Abstract
Bioturbation is an important ecosystem process, and the loss of native digging mammals due to introduced predators and habitat loss may have detrimental consequences for ecosystem health. The mycophagous woylie (Bettongia penicillata ogilbyi) was once widespread across the Australian continent and currently exists in a greatly reduced range, while the omnivorous quenda (Isoodon fusciventer), which once occurred across the southern part of Western Australia (WA), remains common in south west WA over a reduced range. Populations of these two digging marsupials are currently maintained within sanctuaries where they can reach high densities. To assess the influence these digging marsupials have on fungal assemblages, we investigated fungal root associations among seedlings of a key mycorrhizal forest canopy species, Corymbia calophylla, R. Br. K. D. Hill and L. A. S. Johnson. Seedlings were grown in soil collected from inside (heavily-dug soil) and outside (minimally-dug soil) two predator-proof sanctuaries. Our results showed that above-ground seedling biomass was significantly greater for seedlings grown in soil collected from inside the sanctuaries. There were no differences in the diversity or species richness of rhizosphere fungal communities isolated from these seedlings; however, the community composition was significantly different. This was most obvious for the predator-proof enclosure that had been in place for 20 years (Karakamia Sanctuary) compared with the more recently-installed Perup Sanctuary (fenced in 2010; 4 years before this study). At Karakamia, there were greater numbers of putatively hypogeous ectomycorrhizal fungi inside the enclosure and four times the number of operational taxonomic units of arbuscular mycorrhizal fungi outside the enclosure. The differences in fungal communities suggest that digging mammals play a pivotal role in ecosystem functioning by influencing the rhizosphere of this key forest canopy species, which has implications for maintaining the health and persistence of forests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since European settlement, almost half of Australia’s digging mammals have gone extinct or are currently under threat (Fleming et al. 2014), and, given the low population numbers, it is likely that ecosystem functioning provided by these species is greatly reduced. The primary factor that has driven the decline of populations is predation by eutherian predators—the red fox (Vulpes vulpes) and feral cat (Felis catus)—which is exacerbated by other threats such as altered fire regimes and clearing of habitat (Burbidge and McKenzie 1989; Short and Smith 1994; Woinarski et al. 2014). Digging mammals perform important ecosystem services such as soil bioturbation and fungal dispersal, and the broadscale loss of these species is likely to have changed soil dynamics within natural habitats, potentially compromising forest health (Eldridge and James 2009; Fleming et al. 2014; Martin 2003).
Rhizosphere fungi perform a range of important roles in maintaining soil health and function. For example, pathogenic and saprotrophic fungi are key for the creation of dead root material and turnover of soil organic matter, while the symbiosis between plants and mycorrhizal fungi is critical for plant growth and survival. Mycorrhizal associations are generally beneficial, with the fungi providing plants with water, inorganic nutrients and trace elements derived from the decomposition of organic matter (Courty et al. 2010; Finlay 2008). The plant, in turn, provides the fungi with metabolites derived from photosynthesis (Brundrett 2008). The importance of mycorrhizal fungi in temperate forests is summarised by Tommerup and Bougher (2000), with mycorrhizae playing critical roles that lead to increased plant growth and resilience, and protection against plant pathogens.
Many fungi, including ectomycorrhizal and saprotrophic species, form macroscopic fruiting bodies either above ground (epigeous, e.g. mushrooms) or below ground (hypogeous, e.g. truffles). These fruiting bodies are important food resources for many vertebrate and invertebrate species, and digging mammals often serve as important vectors for the spread of fungal spores across the landscape (Lamont et al. 1985). The symbiosis between digging mammals and hypogeous fungi is especially important, as these fungi have limited alternative means of dispersal (Fogel and Trappe 1978). As hypogeous fungi are predominantly ectomycorrhizal, their successful dispersal is key to maintaining plant health in many forested systems. Bandicoots (Peramelidae; Isoodon and Perameles species), bettongs, potoroos, and the rufous rat-kangaroo (Potoroidae; Potorous spp., Bettongia spp., Aepyprymnus rufescens) dig as they forage for hypogeous fungi that can constitute 50–90% of their diet (reviewed by Claridge and May 1994; Johnson 1995; McIlwee and Johnson 1998; Nguyen et al. 2005; Vernes et al. 2001). Mycophagous specialists, especially species from the Potoroidae family, contribute disproportionately to the dispersal of fungi (Nuske et al. 2017). Resulting soil turnover from digging for hypogeous fungi has the potential to influence fungal communities, both by increasing fungal dispersal and by creating new habitat for fungi within digs (e.g. Johnson 1996; Valentine et al. 2017).
The interaction between digging mammals, soil fungi and plants is likely to influence aspects of ecosystem health (Fleming et al. 2014). