Abstract
Anthropogenic disturbance and fragmentation have changed the structure and composition of northern Ethiopian forests. This study was conducted to determine disturbance levels of patches, and examine the effect of anthropogenic disturbance and fragment size on diversity and structural attributes of woody species. A systematic sampling method was used to collect data and 61 nested plots of each 400 m2 were used. The disturbance index was used to assess disturbance intensities. To examine the effects of fragment size and anthropogenic disturbances on woody species diversity and structure, regression analysis was performed. ANOVA was used to examine differences in woody species diversity and structural attributes among fragments. Similarities among patches were assessed by Sorensen’s similarity index. A total of 80 woody species, representing 68 genera and 43 families were recorded. Diversity, evenness, stem density ha−1, and basal area were 2.8 ± 0.47, 0.73 ± 1.10, 1171.21 ± 220.10, and 39.89 ± 17.47 m2 ha−1 respectively. The mean number of species, evenness, and diversity were significantly different among fragments. Diversity, evenness, the mean number of species, and stem density per hectare significantly increased with increasing fragment size. Disturbance intensities varied among fragments. However, the mean basal area, mean seedling, and sapling density per hectare were not significantly affected by patch size and disturbance intensities. Generally, forest fragmentation and anthropogenic disturbance resulted in the reduction of diversity, affect woody species structure, and increased the level of disturbance. Therefore, recognizing the role of small fragments for forest conservation, minimizing further disturbance, and undertaking restoration activities in disturbed patches are strongly needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical forest ecosystems are the largest and richest ecosystems globally (Giam et al. 2011) providing multiple goods and services for humans. They also play a critical role in conserving other organisms belonging to the system (Murphy and Lugo 2007). However, these forests are getting fragmented, over-harvested, and disturbed due to persistent dependence of human livelihood on agriculture, forest, and forest products (Wood et al. 2000). The fragmentation and disturbance of dry Afromontane forests (DAF) in the northern Ethiopian highlands perfectly match this situation.
Forest fragmentation which originally conceived from island bio-geography theory (Macarthur and Wilson 1967), is a landscape-level process. A larger forest area is changed into a number of smaller, isolated, and geometrically complex patches after fragmentation (Laurance 2008). Fragmentation threatens biodiversity (Fahrig 2003; Kurki et al. 2000), alters landscape composition, structure, function, and disturbances (Echeverría et al. 2007; Wilson et al. 2016). Besides, changes in ecosystem processes such as nutrient cycling, (Fahrig 2003), environmental conditions, and genetic exchange (Ibáñez et al. 2014) will take place within newly created fragments. Moreover, forest fragmentation increases the vulnerability of forests to surrounding human pressures (Sagar and Singh 2006). Likewise, plant species reproduction, pollination, and dispersal mechanisms in fragmented landscapes will be altered (Benitez-Malvido 1998) both in the short and long terms (Ewers and Didham 2006).
Two views have been raised regarding the effect of fragment size on plant population. According to Macarthur and Wilson (1967), fragments of the larger area are supposedly assumed to hold more plant species than the smaller fragments. Contrary, Debinski and Holt (2000); Ziter et al. (2013) verified that the network of several smaller fragments has the potential of conserving more plants in a region higher than the larger fragment. Hence, few scientific studies show a positive relationship between forest area and plant population in tropical forests (Debinski and Holt 2000). The effects of forest fragmentation and associated human disturbances could sound different based on site conditions, ecosystem types, and plant species (Drinnan 2005; Ewers and Didham 2006; Echeverria et al. 2007; Haddad et al. 2015). Therefore, human disturbance could have negative or positive effects on the remaining forest stands and may play an important role in determining its composition and structure (Connell 1978).
Fragmentation succeeds disturbance; a discrete event in time and space that alters the structure of forest population, ecosystems, resource availability, and the physical environment (RamõÂrez-Marciala et al. 2001). Disturbance encompasses sudden events affecting the vegetation by increasing the density and independent mortality (Saunders et al. 1991; Stenhouse 2004). It also determines forest dynamics and diversity at both local (Whittaker 1972) and regional scale (Hubbell et al. 1999). However, its effect or consequence varies depending on ecosystems, organisms, and species (Haddad et al. 2015). Therefore, it remains a critical question to what extent anthropogenic disturbance affects the diversity and dendro-metric characteristics of woody species in relation to the level of disturbance and patch size (Godefroid and Koedam 2016). Although human disturbance might be quantified via disturbance indices (based on the ratio of the number of plants that had been cut, the total number of plants within a plot, and canopy cover) (Sagar and Singh 2006), there is no consensus on its influences on forest biodiversity (Miller et al. 2011). Beyond human factors, forests are influenced by natural processes such as species-specific tree mortality, recruitment limitations, natural disturbance by fire, wind, snow, and storms. However, most pressures in forests today are the result of human use. Besides, the consequences of human disturbance are more severe compared to natural disturbances (Kareiva et al. 2007).
The Afromontane region is in the Afro tropic sub-region and it extends from the Knysna forests in the Cape Province of South Africa to the mountains of tropical Africa and East Africa (White 1983). It is in the Afro-tropical territory covering the Arabian Peninsula, Drakensberg Mountains, East African Rift Valleys, and sub-Saharan interior (Linder et al. 2005). There is a strong commonality in plant species between the mountains of Africa and the southern Arabian Peninsula (Miles et al. 2006). However, those species are under intense threats (agricultural practices, fire, and grazing) as they belong to high human population density regions (Chapman et al. 2004).
DAF covers half of the world’s subtropical and tropical forests. Although DAF is a biodiversity conservation priority area and supports the world’s poorest people, it is the least studied (Miles et al. 2006) and has been exposed to disturbances, overexploitation, and fragmentation (Murphy and Lugo 2007). Consequently, DAF needs further investigation, management, and conservation approaches. Similarly, the northern Ethiopian highlands constitute the larger parts of the dry Afromontane regions of Africa which bounce from Cameroon to eastern Africa (White 1983). DAF of northern Ethiopia is a province for biodiversity hot-spots, high endemism, and immense plant diversity (White 1983; Vivero et al. 2006; Kidane et al. 2019). However, there has been frequent extinction, fragmentation, and disturbances of these forests (Aynekulu et al. 2012; Yismaw et al. 2014; Aynekulu et al. 2016) due to their high agricultural productivity, favorable climate, center for ancient human residence, sources of livelihood, and socioeconomic activities (Kidane et al. 2019). Hence, anthropogenic disturbance and fragmentation have affected the structure and composition of northern Ethiopian DAF for centuries (Darbyshire et al. 2003; Bongers et al. 2006; Aynekulu et al. 2012; Tadele et al. 2014; Berhanu et al. 2017) making them highly fragmented (Yismaw et al. 2014; Aynekulu et al. 2016). Consequently, the remaining forest patches in these forests are limited to areas around churches and inaccessible topographies (Wassie et al. 2010).
Nevertheless, northern Ethiopian DAF has rarely been investigated up to date (Tadele et al. 2014; Berhanu et al. 2017). Most of the scientific studies carried out in northern Ethiopian forests focused on the population dynamics, structure, regeneration ecology, and their comparisons along environmental gradients (Wassie et al. 2009; Aynekulu et al. 2012). Therefore, scientific information about the effect of forest fragmentation and disturbance on the diversity and structure of woody species on those DAF is lacking except on “church forests” (Wassie et al. 2010). Furthermore, little is recognized about whether relatively small fragments are particularly vulnerable to ongoing anthropogenic disturbances or not (Echeverria et al. 2007). Besides, the level of disturbance in each of the fragmented patches has not yet been investigated (Tadele et al. 2014). Consequently, the evidence on a link between forest fragmentation and disturbance with plant diversity and structure will contribute to develop management and conservation plans for such forests (Ewers and Didham 2006). Therefore, this study was conducted to determine the disturbance level of patches and examine the effects of fragment size and anthropogenic disturbance on the diversity and structural attributes of woody species.
Materials and methods
Description of the study area
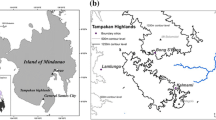
This study was conducted in northern Ethiopia; located between latitudes of 10° 97′ N–11° 01′ N (in ‘UTM’ = north of 1,202,058.91 m–1,234,964.86 m) and longitudes 36° 63′ E–36° 73′ E (in ‘UTM’ = east of 241,391.81 m–295,145.08 m) (Fig. 1). The altitude of the study site ranges from 1800 to 2350 m above sea level.
According to weather data from the Ethiopian national meteorological agency (from 1984 to 2015), the annual monthly temperature range of the study area is 9.4 °C and 29.4 °C. The average temperature of the study area is 18.7 °C. The rainfall distribution is uni-modal with substantial rainfall from June to September (2241 mm). The average annual rainfall of the study area is 1300 mm. The major soil types include; Andisols, Vertisols, Nitosols, and Cambisols (Abere et al. 2017).
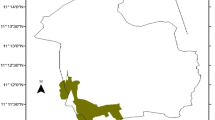
Vegetation types of the area are characterized as DAF (Friis et al. 2010). However, the forests in the area are declining at the rate of 120 ha year−1 (Yismaw et al. 2014). The forest fragments are isolated from each other by agriculture, grazing lands, plantation, rivers, and settlements (Fig. 2). According to a report by Awi zone agricultural office (2017, unpublished data), agricultural activities (encroachment, illegal massive cutting, and removal of plants) and the controlled fire had been reported as the main historical drivers of forest fragmentation and disturbance in the area. However, in 1994, most forest fragments were protected from those activities since Amhara national regional state-designated them as state forests and forest priority areas (Abere et al. 2017). In 2008, the participatory forest management (PFM) approach had been introduced to the area by farm Africa (Ethiopia). The main aims of PFM were to involve communities in forest management, conservation, and benefit-sharing although it was not successful yet. Currently, (2017), most patches are managed by the government. Despite all those activities and efforts, there are still massive pressures on forests because of illegal cutting by residents (Yismaw et al. 2014).
The study area hosts endemic wild avian species such as Poicephalus flaviforns, Agaporni staranta, and Parophasma galinieri. Wild mammals found in the area include Colobus geureza, Tragelabhus scriptus, and Crocuta crocuta (Genet and Ejigu 2017).
Methods
Forest fragment selection and vegetation sampling
In northern Ethiopia, there are several forest patches managed by the government (ex. Figure 2). For our study, a total of nine forest fragments located in the same elevation and climatic conditions were selected. Furthermore, the forest fragments have similar topographic and edaphic conditions (Ma et al. 2015). These fragments were selected using the Google Earth and reconnaissance survey (Panwar and Bhardwaj 2005). The distance between fragments ranged from 50 m to 5 km. The socio-economic attributes; including distance from the market places to all forest fragments is also similar.
The studied remnant forest patches cover a total of 2492 ha. The area coverage of few forest patches was obtained from Awi zone agricultural office. However, Landsat-8 operational land imager sensor data 2016 (http://geography.usgs.gov) and Google maps area calculator tools were used to obtain the area of remaining patches (Yismaw et al. 2014). This is important to predetermine sampling intensity prior to data collection (Sukumar et al. 1992). Therefore, the size of the studied forest fragments ranged from 35 to 747 ha. Based on their relative size, the studied fragments were grouped into 3 fragment size classes (FSC). Consequently, 3 replicate fragments were made for each FSC based on their relative size. They were categorized into small, medium, and large fragment size classes (FSCs) (Tripathi et al. 2010; Majumdar and Datta 2016; Pao and Upadhaya 2017). The size of small, medium and large fragments ranged from 35 to 45 ha, 120 to 325 ha, and 480 to 747 ha respectively. Overall: 6, 15, and 40 main plots were taken from small, medium, and large FSC respectively. Therefore, a total of 2.44 ha of forest fragments or 0.12% of the study area was sampled. Consequently, 0.20%, 0.10%, and 0.09% of small, medium, and large FSC were sampled. This sampling intensity is greater than the required standard minimum sampling intensity recommended to study the effects of fragment size (0.01%) (Sukumar et al. 1992).
Systematic sampling method was used for vegetation sampling in each fragment (Sutherland 2006; Kent 2011; Senbeta et al. 2014; Teshager et al. 2018). Transect lines were laid systematically against the altitude gradient to ensure sufficient representative samples of all the FSC (Tadele et al. 2014; Berhanu et al. 2017). Transect lines are the most covenant sampling design for vegetation sampling. The distance between each transect line and plots (quadrats) were 300 m and 200 m respectively (Tadesse et al. 2017). A total of 61 nested plots (Kent 2011) of each 20 m × 20 m (0.04 ha) size were established within transect lines. Those nested quadrats were established 50 m inward to reduce edge effects in each fragment (Tadele et al. 2014). Similar plot sizes were made in all FSC following Tripathi et al. (2010). The number of plots taken per fragment varied based on their size (e.g. Senbeta et al. 2014).
In each plot, the diameter at breast height (DBH) of all standing woody species with a diameter at breast height (DBH, at 1.3 m above ground level) of ≥ 2 cm were measured using a caliper. Diameter tape was used to measure the DBH of trees that were too big for calipers. The height of woody species was measured using hypsometer. A nested plot design was used depending on the growth forms of plants (Kent 2011). Therefore, all woody species (trees, shrubs, and lianas) were collected, counted, and measured within the main plots (0.04 ha). While five subplots of 5 m × 5 m size (a total of 305) at the four corners and center of the main plot were used to count seedlings and saplings (Kent 2011; Tadesse et al. 2017). Mean seedling and sapling density ha−1 were computed by counting seedlings and saplings. Seedlings in this study are defined as woody species that have a diameter (< 2 cm) and height (< 1 m). Whereas, saplings are woody species with DBH of < 2 cm and height from 1 to 2 m. Shrubs are defined as woody plants that are multi-stemmed at the base of the plant. Therefore, shrubs are multi-stemmed below 1.3 m. Lianas are any long-stemmed, woody vines that use trees or other means for vertical support.
Felling and selective cutting were considered as the most important anthropogenic disturbance symptoms in the area to determine disturbance levels of fragments (Montes et al. 2004; Rudas et al. 2007; Aguilar-santelises and Castillo 2013). Accordingly, old and new cut stumps DSH (diameter at stump height = 30 cm above the ground level) were counted and measured to calculate the basal area of stumps in the main plots. Stumps in this study are defined as the remains of the stems of woody plants measuring DSH of ≥ 2 cm after cutting.
Disturbance level on the basis of the total basal cover (TBC) for each fragment was determined using disturbance index (DI) (%) (e.g. Kanzaki and Yoda 1986; Murali et al. 1998; Aguilar-santelises and Castillo 2013).
where DI is the disturbance index and TBC is the total basal cover. TBC was computed after calculation of basal area for each individual cut and standing stems.
Shannon diversity index and species richness were estimated to determine the diversity of woody species. The structure of woody species in all FSC was determined based on DBH classes. On top of that dendrometric characteristics such as density and basal area were also determined for each FSC (Senbeta et al. 2014). Besides, the frequency for each woody species was computed at each FSC. Other parameters estimated for comparison also include important value index (IVI) and Shannon evenness. IVI is the relative ecological importance of species in an ecosystem (Kent 2011). It was determined from the summation of relative frequency, relative density, and relative dominance.
Identification of woody species was made in the field with help of a local guide familiar with the flora. Woody species that were difficult to identify in the field were identified at the National Herbarium, Addis Ababa University, Ethiopia. Nomenclature followed Flora of Ethiopia and Eritrea (Edwards et al. 2000; Hedberg et al. 2004, 2006).
Statistical analysis
The vegetation data from transects of each fragment were pooled per FSC. Shannon diversity (H´), Shannon’s evenness (E), and species richness (S) were computed to describe and compare the diversity of woody species, evenness, and richness at FSC following Shannon and Weaver (1949); Magurran and Mcgill (2011). Structural parameters of woody species were computed following Mishra et al. (2004). Linear regression analysis was separately run to examine the effects of fragment size and disturbance on woody species diversity and structural attributes. Species richness, structural attributes, and diversity index were used as dependent variables. Whereas the fragment size and disturbance index were used as independent variables (Majumdar and Datta 2016).
