Abstract
Context
Identifying the drivers shaping biological assemblages in fragmented tropical landscapes is critical for designing effective conservation strategies. It is still unclear, however, whether tropical biodiversity is more strongly affected by forest loss, by its spatial configuration or by matrix composition across different spatial scales.
Objectives
Assessing the relative influence of forest patch and landscape attributes on dung beetle assemblages in the fragmented Lacandona rainforest, Mexico.
Methods
Using a multimodel inference approach we tested the relative impact of forest patch size and landscape forest cover (measures of forest amount at the patch and landscape scales, respectively), patch shape and isolation (forest configuration indices at the patch scale), forest fragmentation (forest configuration index at the landscape scale), and matrix composition on the diversity, abundance and biomass of dung beetles.
Results
Patch size, landscape forest cover and matrix composition were the best predictors of dung beetle assemblages. Species richness, beetle abundance, and biomass decreased in smaller patches surrounded by a lower percentage of forest cover, and in landscapes dominated by open-area matrices. Community evenness also increased under these conditions due to the loss of rare species.
Conclusions
Forest loss at the patch and landscape levels and matrix composition show a larger impact on dung beetles than forest spatial configuration. To preserve dung beetle assemblages, and their key functional roles in the ecosystem, conservation initiatives should prioritize a reduction in deforestation and an increase in the heterogeneity of the matrix surrounding forest remnants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rapid loss and degradation of forests throughout the tropics has led to the expansion of fragmented landscapes in which the long-term persistence of biodiversity may be endangered (Melo et al. 2013). Although forest loss is known to have negative impacts on global biodiversity (Fahrig 2003, 2013), the spatial arrangement or configuration of the remaining forest (e.g., forest patch isolation, degree of fragmentation) in such emerging landscapes can also have significant impacts on biological assemblages (Gardner et al. 2008; Thornton et al. 2011; Didham et al. 2012; Newbold et al. 2014; Hanski 2015; Perović et al. 2015). Both the amount of forest in the landscape and the composition of the matrix surrounding the remaining forest patches determine the composition of the landscape—a spatial characteristic with great effects on biodiversity (e.g., Fahrig et al. 2011; Perović et al. 2015). Matrix composition, for example, can affect species’ persistence in fragmented landscapes, by determining the severity of edge effects, as well as the levels of connectivity and resource availability in the landscape (Dunning et al. 1992; Franklin and Lindenmayer 2009; Perfecto et al. 2009; Fahrig et al. 2011; Tscharntke et al. 2012). Yet it is still unclear whether tropical biodiversity is more strongly affected by forest loss, by the spatial configuration of the remaining forest, or by matrix composition. This information is urgently needed to improve conservation strategies, particularly considering unresolved issues about the design of protected areas (Murphy 1989), the role of habitat corridors (Brodie et al. 2015) and the effects of different matrix types (Franklin and Lindenmayer 2009).
While landscape composition is clearly a characteristic that needs to be assessed at the landscape scale, forest loss and forest configuration can be measured at different spatial scales, and consequently, their effects may be scale-dependent. In particular, forest loss can be measured at the patch scale (i.e., forest patch size; MacArthur and Wilson 1967; Hanski 1999) and at the landscape scale (i.e., landscape forest cover; Fahrig 2003). Forest configuration can also be measured at the patch (e.g., patch shape and isolation) and landscape scales (e.g., degree of fragmentation; Cushman et al. 2008; McGarigal et al. 2012). It is unclear, however, which is the scale within which forest loss and configuration has the strongest effect on biodiversity (but see Smith et al. 2011; Thornton et al. 2011; Arroyo-Rodríguez et al. 2013; Fahrig 2013; Ordóñez-Gómez et al. 2015). To address this challenge, multi-scale analyses are needed. Also, because most studies in fragmented landscapes have been carried out in temperate regions (reviewed by McGarigal and Cushman 2002; Fahrig 2003), additional studies in the tropics are urgently needed. Furthermore, because most of the available multi-scale studies in fragmented tropical forests have focused on vertebrates (e.g., Thornton et al. 2011; Arroyo-Rodríguez et al. 2013; Garmendia et al. 2013; Carrara et al. 2015), broadening our focus by studying taxa with different habitat and spatial requirements may increase our ability to reach more general conclusions on these contentious and yet unresolved issues. Focusing on insects may be particularly relevant, as they are highly susceptible to forest spatial changes, with important implications for ecosystem functioning (Didham et al. 1996; Perović et al. 2015).
Dung beetles (Coleoptera: Scarabaeinae) are a diverse and abundant group of insects that perform many important ecological functions in tropical forests (Nichols et al. 2008). Since they are relatively short-lived, have large populations, and are sensitive to environmental alterations, dung beetles can respond quickly to forest changes (Nichols et al. 2007; Larsen et al. 2008; Rös et al. 2012). Dung beetle assemblages in fragmented landscapes have been shown to be affected by forest patch size (Klein 1989; Andresen 2003), landscape forest cover (Rös et al. 2012), edges (Spector and Ayzama 2003), patch isolation (Arellano et al. 2008a; Escobar et al. 2008), matrix type (Klein 1989; Quintero and Roslin 2005), and other spatial variables, such as altitude and slope (Silva and Hernández 2014). Most of these studies evaluate the effects of one or a few attributes at one spatial scale (but see Silva and Hernández 2014), thus limiting the control of potential confounding factors (Ewers and Didham 2006).
Understanding the relative influence of patch and landscape level spatial attributes on dung beetle assemblages is particularly needed for the Lacandona rainforest, Mexico—a biodiversity hotspot that has lost approximately 60 % of the original forest cover (Mora 2008). In this region, only two published studies have assessed dung beetle assemblages, showing that diversity and composition are similar in continuous forest, forest patches, and rustic cocoa plantations, while cattle pastures and rubber tree plantations show a significantly lower species richness, dominated by a few open-area species (Navarrete and Halffter 2008; Barragán et al. 2011). Yet, it is still unclear which patch and landscape spatial attributes affect dung beetles in this region, and what their relative roles in shaping these assemblages are.
The main objective of this study was to determine the relative effects of forest loss versus forest configuration at two spatial scales (patch and landscape), as well as the relative effects of landscape configuration versus landscape composition, in a Neotropical fragmented landscape, using dung beetles as the focal taxon. In particular, we addressed the following questions: (i) Does forest loss have a stronger effect on dung beetle assemblages than forest spatial configuration, and are the relative effects of these drivers consistent across spatial scales (patch and landscape)? and (ii) at the landscape scale, does landscape composition (landscape forest cover and matrix composition) have higher explanatory power than landscape configuration (degree of fragmentation)? Due to findings of previous studies (Fahrig 2003; Nichols et al. 2007; Smith et al. 2011; Fahrig 2013; Carrara et al. 2015), we expected that the amount of forest (i.e., forest patch size and/or landscape forest cover) would be more important than its spatial configuration (i.e., patch shape, patch isolation, and/or degree of fragmentation) in shaping dung beetle assemblages. We also expected that dung beetle assemblages might be more strongly related to patch than to landscape attributes because they are known to be affected by variables such as canopy openness, soil temperature, and resource availability (Halffter and Matthews 1966; Navarrete and Halffter 2008), which are associated with patch size and shape (Murcia 1995). Finally, we expected that dung beetle assemblages would be greatly affected by landscape composition and less by landscape configuration, in accordance with studies showing a negative effect of open areas (i.e., cattle pastures and annual crops) in the matrix (Quintero and Roslin 2005; Halffter et al. 2007; Navarrete and Halffter 2008; Rös et al. 2012).
Methods
Study area
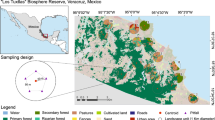
We conducted this study in the Lacandona rainforest located in the Mexican state of Chiapas (Fig. 1). With an extent of 13,000 km2, it represents one of the largest areas of tropical rainforest in Mexico, and a priority area for biodiversity conservation in Mesoamerica (Ceballos et al. 1998). We conducted the study in two adjacent lowlands areas (100–200 m a.s.l.) with similar soil and weather conditions. These areas, the continuous forest of the Montes Azules Biosphere Reserve and the fragmented forest of the Marqués de Comillas Region, are separated by the Lacantún River (Fig. 1). Deforestation outside the Montes Azules Biosphere Reserve, and particularly within the Marqués de Comillas Region, has resulted in heterogeneous landscapes composed of a mosaic of land-cover types (old-growth forest patches, secondary forests, agricultural lands, cattle pastures and human settlements). The Marqués de Comillas Region still maintains its original fauna, although smaller forest patches show compositional and structural alterations of animal assemblages (e.g., Arroyo-Rodríguez et al. 2013; Garmendia et al. 2013; San-José et al. 2014). Annual precipitation averages 2500–3500 mm, and average monthly temperatures are 24–26 °C.
Experimental design and explanatory variables
We adopted a patch–landscape approach (sensu McGarigal and Cushman 2002), i.e., response variables were evaluated within forest patches and landscape variables were measured within a 100 ha landscape (564 m radius from the center of each patch). We selected this landscape size because medium and large herbivorous mammals, which provide the main food resource for dung beetles, respond strongly to forest changes at this scale in the region (Arroyo-Rodríguez et al. 2013; Garmendia et al. 2013). Also, this landscape size is large enough to encompass several populations of the dung beetle species (Halffter and Halffter 1989; Arellano et al. 2008b), as well as large variation in the explanatory variables (Appendix Table A1 in supplementary material), which is needed to make accurate landscape-scale inferences (Eigenbrod et al. 2011). We sampled 24 sites: 21 forest patches embedded in their 100 ha landscapes in the Marqués de Comillas Region and 3 continuous forest sites (100 ha each) in the Montes Azules Biosphere Reserve (Fig. 1). Forest patches ranged from 3 to 92 ha, and distance between any two patches was at least 2 km. The continuous forest sites were separated by at least 4 km, and located ≥1 km from the forest-river edge.
Using recent SPOT 5 satellite images (March 2011) and the SPRING program (Câmara et al. 1996) we first made a supervised classification considering six land-cover types: mature forests, secondary forests, tree crops (i.e., palm and rubber plantations), annual crops (i.e., corn, chili and bean plantations), cattle pastures, and human settlements. Overall, classification accuracy was 77 %. We then used the GIS GRASS program (GRASS 2011) to characterize the patch and landscape attributes. To assess forest loss at the patch and landscape levels we measured patch size and percentage forest cover, respectively. To assess forest configuration we measured patch shape and isolation at the patch level, and degree of fragmentation at the landscape level. Although patch size at the landscape scale (e.g. mean patch size) is considered a measure of landscape configuration (McGarigal et al. 2012), at the patch scale it is more easily viewed as a measure of the amount of forest. Finally, to assess landscape composition we used the above measure of percentage of forest cover, and also measured the percentage of open-areas (i.e. cattle pastures and annual crops) in the matrix.
Patch shape was estimated with the shape index proposed by Patton (1975): \(SI = P/\sqrt {A\pi }\), where P and A are the patch perimeter and area, respectively. The higher the SI values, the higher the shape complexity (perfect circle, SI = 1.0). Patch isolation was measured as the mean nearest-neighbor distance from all patches in the landscape to the focal patch. The degree of fragmentation was evaluated with the splitting index (S) proposed by Jaeger (2000): S = \(A_{t}^{2} /\mathop \sum \nolimits_{i = 1}^{n} A_{i}^{2}\), where A t is the area covered by forest in the landscape and A i is the area of the forest patch i. This index represents the ‘effective number of forest patches’, and being independent of total forest cover in the landscape, it is a measure of fragmentation per se (sensu Fahrig 2003).
Dung beetle sampling and response variables
Beetles were collected using baited pitfall traps (1 L plastic containers buried level with the soil surface). Human dung is known to be an excellent bait to accurately sample dung beetle communities, but due to its short supply and the large amounts necessary for extensive studies, a mixture of pig and human dung (with at least 10 % of the latter) has recently been proposed as a very good alternative (Marsh et al. 2013). Thus, traps were baited with 25 g of a mixture of human and pig excrement (7:3) and they were kept active during 48 h. Beetles were sampled once at each site during the rainy season (July–September 2012), which is the period of greatest activity. Four traps, 50 m apart, were placed along transects. Following Schoereder et al. (2004), sampling effort was proportional to the area sampled. In forest patches we used one transect for every 20 ha; in continuous forest we used five transects per site. Transects were located ≥50 m from patches’ edges, and multiple transects in one site were separated by ≥150 m. A total of 196 pitfall traps were used in this study. All collected individuals were identified to species and counted. Up to 20 individuals per species were oven-dried until constant weight was reached, and then weighed.
We used the coverage estimator recommended by Chao and Jost (2012) to estimate the accuracy of inventories:
where f 1 and f 2 are the number of species with one individual and with two individuals in the sample, respectively, and n is the number of individuals. Sample coverage was very high in all sites (>92 % of the species recorded; Appendix Table A2 in supplementary material), indicating that our sampling effort was adequate to estimate diversity metrics within each site. However, to avoid any potential bias in our results due to differences in sample coverage among sites (see Chao and Jost 2012), we considered not only the observed values of species richness, but also the expected values based on coverage-based extrapolations performed with the entropart package (Marcon and Hérault 2014) for R 3.0.1 (R Core Team 2014).
We determined species diversity metrics using Hill numbers (i.e., numbers equivalent, sensu Jost 2006) with the entropart package (Marcon and Hérault 2014). We used Hill numbers of order 0 (0 D, species richness), 1 (1 D, exponential of Shannon’s entropy), and 2 (2 D, inverse Simpson concentration). 0 D is not sensitive to species abundances and thus gives disproportionate weight to rare species (Jost 2006). 1 D weighs each species according to its abundance in the community; hence it can be interpreted as the number of ‘common’ or ‘typical’ species in the community (Jost 2006). Finally, 2 D can be interpreted as the number of ‘very abundant’ or ‘dominant’ species in the community (Jost 2006). The detailed formulas for the Hill numbers can be found elsewhere (Jost 2006).
To assess changes in community structure we considered dung beetle abundance, total biomass, and the evenness factor (EF) proposed by Jost (2010). EF represents the proportion of dominant species in the community and it is derived from Hill numbers (EF = 2 D/ 0 D; Jost 2010). EF ranges between 1 (when the community is perfectly even) and nearly 1/0 D (when the community is dominated by one species, i.e., 2 D = virtually 1; Jost 2010). Beetle abundance and biomass were averaged per transect.
Data analyses
We used generalized linear models to assess the effects of spatial attributes on each response variable. We fixed a Gaussian error distribution for continuous response variables (i.e., 1 D, 2 D, evenness factor, mean abundance, and mean biomass) after verifying for normality (Shapiro–Wilk test). 0 D (count-dependent variable) was assessed by fixing a Poisson error distribution. To assess collinearity among predictor variables we estimated their variance inflation factors (VIF) using the car package for R version 3.0.1. A VIF > 4 indicates possible collinearity, and a VIF > 10 indicates severe collinearity (Neter et al. 1996). We found severe collinearity between factors at the patch and the landscape scale, particularly between patch size and landscape forest cover (r = 0.95, p < 0.001; VIF = 10.2; Appendix Table A3 in supplementary material). Thus, we decided to carry out separate models for the two spatial scales. We used an information-theoretic approach and multimodel inference to assess the relative effect of each predictor on each response variable (Burnham and Anderson 2002). For each response variable we constructed 8 models, representing all combinations of explanatory variables. For each model we computed the Akaike’s information criterion corrected for small samples (AICc). To correct for overdispersion associated to count data, 0 D was assessed with qAICc instead of AICc values (Calcagno and Mazancourt 2010). Models with a difference in [q]AICc < 2 when compared to the best model (i.e., the one with lowest [q]AICc value) were considered to have similar plausibility (Burnham and Anderson 2002). To obtain model-averaged parameter estimates we used Akaike weights (w i ). The set of models for which Σw i was 0.95 represents a set that has 95 % probability of containing the true best model (Burnham and Anderson 2002). A given spatial attribute was considered an important explanatory variable for a given response variable if: (i) it showed a high sum of Akaike weights (i.e., considering each candidate model in which it appeared); and (ii) the model-averaged unconditional variance was lower than the model-averaged parameter estimate. All models were built using the package glmulti for R version 3.0.1 (Calcagno and Mazancourt 2010). As a post hoc analysis, we constructed rank-abundance curves to see differences in species’ dominance, rarity and community evenness among study sites, and thus better interpret our results.
