Abstract
Edge effect is a key process influencing populations and communities, particularly in fragmented landscapes. A general analytical framework has been proposed to quantify the strength of the edge effects (extent and magnitude); however, factors determining the later remain poorly explored. Using a continuous approach we explore the response of dung beetle species and assemblages to ecotones which differ in environmental dissimilarity in the Southern Atlantic forest of Argentina. Using baited pitfall traps and automatic sensors, we estimated dung beetle abundance, microclimatic conditions and vegetation structure along five different forest-plantations transects. At the assemblages level, the majority of species showed either edge avoidance or preference; however, the response depended on the environmental dissimilarity between habitats (plantation and native forest) and varied from a neutral response on mature plantations (low contrast ecotone) to edge avoidance on recent ones (high contrast ecotone). At the species level, the degree of habitat specialization explains the differential response of species to edge effects; more specialized species showed stronger edge response while generalist species showed softer or neutral responses. Environmental dissimilarity between confronted habitats and species specialization explain the quantitative component of edge effects on species and assemblages. Functional groups (rollers and tunnellers) often showed opposite responses to edge effects. At the landscape level, functional connectivity of forest fragments is probably drastically reduced by high contrasts matrices (such as recent plantations) for native forest species, whereas soft ecotones (such as native forest-mature plantations) maintained functional connectivity. These results are particularly relevant on highly fragmented landscapes, such as the Atlantic forest, where edge effect is probably one the most important mechanisms affecting native species and communities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Edge effects has largely been recognized as a key process influencing individual behavior, population abundance, community structure and ecological interactions in the transitional area (or ecotone) between two or more contrasting habitats (Ries et al. 2004; Murcia 1995; Wimp et al. 2011; Porensky 2011; van Halder et al. 2011). Despite the large number of studies dealing with edge effects (Murcia 1995; Ries et al. 2004), it is only recently that a unified conceptual and analytical framework has been proposed to understand and quantify the biological consequences of this ecological process (Fagan et al. 1999; Ries et al. 2004; Ries and Sisk 2004; Ewers and Didham 2006; Porensky 2011). Mechanisms involved in the response to edge effects include differential availability of resources for individual species (ecological flows, access to complementary resources or organisms mapping onto resource gradients) and changes in the intensity of biotic interactions (such as predation or competition) (Ries et al. 2004; Ries and Sisk 2004; Wimp et al. 2011) which result in a large variety of responses to edge effects among species and communities; moreover, the same species may have a different response depending on the intensity of the edge effect (e.g. “soft vs. hard” ecotones) (Reino et al. 2009; Campbell et al. 2011; Zurita et al. 2012; Kotze et al. 2012). The varied nature of biological responses make generalizations difficult and lead to confusing classifications of species according to their sensitivity to edge effects (Ries and Sisk 2010; Zurita et al. 2012). In spite of biological mechanisms, interior-edge patterns are also affected by non-biological processes, such as geometric constraints and the correlation with patch area that could artificially create edge responses (Soga et al. 2012; Prevedello et al. 2012).
Proposed theoretical models, such as the resource-based model (Ries and Sisk 2004) successfully predict and explain the qualitative response to edge effects. Recently, Ewers and Didham (2006) developed an analytical approach that quantifies the magnitude and extent of edge effects. Through a series of equations, these authors reproduced the theoretical expected patterns of edge response, including edge preference (unimodal response), edge avoidance (sigmoid response) and edge insensitivity (neutral response). This new approach has several advantages: (1) it can be applied to different biological levels of organization (from individual to communities) and geographical scales, (2) it allows comparisons among studies through a standardized calculation of the magnitude and extent of edge effects, (3) it can be applied to both complete (covering the entire range of distances) and incomplete biological responses edge responses. While this methodology provides a useful tool to quantify edge effects, factors explaining the observed differences in both magnitude and extent of the response among species and communities remain poorly understood (Campbell et al. 2011). We expanded the analytical approach proposed by Ewers and Didham (2006) to further explore factors explaining the quantitative component of edge effects on species and assemblages.
Edge effects and habitat use by species have usually been considered separated processes; however, edge effects may influence the dispersal abilities of individuals into non-preferred habitats (e.g. anthropogenic habitats) (Fonseca and Joner 2007; Hansbauer et al. 2010; Campbell et al. 2011). Dispersal abilities and environmental filters are probably the primary mechanisms structuring communities in natural and human-modified habitats (Kraft et al. 2008). As a consequence of the influence of edge effects on individual dispersal abilities, the estimation of habitat suitability for species without taking into account the distance to the native habitat may bias the perception of habitat quality (Zurita et al. 2012). The direction of this bias (positive or negative) will depend on the response of each species to edges; however, as a general trend, the suitability of non-preferred habitats (such as human modified habitats) will be overestimated for species exhibiting edge avoidance (sigmoid response) or edge preference (unimodal response). At the landscape scale, the overestimation of habitat suitability will result in the overestimation of the functional connectivity among remnants of native habitat affecting the performance of models used to study the consequences of habitat loss and fragmentation (such as metapopulation and metacommunity models and individual based models) (Koh et al. 2010; Pe’er et al. 2011). Recently, Zurita et al. (2012) extended the analytical approach proposed by Ewers and Didham (2006) to correct the bias introduced by edge effects on the estimation of habitat suitability for species in both the preferred and non-preferred habitats using the asymptote derivate from unimodal and sigmoid models as a un-biased estimation of habitat suitability. We used this approach to explore the influence of environmental conditions on species dispersion capacities into human-created habitats.
Dung beetles (Coleoptera: Scarabaeinae) have often been used to study the effects of human disturbance including habitat degradation, replacement and fragmentation (Nichols et al. 2007; Qie et al. 2010). Dung beetles are useful models in ecological studies because they play key roles in ecosystem functioning, have a relatively well described taxonomy and show a variety of responses to anthropogenic disturbances (Spector 2006; Nichols et al. 2008). Moreover, different species of dung beetles have showed a broad range of responses to changes in habitat structure, microclimatic conditions and resources abundance caused by human activities (Spector and Ayzama 2003; Navarrete and Halffter 2008; Silva et al. 2010; Lopes et al. 2011).
We proposed that the contrast in environmental conditions (environmental dissimilarity) between habitats and the range of tolerance of individual species to environmental conditions (species specialization) will determine the magnitude and extent of edge effects on assemblages and species. Species specialization (e.g. range of tolerance) has been proposed as an important factor explaining the differential response of species to habitat degradation (specialization-disturbance hypothesis) (Julliard et al. 2006; Devictor et al. 2008), however, it has never been proposed to explain their response to edge effects. We expected a positive relation between the environmental dissimilarity among ecotones and the response of dung beetle assemblages and between specialization and the response of individual species to edge effects. We conducted the study in the southern Atlantic forest, one of the most fragmented and threatened ecosystems (Myers et al. 2000), where recent studies suggested a central role of edge effects in the response of species and assemblages to habitat fragmentation (Banks Leite et al. 2010).
Materials and methods
Study area
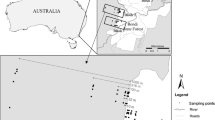
The study was conducted in the subtropical semideciduous Atlantic forest of northeast Argentina (Fig. 1). Mean annual precipitation and temperature are 2,000 mm and 20 °C respectively, with a cold season between June and August; rainfall is evenly distributed throughout the year with no dry season. Native forest is characterized by complex vegetation structure and composition, with three to five tree strata and an understory composed mainly by ferns and bamboo. Landscape in the study area is composed by large tracks of continuous native forest in protected areas, native forest fragments of varied size and shape and commercial tree plantations (Zurita and Bellocq 2010) (Fig. 1).
Pine plantations (mainly Pinus taeda) occupy over 90 % of the tree planted area, but the native Araucaria angustifolia and several species of Eucalyptus spp. are also planted. All plantations in the study area belong to the same forest industry, ensuring consistent forest management. Given that access to private lands is limited, uncontrolled human disturbance (such as hunting, tourism, vehicles, etc.) is very low. The average size of individual plantations stands in the area was 7.4 ha.
Experimental and sampling design
Five tree plantations of different age and type were surveyed to represent a gradient of environmental dissimilarity between human-modified habitats and the native forest where old-growth plantations are more similar to the native forest than recent plantations. As plantations grow, vegetation structure and microclimatic conditions become more similar to the native forest (from hard to soft ecotones) (Zurita and Bellocq 2012). Sampled plantations were: one mature plantation of the native Araucaria angustifolia and one mature plantation of Eucaliptus dunnii (both of 22 years old) and three plantations of Pinus taeda aged 19 (mature), 6 (intermediate) and 2 (recent) years old. Since the objective of this study was not to obtain results which can be extrapolated to a particular situation (ex. mature plantations) we did not include replicates of each plantations type. Instead, as our objective was to explore the response of species and assemblages to edge effects in a series of contrasting habitats, we decided to maximize the number of different ecotones instead of replicating particular ecotone.
In each plantation, a linear transects which starts 300 m inside the native forest and ends 300 m into the plantation (600 m long) was marked. On each 600 m transect, 16 sampling sites comprising both the forest (negative values) and the plantation (positive values) were located at the following distances: 300, 150, 100, 50, 30, 15, 5 and 1 m from the edge between habitats. According to Larsen and Forsyth (2005) a certain degree of interference among traps may occur in the first distances of the gradient (0–30); however Baker and Barmuta (2006) were not able to find autocorrelation in a similar study and conclude that spatial autocorrelation and depletion are unlikely to impair further analyses of edges. In our study, interference among traps will eventually affect the first’s distances of all gradients similarly and, consequently, it will not affect the comparison of species and assemblages responses among gradients.
All transects were, at least, 500 m apart and sampling sites within transects were a minimum of 300 m away from the limit of the stand. To minimize differences related to the origin of native forest species and the interaction between edge effects and area (Gardner et al. 2008; Banks Leite et al. 2010), all transects started at the same continuous protected area (the Peninsula provincial park) (Fig. 1). This protected area, in addition to other national, provincial and private protected areas represent more than 100 km2 of continuous forest.