One-way ANOVA, followed by Tukey HSD Post Hoc tests for unequal sample sizes (Dytham 2009) were used to examine whether fragments of different size classes were significantly different in Shannon diversity and structural attributes (Hammer et al. 2001). Sorensen’s similarity index was estimated to examine the similarity in species composition among the FSC. A PAST software package (ver.3.) was used to calculate the similarity, diversity, and evenness of woody species in each fragment.
Results and discussion
Species diversity
A total of 80 woody species, representing 68 genera, and 43 families were recorded in all studied fragments (Table 1; Online Appendix 1). Out of these species, 43 (53.75%), 27 (33.75%), and 10 (12.5%) were trees, shrubs, and lianas respectively in terms of life forms. Fabaceae (nine species), Celastraceae (five species), Moraceae, and Rubiaceae (four species) are the families with the highest number of species respectively. Apocynaceae, Asteraceae, Euphorbiaceae, Myrsinaceae, Rosaceae, Oleaceae, and Solanaceae were equally represented by three species. Whereas, Acanthaceae, Boraginaceae, Flacourtiaceae, Meliaceae, Rutaceae, and Salicaceae were represented by two species. The remaining 25 families were represented by a single species. The highest and lowest number of families, genera, and species were recorded in large and small FSC respectively (Table 1). ‘Fabaceae’ is the most dominant family elsewhere (Atkinson and Marín-Spiotta 2014). Moreover, Tadele et al. (2014) and Berhanu et al. (2017) reported ‘Fabaceae’ as the most diverse family in DAF of northwestern Ethiopia. The family ‘Apiaceae’ had been reported as a diverse family in central Ethiopia by Shiferaw et al. (2018) in line with the result of this study.
Out of the total woody species recorded 16 (20%), 10 (12.5%), and 5 (6.25%) were recorded from large, medium, and small FSC respectively. The result indicated that woody species showed a varied response to fragmentation and disturbance. Species that are possibly shade-intolerant, light-demanding, pioneer, and associated with disturbance were restricted to highly disturbed small fragments. While relative proportions of species that are shade-loving and are not light-demanding increased with increasing the size of fragments. However, those shade-loving woody species declined and wiped-out at disturbed fragments. Besides, some species may require moderate openings, shade, and disturbance. This could be the possible reason for the restriction of 5, 10, and 16 woody species in small, medium, and large FSC separately, and 10 species without being shared by large and small FSC. The result of the present study agrees with Rodríguez-loinaz et al. (2012) and Krauss and Klein (2004) stating that the ‘effect of the patch size, fragmentation, and spatial isolation of patches did not affect all the growth and life forms of plants in the same way’. According to Ries et al. (2004), a decrease in fragment size results in an increased edge to core ratio that is disadvantageous to habitat specialist species. Therefore, though small FSC are low in species richness and contribute little to plant diversity conservation separately, they may contain high plant diversity as a network. Similar results were reported by Ma et al. (2015).
The three most abundant species recorded in the study area were Lepidotrichilia volkensii (17.3%), Albizia gummifera (16.3%), and Pavetta abyssinica (8.6%). Those species constituted 42% of the total abundance of the area. The Shannon diversity index and evenness of the studied fragments were 2.8 ± 0.47 and 0.73 ± 1.10 respectively. The mean number of species (DBH ≥ 2 cm), evenness, and Shannon (H’) were significantly different (P < 0.05) among FSC (Table 2). The result shows that there is a relationship between plant diversity and fragment size. This result is in line with other studies in tropical forests. For example, Rosenzweig (1995), noted that a decrease in fragment size results in lower species richness. In contrary to this finding, Pao and Upadhaya (2017) reported no significant increase in species richness and Shannon diversity index with the patch size.
Disturbance levels of forest fragments
The disturbance level in each FSC revealed a statistically significant difference (Table 2). The highest disturbance index was computed in small FSC (DI = 31.42 ± 2.1) compared to the medium (20.34 ± 2.80) and large (10.93 ± 2.67) FSCs. The highest disturbance index at small FSC substantiated by its highest number of cut stumps (DSH ≥ 2 cm) than medium and large FSC (Table 2).
During field-work and transect walk, it was observed that species with various size ranges were cut in all forest fragments for fuel, lumber, construction, pole, farm tools, and fencing (Fig. 3). On the other hand, there was a difference in the mean number of plants cut in each of FSC (Table 3). According to forest guards and cut stump count, Albizia gummifera being easily chopped, having high calorific value, and extremely needed for lumber making was frequently visited by illegal tree cutters. Rhus glutinosa being termite resistant, extremely needed for house construction, and rare stood second in being cut illegally (e.g. Fig. 3). It was also noticed that species with the highest timber quality and strength for construction have only a few numbers of individuals in the study area. Those species include Cordia africana, Prunus africana, and Rhus glutinosa which were cut illegally, leading to possible local extinction.
As shown in Fig. 3, tree species with different ages and size ranges were cut down illegally in the study area (e.g. Fig. 3a, c, and e). This indicates that the DAF fragments of northern Ethiopia are under heavy ongoing anthropogenic disturbance. However, the severity of human disturbance and selective logging is high in small FSC which are situated near settlement areas. Pao and Upadhaya (2017), reported greater cut stumps numbers in smaller fragments similar to the result of this study. Furthermore, lumbering inside forests (Fig. 3h), pit-sawing, and taking them home (Fig. 3c and g), and an attempt to fell a big tree (Fig. 3f) were noted during fieldwork in the study area. According to forest guards and Awi zone agricultural office experts, the effects of selective logging and firewood collection are different here from somewhere else. This may due to a lack of alternative energy sources and economic incentives from the forest for the local communities. Besides, land encroachment activities due to the shortage of agricultural land, lack of employment, and alternative livelihood opportunities were also reported and noted in the study area, these resulted in forest disturbance and fragmentation.
The Shannon diversity index (H’) in small FSC is low (2.3 ± 0.10) compared to medium (2.7 ± 0.11) and large (3.33 ± 0.25) FSC (Table 2). The difference in diversity index is attributed to variable disturbance intensities in the fragments. There was the highest disturbance index (31.42 ± 2.10) on small FSC in this study. This is because of their size and proximity to human settlement as the first three sites were relatively very close to human settlements and easily accessible. This could have hastened the degree of alteration in conditions of those small forest fragments. The highest disturbance index (Table 3) in small forest fragments constrained recruitment of most of species in the present study (Online Appendix 2). For example, most species such as Allophylus abyssinicus, Apodytes dimidiate, Ehretia cymosa, Ekebergia capensis, Embelia schimperi, Juniperus procera, Olea capensis, and Olea europaea were absent at small fragments as they couldn’t endure the highest disturbance intensities confronted. In contrast, only some species such as Acacia abyssinica, Acacia lahai, Vernonia auriculifera, Maytenus obscura, and Maytenus undata are able to survive in small fragments as they might tolerate such disturbance intensities (Online Appendix 2).
The result of the present study is in line with Magurran and Mcgill (2011), which found out Shannon diversity is high in fragments where anthropogenic disturbance is low and vice versa. According to Macarthur and Wilson (1967), the smaller fragments had a lower number of plant species than larger fragments. Sagar and Singh (2006) noted that forests nearer to settlements are invariably more intensively exploited and are subjected to greater human pressure and over-exploited than remote forests. Furthermore, Aynekulu et al. (2016) reported that the cause for the low plant species diversity in the small fragment is the steady selective logging of useful species. Terborgh (2015) has emphasized that the activities of humans do more to accelerated species loss than the operations of internal biological processes. Therefore, anthropogenic disturbances via selective logging may usually result in a direct decline in species diversity. Furthermore, Echeverria et al. (2007), reported that the dissimilarities in species richness and diversity within the fragmented patches are because of prevailing anthropogenic disturbances.
Relatively, the highest Shannon diversity index in large FSC (Table 2) was due to low disturbance index, large fragment size, habitat conditions, species characteristics, and high evenness values (Zegeye et al. 2006; Tadele et al. 2014). According to the intermediate disturbance hypothesis theory (IDH), low disturbance intensity close to natural disturbance levels cause’s positive effects on plant diversity (Connell et al. 2008). Moreover, Macarthur and Wilson (1967) stated that the largest fragments support the largest populations associated with lower extinction rates and disturbance.
The largest forest fragments have the highest habitat heterogeneity and suitable conditions to hold greater plant diversity. Therefore, species richness declines over time following forest fragmentation. Furthermore, a positive relationship between Shannon diversity and fragment size could also be due to more even abundances of woody species in large fragments. Removal of more woody species in small FSC might increase the population dominance of few species. This may have resulted in decreased evenness and lower species diversity in those fragments. Therefore, logging in forests for different purposes caused a severe deterioration, especially for those small fragments situated near settlements. Similar findings had reported by Malik et al. (2014) stating that anthropogenic disturbance causes disruption of forests and changes species composition which ultimately leads to the reduction of plant species evenness and diversity. Low Shannon evenness index in small FSC may also due to the widening of canopy gaps that arises from anthropogenic disturbance pressure which hampered the recruitment of shade-loving species. According to Hill and Curran (2003), fragment size contributed up to 92% of vegetation variation. Therefore, larger forest size possibly supports the larger pool of species available. Hillebrand et al. (2008) confirmed that anthropogenically induced disturbances affect diversity, the number, and the distribution of plants. Likewise, Wekesa et al. (2019) reported that plant diversity is highly affected by forest fragmentation too.
Woody species similarities among forest fragments
Woody species retained on small highly disturbed FSCs and on large FSCs varied in similarity. However, medium and large FSCs exhibited the highest similarity (Table 4).
There was low similarity (40–72%) among the fragments though there were close proximity, relatively comparable elevation, and climatic condition. This could be due to the difference in fragment sizes and level of disturbance intensities (Table 3). Pao and Upadhaya (2017) reported a similarity index ranging from 52 to 77% among small-sized disturbed fragments and very large-sized undisturbed fragments. According to Ayyappan and Parthasarathy (2001), species similarity in a given forest depends on forest size, climate, and geographic location. However, human interaction in the past and present could also affect it. Therefore, following fragmentation and disturbance, species richness and composition declined. Consequently, this could have direct effects on the dissimilarity of fragments. Since small fragments lost more species than medium and large, Sørenson’s similarity index decreased when the fragments were compared for similarity. Thus, disturbance plays an important role for the differences in the similarity between forest fragments in the northern Ethiopian highlands.
Woody species structure
Density and basal area
The overall mean density ha−1 (DBH ≥ 2 cm) of all the studied fragments for 3,305 total individuals was 1171.21 (Online Appendix 2, 3, 4) respectively. Vernonia auriculifera (225 ha−1) had the highest stem density in small FSC (Online Appendix 2). This is because V. auriculifera is a pioneer species (Lemenih and Teketay 2006). While Albizia gummifera had the highest stem density in both medium (251 ha−1) and large (268 ha−1) fragment size classes (Online Appendix 3, 4).
Mean stem density ha−1 ranged from 992 ± 76.37 to 1411.33 ± 48.4 stem ha−1 among the FSCs. However, stem density difference was statistically significant only between small and large FSCs (F2, 6 = 7.9, p < 0.05) (Table 6). This might be due to the presence of the highest number of woody species per ha−1 in large FSC or low prevailing disturbance index in large FSC. Most of the time woody species with the highest stem density ha−1 in small-sized fragments had also the highest seedling and sapling density ha−1. Pioneer species such as Maytenus arbutifolia, Justicia schimperiana, and V. auriculifera are favored by disturbance and sudden openings for their growth and establishment (Bekele 2007; Berhanu et al. 2017). However, seedlings of A. gummifera were plentiful in openings adjoining to mother trees at low to medium disturbed FSCs (Table 3).
Mean basal area (DBH ≥ 2 cm) ha−1 ranged from 31.83 ± 21.19 m2 ha−1 to 53.5 ± 11.24 m2 ha−1. Nevertheless, it was not significantly different between FSCs (F2, 6 = 1.6; p > 0.05), (Table 5). Even though the number of individuals was higher in the large FSC, their contribution to the basal area was low due to lower DBH values. Besides, a relatively higher number of old individuals associated with disturbance in small FSC probably have attributed to the insignificant difference in basal area ha−1 among fragments. 69% of woody species have seedlings ranging from 0.5 to 52% in the study area. Whereas, 31% of woody species lack recruitment (Online Appendix 5, 6, 7). Woody species such as Rhus glutinosa, Olea europaea, Dracaena steudneri, Cordia africana, Ekebergia capensis, Erythrococca trichogyne, Juniperus procera, and Syzygium guineense lacked seedlings. This could be attributed to the life strategies of those species, seed dormancy, germination problem, and site conditions. Moreover, a destructive seedling collection of economically, culturally, and socially important plant species (such as Ehretia cymosa, C. africana, Ficus thonningii, Ficus sur, Embelia schimperi, O. europaea, and Olea capensis) from forests to settlement areas were reported by forest guards as a common practice in the study area.
The mean number of seedlings ha−1 ranged from 4282 ± 267.76 ha−1 to 3785 ± 779.77 ha−1. While the mean number of saplings ha−1 ranged from 917 ± 152.75 to 586 ± 279 ha−1 (Table 5). Relatively, the highest mean seedling and sapling density ha−1 were observed in the small FSC. The presence of wider and frequent gaps in small FSC might be a reason for the relative difference in the mean number of seedling and sapling density ha−1 among fragments. However, the difference between seedling and sapling density ha−1 was not statistically significant among FSC (F2, 6 = 1; F2, 6 = 3.4, (P > 0.05) (Table 5). This insignificant difference in the mean seedling and sapling density ha−1 among FSC might be attributed to low light intensity on the forest floor of large fragments. Moreover, a dense overhead canopy, low disturbance intensities, and high established seedling mortality in the larger fragments could also be the other reason too. Similar results were reported by Pandeya et al. (2007) in line with the finding of the present study. Wider frequent gaps and openings created by logging in small FSC were observed in the present study. Those gaps and openings may have enhanced fast regeneration, growth of light-demanding, shade-intolerant, and disturbance tolerant seedlings and saplings in small FSC.
Relatively, the highest stem density ha−1 in large FSC possibly could be due to patch size as the larger forest areas support more individuals. According to Hill and Curran (2003), the larger fragments exhibited a higher total number of individuals which could be attributed to ecological processes. Furthermore, the smaller fragments were not mostly emphasized by the management authorities. Likewise, Majumdar and Datta (2016) reported that smaller fragments had lower stem density ha−1 than the larger fragments. Bhat et al. (2011) stated that the removal of trees by people in small fragments was the prime reason for decreasing tree density too. However, Atkinson and Marin-Spotta (2014) and Pao and Upadhaya (2017) reported selective removal in disturbed forests may lead to an increase in stem density reverse to the result of this study.
Relatively, a comparable amount of basal area ha−1 in the small fragments with medium and large in the present study (Table 5) possibly might be due to a stimulated growth effect on surviving trees on small and medium-sized fragments helping to rebuild the forest structure. According to Bhat et al. (2011) removal of trees by people in the forest sites, facilitates the availability of light, nutrients, and moisture to surviving trees. This stimulates tree growth, leading to an increase in the basal area. Wassie et al. (2010) documented that the basal area is insignificantly related to the forest size in agreement with the result of the current study. However, RamõÂrez-Marciala et al. (2001) found out that the mean basal area is high at intermediate and severely disturbed sites in contrast to the finding of this study. Besides, an insignificant variation in basal area ha−1 with fragment size and disturbance was also reported by Pao and Upadhaya (2017). Furthermore, Tripathi et al. (2010) indicated that small FSC contains a greater number of dominant and disturbance tolerant woody species.
