Abstract
This study assesses the effects of human disturbance on: (1) floristic composition, diversity, and plant community types; and, (2) the regeneration potential of woody species in the Debrelibanos Monastery forest patch in the North Showa zone of Oromia National Regional State, Ethiopia. Fifty sample quadrats were placed on parallel transect lines along altitudes in the forest using a systematic sampling design. Quadrats of 1 m2, 100 m2 and 400 m2 were used for recording herbaceous/seedling, sapling and tree species, respectively, with symptoms of different human disturbance effects. One hundred and thirteen species belonging to 52 families were recorded in the forest patch. The results show 459.5 stems ha−1 of tree/shrub species present. Four community types, namely Juniperus procera–Acacia lahai, Carissa spinarum–Acacia abyssinica, Olea europaea subsp. cuspidata–Euphorbia abyssinica and Ficus sur–Allophylus abyssinicus were identified. The overall Shannon–Wiener diversity index was 3.3. The regeneration patterns of some species were poor. Slope, altitude and human activities had significant effects on vegetation patterns and imply that current management practices are not satisfactory to sustain the forest. Unless improved management interventions are made, the sustainability of forest services will be at stake.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Forests are among the most important ecosystems and cover about one-third of the earth’s land surface (Thies et al. 2011). Tropical forests are habitats of numerous species, constituting biological diversity through many Webs of Life. They support various life forms, including humans who dwell in settlements in and around forests (Mukhopadhyay et al. 2007). Between 1990 and 2000, 1410 km2 forests of Ethiopia were lost each year, equaling an average annual deforestation of 0.9%. However, between 2000 and 2005, deforestation increased by 1.0% to 10.4% per year, i.e., 21,140 km2 lost in 15 years between 1990 and 2005. Estimates by Shiferaw et al. (2018) showed that the area of closed forest declined to about 3.0–4.0% of the country. A recent report (FAO 2007) showed that 124,990 km2 (11.4%) of the total land area of Ethiopia (1,096,310 km2) was covered by forests.
Forest resources, particularly in central and northern Ethiopia, have been denuded in the last several decades and are shrinking mainly towards the south (Dessie and Kleman 2007) and southwestern parts of the country (Aynekulu 2011). In the northern and central highlands, only a few isolated patches can be found in inaccessible areas and around the Ethiopian Orthodox Tewahido churches (Wassie 2007; Bekele 1994). Despite declining vegetation cover, Ethiopia hosts a rich biodiversity. The Ethiopian flora consists of more than 5750 species, including subspecies and varieties of species (Kelbessa and Demissew 2014). About 12% are possibly endemic (Abera 2014). However, unsustainable utilization of plant resources has resulted in the decline of the country’s biodiversity at a rapid rate.
Deforestation of tropical forests for agriculture is one of the major forces shaping the Earth’s surface (Lambin and Meyfroidt 2011). Population growth coupled with traditional farming practices have posed tremendous impact on land resources in the Ethiopian highlands where climatic conditions are favorable for life and agricultural production. The high concentration of farming population results in land shortage, habitat fragmentation, loss of vegetative cover, and exposure of extensive areas to erosion (Teshome 2014). Antagonism between forestry practices and grazing has given rise to a fragmented landscape, with remnant patches of native forests on the top of hills and mixed in the grasslands in the valleys (Sanchez-Jardon et al. 2010). As a result, deforestation and fragmentation influence the microclimate, composition, diversity, and vegetative structure of these remaining patches of tropical forest (Nagy et al. 2015). The effects of deforestation and degradation are continuing in the highlands, particularly within the Debrelibanos Monastery forest patch (henceforth referred to as the DMFP).
Except for a few research reports by Getaneh and Girma (2014), no detailed scientific study has been carried out regarding the effects of human disturbance in the DMFP. This study assesses the effects of human disturbances on: (1) the floristic composition, diversity, and plant community types; and, (2) the regeneration potential of woody species in the DMFP in the North Showa zone of Oromia National Regional State. These objectives are designed to prove the following hypothesis: (1) anthropogenic effects change vegetation patterns; (2) anthropogenic affects regeneration patterns; and, (3) some physiographic variables affect vegetation patterns.