Results
We collected 9418 individuals belonging to 43 species (Appendix Table A4 in supplementary material). In the Montes Azules Biosphere Reserve we collected 39 species, including three (7 %) unique species (Canthon angustatus, Eurysternus angustulus and Sulcophanaeus chryseicollis). Copris laeviceps (42 % of sampled individuals in the reserve), Eurysternus caribaeus (17 %) and Uroxys microcularis (7 %) were the most representative species in the reserve (Fig. 2). In the Marqués de Comillas Region we collected 40 species, including 3 unique species. As in the continuous forest, Copris laeviceps and Eurysternus caribaeus also dominated in the Marqués de Comillas Region. Other species dominating dung beetle assemblages in patches were: Ateuchus chrysopyge, Bdelyropsis bowditchi, Dichotomius amplicollis, Deltochilum gibbosum, Deltochilum pseudoparile, and Uroxys micros (Fig. 2, Table A4 in supplementary material).
Relative abundance of dung beetle species in fragments (F) and continuous forest sites (CF) in the Lacandona rainforest, Mexico. Fragments were ordered from the smallest (F1) to the largest (F21) and then pooled in groups of three (triplets) according to their size; the three continuous forest sites were also pooled into a triplet. Each curve represents the dung beetle assemblage in a triplet. The dashed line indicates the number of species representing <5 % of individuals sampled in each triplet. The identity of dominant species within each triplet is also indicated: Ach Ateuchus chrysopyge; Bbo Bdelyropsis bowditchi; Cfe Canthon femoralis; Cla Copris laeviceps; Dam Dichotomius amplicollis; Dgi Deltochilum gibbosum; Dps Deltochilum pseudoparile; Eca Eurysternus caribaeus; Umi Uroxys micros; Umic Uroxys microcularis
Associations between species diversity (0 D, 1 D, and 2 D) and explanatory variables were quite similar when diversity metrics were calculated using the observed number of species per site, and when they were based on the expected number of species per site. Hence, only the results based on observed values are described here (results for analyses based on expected values are shown in Appendix Figure A1 in supplementary material). Overall, the explanatory variables that best predicted changes in dung beetle assemblages were those associated with forest loss at both spatial scales, i.e., patch size and landscape forest cover (Fig. 3, Appendix Tables A5 and A6 in supplementary material). In particular, considering the response variables for which complete models showed the highest percentage of explained deviance, we found that patch size and landscape forest cover were the main attributes positively affecting 0 D (69 and 76 % of explained deviance at the patch and landscape scales, respectively; Fig. 3a), mean abundance (28 and 38 %; Fig. 3e) and mean biomass (36 and 36 %; Fig. 3f) of dung beetles. Because 1 D and 2 D remained almost constant across the forest amount gradient at both spatial scales (Fig. 3b, c), the positive relationships between 0 D and the amount of forest were associated to high numbers of rare species. In other words, the number of rare species increased in larger patches and in patches imbedded in landscapes with higher forest cover, which in turn reduced community evenness in these patches (Fig. 3d, Appendix Tables A5 and A6 in supplementary material). This was supported by the fact that the number of species representing <5 % of individuals was notably higher in larger patches and in continuous forest areas (Fig. 2).
Predictor variables included in the ΔAICc < 2 set of models (black bars) and 95 % set of models (gray bars) for species diversity (Hill numbers of order 0, 1, and 2), evenness, mean abundance and mean biomass of dung beetle assemblages in the Lacandona rainforest, Mexico. The importance of each variable is shown by the sum of Akaike weights (Σw i , panels in the left side). Panels in the right side indicate the values of model-averaged parameter estimates (β) and unconditional variance of information-theory-based model selection and multimodel inference. We tested separately the impact of forest patch spatial attributes (patch size [PS], shape [SI] and isolation [PI]) and landscape spatial attributes (landscape forest cover [FC], fragmentation level [S], and the percentage of the matrix covered by open areas [OA]). The sign (±) of parameter estimates represents a positive or negative effect of the predictor on the response variable. The goodness-of-fit of each complete model (i.e., the percentage of deviance explained by each complete model) is also indicated, in parenthesis
The percentage of open-area matrix in the landscape was negatively related to 0 D (Fig. 3a), mean abundance (Fig. 3e) and mean biomass (Fig. 3f), but positively related to 2 D (Fig. 3c) and community evenness (Fig. 3d, Appendix Table A6 in supplementary material). The number of dominant species (2 D) tended to be positively related to landscape fragmentation (Fig. 3c), and as a consequence, there was also a tendency for community evenness to increase in landscapes with higher degree of fragmentation (Fig. 3d). Patches with more complex shapes showed lower 0 D (Fig. 3a), and more isolated patches showed increased mean abundance (Fig. 3e). However, because fragment shape and isolation were each related to only one response variable (Appendix Table A5 in supplementary material), we conclude that they had lower impact on dung beetle assemblages, compared to the other predictors.
Discussion
This study contributes to improving our understanding on the main drivers of dung beetle diversity in fragmented tropical landscapes. Two main findings deserve special attention: (i) forest amount at the patch (patch size) and landscape scales (landscape forest cover) showed a stronger impact on dung beetles than forest configuration at the patch (patch shape, patch isolation) and landscape scales (degree of landscape fragmentation); and (ii) landscape composition (percentage of forest cover and percentage of the matrix covered by cattle pastures and annual crops) had higher explanatory value than landscape configuration (degree of fragmentation). Therefore, our results support the idea that species’ persistence and distribution in fragmented landscapes are more strongly affected by attributes related to forest loss across spatial scales and by landscape composition, than by those related to patch and landscape configuration (e.g., Fahrig 2003; Ethier and Fahrig 2011; Smith et al. 2011).