Diameter and height class distribution
According to Condit et al. (1998) diameter class distribution indicates the variations in population structure and species arrangement in a forest. Consequently, the highest DBH was recorded for Prunus africana (170 cm) and A. gummifera (157 cm). The tallest species were P. africana and A. gummifera reaching up to 45 m. The highest proportion of woody species (83.3%) in each FSC were observed in lower diameter (2–16 cm) and height (2–17 m) classes respectively. Therefore, the number of individuals gradually declines with increasing diameter and height classes (Fig. 4). However, each DBH and height classes were represented at least by a few individuals. The result revealed that the DBH and height class distribution of woody species ha−1 (density) in each of FSC showed an Inverted-J shaped structure (Fig. 4 a and b). However, the difference in mean number of individuals ha−1 in the lower DBH class (2–16 cm) among FSCs and disturbance intensities were not statistically significant (R2 = 0.025, P > 0.5; R2 = 0.0003, P > 0.5). Consequently, the removal of more upper canopy trees reduces gaps in the lowest DBH classes. This may also favor the occurrence of more species in the lower DBH classes. Therefore, those forests could be under secondary development after selective logging (Fashing et al. 2004; Berhanu et al. 2017; Pao and Upadhaya 2017). On the other hand, an inverted-J-shaped pattern could be an indicator of good biological functions, recruitment capacity, health status, and stable population (Tadele et al. 2014; Senbeta et al. 2014; Berhanu et al. 2017). According to Pao and Upadhay (2017), the reverse J-shaped pattern is an indicator of forest regeneration. Denslow (1980) also showed that canopy openings formed by disturbances are known to offer favorable micro-environments and growth of early secondary species.
Woody species population structure
Population structure is the way in which the attributes of plants are distributed within forests. It helps to prepare forest management plans, to analyze disturbance, and harvest events. Furthermore, structural data provide evidence on disturbance history, environment, and the future tendency of forests (Franklin et al. 2002; Wilson and Witkowski 2003; Wale et al. 2012).
The population structure of woody species selected based on their IVI in small FSC showed three patterns. The first pattern was an inverted-J-shaped where a greater number of individuals were presented in the lower DBH classes (Fig. 5c). The number of individuals decreased at the higher diameter classes in this pattern. This indicated that the selective removal of individuals of preferred size was made at the higher classes. Accordingly, A. gummifera and Croton macrostachyus were comprised in the reverse J-shaped pattern. Those species behave to have healthy regeneration. On the other hand, the skewed inverted-J-shaped pattern could show abnormal population distributions of a species (Berhanu et al. 2017). The second pattern was bell-shaped. In this pattern, the number of individuals presented in the middle diameter class was higher than the lower and higher classes (Fig. 5b). Acacia abyssinica was included in this category. According to Teshager et al. (2018), a bell-shaped pattern is an indicator of the poor and irregular requirements for species reproduction due to intense disturbance. Therefore, human disturbance, biotic, and abiotic factors may have caused a problem in those forests. Senbeta et al. (2014) also reported that a bell-shaped pattern could result from an intensive competition of trees in an ecosystem. Therefore, the disturbance may have delayed regeneration and normal health status of species in those sites (Senbeta et al. 2014). According to Cotler and Ortega-Larrocea (2006), anthropogenic disturbances determine the vegetation structure through their influences on regeneration success. J-shaped was the third pattern observed in small FSC. Accordingly, a few individuals at lower and middle DBH classes were presented in this pattern. Besides, more mature individuals were presented at the highest DBH classes (Fig. 5a). P. africana was included in this category. This pattern indicated low regeneration potential and conservation measure requirements for a particular species.
Likewise, the population structure of individuals in medium FSC showed three patterns. The first pattern was a broken inverted-J-shaped curve. There were the highest numbers of individuals at the lowest diameter classes in this pattern. Besides, the numbers of individuals were lacking at the highest diameter classes. Lepidotrichilia volkensii was under this category (Fig. 6). The second pattern observed in medium FSC was an inverted-J-shaped curve. The highest numbers of individuals were presented in the lower diameter classes at this pattern. However, the number of individuals gradually decreased in the highest diameter classes. A. gummifera, C. macrostachyus, and Celtis africana were included in this category (see Fig. 5c). The third pattern was J shaped curve where only a few individuals were presented at a lower and middle DBH class. On the other hand, more mature individuals were offered at the highest DBH class. P. africana was comprised in this set (see J-shaped pattern indicated in Fig. 5a).
The first population structure pattern in large FSC was J-shaped where the higher proportions of individuals were presented at the higher DBH classes. However, their trend decreased towards the lower DBH classes. Apodytes dimidiata, Ekebergia capensis, Allophylus abyssinicus, and P. africana were indicated under this category (Fig. 7a). The second pattern in large FSC lacked individuals in the lower and medium DBH classes. On the other hand, only a few matured individuals were presented at the highest DBH classes in this category. For example, Juniperus procera, Syzygium guineense, and Schefflera abyssinica exhibited this structure (Fig. 7b). The third pattern exhibited by A. gummifera and C. macrostachyus was an inverted-J-shaped structure (see Fig. 5c). The fourth pattern was a broken inverted-J shaped where individuals were absent at intermediate and higher DBH classes. While a higher percentage of individuals were presented at the lower DBH classes. Accordingly, Ehretia cymossa, L. volkensii, and O. capensis showed this pattern (see Fig. 6). Tadele et al. (2014) point out that an inverted J-shaped curve is an indicator of good biological function and recruitment capacity for a species. Similar population structure patterns were reported by Berhanu et al. (2017) at Kuandisha DAF fragment in northwestern Ethiopia. Therefore, the difference in the distribution pattern of DBH classes could be attributed to selective removal, site conditions, fragment size, and species characteristics.
Woody species frequency
A variation in frequency among woody species in all studied fragments was observed in this study (Online Appendix 2, 3, and 4). Accordingly, 9.35%, 7.5%, and 0.82% of woody species in small, medium, and large FSC were restricted to a single plot. Therefore, species which have low-frequency distribution and values are narrowly distributed, rare, and restricted to a specific location (Tadele et al. 2014). Consequently, those species are prioritized for conservation (Berhanu et al. 2017). Species characteristics, disturbance, regeneration potential, and life strategies may lead to low-frequency distribution (Zegeye et al. 2006; Cotler and Ortega-Larrocea 2006). The variation in frequency between species among FSC could be also attributed to alterations in species requirement, disturbance, physiognomies, and fragment sizes. Therefore, some species were more frequent in small FSC while other species were frequent in medium to large FSC. Accordingly, A. abyssinica (12.25%), V. auriculifera (10%), M. arbutifolia (10%), and A. lahai (9.5%) were the most frequent woody species in small FSC (Online Appendix 2). According to Bekele (2007), those species are common in disturbed and secondary DAF. They are also gap plasters, pioneers, and favored and associated with disturbance. On the other hand, species such as L. volkensii (13.25%), A. gummifera (8%), P. abyssinica (6.9%), and C. macrostachyus (5.78) were the most frequent species in medium FSC (Online Appendix 3).
The most frequent species in the large FSC were, A. gummifera (8.2%), P. abyssinica 5.63%), L. volkensii (5.56%), Erythrococca trichogyne (5.5%), and C. macrostachyus (5.1%) (Appendix 4). A high-frequency distribution of those species denotes their wider distribution within forests (Tadele et al. 2014). Similar results were reported by Berhanu et al. (2017), where the highest frequency was documented for C. macrostachyus, M. arbutifolia, and A. abyssinica in Kuandisha DAF fragments of northwestern Ethiopia.
Important value index of woody species
The result of the study shown the IVI of species at each FSC (Appendix 2, 3, 4). Accordingly, Entada abyssinica (3.13%) and A. gummifera (39.4%) were species with the lowest and highest IVI values in small FSC respectively (Appendix 2). On the other hand, Cissus petiolata, Hibiscus macranthus, and M. undata (0.52% each), and A. gummifera (64.53%) were species with the lowest and highest IVI values in medium FSC (Appendixes 3). Likewise, Maesa lanceolata (0.21%) and A. gummifera (76.94%) were species with the lowest and highest IVI at large FSC (Online Appendix 4). The top-ranked woody species by IVI in the studied FSC is indicated in Table 6 below (see more at Online Appendix 2, 3, 4).
Berhanu et al. (2017) documented higher IVI values for Croton macrostachyus in DAF fragments of northwestern Ethiopia similar to the result of the current study. Furthermore, Shiferaw et al. (2018) reported the highest IVI values for Acacia abyssinica and Acacia lahai in remnant forests of central Ethiopia in line with this study. Besides, Tadele et al. (2014), reported the higher IVI for Prunus africana and Albizia gummifera species in DAF forests of northern Ethiopia. However, Aynekulu et al. (2016) reported higher IVI values for Juniperus procera, Olea europaea, Carissa edulis, and Rhus glutinosa in reverse to the result of the current study. Besides, Tadele et al. (2014) documented higher IVI for A. abyssinicus and Bersama abyssinica which this study did not. This could be because of the higher anthropogenic effects at the higher DBH classes in most fragments at the current study area.
Effects of forest fragmentation and disturbance on species diversity and structure
The regression analysis in this study (R2 = 0.90, p < 0.05) showed a negative significant relationship between the fragment size and disturbance intensities (Figs. 8, 9). Accordingly, the level of disturbances significantly decreased as the fragment size increased. However, Shannon index (H’), Shannon evenness index (E), the mean number of woody species, and the mean stem density ha−1 exhibited a reverse trend where each of them increased as the fragment size increased (p < 0.05). In contrast, all of those parameters significantly decreased as disturbance index (DI) increased (p < 0.05). Consequently, the result of the study declared that human-induced disturbance (encroachment and selective cutting) along the forest fragments leads to the reduction of fragment sizes. Furthermore, anthropogenic disturbance in the study area affected forest structure, diversity, and disturbance levels of forest fragments too.
Effects of disturbance intensity on (a Shannon diversity index, b number of species, c stem density ha−1, and d evenness index), northern Ethiopia. The regression graphs for both effects of fragment size and disturbance on diversity and structure were sketched for parameters where regression analysis was statistically significant
Similar results were documented by Mohandass et al. (2018) reporting that the total species richness and abundance were significantly and positively correlated with increasing patch size. According to Ma et al. (2015), fragment sizes have a significantly positive relation to forest composition. Majumdar and Datta (2016) reported a significant increase in species richness, diversity, and forest structure along with fragment size. However, Aguilar-santelises and Castillo (2013) reported a positive relationship between disturbance and diversity where small disturbed fragments tend to be more diverse than large fragments which this study did not.
On the other hand, mean basal area ha−1 (DBH ≥ 2 cm), mean seedling, and sapling density ha−1 were not significantly affected by fragment size and deference in disturbance intensities (Figs. 8, 9). This indicates that there is a higher response of mature plants to fragment size and disturbance intensities than smaller immature plants. Besides, this could probably due to small fragments deliver the establishment of mature species comparable to large-sized fragments. Moreover, this may indicate that small FSC are benefited by open, less dense habitats, resilient, and pioneer species (Aguilar-santelises and Castillo 2013).
Conclusions and recommendations
Although the result of this study showed a total of 80 woody species, differences in woody species diversity and structural attributes were observed among fragment sizes and disturbance intensities. Accordingly, Species diversity and density increased with fragment size and vice-versa. However, this was not the case for seedling and saplings. Despite the intensity of disturbance in the study area, forest fragments maintain higher potential for regeneration. Besides, disturbance played an important role in the variation between forest fragments and many of the forest structural attributes. Conversely, the responses of species to forest fragmentation and disturbance were specific to species and its parameters. Therefore, the abundance, frequency, evenness, richness, density, and basal area of some species were strongly related to changes in fragment size and disturbance. Whereas others did not exhibit significant changes.
Density−1 of woody species in DBH and height classes revealed that the forest populations of the area had a relatively higher potential for regeneration capacity. However, the structure of the selected woody species exhibited different trends from species to species among FSCs. Furthermore, the similarity among forest fragments differed based on fragment sizes and disturbances levels. Therefore, there is a difference in the level of anthropogenic disturbance among forest fragments. Largely, forest fragmentation and disturbance resulted in the reduction of woody species diversity, affected many of the structural attributes, and increased the levels of disturbance in the study area. Moreover, large size fragments harbored species that are not found in the small fragments. The smallest fragments intern privileged those species which are shade-intolerant and associated with disturbance. However, those species were absent in the large fragments. Accordingly, conservation plans in fragmented forests ought to reveal fragment size, disturbance intensities, and the species to be conserved. Our major findings provided the following sound recommendations for conservation, sustainable management, and restoration of those remnant fragmented patches.
Recognizing the potential role of small remnant forest patches for species conservation is suggested. Sustainable forest management and conservation plan should be developed and implemented to reduce further forest fragmentation, disturbance, and illegal cutting. All stockholders should work together to reduce forest fragmentation and disturbance that led to local extinctions. Farming inside and surroundings of forest patches and human encroachment must be addressed in collaboration with local communities. Restoration and rehabilitation programs should be carried out at a larger scale in collaboration with the local communities and concerned stakeholders. Further studies are required to investigate the effects of forest fragmentation and disturbance on seed dispersal, pollination, regeneration, germination, seed survival, and ecosystem functioning. Involving communities via the sense of ownership and benefit-sharing is useful to sustainably conserve, manage, and utilize forests. Besides, providing seedlings of socially, environmentally, and economically useful species for communities to establish plantation forests either in privately owned or communal land will be useful to reduce further anthropogenic pressure and forest distraction. Otherwise, further fragmentation, disturbance, forest loss, and local extinction will take place on a larger scale.
Data availability
The authors declare that data supporting the findings of this study are available within the article and its supplementary information files.
References
Abere F, Belete Y, Kefalew A (2017) Carbon stock of Banja forest in Banja district, Amhara region, Ethiopia: an implication for climate change mitigation. J Sustain For 9811:16. https://doi.org/10.1080/10549811.2017.1332646
Aguilar-santelises R, Castillo RF (2013) Factors affecting woody plant species diversity of fragmented seasonally dry oak forests in the Mixteca Alta, Oaxaca, Mexico. Rev Mex Biodivers 84:575–590. https://doi.org/10.7550/rmb.30458
Atkinson EE, Marín-Spiotta E (2014) Land use legacy effects on structure and composition of subtropical dry forests in St. Croix, U.S. Virgin Islands For Ecol Manage 335:270–280. https://doi.org/10.1016/j.foreco.2014.09.033
Aynekulu E, Aerts R, Moonen P et al (2012) Altitudinal variation and conservation priorities of vegetation along the Great Rift Valley escarpment, northern Ethiopia. Biodivers Conserv 21:2691–2707. https://doi.org/10.1007/s10531-012-0328-9
Aynekulu E, Aerts R, Denich M et al (2016) Plant diversity and regeneration in a disturbed isolated dry Afromontane forest in northern Ethiopia. Folia Geobot 51:115–127. https://doi.org/10.1007/s12224-016-9247-y
Ayyappan N, Parthasarathy N (2001) Composition, population structure and distribution of dipterocarps in a tropical evergreen forest at Varagalaiar, Anamalais, Western Ghats, South India. J Trop For Sci 13:311–321
Bekele A (2007) Useful trees and shrubs of Ethiopia: Identification, Propagation, and Management for 17 Agroclimatic Zones, 2nd edn. World Agroforestry Centre, Nairobi
Benitez-Malvido J (1998) Impact of forest fragmentation on seedling abundance in a tropical rain forest. Conserv Biol 12:380–389. https://doi.org/10.2307/2387508