Materials and methods
Description of the study area
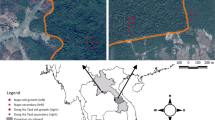
This study was conducted April 15 to 30, 2017 in the DMFP located 110 km north of Addis Ababa. The Debrelibanos Monastery is situated between 2341 and 2500 masl with an area of 0.85 km2 (Getaneh and Girma 2014). The monastery is located 38°50′51″E to 9°42′43″N (Fig. 1).
Since there was no meteorological station for the Debrelibanos district, climate data from the nearest meteorological station was used. Thirty-seven years of climate data were drawn using the Walter climadiagram program of R software version 3.4.2 (R Software Developing Team 2017) and library climatol (Woldu 2017). The highest monthly rainfall is in July and the lowest in November and December. Average monthly maximum temperatures were in June (20.9 °C) and monthly average minimum temperatures in December (5.7 °C). Annual rainfall and temperatures were 1097 mm and 13.3 °C, respectively (Fig. 2).
According to the WRB (2006) soil classification, the most common soil type in the area is vertisol (with a high content of expansive clay) which constitutes about 56% or 166.8 km2 of the area, while nitosol is 38% (113.4 km2), and the rest 6% (17.9 km2) a mixture of different soil types. The soil texture is 63% clay, 27% silt and 10% sand (Getaneh and Girma 2014).
The vegetation is characterized by remnant Afromontane forest in the middle altitudes and grass lands at higher elevations in the district. The old Afromontane forest in the middle altitude is owned by the Debrelibanos Monastery of the Ethiopian Orthodox Church. The common vegetation is remnants of trees in agricultural fields, shrubs and secondary forests surrounding the forest patch.
The population of the Debrelibanos district was over 45,000, and crop production, rearing livestock and petty trade are the main activities around the forest patch. In the forest, the Orthodox Tewahido Church Saints and other believers have settled for giving spiritual services in the monastery church.
Sampling design
After a reconnaissance survey of the forest area, nine parallel transect lines with a 500-m interval between lines were laid across the forest from southwest to northeast (Fig. 1). Fifty quadrats 20 m × 20 m (400 m2) were placed systematically on transects with 10-m altitude changes. Therefore, for the tree inventory, 20 m × 20 m size quadrants were used; for the sapling and seedling and herbaceous species inventories, 10 m × 10 m and 1 m × 1 m were set out in each main plot, respectively (Fig. 1).
Data collection
Diameter at breast height (DBH) for trees or diameters at stump height (DSH) for shrubs were measured in the plots. Individual woody categorizations were made at height less than 1 m and DBH/DSH less than 1-cm for seedlings. The 1.0–2.0 heights and DBH/DSH 1.0–5.0 cm for saplings, and heights greater than 2.0 m and DBH/DSH ≥ 5 cm for tree/shrub species were recorded. For saplings and seedlings, only their numbers were recorded. Climbers germinate on the forest floor and anchor or adhere to other plants for part of their life cycle to attain greater stature (Swaine et al. 2005). Accordingly, climbers were considered in each of the life forms.
The equivalent shrub diameters are for individuals with above ground stems growing in a cluster according to Chojnacky and Milton (2008), and field experience, Eq. (1). Saplings were sampled using 10 m × 10 m subplots established in the center of the plot, whereas seedlings were counted in 1 m × 1 m plots at the center and four corners of the main plot. The presence/absence of species was registered by visual estimation. All plots were geo-referenced using GPS for coordinates and altitudes, and a clinometer for slopes.
In all quadrants, species were identified by their names, pressed, coded and grouped as trees, shrubs, herbs and climbers. Scientific name identification was carried out in the herbarium, and voucher specimens prepared and placed in the National Herbarium (ETH), Addis Ababa University for further identification. Nomenclatures followed the published flora of Ethiopia and Eritrea from volumes 1–8.
where DSH is Diameter at Stump Height, DSHi is Diameter at Stump Height of individual shrubs.
Physiographic and anthropogenic variables
Physiographical variables such as altitude, geographical coordinates, slope and aspects were recorded for each plot. As a possible indicator of total solar energy, the aspect was coded according to Woldu et al. (1989). Thus, N = 0; E = 2; S = 4; W = 2.5; NE = 1; SE = 3; SW = 3.3 and NW = 1.3. Grazing intensity was estimated based on visual observation of different symptoms of livestock effects such as dung droppings and herbage cuttings following scales designed by Tekle et al. (1997) and Woldu and Backeus (1991). The degree of human interference in each plot was estimated following Hadera (2000). A 0–3 subjective scale was used to record the presence or absence of stumps, logs, and signs of fuel wood collection. The magnitude of the impacts was quantified following 0 = nil; 1 = low; 2 = moderate; and 3 = heavy.
Data analysis
The analysis of vegetation patterns and environmental relations was organized by recording and arranging the data on the Excel Microsoft data sheet. All vegetation data were verified and analyzed using cluster and multivariate data analysis in base R Software programs to compute the diversity, environmental and anthropogenic variables versus vegetation relationships using ecological indicators and multivariate ordination programs of Canonical Correspondence Analysis (CCA).
Diversity analysis
Plant diversity can be measured using Shannon–Weiner diversity index and species richness using Eq. (2) (Magurran 2004; Kent and Coker 1992). Evenness (E’) was calculated from the ratio of observed diversity to maximum diversity following Pielou (1966), Eq. (3).
The cover/abundance values of all plant species in each plot were visually estimated using a Braun-Blanquet scale, modified 1–9 following Van der Maarel (1979). The basal area per tree was calculated using Eq. (4). Frequency is described as the occurrence or absence of a given species per sampling unit. It is expressed as an absolute frequency, which refers to the percentage of the total number of sampling units containing the species to all the plots or as a relative frequency showing the percentage of absolute frequency of a species compared to the total absolute frequencies of all the plots added up (Lamprecht 1989). The IVI indicates the importance of species in the system and calculated with three components as following Kent and Coker (1992) Eq. (5).
where ni= total number of individuals in each species; H is observed index of species diversity; N = total number of individuals in all species.
where H is observed index of species diversity; Hmax = the maximum level of diversity possible within a given population, given S species and N individuals
where d is diameter at breast height, BA is basal area.
where IVI is Importance Value Index; R1 is Relative Density, R2 is Relative Dominance, and R3 is Relative Frequency. For separation of community types and environmental and anthropogenic variables versus vegetation relationships, R software version 3.4.2 was used. The similarity ratio (SR) with colored leaves and labels in the working directory using Ward’s method were applied (Woldu 2017). The groups were tested for the hypothesis of no difference between groups using the multi-response permutation procedure (Senbeta 2006). Both presence-absence and quantitative data were entered into a spread sheet, classified, and ordinated various multivariate methods. Synoptic cover abundance values for each species in all communities were calculated following Woldu (2017).
For the analysis of environmental and anthropogenic variables versus vegetation relationships, the Canonical Correspondence Analysis (CCA) ordination technique was used (Senbeta 2006; Naderi and Sharafatmandrad 2017). The regeneration patterns of the species were determined based upon the population size of seedlings, saplings and trees. Histograms were drawn using Microsoft Excel Software.
Results and discussion
Effects of selected anthropogenic and physiographic variables on vegetation patterns
Plant species experience the conditions provided by environmental properties (Cajo and Braak 1987). The results indicate that altitude and grazing intensity (P < 0.05) and slope (P < 0.01) had significant effects on vegetation patterns in the DMFP. However, human impacts and disturbance factors did not show any significant effects (P < 0.05) on vegetation patterns (Table 1; Fig. 3).
Floristic composition and structure
Species composition influences ecosystem properties in terms of species richness and diversity (Downing 2005). In this study, 113 plant species belonging to 52 families in sampled quadrants were recorded, including trees, shrubs and herbaceous plants, which is approximately 2% of the plant species in the flora of Ethiopia (Table S1). A comparison of species composition of dry Afromontane forests is difficult due to the inherent heterogeneity of the forests (Aynekulu 2011). However, to give a general view of the DMFP for instance, the number of species was less by 26.1% than the number Aynekulu (2011) recorded in dry Afromontane forests of fragmented landscapes in northern Ethiopia, and higher by 38.9% than the number recorded by Pare et al. (2009) in the Sudanian dry forest of Burkina Faso.