The importance of forest loss
Patch isolation and degree of landscape fragmentation were weakly related to dung beetle assemblages, whereas both forest patch size and landscape forest cover were strongly and positively related to species richness, mean abundance, and mean biomass of dung beetles. Further, the loss of rare species increased community evenness in smaller patches surrounded by a lower percentage of forest cover. Other studies have also found that species richness, abundance (Estrada et al. 1998; Arellano et al. 2008a; Escobar et al. 2008), and biomass (Larsen et al. 2008) of dung beetles are positively related to the amount of remaining forest. This could be related to the fact that larger forests can sustain greater environmental heterogeneity and resources, thus supporting more species (Tscharntke et al. 2002; Navarrete and Halffter 2008). Furthermore, larger forest remnants can maintain a higher number of forest-specialists (Halffter and Halffter 1989). Finally, larger fragments also have a higher richness of mid- and large-sized mammal species (Garmendia et al. 2013), whose biomass has been related to increased dung beetle richness (Culot et al. 2013).
The size of forest remnants is also related to edge effects. Negative edge effects are expected to be weaker in larger patches as they show a higher proportion of core area unaffected by edge (Murcia 1995; Ewers and Didham 2002). Patches with more complex shapes also have stronger edge effects (Murcia 1995). Although we did not measure edge effects directly, our results agree with the idea that edge effects have negative impacts on biodiversity, as we found decreased species richness in smaller patches and in those with more complex shapes. Forest edges usually show lower plant biomass, diversity, and stratification, which in turn contributes to lower relative humidity and higher temperature compared to the forest interior (Laurance et al. 2002). These environmental changes can contribute to dung desiccation, negatively affecting the survival of dung beetle larvae (Klein 1989). In fact, many forest-interior species respond negatively to forest edges, with studies reporting declines in species richness, abundance and total biomass (Spector and Ayzama 2003; Barnes et al. 2014).
The importance of the matrix
As we expected, and consistent with previous studies on dung beetles in several Neotropical landscapes (Quintero and Roslin 2005; Halffter et al. 2007; Navarrete and Halffter 2008; Díaz et al. 2010; Rös et al. 2012), the matrix surrounding forest patches had a significant effect on dung beetle assemblages. When open areas (i.e., cattle pastures and annual crops) composed a larger proportion of the matrix, species richness, mean abundance, and mean biomass decreased. Yet, due to the increase in the number of dominant species (2 D), the abundances of species became more evenly distributed in landscapes with open-area dominated matrices. In particular, we found that rare species were lost (e.g., Phanaeus sallei, Uroxys platypyga, and Megathoposoma candezei), whereas disturbance-adapted species (e.g., Copris laeviceps and Deltochilum pseudoparile) were dominant in landscapes with a matrix dominated by open habitat.
The composition of the matrix is also related to the severity of edge effects (Murcia 1995). Open areas represent important barriers to dung beetle dispersal in fragmented tropical landscapes (Díaz et al. 2010), most probably because of the microclimatic differences between open and forest-interior areas (Klein 1989). As the structural complexity of the vegetation in the surrounding matrix increases (e.g., through secondary succession), the barrier effect for dung beetles drastically diminishes (Quintero and Roslin 2005). Also, according to the ‘landscape insurance hypothesis’ (sensu Tscharntke et al. 2012), landscapes with a homogeneous matrix, such as those dominated by open areas (e.g. pastures, annual crops), support a lower number of species than landscapes with heterogeneous matrices because they provide lower resilience and stability of ecological processes in human-modified landscapes (also see Perović et al. 2015). In fact, the availability of complementary and/or supplementary resources present in the matrix is higher in landscapes with heterogeneous matrices (Dunning et al. 1992).
Ecological and conservation implications
We found that, in contrast to other taxonomic groups in the region (e.g., primates: Arroyo-Rodríguez et al. 2013; birds: Carrara et al. 2015; mid- and large-sized terrestrial mammals: Garmendia et al. 2013; small rodents: San-José et al. 2014; trees: Hernández-Ruedas et al. 2014), dung beetles appear to be much more sensitive to forest spatial changes. This finding supports the use of Scarabaeinae subfamily as a good indicator of habitat modifications, both at the patch and landscape levels (Larsen et al. 2008; Nichols and Gardner 2011; Rös et al. 2012). This result also stresses the importance of including multiple taxa when trying to generalize on the effects of fragmentation-related drivers of biodiversity patterns.
Deforestation in the Marqués de Comillas Region is relatively recent (~40 years), but it has suffered the highest rates of forest loss in the Mesoamerican Biological Corridor (Mora 2008). Thus, if current trends of deforestation and defaunation continue in the region, the abundance, diversity and biomass of this group of insects, and consequently their functional roles in the ecosystem (e.g., nutrient cycling, soil conditioning, parasite/pest suppression, secondary seed dispersal, and seed-bank dynamics; Andresen and Feer 2005; Nichols et al. 2008; Santos-Heredia and Andresen 2014) will be severely threatened. Studies that have measured the effects of habitat modification on the ecological functions performed by dung beetle assemblages have indeed found strong negative effects (e.g., Braga et al. 2013). Therefore, management and conservation strategies focused on protecting dung beetle assemblages can be expected to have positive effects for ecosystem functioning in natural habitats and for ecosystem services in anthropogenic habitats (Losey and Vaughan 2006). In this sense, we found that forest loss and the increment of open areas in the matrix represented the highest threats to the conservation of dung beetle assemblages. Thus, conservation initiatives should be focused on protecting the largest remaining forest patches, increasing the area of smaller forest patches (Fahrig 2003) and improving the quality of the matrix.