Berhanu A, Demissew S, Woldu Z, Didita M (2017) Woody species composition and structure of Kuandisha afromontane forest fragment in northwestern Ethiopia. J For Res 28:343–355. https://doi.org/10.1007/s11676-016-0329-8
Bhat DM, Hegde GT, Shetti DM et al (2011) Impact of disturbance on composition, structure, and floristics of tropical moist forests in Uttara Kannada District, Western Ghats, India Ecotropica: Society. Trop Ecol 17:1–14
Bongers F, Wassie A, Sterck F et al (2006) Ecological restoration and church forests in northern Ethiopia. J Dry Lands 1:35–44
Chapman HM, Olson SM, Trumm D (2004) An assessment of changes in the montane forests of Taraba State, Nigeria, over the past 30 years. Oryx 38:282–290. https://doi.org/10.1017/S0030605304000511
Connell JH (1978) Diversity in tropical rain forests and coral reefs high diversity of trees and corals is maintained only in a nonequilibrium state. Science 199:1302–1310
Cotler H, Ortega-Larrocea MP (2006) Effects of land use on soil erosion in a tropical dry forest ecosystem, Chamela watershed, Mexico. Catena 65:107–117. https://doi.org/10.1016/j.catena.2005.11.004
Darbyshire I, Lamb H, Umer M (2003) Forest clearance and regrowth in northern Ethiopia during the last 3000 years. Holocene 13:537–546. https://doi.org/10.1191/0959683603hl644rp
Debinski DM, Holt RD (2000) A survey and overview of habitat fragmentation experiments. Conserv Biol 14:342–355. https://doi.org/10.1046/j.1523-1739.2000.98081.x
Denslow JS (1980) Oecologia during succession under different disturbance regimes. Oecologia 21:18–21
Drinnan IN (2005) The search for fragmentation thresholds in a Southern Sydney Suburb. Biol Conserv 124:339–349. https://doi.org/10.1016/j.biocon.2005.01.040
Dytham C (2009) Evolved dispersal strategies at range margins. Proc R Soc B 27:1407–1413. https://doi.org/10.1098/rspb.2008.1535
Echeverría C, Newton AC, Lara A et al (2007) Impacts of forest fragmentation on species composition and forest structure in the temperate landscape of southern Chile. Glob Ecol Biogeogr 16:426–439. https://doi.org/10.1111/j.1466-8238.2007.00311.x
Edwards S, Kelbessa E, Demissew S (2000) Flora of Ethiopia and Eritrea, 1st edn. Volume 2, part 1: Magnoliaceae to Flacourtiaceae. National Herbarium, Addis Ababa, Ethiopia and Uppsala, Sweden
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev Camb Philos Soc 81:117–142. https://doi.org/10.1017/S1464793105006949
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Annu Rev Ecol Evol Syst 34:487–515. https://doi.org/10.1146/annurev.ecolsys.34.011802.132419
Franklin JF, Spies TA, Van Pelt R, Carey AB, Thornburgh DA, Berg DRLD, Harmon ME, Keeton WS, Shaw DC, Bible KCJ (2002) Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. For Ecol Manag 155:399–423
Friis I, Demissew S, van Breugel P (2010) Atlas of the potential vegetation of Ethiopia, 1st edn. Det Kongelige Danske Videnskabernes Selskab, Gothersgade
Genet Y, Ejigu D (2017) Community composition, relative abundance and habitat association of avian species in Apini and Dikuma forest patches, Awi Administrative Zone, Ethiopia. Ethiop J Sci Technol 10:33–50
Giam X, Scheffers BR, Sodhi NS et al (2011) Reservoirs of richness: least disturbed tropical forests are centres of undescribed species diversity. Proc R Soc B Biol Sci 279:67–76. https://doi.org/10.1098/rspb.2011.0433
Godefroid S, Koedam N (2016) How important are large vs. small forest remnants for the conservation of the woodland flora in an urban context ? Glob Ecol Biogeogr 12:287–298
Haddad NM, Brudvig LA, Clobert J et al (2015) Habitat fragmentation and its lasting impact on Earth’ s ecosystems. Sci Adv 1:1–9. https://doi.org/10.1126/sciadv.1500052
Hammer Ø, Harper DAT, Ryan PD (2001) Past: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Hedberg L, Friis I, Edwards S (2004) Flora of Ethiopia and Eritrea, 1st edn. Addis Ababa Univcnity, Addis Ababa
Hedberg I, Kelbessa E, Edwards S, et al (2006) Flora of Ethiopia and Eritrea, 2nd edn. The National Herbarium, Biology Department, Science Faculty, Addis Ababa University, Ethiopia; and the Department of Systematic Botany Uppsala University, Sweden, Addis Ababa
Hill JL, Curran PJ (2003) Area, shape and isolation of tropical forest fragments: effects on tree species diversity and implications for conservation. J Biogeogr 30:1391–1403. https://doi.org/10.1046/j.1365-2699.2003.00930.x
Hillebrand H, Bennett M, Cadotte W (2008) Consequences of dominance: a review of evenness effects on local and regional ecosystem processes. Ecology 89:1510–1520
Hubbell SP, Foster RB, O’Brien ST et al (1999) Light-gap disturbances, recruitment limitation, and tree diversity in a neotropical forest. Science 283:554–557. https://doi.org/10.1126/science.283.5401.554
Ibáñez I, Katz DSW, Peltier D et al (2014) Assessing the integrated effects of landscape fragmentation on plants and plant communities: the challenge of multiprocess-multiresponse dynamics. J Ecol 102:882–895. https://doi.org/10.1111/1365-2745.12223
Kanzaki M, Yoda K (1986) Regeneration in subalpine coniferous forests – II. Mortality and the pattern of death of canopy trees. Bot Mag Tokyo 99:37–51. https://doi.org/10.1007/BF02488621
Kareiva P, Watts S, McDonald R, Boucher T (2007) Domesticated nature: shaping landscapes and ecosystems for human welfare. Science 316:1866–1869. https://doi.org/10.1126/science.1140170
Kent M (2011) Vegetation description and data analysis: a practical approach, 2nd edn. Wiley, Landon
Kidane YO, Steinbauer MJ, Beierkuhnlein C (2019) Dead end for endemic plant species? A biodiversity hotspot under pressure. Glob Ecol Conserv 19:e00670. https://doi.org/10.1016/j.gecco.2019.e00670
Krauss J, Klein A (2004) Effects of habitat area, isolation, and landscape diversity on plant species richness of calcareous grasslands. Biodivers Conserv 13:1427–1439
Kurki S, Nikula A, Helle P, Linden H (2000) Landscape eragmentation and forest composition effects on grouse breeding success in Boreal forests. Ecology 81:1985–1997
Laurance WF (2008) Theory meets reality: how habitat fragmentation research has transcended island biogeographic theory. Biol Conserv 141:1731–1744. https://doi.org/10.1016/j.biocon.2008.05.011
Linder HP, Lovett JC et al (2005) A numerical re-evaluation of the sub-Saharan phytochoria of mainland Africa. Biol Skr 55:229–252
Ma L, Huang M, Shen Y et al (2015) Species diversity and community structure in forest fragments of Guangzhou, South China. J Trop For Sci 27:148–157
MacArthur RH, Wilson EO (1967) The theory of island biogeography, illustrate. Princeton University Press, Princeton
Magurran AE, Mcgill BJ (2011) Frontiers in measurement and assessment. In: Magurran AE, Mcgill BJ (eds) Compositional similarity and beta diversity, 2nd edn. Oxford University Press, New York, p 359
Majumdar and Datta (2016) Effect of patch size, disturbances on diversity and structural traits of tropical semi-every green forest in the lowland IndoBurma hotspot: implication on conservtion of the threatened tree spcies. J Mt Sci 13:1397–1410
Malik ZA, Hussain A, Iqbal K, Bhatt AB (2014) Species richness and diversity along the disturbance gradient in Kedarnath Wildlife Sanctuary and its adjoining areas in Garhwal Himalaya, India. Int J Curr Res 6:10918–10926
Miles L, Newton AC, DeFries RS et al (2006) A global overview of the conservation status of tropical dry forests. J Biogeogr 33:491–505. https://doi.org/10.1111/j.1365-2699.2005.01424.x
Miller AD, Roxburgh SH, Shea K (2011) How frequency and intensity shape diversity-disturbance relationships. Proc Natl Acad Sci USA 108:5643–5648. https://doi.org/10.1073/pnas.1018594108
Mishra BP, Tripathi OP, Tripathi RS, Pandey HN (2004) Effects of anthropogenic disturbance on plant diversity and community structure of a sacred grove in Meghalaya, northeast India. Biodivers Conserv 13:421–436. https://doi.org/10.1023/B:BIOC.0000006509.31571.a0
Mohandass D, Campbell MJ, Davidar P (2018) Impact of patch size on woody tree species richness and abundance in a tropical montane evergreen forest patches of south India. J For Res 29:1675–1687. https://doi.org/10.1007/s11676-018-0592-y
Montes AH, Olaya-M LA, Castro-H F (2004) Incident of human perturbance on diversity, richness, and distribution of Eleutherodactylus (Anura: Leptodactylidae) in a cloud forest of southwest Colombia. Caldasia 26:265–274