Among the species recorded 45 (39.8%) were herbs, 26 (23%) trees, 36 (31.9%) shrubs and 6 (5.3%) climbers (Fig. 4). The vegetation cover in the study site is dominated by shrub and herb species. The cause for the low tree species diversity is due to the gradual selective logging of some useful species (Aynekulu 2011). Among the families recorded, Asteraceae contributed the highest number of species, 28 (15.6%), followed by Poaceae16 (8.9%), Lamiaceae 10 (5.6%), and Fabaceae and Apiaceae, 8 (4.5%).
The collection of information for the conservation of endemic plants is vital for further research and planning conservation actions (Vivero et al. 2005). According to IUCN Red List Categories, twelve endemic plant species were recorded from the study area (Table 2), and shared 10.6% of the total floristic composition of the forest.
Ecological availability of woody species
Stand density is an important forest management parameter along with other forest structure parameters, e.g., crown closure and crown diameter, and is used to evaluate the regeneration status of a forest (Quackenbushg et al. 2000). In this study, the overall density of the DMFP was 459.5 stems ha−1 of woody species (Table S2). However, Demie et al. (2013) reported 325.0 stems ha−1 from the same forest patch. This is 29.3% lower than in the present study. Stand density of the DMFP was lower by 79.1% than for other dry Afromontane forests in Ethiopia, for example, the Lake Zengena forest remnant montane forest (2202 stems ha−1) in northwestern Ethiopia (Tadele et al. 2014). The low density in this study is probably due to livestock grazing.
IVI is a good index for summarizing vegetation characteristics and ranking species for management and conservation practices (Kent and Coker 1992). The species IVI in this study showed that Olea europaea subsp. cuspidate (Wall. & G.Don) Cif. (100.0%), Juniperus procera Hoechst. ex Endl. (69.1%), Euphorbia abyssinica (18.7%), Acacia abyssinica (16.5%), Acacia lahai (9.2%), Carissa spinarum L. (15.6%), Allophylus abyssinicus (15.4%), Ficus sur Forssk. (7.9%), Calpurnia aurea (6.5%) and Clutia abyssinica (7.4%) species were the dominant species in the forest). Rhus glutinosa (0.9%), Ekebergia capensis (0.1%), Buddleja polystachya (0.9%), Croton macrostachyus (3.0%), Grewia trichocarpa (1.2%), Prunus africana (1.4%), Rhus natalensis (5.0%), and Urera hypselodendron (1.6%) had lower IvI values (Table S2).
Plant communities
Plant communities provide useful clues for analyzing patterns of plant species which have similar environmental requirements for their existence (Aynekulu 2011). In Fig. 5, four plant community types, Juniperus procera–Acacia lahai, Carissa spinarum–Acacia abyssinica, Olea europaea subsp. cuspidata–Euphorbia abyssinica and Ficus sur–Allophylus abyssinicus, were represented on 11, 10, 15, and 14 plots, respectively.
Variations in a particular species are used to interpret the relative variations between and within the species and help to explain the underlying reasons for such differences (Kent and Coker 1992). In this study, the results show that four community types differed in species diversities (Table 4). The Juniperus procera–Acacia lahai community had a higher Shannon–Wiener diversity index (3.1) than Carissa spinarum–Acacia abyssinica (3.0), Olea europaea subsp cuspidata–Euphorbia abyssinica (2.6) and Ficus sur–Allophylus abyssinicus (2.9).
However, the results in Table 4 show that the species richness of the Carissa spinarum–Acacia abyssinica community (72; 31.17%) was higher than the Juniperus procera–Acacia lahai (55; 23.81%) and Olea europaea subsp. cuspidate–Euphorbia abyssinica (55; 23.81%) communities and higher than the Ficus sur–Allophylus abyssinicus community (49; 21.21%). The results indicate that a community type with high species richness is not necessarily one having high species diversity because diversity is the combination of species richness and relative species abundance in space and time (Magurran 2004).
C1: Juniperus procera–Acacia lahai type
The most dominant species in this community were Juniperus procera and Acacia lahai. They were not characteristic species of the canopy layer. The main associated species were Dodonaea angustifolia; Maytenus arbutirolia, Clutia abyssinica, and a woody climber Urera hypselodendron (Hochst. ex A. Rich.) Wedd. in the middle story, and the herbaceous species Cynodon dactylon, Hyparrhenia hirta, Fimbristylis complanata, Geranium elamellatum, Cenchrus ciliaris and Crassula alsinoides in the understory (Table 3).