At each sampling site (16 along each forest-plantation transect), dung beetles were sampled during January–February 2009 using baited pitfall traps for a total of 15 consecutive days. Traps were baited with approximately 20 gr of human dung and renewed every 72 h. Human dung is widely used in dung beetle studies in tropical and subtropical ecosystems because it is attractive to the majority of dung beetle species (Nichols et al. 2007). Traps were filled with a 20 % solution of water and ethylene glycol, to prevent decomposition. Collected specimens were identified to species, when possible, using taxonomic keys and the assistance of a specialized entomologist (Vaz-de-Mello et al. 2011).
Additionally, vegetation structure and microclimatic conditions were described on each sampling site. Canopy and understory cover were estimated on digital photos taken from 1.5 m above the ground. The percentages of canopy cover and understory vegetation in each photo were estimated with Scion-Image Alpha 4.0.3.2. To estimate litter biomass we collected leaf litter and fine wood debris from a 15 × 15 cm plot and stored it in paper bags. Litter was dried at 60 °C for 72 h until weight was constant and then weighed. Ground temperature was recorded every 15 min with a data logger (iButton Termocron) for 10 days of the 15-day sampling period. Average, maximum and minimum daily temperatures and thermal amplitude (maximum–minimum) were calculated from temperature records.
Data analysis
We calculated the relative abundance of each dung beetle species as the total number of individuals caught per pitfall trap over the 15 days sampling period. At the assemblages level differences in species composition (beta diversity) among sampling sites on each forest-plantations transect was estimated with the following procedure: First, an independent multidimensional scaling analysis (MDS) was performed on each forest-plantation transect to ordinate sampling sites according to their similarity on species compositions (using the Morisita-Horn index). Then, we calculated the Euclidian distance on the bi-plot (axis I and II of the MDS) between each sampling site and the −300 m sampling site (interior forest). This Euclidian distance represents the dissimilarity on assemblage composition in relation to the interior forest (−300 m) dung beetles assemblage (beyond edge effects) (Banks Leite et al. 2010). Additionally, we classified dung beetles into functional groups based on the general pattern of dung manipulation (Simmons and Ridsdill-Smith 2011): (1) rollers: species usually roll the ball away from the dropping before burring; and (2) tunnellers: species usually burry directly below the dropping (without rolling away).
The relative abundance of each species, dissimilarity in relation to the forest assemblage (Euclidian distance), abundance within functional groups (rollers and tunnellers) and environmental conditions (temperature, understory, canopy and litter cover) along each 600 m forest-plantations transect were analyzed using the procedure described by Ewers and Didham (2006) and the modifications introduced by Zurita et al. (2012). First, the best fit of each dependent variable to five models (mean, lineal, power, sigmoid and unimodal) was evaluated using the Akaike’s information criteria with a correction for small sample size (AICc). The five models represented the possible theoretical edge responses, they were:
-
1.
Mean: the dependent variable is constant along the forest-plantation transect (neutral response).
-
2.
Lineal: it represents an incomplete edge response; the dependent variable is higher in one habitat type and species response to edges extends beyond the sampled range on both sides of the ecotone.
-
3.
Power: similar to the lineal model, it represents a partially incomplete edge response, the response variable reaches an asymptote only on one side of the ecotone.
-
4.
Sigmoid model: in this case, the dependent variable is higher in one habitat type (in the case of species, one habitat is preferred over the other). Ymax and Ymin represent the recalculated variable in both habitat types beyond the influence of edge effects (lower and upper asymptotes). β0 and β1 are constants influencing the shape of the sigmoid between asymptotes. Distance is the independent variable (−300 to 300 m).
$$ {\text{Dependant}}\,{\text{variable}} = {\text{Y}}\hbox{min} \, + \, \left( {\left( {{\text{Y}}\hbox{max} - {\text{Y}}\hbox{min} } \right)/\left( {1 + {\text{e}}^{{\left( {\beta 0 \, - \, {\text{Distance}}} \right) \times \beta 1}} } \right)} \right) $$ -
5.
Unimodal model: the dependent variable increases approaching the edge between both habitats (edge preference). When variables fitted to this model, we performed two separate power regression analysis to recalculate the value of the dependent variable on each habitat type beyond edge effects (Ymax and Ymin) (Zurita et al, in press). We applied this correction because of the symmetrical nature of the function that tends to bias the value of the dependent variable in both habitats. β0 is a constant indicating the peak of the function while, β1, β2, and β2 are constants determining the amplitude of the curve between the asymptotes.
$$ {\text{Dependant}}\,{\text{variable}} = {\text{Y}} \hbox{min} + \left( {\left( {\beta 0 - {\text{Y}}\hbox{min} } \right)/\left( {1 + {\text{e}}^{{\left( {\beta 1 - {\text{Distance}}\,{ + } \,\beta 2 \times {\text{Distance2}}} \right) \, \times \beta 3}} } \right)} \right) \, $$
To describe the quantitative component of edge effect, we estimated its magnitude and the extent on species assemblages and environmental variables. In the sigmoid model, the magnitude was calculated as the percentual (or the percentage of difference) difference between the lower and upper asymptote ((Ymax − Ymin)/Ymax*100) and the extent as the distance between the two inflection points of the second derivate (Ewers and Didham 2006). In the unimodal model, Ymax was calculated from the inflection point of the first derivate to estimate the magnitude of edge effect; the extent was calculated as the distance between the two maxima of the second derivative of this function (for more details on the used procedure, see Ewers and Didham 2006 and Zurita et al. 2012).
We compared the proportion of species which showed different patterns of response to edge effects (ecotone preference, avoidance or neutral) among forest-plantation ecotones (three mature plantations, one intermediate and one recent plantation) through a 5 × 3 Chi2 independency table. We performed ANOVA to compare the extent and magnitude of edge effects among forest-plantation transects. We used multiple and partial regression analysis to explore the relation between beta diversity (dissimilarity with either the forest or the plantation interior assemblages) and environmental variables along the forest-plantations transects. We excluded canopy cover, maximum temperature and thermal amplitude from regression analysis due to their high correlation with the average temperature (R = 0.83, R = 0.65, R = −0.79, p < 0.01 for all cases).
To test the hypothesis that environmental dissimilarity between habitats will determine the quantitative component of edge effects on assemblage composition, we performed non-parametric correlation analysis between the magnitude or the extent of edge effects on assemblage composition (Euclidian distance) and on environmental conditions. We used a non-parametric correlation instead of a regression analysis to account for the low number of independent points (10). We expected a positive relationship between the magnitude or the extent of edge effects on environmental conditions and on assemblage compositions.
At the species level, we expected a positive relation between species specialization and their quantitative response to edge effects (the more specialized species, the stronger the magnitude and extent of edge effects). To test this relation, we first calculated the coefficient of variation (SD/Mean) of species abundance on the six sampled habitats (plantations and native forest) as a measure of habitat specialization (SSI). This index has been proposed and tested by Julliard et al. (2006) as a proxy to study the influence of specialization on the response of species to disturbances. To calculate the index we used the average abundance of each species on each habitat on pitfall traps located at 100, 150 and 300 m (beyond edge effects). Higher values of SSI indicate higher levels of habitat specialization. Then, we performed a simple regression analysis between the index and the magnitude of edge effects.
Results
Environmental conditions in native forest and tree plantations
In general, environmental variables showed either a neutral or a sigmoid response to the forest-plantation gradient (57 and 33 % of the cases, respectively). Mature plantations (Araucaria, Eucalypt and Pine) and native forest differed only in a higher litter biomass in the former; vegetation cover (understory and canopy) and microclimatic conditions (average and maximum temperature and thermal amplitude) were similar in mature plantations and native forest. Ground temperature was lower on the intermediate aged pine plantation than in native forest while the recent pine plantation showed the opposite pattern. Also litter biomass and vegetation cover differed between native forest and the recent plantation (Table 1). In short, mature plantations were environmentally similar to native forest while the recent plantation differed markedly and the intermediate plantation represents the intermediate situation.
Species and assemblage responses
A total of 9682 individuals from 28 species were captured on the 80 baited pitfall traps (16 per forest-plantation gradient) (Table 2). The number of captured individuals was similar or higher to that found in recent studies in tropical areas (Gardner et al. 2008); however the number of captured species was lower, which is an expectable pattern for a subtropical forest. The majority of individuals were identified to the species level, with the general exception of species from the genus Dichotomius and Canthidium. After excluding species with less than 20 records (i.e., insufficient data for regression analysis), 10 species were left for statistical analysis at the species level (9,403 individuals) (Table 2).
A total of 39 % of species showed a neutral response to ecotones (mean model), 36 % preferred one habitat type (sigmoid and lineal models) and 25 % preferred ecotones (unimodal model) (Fig. 2; Table 2). When comparing forest-plantation ecotones, the relative importance of each response type differed (n = 8, χ2 = 21.5, p = 0.005). A neutral response was the most common pattern observed in mature plantations (Pine, Eucalypt and Aracuaria) while ecotone preference (unimodal model) was the most common response on the intermediate pine plantation. Finally, ecotone avoidance (sigmoid and lineal responses) was the most common pattern on the recent tree plantation (Fig. 3). In average, the extent of the edge effect was higher on the intermediate tree plantation compared to other forest-plantation gradients (ANOVA, F4,27 = 3.3, p = 0.03, Table 3), while the magnitude was higher in the recent and the intermediate plantations than in mature plantations (F4,27 = 4.5, p = 0.006, Table 3).
Patterns of dung beetle response to the forest-plantation gradient in the Atlantic forest of Argentina. a Neutral response (Dichotomius sp.) fitting to the mean model, b Edge avoidance (Dichotomus mormon) fitting to the sigmoid model and c Edge preference (Eurysternus caribaeus) fitting to the unimodal model. Negative values represent the native forest and positive values tree plantations
Sampling sites on the multidimensional scaling analysis (MDS) ordinate following consecutive distances (from −300 to 300 m) on the intermediate and the recent pine plantation gradients, while no clear pattern of ordination was observed in mature plantations (Fig. 4). In the regression analysis, the dissimilarity on dung beetle assemblages in relation to the forest assemblage (Euclidian distance to the −300 m sampling site in the MDS bi-plot) remained constant along the forest-plantation gradient (best fit to the mean model) in mature plantations and showed a sigmoid response in the intermediate and the recent pine plantations (Fig. 5). This result indicates that the composition of the dung beetle assemblage remains constant along forest-mature plantations gradients, but changes along forest-plantation transition in the recent and the intermediate plantations.
Multidimensional scaling analysis (first and second axis) based on the Morisita-Horn index of similarity in five different forest-plantation gradients in the Atlantic forest of Argentina. Negative values indicate distances inside forest and positive values distances inside plantations. a–c Araucaria, Eucalypt and Pine mature plantations respectively; d 7-year-old (filled circles) and 2-year-old (open circles) pine plantations
Patterns of dung beetle assemblages response along five different forest-plantation gradients in the Atlantic forest of Argentina. a–c Mature Araucaria, Eucalypt and Pine plantations, d 7-year-old pine plantation, e 2-year-old pine plantation. Y axis (MDS Distance) represents the Euclidian distance between the forest interior assemblage (−300 m) and sampling sites along the forest-plantation transect on the MDS. Negative values indicate distances inside forest while positive values indicate distances inside plantations
In the analysis of functional groups, the abundance of rollers and tunnellers showed different pattern of response among and within ecotones. Rollers showed no response (mean model) on the ecotones between native forest and the Eucalypt or the mature Pine plantations, a sigmoid response on the ecotone with the Araucaria or the recent Pine stands (with opposite direction), and a unimodal response on the intermediate Pine stand. Tunnellers exhibited no response in the ecotone with either Eucalypt or Araucaria stands and a sigmoid response on Pine stands (Fig. 6).
On multiple regression analysis, the dissimilarity of dung beetle assemblages in relation to the forest interior assemblages was not related to environmental variables on the Araucaria and the mature Pine plantation (Table 2). On the Eucalypt plantation, dissimilarity with the forest interior assemblage increased with understory and litter biomass (Table 3). Finally, for the recent plantation, average temperature (primarily) and understory cover (secondarily) were the main determiners of the dissimilarity with both the forest interior assemblage (Table 2).
The magnitude and extent of edge effects on assemblage composition was positively related to the magnitude and extent of temperature change (rs = 0.99 and rs = 0.81; p < 0.001 in both cases) but not to understory and litter biomass (rs < 0.5 and p > 0.05 in both cases). Also, as expected, at the species level the magnitude of edge effects increased with the degree of habitat specialization (SSI) (R2 = 0.47, p = 0.04); however, the extent of the edge effect was not related to habitat specialization (R2 = 0.14, p = 0.32).
Discussion
As we proposed, environmental dissimilarity and the degree of species specialization successfully explained the magnitude (not the extent) of the biological response of dung beetle assemblages and species to edge effects. Results of this study contribute to the current theoretical edge effect framework (Fagan et al. 1999; Ries et al. 2004; Ries and Sisk 2004); explanatory and predictive models, such as the resource based model (Ries and Sisk 2004), can be improved through the incorporation of the present results to perform more precise predictions.
The resource-based model predicts the qualitative response of species to edge effects (it predicts edge avoidance, preference or a neutral response) based on the spatial distribution of resources and environmental conditions between habitats (Ries and Sisk 2004). This model excludes changes on biotic interactions that may be an important mechanism in specific cases (Wimp et al. 2011). When complementary resources from both habitats are available on the ecotone, edge preference is the most probable response; in contrast, when habitats offer supplementary resources, a neutral or edge avoidance response are the most plausible edge responses. The qualitative response of dung beetle species and assemblages in this study can be directly related to the resource-based model considering the temperature as an important environmental filter for these taxa (Scheffler 2005; Verdú et al. 2007). Previous studies showed that tropical dung beetle species are, in general, unable to exploit modified habitats exhibiting high temperatures (Klein 1989; Navarrete and Halffter 2008). Similar temperature between native forest and mature tree plantations may explain the neutral response observed in most species and assemblages. In contrast, the extreme temperature in the recent plantation created an unsuitable habitat for most native forest species; explaining the sigmoid response exhibited by the majority of forest species and assemblages. The same process probably occurs in the opposite direction, native forest represents a low quality habitat for open habitat species occurring in the recent plantation. Similarly to our results, Pawson et al. (2008) and Kotze et al. (2012) found stronger edge responses of different arthropod assemblages to high contrast ecotones (open habitats) compared to low contrast ecotones (tree plantations) in New Zealand. Also, consistent with the observed patterns and the scale of dung beetles response to edge effects, previous studies in Costa Rica have shown that major changes on microclimatic conditions occur within the first 300 m from the edge (Didham and Lawton 1999; Harper et al. 2005).
Functional groups based on dung manipulation exhibited opposite response patterns to the same ecotone. The importance of dung beetles on ecosystem functioning and the role of disturbance on the functional composition of assemblages has been recognized in several studies (Nichols et al. 2007; Barragán et al. 2011). Our study showed that changes in functional groups occurred gradually from the ecotone between habitats; that may affect differentially ecological functions in both the native and the human-created habitat. Effects of functional changes on dung beetles assemblages are unclear; however variations in the relative abundance of rollers and tunnellers will probably modify spatial patterns of secondary seed dispersal and nutrient cycling affecting future vegetation structure and composition on the ecotone between habitats. A marked increase on the abundance of rollers on the recent plantation was primarily associated to a drastic increase on the abundance of species on the genus Canthon (in particular, Canthon quinquemaculatus and Canthon virens); however, species in that habitat were observed leaving dung balls on the surface (Zurita per. obs.), presumably reducing seed burial and germination due to high soil temperatures.
The varied nature of contrasts between habitats (or “not all ecotones are the same”) has been recognized as an important factor influencing the response of species and assemblages to edge effects (Reino et al. 2009; Pawson et al. 2008). However, our results support the idea of a gradient of hardness (or softness) rather than a dichotomist classification (soft vs. hard ecotones) (Ries and Sisk 2010). The ecotone between tree plantation and native forest become “softer” for dung beetles as plantations grow in association with a decrease in ground temperature and increasing understory cover. As a consequence, through the plantation cycle the most common response switches from edge avoidance (sigmoid model) to a neutral response (mean model).While changes in environmental conditions probably have a central role on the response of dung beetles to edges and on the use of anthropogenic habitats; differences in the period of time since disturbance started may also influence richness and composition of dung beetles assemblages on tree plantations (Pawson et al. 2008); stochastic colonization of plantations increases with plantation age. Recently Prevedello et al. (2012) suggested that edge avoidance could simply be explained by geometric constraints; areas in the center of the patch receive individuals from all directions whereas areas near the edge receive individuals only from the center of the patch. In our study, geometric constraints can inflate the proportion of species showing edge avoidance; however, because of all transects limited with the same large continuous area (protected area), the comparison among transects will not be affected.
Interestingly, the high permeability of mature tree plantations for native species was independent of the plantation type (native Araucaria and exotic Pine and Eucalypt) which is consistent with the results from the meta-analysis of Nichols et al. (2007) but contradicts the results of Gardner et al. (2008) who found a severely impoverished dung beetle assemblage in Eucalypt plantations in relation to native forest. Some methodological limitations suggested by Gardner et al. (2008) to explain differences with the results of Nichols et al. (2007) were overcome in our study by including the distance to edges, the source of native species and the landscape context. A plausible explanation is the difference in plantation age between the results of Nichols et al. (2007), and those of Gardner et al. (2008) and our study; Pine and Eucalypt plantations surveyed in our study and the revision of Nichols et al. (2007) were at least 10 year-old, while Eucalypt plantations sampled by Gardner et al. (2008) were only 4–5 years-old. As we previously mentioned, this difference in plantation age may drastically change the suitability of tree plantations for native species. An alternative explanation is related to biogeographic differences in the regional pool of species; species in tropical forest (such as the Amazon) are probably more specialized and less capable of exploiting modified habitats compared to species in subtropical forests, such as the semidecidous Atlantic forest (Rös et al. 2012). A differential distribution of dung along the forest-plantation gradient is an alternative hypothesis to explain the response of dung beetle species to edge effects. In this case, the magnitude and extent of edge effects on medium and large mammals should be similar to those of dung beetles. However, it is unlikely that a differential distribution of dung is the main factor determining dung beetle responses to ecotones for at least three reasons: (1) the extent of edge effects on dung beetles occurs at a small scale (meters), while medium and large mammals respond to larger scales; (2) edge effects on medium and large mammals typical from the study area (Tapirus terrestres, Tayassu pecari, Pecari tajacu, Mazama spp, Cerdocyon thous and several species of Felidae) result mainly from an increase of the interaction with humans (hunters) and domestic dogs rather than from changes on habitat structure (Lacerda et al. 2009); however, all gradients were located in a highly protected area and subjected to similar human pressure; and (3) the majority of these mammals are capable of using, or at least freely move, along forest and open habitats.
While the quantitative response of assemblages depends on the contrast of resources and conditions between habitats, the response of species depends on the degree of species specialization (or niche amplitude). Confronted with the same ecotone, specialized species exhibited stronger edge response compared to generalist species. This selection of species along the ecotone based on the range of tolerance probably occurs through environmental filtering and results in different dung beetle assemblages in young plantations. The positive relation between specialization and the response to habitat disturbance is, in fact, a prediction of the specialization-disturbance hypothesis (Vázquez and Simberloff 2002; Colles et al. 2009); however, the hypothesis has never been tested on the species edge response. One limitation of this hypothesis is the complexity encountered to identify the specific component of the niche in relation to the response to disturbance and the measurement of the niche amplitude. This limitation was partially solved by the index proposed by (Julliard et al. 2006) which successfully explained the response of species to habitat disturbance and fragmentation (Devictor et al. 2008). While more research is needed to identify the specific component of the niche responsible for the response of dung beetles to edge effects, we found evidence that tolerance to different temperatures is probably an underlying mechanism.