Murali KS, Shankar U, Shaanker RU et al (1998) Extraction or non-timber forest products in the forests of Biligiri Rangan Hills, India. 6. Fuelwood pressure and management options. Econ Bot 52:320–336. https://doi.org/10.1007/BF02862151
Murphy PG, Lugo AE (2007) Ecology of tropical dry. Ecology 17:67–88
Pandeya SC, Chandra A, Pathak PS (2007) Genetic diversity in some perennial plant species with-in short distances Lucknow Agra ( A ) Dalbergia sissoo Delonix regia Cassia fistula Calotropis procera ( B ). J Environ Biol 28:83–86
Panwar P, Bhardwaj SD (2005) Variation in natural regeneration of shorea robusta in the vicinity of a cement factory. For Trees Livelihoods 15:337–347. https://doi.org/10.1080/14728028.2005.9752535
Pao NT, Upadhaya K (2017) Effect of fragmentation and anthropogenic disturbances on floristic composition and structure of subtropical broad leaved humid forest in Meghalaya, Northeast India. Appl Ecol Environ Res 15:385–407. https://doi.org/10.15666/aeer/1504_385407
RamõÂrez-Marciala N, GonzaÂlez-Espinosab M, Williams-Linerac G (2001) Anthropogenic disturbance and tree diversity in Montane Rain Forests in Chiapas, Mexico. For Ecol Manage 154:311–326
Rodríguez-loinaz G, Amezaga I, Onaindia M (2012) Does forest fragmentation affect the same way all growth-forms ? J Environ Manage 94:125–131. https://doi.org/10.1016/j.jenvman.2011.06.024
Rosenzweig ML (1995) Species diversity in space and time, illustrate. Cambridge University Press, New York
Rudas G, Marcelo D, Armenteras D, et al (2007) Biodiversity and human activity: relationships in sub-Andean forest ecosystems in Colombia, 1st edn. Bogotá: Alexander von Humboldt Biological Resources Research Institute, Alexander von Humbold Bogotá D.C., Colombia
Sagar R, Singh JS (2006) Tree density, basal area and species diversity in a disturbed dry tropical forest of northern India: implications for conservation. Environ Conserv 33:256–262. https://doi.org/10.1017/S0376892906003237
Saunders DA, Hobbs RJ, Margules CR (1991) Society for conservation biology biological consequences of ecosystem fragmentation: a review biological consequences of ecosystem fragmentation: a review. Source Conserv Biol Conserv Biol 5:18–32
Senbeta F, Schmitt C, Woldemariam T et al (2014) Plant diversity, vegetation structure and relationship between plant communities and environmental variables in the afromontane forests of Ethiopia. Sinet Ethiop J Sci 37:113–130
Shannon E, Weaver W (1949) A mathematical model of communication. University of illinois Press, Illinois
Shiferaw W, Bekele T, Demissew S (2018) Anthropogenic effects on floristic composition, diversity and regeneration potential of the Debrelibanos Monastery forest patch, central Ethiopia. J For Res 30:2151–2161. https://doi.org/10.1007/s11676-018-0782-7
Stenhouse RN (2004) Fragmentation and internal disturbance of native vegetation reserves in the Perth metropolitan area, Western Australia. Landsc Urban Plan 68:389–401. https://doi.org/10.1016/S0169-2046(03)00151-8
Sukumar R, Dattaraja HS, Suresh H et al (1992) Long-term monitoring of vegetation in a tropical deciduous forest in Mudumalai, southern India. Curr Sci 62:608–616
Sutherland WJ (2006) Ecological census techniques a handbook. Cambridge University Press, New York
Tadele D, Lulekal E, Damtie D, Assefa A (2014) Floristic diversity and regeneration status of woody plants in Zengena Forest, a remnant montane forest patch in northwestern Ethiopia. J For Res 25:329–336. https://doi.org/10.1007/s11676-013-0420-3
Tadesse S, Woldetsadik M, Senbeta F (2017) Effects of participatory forest management on livelihood assets in Gebradima forest, southwest Ethiopia. For Trees Livelihoods 35:604–622. https://doi.org/10.1080/14728028.2017.1322920
Terborgh JW (2015) Toward a trophic theory of species diversity. Proc Natl Acad Sci USA 112:11415–11422. https://doi.org/10.1073/pnas.1501070112
Teshager Z, Argaw M, Eshete A (2018) Woody species diversity, structure and regeneration status in Weiramba forest of Amhara region, Ethiopia : implications of managing forests for biodiversity conservation. J Nat Sci Res 8:26
Tripathi OP, Upadhaya K, Tripathi RS, Pandey HN (2010) Diversity, dominance and population structure of tree species along fragment-size gradient of a subtropical humid forest of Northeast India. Res J Environ Earth Sci 2:97–105
Vivero JL, Ensermu K, Sebsebe D (2006) Progress on the red list of plants of Ethiopia and Eritrea: conservation and biogeography of endemic flowering taxa, 1st edn. Royal Botanic Gardens, Landon
Wale HA, Bekele T, Dalle G (2012) Floristic diversity, regeneration status, and vegetation structure of woodlands in Metema Area, Amhara National Regional State, Northwestern Ethiopia. J For Res 23:391–398. https://doi.org/10.1007/s11676-012-0275-z
Wassie A, Sterck FJ, Teketay D, Bongers F (2009) Effects of livestock exclusion on tree regeneration in church forests of Ethiopia. For Ecol Manage 257:765–772. https://doi.org/10.1016/j.foreco.2008.07.032
Wassie A, Sterck FJ, Bongers F (2010) Species and structural diversity of church forests in a fragmented Ethiopian Highland landscape. J Veg Sci 21:938–948. https://doi.org/10.1111/j.1654-1103.2010.01202.x
Wekesa C, Kirui BK, Maranga EK et al (2019) Forest Ecology and Management Variations in forest structure, tree species diversity, and above-ground biomass in edges to interior cores of fragmented forest patches of Taita Hills, Kenya. For Ecol Manage 440:48–60. https://doi.org/10.1016/j.foreco.2019.03.011
White F (1983) The vegetation of Africa. Nat Resour Res 20:356
Whittaker RH (1972) Evolution and measurement of species diversity. Wiley 21:213–251
Wilson BG, Witkowski ETF (2003) Seed Banks, Bark Thickness and Change in Age and Size Structure (1978–1999) of the African Savanna Tree, Burkea africana 1978–1999) of the African savanna tree, Burkea africana. Plant Ecol 167:151–162
Wilson MC, Chen XY, Corlett RT et al (2016) Habitat fragmentation and biodiversity conservation: key findings and future challenges. Landsc Ecol 31:219–227. https://doi.org/10.1007/s10980-015-0312-3
Wood A, Stedman-Edwards P, Mang J (2000) The root causes of biodiversity loss, illustrate. Earthscan, Washington DC
Yismaw A, Gedif B, Addisu S, Zewudu F (2014) Forest cover change detection using remote sensing and GIS in Banja District, Amhara Region, Ethiopia. Int J Environ Monit Anal 2:354. https://doi.org/10.11648/j.ijema.20140206.19
Zegeye H, Teketay D, Kelbessa E (2006) Diversity, regeneration status and socio-economic importance of the vegetation in the islands of Lake Ziway, south-central Ethiopia. Flora 201:483–498. https://doi.org/10.1016/j.flora.2005.10.006
Ziter C, Bennett EM, Gonzalez A (2013) Functional diversity and management mediate aboveground carbon stocks in small forest fragments. Ecosphere 4:1–21. https://doi.org/10.1890/ES13-00135.1
Acknowledgements
We would like to thank Wollo University through the ministry of science and higher education of Ethiopia for funding support to undertake this study.
Funding
Financial support to undertake the study was received from Wollo University through ministry of science and higher education of Ethiopia.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [MGM]. The first draft of the manuscript was written by [MGM] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The authors have no Ethical or conflicts of interest to declare that are relevant to the content of this article.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in Fig. 3d and g.
Additional information
Communicated by Daniel Sanchez Mata.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Forest and plantation biodiversity.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muluneh, M.G., Feyissa, M.T. & Wolde, T.M. Effect of forest fragmentation and disturbance on diversity and structure of woody species in dry Afromontane forests of northern Ethiopia. Biodivers Conserv 30, 1753–1779 (2021). https://doi.org/10.1007/s10531-021-02167-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-021-02167-x