C2: Carissa spinarum–Acacia abyssinica type
Acacia abyssinia in the canopy layer with the shrub Carissa spinarum were the most dominant species and were associated with Rhus natalensis in the canopy, and the tree, Vernonia bipontini and the shrub Calpurnia aurea in the middle layer. Characteristic herbaceous species were Achyranthes aspera var pubescen, Asparagus africanus Lam., Ocimum lamiifolium Taraxacum officinale, and one herbaceous climber, Clematis simensis Fres in the understory (Table 3).
In order to compare the diversity status of the DMFP with the diversity of other dry Afromontane forests, it is necessary to analyze the overall diversity indices of the forest patch. The results show that the forest had a species richness of 113, a Shannon–Weiner diversity index of 3.3, and an evenness of 0.7 (Table 4). The Shannon–Weiner diversity of the study site was comparable with those found by Wassie (2007) in church forests of Gedamselase and Qorata in northern Ethiopia. However, the DMFP was higher in Shannon–Weiner diversity than Woji, Alember and Debresena church forests in northern Ethiopia. However, Shannon–Weiner diversity was less than Wonkesht and Shamo church forests in northern Ethiopia.
C3: Olea europaea subsp. cuspidata–Euphorbia abyssinica type
In this community, the most dominant tree species were Olea europaea subsp. cuspidata and Euphorbia abyssinica in the canopy layer. However, no characteristic tree species were associated with the community. Jasminum stans, Leonotis ocymifolia, Lippia adoensis and Entada abyssinica were associated shrub species in middle layer. Snowdenia polystachya, Hypoestes triflora, Galium simense, and Hibiscus macranthus were characteristic understory herbaceous species (Table 3).
C4: Ficus sur–Allophylus abyssinicus type
The dominant species in this type were Ficus sur and Allophylus abyssinicus. The dominance value revealed that associated characteristic tree species in the upper canopy did not exist in the community except the two existing in the community name. The main associated species were the tree species, Brucea antidysenterica and the shrub, Sparmannia ricinocarpa in the middle story. The understory consisted of the herbaceous Achyranthes aspera var. argentea, and the herbaceous climbers Cyphostemma cyphopetalum and Stephania abyssinica. (Table 3).
Regeneration patterns of woody species
The population structure can help in understanding the regeneration patterns of species and forest stands (Worku et al. 2012). According to Worku et al. (2012), the pattern of population structure for a given species can be roughly grouped in one of three basic types (I, II, III). Type I is a pattern in which a diameter size class distribution displays a greater number of smaller trees than large ones. Type II is characteristic of species that show discontinuous, irregular and or periodic recruitment. Type III reflects species whose regeneration is severely limited. The results in this study show that the populations of Allophylus abyssinicus, Acacia abyssinica, Acacia lahai, Juniperus procera, Olea europaea subsp. cuspidata and Ficus sur, the regeneration patterns had J-shape distributions (Type III). The density value of populations increased from seedlings to saplings, and then trees (Fig. 6a). Moreover, the results also reveal that trees were at climax growth conditions and either contributed few seeds or there might be seed predators which affected seed dispersal. Human activities such as the grazing of livestock and lack of seed rain dispersal could also be reasons for a lack of seedlings and saplings of the above mentioned species (Wasssie 2007).
The regeneration patterns of Calpurnia aurea, Maytenus senegalensis, Carissa spinarum, Ficus thonningii and Maytenus arbutirolia were bell–shaped (Type II) and had the following order: saplings > seedlings > trees (Fig. 6b). The bell-shaped distribution suggests that something inhibited regeneration (Worku et al. 2012; Shiferaw et al. 2018). This may be inherent to the species or due to external factors, and requires further investigation. The pattern might be due to cutting of the species for fuel wood for baking bread (‘Daabbe’) and cooking broad bean (‘Nifro’), which are recognized by Tewahido Orthdox church followers for spiritual purposes. The regeneration pattern of Prunus africana species showed a different shape from Type I pattern but discontinued (U-shape) in the mid–diameter classes (Fig. 6c).