The role of edge effects influencing populations and communities is currently a central area of study, particularly in tropical and subtropical forests where edge effect is now recognized a central process explaining the response of species to habitat fragmentation (Banks Leite et al. 2010). A general edge effects framework which could predict the strength will increase the explanatory and predictive power of theoretical models dealing with edge effects and functional connectivity. This is particularly important to improve land management both at a local (stand) and landscape context on hyper fragmented landscapes, such as the Atlantic forest.
References
Baker SC, Barmuta LA (2006) Evaluation spatial autocorrelation and depletion in pitfall-traps studies of environmental gradients. J Insect Conserv 10:269–276
Banks Leite C, Ewers RM, Metzger JP (2010) Edge effects as the principal cause of area effects on birds in fragmented secondary forest. Oikos 119:918–926
Barragán F, Moreno CE, Escobar F, Halffter G, Navarrete D (2011) Negative impacts of human land use on dung beetle functional diversity. PLoS ONE 6:e17976
Campbell RE, Harding JS, Ewers RM, Thorpe S, Didham RK (2011) Production land use alters edge response functions in remnant forest invertebrate communities. Ecol Appl 21:3147–3161
Colles A, Liow LH, Prinzing A (2009) Are specialists at risk under environmental change? Neoecological, paleoecological and phylogenetic approaches. Ecol Lett 12:849–863
Devictor V, Julliard R, Jiguet F (2008) Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 117:507–514
Didham RK, Lawton JH (1999) Edge structure determines the magnitude of changes in microclimate and vegetation structure in tropical forest fragments. Biotropica 31:17–30
Ewers RM, Didham RK (2006) Continuous response functions for quantifying the strength of edge effects. J Appl Ecol 43:527–536
Fagan WF, Cantrell RS, Cosner C (1999) How habitat edges change species interactions. Am Nat 153:165–182
Fonseca CR, Joner F (2007) Two sided edge effect studies and the restoration of endangered ecosystems. Restor Ecol 15:613–619
Gardner TA, Hernandez MIM, Barlow J, Peres CA (2008) Understanding the biodiversity consequences of habitat change: the value of secondary and plantation forests for neotropical dung beetles. J Appl Ecol 45:883–893
Hansbauer MM, Storch I, Knauer F, Pilz S, Küchenhoff H, Végvári Z, Pimentel RG, Metzger JP (2010) Landscape perception by forest understory birds in the Atlantic Rainforest: black-and-white versus shades of grey. Land Ecol 25:407–417
Harper KA, Macdonald SE, Burton PJ, Chen J, Brosofske KD, Saunders SC, Euskirchen ES, Roberts DAR, Jaiteh MS, Esseen PERA (2005) Edge influence on forest structure and composition in fragmented landscapes. Conserv Biol 19:768–782
Julliard R, Clavel J, Devictor V, Jiguet F, Couvet D (2006) Spatial segregation of specialists and generalists in bird communities. Ecol Lett 9:1237–1244
Klein BC (1989) Effects of forest fragmentation on dung and carrion beetle communities in central Amazonia. Ecology 70:1715–1725
Koh LP, Lee TM, Sodhi NS, Ghazoul J (2010) An overhaul of the speciesûarea approach for predicting biodiversity loss: incorporating matrix and edge effects. J Appl Ecol 47:1063–1070
Kotze DJ, Lehvävirta S, Koivula M, O’Hara RB, Spence JR (2012) Effects of habitat edges and trampling on the distribution of ground beetles (Coleoptera, Carabaidae) in urban forests. J Insect Conserv. doi:10.1007/s1084101294752
Kraft NJB, Valencia R, Ackerly DD (2008) Functional traits and niche-based tree community assembly in an Amazonian forest. Science 322:580–582
Lacerda ACR, Tomas WM, Marinho-Filho J (2009) Domestic dogs as an edge effect in the Brasília National Park, Brazil: interactions with native mammals. Animal Conserv 12:477–487
Larsen TH, Forsyth A (2005) Trap spacing and transect design for dung beetle biodiversity studies. Biotropica 37:322–325
Lopes J, Korasaki V, Catelli LL, Marcal VVM, Nunes MPBP (2011) A comparison of dung beetle assemblage structure (Coleoptera: Scarabaeidae: Scarabaeinae) between an Atlantic forest fragment and adjacent abandoned pasture in Paraná. Brazil. Zoologia 28:72–79
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10:58–62
Myers N, Mittermeier RA, Mittermeier CG, da Fonseca GAB, Kent J (2000) Biodiversity hotspots for conservation priorities. Nature 403:853–858
Navarrete D, Halffter G (2008) Dung beetle (Coleoptera: Scarabaeidae: Scarabaeinae) diversity in continuous forest, forest fragments and cattle pastures in a landscape of Chiapas, Mexico: the effects of anthropogenic changes. Biodivers Conserv 17:2869–2898
Nichols E, Larsen T, Spector S, Davis AL, Escobar F, Favila M, Vulinec K (2007) Global dung beetle response to tropical forest modification and fragmentation: a quantitative literature review and meta-analysis. Biol Conserv 137:1–19
