
Costin AB (1954) A study of the ecosystems of the Monaro region of New South Wales with special reference to soil erosion. Soil Conservation Service of New South Wales, Sydney
Costin AB, Gray M, Totterdell CJ, Wimbush DJ (2000) Kosciuszko alpine flora, 2nd edn. CSIRO, Melbourne
Crimmins SM, Dobrowski SZ, Greenberg JA, Abatzoglou JT, Mynsberge AR (2011) Changes in climatic water balance drive downhill shifts in plant species’ optimum elevations. Science 331:324–327
Dirnböck T, Dullinger S (2004) Habitat distribution models, spatial autocorrelation, functional traits and dispersal capacity of alpine plant species. J Veg Sci 15(1):77–84
Dullinger S, Dirnböck T, Grabherr G (2003) Patterns of shrub invasion into high mountain grasslands of the northern Calcareous Alps, Austria. Arct Antarct Alp Res 35(4):434–441
Dullinger S, Gattringer A, Thuiller W, Moser D, Zimmermann NE, Guisan A, Willner W, Plutzar C, Leitner M, Mang T, Caccianiga M, Dirnböck T, Siegrum E, Fischer A, Lenoir J, Svenning J-C, Psomas A, Schmatz DR, Silc U, Vittoz P, Hülber K (2012) Extinction debt of high mountain plants under twenty-first century climate change. Nat Clim Change. doi:10.1038/NCLIMATE1514
Edmonds T, Lunt ID, Roshier DA, Louis J (2006) Annual variation in the distribution of summer snowdrifts in the Kosciuszko alpine area, Australia, and its effect on the composition and structure of alpine vegetation. Austral Ecol 31:837–848
Engler R, Randin C, Thuiller W, Dullinger S, Zimmermann NE, Araújo MB, Pearman PB, Le Lay G, Piédallu C, Albert CH, Choler P, Coldea G, de Lamo X, Dirnböck T, Gégout J-C, Gómez-García D, Grytnes J-A, Heegaard E, Høistad F, Nogués-Bravo D, Normand S, Pucas M, Sebastià MT, Stanisci A, Theurillat J-P, Trivedi M, Vittoz P, Guisan A (2011) 21st climate change threatened European mountain flora. Glob Change Biol 17:2330–2341
Erschbamer B, Unterluggauer P, Winkler E, Mallaun M (2011) Changes in plant species diversity revealed by long-term monitoring on mountain summits in the Dolomites (northern Italy). Preslia 88:387–401
Gottfried M, Pauli H, Futschik A, Akhalkatsi M, Barancok P, Benito Alonso JL, Coldea G, Dick J, Erschbamer B, Fernandez Calzado MR, Kazakis G, Krajci J, Larsson P, Mallaun M, Michelsen O, Moiseev D, Moiseev P, Molau U, Merzouki A, Nagy L, Nakhutsrishvili G, Pedersen B, Pelino G, Puscas M, Rossi G, Stanisci A, Theurillat J-P, Tomaselli M, Villar L, Vittoz P, Vogiatzakis I, Grabherr G (2012) Continent-wide response of mountain vegetation to climate change. Nat Clim Change 2:111–115
Grabherr G, Gottfried M, Pauli H (1994) Climate effects on mountain plants. Nature 369(9):448
Grabherr G, Gottfried M, Gruber A, Pauli H (1995) Patterns and current change in alpine plant diversity. In: Chapin FS, Körner C (eds) Arctic and alpine biodiversity: patterns, causes and ecosystem consequences. Springer, Berlin, pp 167–181
Green K (2010) Alpine taxa exhibit differing responses to climate warming in the Snowy Mountains of Australia. J Mt Sci 7:167–175
Green K, Pickering CM (2009) The decline of snowpatches in the Snowy Mountains of Australia: importance of climate warming, variable snow and wind. Arct Antarct Alp Res 41:212–218
Guisan A, Theurillat J-P (2000) Assessing alpine plant vulnerability to climate change: a modelling perspective. Integr Assess 1:307–320
Hennessey K, Whetton P, Smith I, Bathols J, Hutchinson M, Sharples J (2003) The impact of climate change on snow conditions in mainland Australia. CSIRO Atmospheric Research, Aspendale
Hoffmann AA, Camac JS, Williams RJ, Papst W, Jarrad FC, Wahren C-H (2010) Phenological changes in six Australian subalpine plants in response to experimental warming and year-to-year variation. J Ecol 98:927–937
Holzinger B, Hüber K, Camenisch M, Grabherr G (2008) Changes in plant species richness over the last century in the eastern Swiss Alps: elevational gradient, bedrock effects and migration rates. Plant Ecol 195:179–196