As a rule, cutting of other tree species in the Monastery forest is forbidden except for Carissa spinarum and other shrubs for fuel wood. Cutting these species prevented their growth to higher DBH and height classes (personal communication with assigned for resources management of the Monastery). Despite indications of inhibited regeneration, most of the species in these groups had a considerable number of individuals in the middle diameter classes that could be managed to improve their regeneration (Worku et al. 2012).
Conclusions
In this study, the results show that the Debrelibanos Monastery forest patch has 113 plant species, with herbs contributing the highest number, followed by shrubs and trees. This suggests that the regeneration of woody tree species is more vulnerable to human activities in the form of gradual cutting and livestock grazing. As a result, herbs and shrubs dominate the lower strata of the forest.
Densities and importance value indices of some woody species were comparable with some woody species in dry Afromontane forests in Ethiopia. This implies that the size of the forest was small and the presence of large woody species was severely impacted by human activities. For instance, the results reveal that density and importance value indices of Olea europaea subsp. cuspidata, Juniperous procera, Acacia abyssinica, Acacia lahai, Ficus sur and Allophylus abyssinicus were better than for Prunus africana, Ekebergia capensis, Ficus thonningii, Buddleja polystachya and Calpurnia aurea. The variations in densities and importance value indices were associated with anthropogenic activities, implying that the species were endangered.
The basal area of the Debrelibanos forest was lower than that of some dry Afromontane forests in Ethiopia. This indicates that the diameter at breast height and number of individuals were lower in the forest, and implies that the forest was under severe disturbances by human activities.
Juniperus procera–Acacia lahai, Carissa spinarum–Acacia abyssinica, Olea europaea subsp. cuspidata–Euphorbia abyssinica and Ficus sur–Allophylus abyssinicus communities differed in species diversities, which ranged from Shannon–Weiner diversity values of 2.6 to 3.1. The overall Shannon–Weiner diversity of the forest was 3.3. On the other hand, the evenness index was 0.7. The number of families recorded was 52, which is near to the upper limits of the number of families for tropical forests (Campbell et al. 1992, cited in Swamy et al. 2000). This suggests that the forest had habitat heterogeneity.
The results indicate that altitude, slope and human activities had significant impact among community types on vegetation patterns. However, human activities (cutting for fuel wood) did not show any significant impact on community types in the forest. This showed that cutting woods in the forest is inhibited in the forest.
The distribution of Acacia lahai, Acacia abyssinica, Euphorbia abyssinica and Olea europaea subsp. cuspidata showed J-shape distribution, revealing that they had not in good regeneration potential. Whereas Prunus africana, Allophylus abyssinicus, Croton macrostachyus and Rhus natalensis showed that their regeneration was impeded or irregular in some diameter or height classes. The population pattern of Juniperus procera also showed irregular distribution and its capacity to regenerate was inhibited. The presence of some large trees and the prevalence of small to medium-sized individuals showed that it was exposed to long-term, gradual human activities (animal grazing and cutting), and there were problems of seed dispersal. In general, the dynamics of forest conditions in the Debrelibanos forest patch needs detailed studies in the future, as the forest harbors a significant number of endemic species.
However, it can be seen that better management is needed so that the resources can be effectively utilized on a sustainable basis. Therefore, the following recommendations are made to meet these requirements: (1) Create awareness of the various uses of non-timber forest products (NTFPs) that could be utilized from the forest so that local communities will be in agreement for forest management; (2) Grazing land management in the surrounding kebeles should be encouraged so that grazing pressure in the forest can be reduced; (3) There should a program of awareness for local communities in the utilization of the forest; (4) Feeding of livestock in the forest should be reduced so that regeneration can be encouraged; (5) Forest management techniques such as enrichment planting, especially with indigenous species, should be encouraged; and, (6) Additional research should be undertaken to improve conservation of the forest resources.