These management strategies not only increase the amount of suitable habitat, but they would also enhance landscape connectivity, reduce the proportion of forest exposed to edge effects and allow for the persistence of species with large home range requirements. To improve matrix quality, conservation practices should aim at reducing the amount of open area, for example, by diversifying productive alternatives in the region and increasing the economic feasibility of agroecosystems with a forest-like structure (e.g., shade-grown crops and silvopastoral systems). For example, shade cocoa plantations under rustic management in the study region have proven to be good habitat for dung beetles (C. Santos-Heredia unpubl. data) and other animal groups (e.g., primates: Zárate et al. 2014). Overall, three attributes related to forest loss and to landscape composition (size of forest fragments, the amount of forest cover in the landscape, and the amount of open area in the matrix), seem to be acting interdependently to determine the structure and composition of the dung beetle assemblages in the Lacandona rainforest. Thus, conservation efforts will also require multiple but interdependent management actions at both the patch and landscape level in order to attain long-term success.
References
Andresen E (2003) Effect of forest fragmentation on dung beetle communities and functional consequences for plant regeneration. Ecography 26:87–97
Andresen E, Feer F (2005) The role of dung beetles as secondary seed dispersers and their effect on plant regeneration in tropical rainforests. In: Forget PM, Lambert J, Hulme P, Vander Wall SB (eds) Seed fate: predation, dispersal and seedling establishment. CABI Publishing, Oxon, pp 331–349
Arellano L, León-Cortés J, Halffter G (2008a) Response dung beetle assemblages to landscape structure in remnant natural and modified habitats in southern Mexico. Insect Conserv Divers 1:253–262
Arellano L, León-Cortés JL, Ovaskainen O (2008b) Patterns of abundance and movement in relation to landscape structure: a study of a common scarab (Canthon cyanellus cyanellus) in Southern Mexico. Landscape Ecol 23:69–78
Arroyo-Rodríguez V, González-Perez IM, Garmendia A, Solà M, Estrada A (2013) The relative impact of forest patch and landscape attributes on black howler monkey populations in the fragmented Lacandona rainforest, Mexico. Landscape Ecol 28:1717–1727
Barnes AD, Emberson RM, Chapman HM, Krell F-T, Didham RK (2014) Matrix habitat restoration alters dung beetle species responses across tropical forest edges. Biol Conserv 170:28–37
Barragán F, Moreno CE, Escobar F, Halffter G, Navarrete D (2011) Negative impacts of human land use on dung beetle functional diversity. PLoS ONE 6:e17976
Braga RF, Korasaki V, Andresen E, Louzada J (2013) Dung beetle community and functions along a habitat-disturbance gradient in the Amazon: a rapid assessment of ecological functions associated to biodiversity. PLoS ONE 8:e57786
Brodie JF, Giordano AJ, Dickson B, Hebblewhite M, Bernard H, Mohd-Azlan J, Anderson J, Ambu L (2015) Evaluating multispecies landscape connectivity in a threatened tropical mammal community. Conserv Biol 29:122–132
Burnham KP, Anderson DR (2002) Model selection and multi-model inference. A practical information-theoretic approach, 2nd edn. Springer, New York
Calcagno V, Mazancourt C (2010) glmulti: an R package for easy automated model selection with (Generalized) Linear Models. J Stat Softw 34:1–29
Câmara G, Souza RCM, Freitas UM, Garrido J, Mitsuo F II (1996) SPRING: integrating remote sensing and GIS by object-oriented data modeling. Comput Graph 20:395–403
Carrara E, Arroyo-Rodríguez V, Vega-Rivera JH, Schondube JE, de Freitas SM, Fahrig L (2015) Impact of landscape composition and configuration on forest specialist and generalist bird species in the fragmented Lacandona rainforest, Mexico. Biol Conserv 184:117–126
Ceballos G, Rodríguez P, Medellín R (1998) Assessing conservation priorities in megadiverse Mexico: mammalian diversity, endemicity and endangerment. Ecol Appl 8:8–17
Chao A, Jost L (2012) Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology 93:2533–2547
Culot L, Bovy E, Vaz-de-Mello FZ, Guevara R, Galetti M (2013) Selective defaunation affects dung beetle communities in continuous Atlantic rainforest. Biol Conserv 163:79–89
Cushman SA, McGarigal K, Neel MC (2008) Parsimony in landscape metrics: strength, universality, and consistency. Ecol Indic 8:691–703
Díaz A, Galante E, Favila ME (2010) The effect of the landscape matrix on the distribution of dung and carrion beetles in a fragmented tropical rain forest. J Insect Sci 10:81. Available online: insectscience.org/10.81
Didham RK, Ghazoul J, Stork NE, Davis AJ (1996) Insects in fragmented forest: a functional approach. Trends Ecol Evol 11:255–260
Didham RK, Kapos V, Ewers RM (2012) Rethinking the conceptual foundations of habitat fragmentation research. Oikos 121:161–170
Dunning JB, Danielson BJ, Pulliam HR (1992) Ecological processes that affect populations in complex landscapes. Oikos 65:169–175
Eigenbrod F, Hecnar SJ, Fahrig L (2011) Sub-optimal study design has major impacts on landscape-scale inference. Biol Conserv 144:298–305
Escobar F, Halffter G, Solis A, Halffter V, Navarrete D (2008) Temporal shifts in dung beetle community structure within a protected area of tropical wet forest: a 35-year study and its implications for long term conservation. J Appl Ecol 45:1534–1592