Hughes L (2003) Climate change and Australia: trends, projections and impacts. Austral Ecol 28(4):423–443
Klanderud K, Birks HJB (2003) Recent increases in species richness and shifts in altitudinal distributions of Norwegian mountain plants. Holocene 13(1):1–6
Körner C (1992) Response of alpine vegetation to global climate change. Catena 22:85–96
Körner C, Larcher W (1988) Plant life in cold climates. Symp Soc Exp Biol 42:25–57
Körner C (2003) Alpine plant life, 2nd edn. Springer, Berlin
Kullman L (2002) Rapid recent range-margin rise of tree and shrub species in the Swedish Scandes. J Ecol 90:68–77
Laurance WF, Dell B, Turton SM, Lawes MJ, Hutley LB, McCallume H, Dale P, Bird M, Hardyb G, Prideaux G, Gawneg B, McMahond CR, Yuh R, Hero J-M, Schwarzkopf L, Krockenberger A, Setterfield SA, Douglas M, Silvester E, Mahonyl M, Vellam K, Saikia U, Wahren C-H, Xue Z, Smith B, Cocklin C (2011) The 10 Australian ecosystems most vulnerable to tipping points. Biol Conserv 144:1472–1480
Lenoir J, Gégout JC, Marquet PA, P. dR, Brisse H (2008) A significant upward shift in plant species optimum elevation during the 20th century. Science 320:1768–1771
Lesica P, McCune B (2004) Decline of arctic-alpine plants at the southern margins of their range following a decade of climatic warming. J Veg Sci 15(5):679–690
Lesica P, Steele BM (1996) A method for monitoring long term population trends: an example using rare arctic-alpine plants. Ecol Appl 6:879–887
McDougall KL (2003) Aerial photographic interpretation of vegetation changes on the Bogong High Plains, Victoria, between 1936 and 1980. Aust J Bot 51:251–256
McDougall KL, Brookhouse MT, Broome LS (2012) Dendroclimatological investigation of mainland Australia’s only alpine conifer, Podocarpus lawrencei Hook.f. Dendrochronologia 30:1–9
Milberg P, Hansson ML (1993) Soil seed bank and species turnover in a limestone grassland. J Veg Sci 4:35–42
Moiseev PA, Shiyatov SG (2003) Vegetation dynamics at the treeline ecotone in the Ural highlands, Russia. Alpine biodiversity in Europe. In: Nagy L, Grabherr G, Körner C, Thompson DBA (eds) Alpine biodiversity in Europe—a Europe-wide assessment of biological richness and change. Ecological studies, vol 167. Springer, Berlin, pp 423–435
Myers-Smith IH, Forbes BC, Wilmking M, Hallinger M, Lantz T, Blok D, Tape KD, Macias-Fauria M, Sass-Klaassen U, Lévesque E, Boudreau S, Ropars P, Hermanutz L, Trant A, Collier LS, Weijers S, Rozema J, Rayback SA, Schmidt NM, Schaepman-Strub G, Wipf S, Rixen C, Ménard CB, Venn S, Goetz S, Andreu-Hayles L, Elmendorf S, Ravolainen V, Welker J, Grogan P, Epstein HE, Hik DS (2011) Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ Res Lett 6(4):045509
Nicholls N (2005) Climate variability, climate change and the Australian snow season. Aust Meteorol Mag 54:177–185
Pauli H, Gottfried M, Grabherr G (2003a) The Piz Linard (3411 m), the Grisons, Switzerland—Europe’s oldest mountain vegetation study site. In: Nagy L, Grabherr G, Körner C, Thompson DBA (eds) Alpine biodiversity in Europe—a Europe-wide assessment of biological richness and change. Ecological studies, vol 167. Springer, Berlin, pp 443–448
Pauli H, Gottfried M, Dirnböck T, Dullinger S, Grabherr G (2003b) Assessing the long-term dynamics of endemic plants at summit habitats. In: Nagy L, Grabherr G, Körner C, Thompson DBA (eds) Alpine biodiversity in Europe—a Europe-wide assessment of biological richness and change. Ecological studies, vol 167. Springer, Berlin, pp 195–207
Pauli H, Gottfried M, Hohenwallner D, Reiter K, Casale R, Grabherr G (2004) The GLORIA field manual—multi-summit approach. European Commission DG Research, EUR 21213, Office for Official Publications of the European Communities, European Commission, Luxembourg
Pauli H, Gottfried M, Reiter K, Klettner C, Grabherr G (2007) Signals of range expansions and contractions of vascular plants in the high Alps: observations (1994–2004) at the GLORIA master site Schrankogel, Tyrol, Austria. Glob Change Biol 13(1):146–156