References
Abera B (2014) Medicinal plants used in traditional medicine by Oromo people, Gimbi District, Southwest Ethiopia. J Ethnobiol Ethnomed 10(4):1–15
Aynekulu EB (2011) Forest diversity in fragmented landscapes of northern Ethiopia and implications for conservation. PhD Thesis, Ecology and Development Series No. 76, Bonn, Germany, pp 1–141. http://hss.ulb.uni-bonn.de/2011/2294/2294a
Bekele T (1994) Phytosociology and ecology of a humid afromontane forest on the Central Plateau of Ethiopia. J Veg Sci 5(1):87–98
Cajo JF, Braak T (1987) The analysis of vegetation-environment relationships by canonical correspondence analysis. Vegetatio 69:69–77
Chojnacky DC, Milton M (2008) Field measurements for forest carbon monitoring, measuring carbon in shrubs. In: Hoover CM (ed) Springer Science + Business Media B.V. pp 45–72. https://springerlink.bibliotecabuap.elogim.com/chapter/10.1007/978-1-4020-8506-2_5/
Demie G, Limenih M, Belliethanthan S (2013) Plant community types, vegetation structure and regeneration status of remnant dry afromontane natural forest patch within debrelibanos monastry. Natural Resources and Conservation, Ethiopia, pp 1–24
Dessie G, Kleman J (2007) Pattern and magnitude of deforestation in the South Central Rift Valley Region of Ethiopia. Mt Res Dev 27(2):162–168
Downing AL (2005) Relative effects of species composition and richness on ecosystem properties in ponds. Ecology 86(3):701–715
FAO (2007) State of the world’s forests, Food and Agriculture Organisation of the United Nations, Rome. http://www.fao.org/3/a-a0773e.pdf
Getaneh S, Girma Z (2014) An ethnobotanical study of medicinal plants in Debrelibanos Wereda, Central Ethiopia. Afr J Plant Sci 8(7):366–379
Hadera G (2000) A study on the ecology and management of the Dessa forest in the northeastern escarpment of Ethiopia, Unpublished MSc Thesis, Addis Ababa University
Kelbessa E, Demissew S (2014) diversity of vascular plant taxa of the flora of Ethiopia and Eritrea. Ethiop J Biol Sci 13:37–45
Kent M, Coker P (1992) Vegetation descrption and analysis: a practical approach, England, pp 1–363. https://www.jstor.org/stable/4201426
Lambin EF, Meyfroidt P (2011) Global land use change, economic globalization, and the looming land scarcity. Proc Natl Acad Sci USA 108(9):3465–3472
Lamprecht H (1989) Tropical forest ecosystems and their tree species. In: Siliviculture in Tropics, possibilities and methods for their long-term utilization, Institute of for siliviculture of the University of Gottingen. Technical Cooperation of Federal Republic of Germany, Berlin
Magurran AE (2004) Measuring biological diversity, UK, pp 1–132. https://www2.ib.unicamp.br/profs/thomas/.pdf
Mukhopadhyay D, Tewari TH, Roy SB (2007) Role of community institutions in joint forest management. J Hum Ecol 21:37–42
Naderi H, Sharafatmandrad M (2017) Change of species diversity in vascular plants across ecological species groups. J Rangel Sci 7(2):107–121
Nagy CR, Porder SC, Paulo NB, Mota RQ, Avizdo SN (2015) Structure and composition of altered riparian forests in an agricultural Amazonian landscape. Ecol Appl 25(6):1725–1738
Pare S, Tigabu M, Savadogo P, Ode PC, Ouadba JM (2009) Does designation of protected areas ensure conservation of tree diversity in the Sudanian dry forest of Burkina Faso? Afr J Ecol 48:347–360
Pielou EC (1966) Species diversity and pattern diversity in the study of ecological succession. J Theor Biol 10:370–383
Quackenbushg L, Hopkins P, Kinn G (2000) Developing forestry products from high resolution digital aerial imagery. Photogramm Eng Remote Sens 66(11):1337–1346
Sanchez-Jardon L, Acosta B, del Pozo A, Casado MA, Ovalle C, Elizalde HF, Hepp C, de Miguel JM (2010) Grassland productivity and diversity on a tree cover gradient in Nothofagus pumilio in NW Patagonia. Agr Ecosyst Environ 137:213–218
Senbeta F (2006) Biodiversity and ecology of afromontane rainforests with wild coffee arabica L. Populations in Ethiopia, PhD thesis, Ecology and Development Series, 38, University of Bonn, ZEF Bonn, Germany, pp 1–152. https://www.zef.de/fileadmin/webfiles/…ecology_development/ecol_dev_38_text.pdf