Estrada A, Coates-Estrada R, Anzures A, Cammarano P (1998) Dung and carrion beetles in tropical rain forest fragments and agricultural habitats at Los Tuxtlas, Mexico. J Trop Ecol 14:577–593
Ethier K, Fahrig L (2011) Positive effects of forest fragmentation, independent of forest amount, on bat abundance in eastern Ontario, Canada. Landscape Ecol 26:865–876
Ewers RM, Didham RK (2002) The effect of fragment shape and species’ sensitivity to habitat edges on animal population size. Conserv Biol 21:926–936
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev 81:117–142
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. Ann Rev Ecol Evol Syst 34:487–515
Fahrig L (2013) Rethinking patch size and isolation effects: the habitat amount hypothesis. J Biogeogr 40:1649–1663
Fahrig L, Baudry J, Brotons L, Burel FG, Crist TO, Fuller RJ, Sirami C, Siriwardena GM, Martin JL (2011) Functional landscape heterogeneity and animal biodiversity in agricultural landscapes. Ecol Lett 14:101–112
Franklin JF, Lindenmayer DB (2009) Importance of matrix habitats in maintaining biological diversity. Proc Natl Acad Sci USA 106:349–350
Gardner TA, Hernandez MIM, Barlow J, Peres CA (2008) Understanding the biodiversity consequences of habitat change: the value of secondary and plantation forests for Neotropical dung beetles. J Appl Ecol 45:883–893
Garmendia A, Arroyo-Rodríguez V, Estrada A, Naranjo E, Stoner KE (2013) Landscape and patch attributes impacting medium- and large-sized terrestrial mammals in a fragmented rain forest. J Trop Ecol 29:331–344
GRASS Development Team (2011) Geographic resources analysis support system (GRASS) Software. Open Source Geospatial Foundation Project
Halffter G, Matthews EG (1966) The natural history of dung beetles of the subfamily Scarabaeinae (Coleoptera, Scarabaeidae). Folia Entomol Mex 12:1–312
Halffter G, Halffter V (1989) Behavioral evolution of the non-rolling roller beetles (Coleoptera: Scarabaeidae). Acta Zool Mex 32:1–53
Halffter G, Pineda E, Arellano L, Escobar F (2007) Instability of copronecrophagous beetle assemblages (Coleoptera: Scarabaeinae) in a mountainous tropical landscape of Mexico. Environ Entomol 6:1367–1407
Hanski I (1999) Metapopulation ecology. Oxford University Press, Oxford
Hanski I (2015) Habitat fragmentation and species richness. J Biogeogr 42:989–994
Hernández-Ruedas MA, Arroyo-Rodríguez V, Meave JA, Martínez-Ramos M, Ibarra-Manríquez G, Martínez E, Melo FPL, Santos BA (2014) Conserving tropical tree diversity and forest structure: the value of small rainforest patches in moderately-managed landscapes. PLoS ONE 9:e98932
Jaeger JAG (2000) Landscape division, splitting index, and effective mesh size: New measures of landscape fragmentation. Landscape Ecol 15:115–130
Jost L (2006) Entropy and diversity. Oikos 113:363–375
Jost L (2010) The relation between evenness and diversity. Diversity 2:207–232
Klein BC (1989) Effects of forest fragmentation on dung and carrion beetle communities in central Amazonia. Ecology 70:1715–1725
Larsen TH, Lopera A, Forsyth A (2008) Understanding trait-dependent community disassembly: dung beetles, density functions and forest fragmentation. Conserv Biol 22:1288–1298
Laurance WF, Lovejoy TE, Vasconcelos HL, Bruna EM, Didham RK, Stouffer PC, Gascon C, Bierregaard RO, Laurance SG, Sampiao E (2002) Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conserv Biol 16:605–618
Losey JE, Vaughan M (2006) The economic value of ecological services provided by insects. Bioscience 56:311–323
MacArthur RH, Wilson EO (1967) The theory of island biogeography. Princeton University Press, Princeton
Marcon E, Hérault B (2014) entropart: an R package to measure and partition diversity. Institute for Statistics and Mathematics, Wirtschaftsuniversität Wien
Marsh CJ, Louzada J, Beiroz W, Ewers RM (2013) Optimising bait for pitfall trapping of Amazonian dung beetles (Coleoptera:Scarabaeinae). PLoS ONE 8(8):e73147
McGarigal K, Cushman SA (2002) Comparative evaluation of experimental approaches to the study of habitat fragmentation effects. Ecol Appl 12:335–345
McGarigal K, Cushman SA, Ene E (2012) FRAGSTATS v4: Spatial pattern analysis program for categorical and continuous Maps. Computer software program produced by the authors at the University of Massachusetts, Amherst. http://www.umass.edu/landeco/research/fragstats/fragstats.html
Melo FPL, Arroyo-Rodríguez V, Fahrig L, Martínez-Ramos M, Tabarelli M (2013) On the hope for biodiversity-friendly tropical landscapes. Trends Ecol Evol 28:461–468
Mora F (2008) Caracterización de la cobertura forestal en el Corredor Biológico Mesoamericano-México: patrones espaciales en la pérdida y fragmentación de bosques. In: CONABIO (ed) Importancia del capital ecológico de la región del Corredor Biológico Mesoamericano-México: evaluación de la biodiversidad, ciclo hidrológico y dinámica de la cobertura forestal. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad; Corredor Biológico Mesoamericano México, Mexico City, pp 55–83
Murcia C (1995) Edge effects in fragmented forests: implications for conservation. Trends Ecol Evol 10:58–62
Murphy DD (1989) Conservation and confusion–wrong species, wrong scale, wrong conclusions. Conserv Biol 3:82–84
Navarrete D, Halffter G (2008) Dung beetle (Coleoptera: Scarabaeidae: Scarabaeinae) diversity in continuous forest, forest fragments and cattle pastures in a landscape of Chiapas, Mexico: the effects of anthropogenic changes. Biodivers Conserv 17:2869–2898
Neter J, Kutner MH, Nachtsheim CJ, Wassermen W (1996) Applied linear statistical models, 4th edn. Irwin, Chicago
Newbold T, Hudson LN, Phillips HRP, Hill SLL, Contu S, Lysenko I, Blandon A, Butchart SHM, Booth HL, Day J, De Palma A, Harrison MLK, Kirkpatrick L, Pynegar E, Robinson A, Simpson J, Mace GM, Scharlemann JPW, Purvis A (2014) A global model of the response of tropical and sub-tropical forest biodiversity to anthropogenic pressures. Proc R Soc B 281:20141371
Nichols E, Larsen T, Spector S, Davis AL, Escobar F, Favila M, Vulinec K (2007) Global dung beetle response to tropical forest modification and fragmentation: a review and meta-analysis. Biol Conserv 137:1–19
Nichols E, Larsen T, Spector S, Amezquita S, Favila ME (2008) Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol Conserv 141:1461–1474
Nichols ES, Gardner TA (2011) Dung beetles as a candidate study taxon in applied biodiversity conservation research. In: Simmons LW, Ridsdill-Smith TJ (eds) Ecology and evolution of dung beetles. Wiley, Chichester, pp 267–291
Ordóñez-Gómez JD, Arroyo-Rodríguez V, Nicasio-Arzeta S, Cristóbal-Azkarate J (2015) Which is the appropriate scale to assess the impact of landscape spatial configuration on the diet and behavior of spider monkeys? Am J Primatol 77:56–65
Patton DR (1975) A diversity index for quantifying habitat “edge”. Wild Soc B 3:171–173
Perfecto I, Vandermeer J, Wright A (2009) Nature’s matrix: Linking agriculture, conservation and food sovereignty. Earthscan
Perović D, Gámez-Virués S, Börschig C, Klein AM, Krauss J, Steckel J, Rothenwöhrer C, Erasmi S, Tscharntke T, Westphal C (2015) Configurational landscape heterogeneity shapes functional community composition of grassland butterflies. J Appl Ecol 52:505–513
Quintero I, Roslin T (2005) Rapid recovery of dung beetle communities following habitat fragmentation in Central Amazonia. Ecology 86:3303–3311
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rös M, Escobar F, Halffter G (2012) How dung beetles respond to a human-modified variegated landscape in Mexican cloud forest: a study of biodiversity integrating ecological and biogeographical perspectives. Divers Distrib 18:377–389
San-José M, Arroyo-Rodríguez V, Sánchez-Cordero V (2014) Association between small rodents and forest patch and landscape structure in the fragmented Lacandona rainforest, Mexico. Trop Conserv Sci 7:403–422
Santos-Heredia C, Andresen E (2014) Upward movement of buried seeds: another ecological role of dung beetles promoting seedling establishment. J Trop Ecol 30:409–417
Schoereder JH, Galbiati C, Ribas CR, Sobrinho TG, Sperber CF, DeSouza O, Lopes-Andrade C (2004) Should we use proportional sampling for species-area studies? J Biogeogr 31:1219–1226
Silva PG, Hernández MIM (2014) Local and regional effects on community structure of dung beetles in a mainland-island scenario. PLoS ONE 9:e111883
Smith AC, Fahrig L, Francis CM (2011) Landscape size affects the relative importance of habitat amount, habitat fragmentation, and matrix quality on forest birds. Ecography 34:103–113
Spector S, Ayzama S (2003) Rapid turnover and edge effects in dung beetle assemblages (Scarabaeidae) at a Bolivian Neotropical forest–savanna ecotone. Biotropica 35:394–404
Thornton DH, Branch LC, Sunquist ME (2011) The relative influence of habitat loss and fragmentation: do tropical mammals meet the temperate paradigm? Ecol Appl 21:2324–2333
Tscharntke T, Steffan-Dewenter I, Kruess A, Thies C (2002) Characteristics of insect populations on habitat fragments: a mini review. Ecol Res 17:229–239
Tscharntke T, Tylianakis M, Rand TA, Didham RK, Fahrig L, Batáry P, Bengtsson J, Clough Y, Crist TO, Dormann CF, Ewers RM, Fründ J, Holt RD, Holzschuh A, Klein AM, Kleijn D, Kremen C, Landis DA, Laurance W, Lindenmayer D, Scherber C, Sodhi N, Steffan-Dewenter I, Thies C, van der Putten WH, Westphal C (2012) Landscape moderation of biodiversity patterns and processes - eight hypotheses. Biol Rev 87:661–685
Zárate DA, Andresen E, Estrada A, Serio-Silva JC (2014) Black howler monkey (Alouatta pigra) activity, foraging and seed dispersal patterns in shaded cocoa plantations versus rainforest in southern Mexico. Am J Primatol 76:890–899
Acknowledgments
We thank the insightful comments and suggestions provided by the Subject Editor (Dr. Jochen Krauss) and two anonymous reviewers. This research was funded by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT), DGAPA-UNAM (Projects IA-203111 and IB-200812). H.A.S.J. obtained a scholarship from the CONACyT, Mexico to make her MSc studies in the Posgrado en Ciencias Biologicas, UNAM. The Instituto de Investigaciones en Ecosistemas y Sustentabilidad (UNAM) provided logical support. H. Ferreira, A. Palencia and A. López provided technical support, and A. Navarrete provided the SPOT images. C. Dobler digitized the maps, F. Escobar helped in the identification of specimens and S. Nicasio-Arzeta elaborated the Fig. 1. H.A.S.J. is grateful to Audón Jamangapé for his invaluable field assistance. We are indebted to the local people of the Marqués de Comillas region.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sánchez-de-Jesús, H.A., Arroyo-Rodríguez, V., Andresen, E. et al. Forest loss and matrix composition are the major drivers shaping dung beetle assemblages in a fragmented rainforest. Landscape Ecol 31, 843–854 (2016). https://doi.org/10.1007/s10980-015-0293-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-015-0293-2