Pauli H, Gottfried M, Dullinger S, Abdaladze O, Akhalkatsi M, Alonso JLB, Coldea G, Dick J, Erschbamer B, Calzado RF, Ghosn D, Holten JI, Kanka R, Kazakis G, Kollar J, Larsson P, Moiseev P, Moiseev D, Molau U, Mesa JM, Nagy L, Pelino G, Puscas M, Rossi G, Stanisci A, Syverhuset AO, Theurillat J-P, Tomaselli M, Unterluggauer P, Villar L, Vittoz P, Grabherr G (2012) Recent plant diversity changes on Europe’s mountain summits. Science 336:353–355
Pickering CM, Green K (2009) Vascular plant distribution in relation to topography, soils and micro-climate at five GLORIA sites in the Snowy Mountains, Australia. Aust J Bot 57:189–199
Pickering CM, Hill W, Green K (2008) Vascular plant diversity and climate change in the alpine zone of the Snowy Mountains, Australia. Biodivers Conserv 17:1627–1644
Scherrer P (2003) Monitoring vegetation change in the Kosciuszko alpine zone, Australia. PhD thesis, Griffith University, Gold Coast
Scherrer P, Pickering CM (2005) Recover of alpine vegetation from grazing and drought: data from long-term photoquadrats in Kosciuszko National Park, Australia. Arct Antarct Alp Res 37(4):574–584
Theurillat J-P, Guisan A (2001) Potential impact of climate change on vegetation in the European Alps: a review. Clim Change 50:77–109
Venn SE (2007) Plant recruitment across alpine summits in south-eastern Australia. PhD thesis, La Trobe University, Melbourne
Venn SE, Morgan JW (2005) Patterns in alpine vegetation across an altitudinal gradient in Victoria, Australia: an example of ‘space for time substitution’ in order to assess the potential effects of climate change. In: Price MF (ed) Global change in mountain regions. Sapiens Publishing, Duncow
Venn SE, Morgan JW (2009) Patterns in alpine seedling emergence and establishment across a stress gradient of mountain summits in south-eastern Australia. Plant Ecol Divers 2(1):5–6
Venn SE, Morgan JW (2010) Soil seedbank composition and dynamics across alpine summits in south-eastern Australia. Aust J Bot 58:349–362
Venn SE, Morgan JW, Green PT (2009) Do facilitative interactions with neighboring plants assist the growth of seedlings at high altitudes in alpine Australia? Arct Antarct Alp Res 41(3):381–387
Venn SE, Green K, Pickering CM, Morgan JM (2011) Using plant functional traits to explain community composition across a strong environmental filter in Australian alpine snowpatches. Plant Ecol 212:1491–1499
Vittoz P, Bayfield N, Brooker R, Elston DA, Duff EI, Theurillat J-P, Guisan A (2010) Reproducibility of species lists, visual cover estimates and frequency methods for recording high-mountain vegetation. J Veg Sci 21(6):1035–1047
Walther G-R, Beißner S, Burga CA (2005) Trends in the upwards shift of alpine plants. J Veg Sci 16:541–548
Wearne LJ, Morgan JW (2001) Floristic composition and variability of subalpine grasslands in the Mt Hotham region, north-eastern Victoria. Aust J Bot 49:721–734
Zobel MM, Otsus JL, Moora M, Möls T (2000) Is small-scale species richness limited by seed availability or microsite availability. Ecology 81:3274–3282
Acknowledgments
This project was supported by the Australian Alps Liaison Committee, the National Climate Change and Adaptation Research Facility and the New South Wales National Parks and Wildlife Service. Sarah Butler, Nicole Beutel, Rochelle Steven and Craig Hyde assisted with fieldwork. Special thanks go to Georg Grabherr and the GLORIA Vienna team. Sarah Butler commented on early versions of the manuscript. We also thank two anonymous referees for their valuable and insightful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Venn, S., Pickering, C. & Green, K. Short-term variation in species richness across an altitudinal gradient of alpine summits. Biodivers Conserv 21, 3157–3186 (2012). https://doi.org/10.1007/s10531-012-0359-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-012-0359-2