Shiferaw W, Lemenih M, Gole TWM (2018) Analysis of plant species diversity and forest structure in Arero dry Afromontane forest of Borena zone, South Ethiopia. Trop Plant Res 5(2):129–140
Swaine MD, Hawthorne WD, Bongers F, Toledo Aceves M (2005) Climbing plants in Ghanaian forests. In: Bongers F, Parren MPE, Traore D (eds) Forest climbing plants of West Africa. diversity, ecology and management, CAB International, Wallingford, Oxfordshire, UK, pp 93–107. https://www.researchgate.net/…Swaine/…Climbing_plants_in_Ghanaian_forests/
Swamy PS, Sundarapandian SM, Chandrasekar P, Chandrasekaran S (2000) Plant species diversity and tree population structure of a humid tropical forest in Tamil Nadu, India. Biodivers Conserv 9:1643–1669
Tadele D, Lulekal E, Damtie D, Assefa A (2014) Floristic diversity and regeneration status of woody plants in Zengena Forest, a remnant montane forest patch in northwestern Ethiopia. J For Res 25(2):329–336
Tekle K, Backeus I, Skuglund J, Woldu Z (1997) Vegetation on hill slopes of Wello, Ethiopia: degradation and regeneration. Nord. J Bot 17(5):483–493
Teshome M (2014) Population growth and cultivated land in Rural Ethiopia: land use dynamics, access, farm size, and fragmentation. Resour Environ 4(3):148–161
Thies C, Romsoman G, Cotter J, Meaden S (2011) Intact forest landscapes: why it is crucial to protect them from industrial exploitation. Green Space Research Laboratories Technical Note No. 5, Greenpeace International, 1066 AZ Amsterdam, The Netherlands, pp 1–20. http://www.greenpeace.org/eu-unit/en/campaigns/forests/
Van der Maarel E (1979) Transformation of cover abundance in phytosociology and its effects on community similarity. Vegetation 39:97–114
Vivero JL, Kelbessa E, Demissew S (2005) The red list of endemic trees and shrubs of Ethiopia and Eritrea. In: Magin G, Oldfield S (eds), ISBN: 1 903703 19 0, Fauna & Flora International, Cambridge, UK, pp 1–28. https://www.academia.edu/1440709/
Wassie AE (2007) Ethiopian church forests: opportunities and challenges for restoration. PhD thesis. Wageningen Univeristy, Wageningen, The Netherlands, ISBN: 978-90-8504-768-1, pp 1–212
Woldu Z (2017) Comprehensive analysis of vegetation and ecological data-basic, concepts, methods. Addis Ababa University, Addis Ababa University Press, Rehobot Printing, Addis Ababa, Ethiopia, pp 1–482. www.aau.edu.et/offices/v_president-office/
Woldu Z, Backeus I (1991) The shrub land vegetation in western shewa, ethiopia and its possible recovery. J Veg Sci 2:173–180
Woldu Z, Feoli E, Nigatu L (1989) Partitioning an elevation gradient of vegetation from southeastern ethiopia by probabilistic methods. Vegetation 81:189–198
Worku A, Teketay D, Lemenih M, Fetene M (2012) Diversity, regeneration status, and population structures of gum and resin producing woody species in Borana, Southern Ethiopia. For Trees Livelihoods 21(2):85–96
World Reference Base for Soil Resources (WRB) (2006) World reference base for soil resources: a framework for international classification, correlation and accumulation. World Soil Resource No. 103. 2nd ed., FAO, Rome, pp 1–145. http://www.fao.org/3/a-a0510e.pdf
Acknowledgements
The first author is thankful to Mr. Bahiru Mekonnen and Mr. Kaletsidik Yifru for their assistance during data collection. Special thanks also go to Abba Amdemichael Delelegn for hosting us and providing shelter during data collection. All the members of the National Herbarium of Addis Ababa University are appreciated for their facilitation of materials in the herbarium.
Author information
Authors and Affiliations
Corresponding author
Additional information
Project funding
The work was supported by Arba Minch and Addis Ababa Universities.
The online version is available at http://www.springerlink.com
Corresponding editor: Zhu Hong.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shiferaw, W., Bekele, T. & Demissew, S. Anthropogenic effects on floristic composition, diversity and regeneration potential of the Debrelibanos Monastery forest patch, central Ethiopia. J. For. Res. 30, 2151–2161 (2019). https://doi.org/10.1007/s11676-018-0782-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-018-0782-7