Nichols E, Spector S, Louzada J, Larsen T, Amezquita S, Favila ME (2008) Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol Conserv 141:1461–1474
Pawson SM, Brockerhoff EG, Meenken ED, Didham RK (2008) Non-native plantation forests as alternative habitat for native forest beetles in a heavily modified landscape. Biodivers Conserv 17:1127–1148
Pe’er G, Henle K, Dislich C, Frank K (2011) Breaking functional connectivity into components: a novel approach using an individual-based model, and first outcomes. PLoS ONE 6:e22355
Porensky LM (2011) When edges meet: interacting edge effects in an African savanna. J Ecol 99:923–934
Prevedello JA, Figueiredo MSL, Grelle CEV, Vieira MV (2012) Rethinking edge effects: the unaccounted role of geometric constraints. Ecography. doi:10.1111/j.1600-0587.2012.07820.x
Qie L, Lee TM, Sodhi NS, Lim SLH (2010) Dung beetle assemblages on tropical land bridge islands: small island effect and vulnerable species. J Biogeogr 38:792–804
Reino L, Beja P, Osborne PE, Morgado R, Fabipo A, Rotenberry JT (2009) Distance to edges, edge contrast and landscape fragmentation: Interactions affecting farmland birds around forest plantations. Biol Conserv 142:824–838
Ries L, Sisk TD (2004) A predictive model of edge effects. Ecology 85:2917–2926
Ries L, Sisk TD (2010) What is an edge species? The implications of sensitivity to habitat edges. Oikos 119:1636–1642
Ries L, Fletcher RJ Jr, Battin J, Sisk TD (2004) Ecological responses to habitat edges: mechanisms, models, and variability explained. Annu Rev Ecol Evol Syst 35:491–522
Rös M, Escobar F, Halffter G (2012) How dung beetles respond to a human modified variegated landscape in Mexican cloud forest: a study of biodiversity integrating ecological and biogeographical perspectives. Divers Distrib 18:377–389
Scheffler PY (2005) Dung beetle (Coleoptera: Scarabaeidae) diversity and community structure across three disturbance regimes in eastern Amazonia. J Trop Ecol 21:9–19
Silva FAB, Costa CMQ, Moura RC, Farias AI (2010) Study of the dung beetle (Coleoptera: Scarabaeidae) community at two sites: Atlantic Forest and Clear-Cut, Pernambuco, Brazil. Environ Entomol 39:359–367
Simmons LW, Ridsdill-Smith TJ (2011) Ecology and evolution of dung beetles. Wiley-Blackwell, Oxford
Soga M, Kanno N, Yamura Y, Koike S (2012) Patch size determines the strength of edge effects on carabid beetle assemblages in urban remnant forest. J Insect Conserv. doi:10.1007/s108410129524x
Spector S (2006) Scarabaeine dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae): an invertebrate focal taxon for biodiversity research and conservation. Coleopt Bull 60:71–83
Spector S, Ayzama S (2003) Rapid turnover and edge effects in dung beetle assemblages (Scarabaeidae) at a Bolivian neotropical forest savanna ecotone. Biotropica 35:394–404
Van Halder I, Barbaro L, Jactel H (2011) Conserving butterflies in fragmented plantation forests: are edge and interior habitats equally important. J Insect Conserv 15:591–601
Vaz-de-Mello FZ, Edmonds WD, Ocampo FC, Schoolmeesters P (2011) A multilingual key to the genera and subgenera of the subfamily Scarabaeinae of the New World (Coleoptera: Scarabaeidae). Zootaxa 2854:1–73
Vázquez DP, Simberloff D (2002) Ecological specialization and susceptibility to disturbance: conjectures and refutations. Am Nat 159:606–623
Verdú JR, Arellano L, Numa C, Micó E (2007) Roles of endothermy in niche differentiation for ball rolling dung beetles (Coleoptera: Scarabaeidae) along an altitudinal gradient. Ecol Entomol 32:544–551
Wimp GM, Murphy SM, Lewis D, Ries L (2011) Do edge responses cascade up or down a multi-trophic food web? Ecol Lett 14:863–870
Zurita GA, Bellocq MI (2010) Spatial patterns of bird community similarity: bird responses to landscape composition and configuration in the Atlantic forest. Landsc Ecol 25:147–158
Zurita GA, Bellocq MI (2012) Bird assemblages in anthropogenic habitats: identifying a suitability gradient for native species in the Atlantic forest. Biotropica 44:412–419
Zurita GA, Pe’er G, Bellocq MI, Hansbauer MM (2012) Edge effects and their influence on habitat suitability calculations: a continuous approach applied to birds of the Atlantic forest. J Appl Ecol 49:503–512
Acknowledgments
Misiones provincial government (MERNyT of Misiones) and Alto Paraná S.A. gave the appropriate permissions for collecting dung-bettles. This project was funded by the Agencia Nacional de Promoción Científica y Tecnologica (PICT), CONICET and the Universidad de Buenos Aires. Federico Ocampo provided assistance on species identification.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peyras, M., Vespa, N.I., Bellocq, M.I. et al. Quantifying edge effects: the role of habitat contrast and species specialization. J Insect Conserv 17, 807–820 (2013). https://doi.org/10.1007/s10841-013-9563-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10841-013-9563-y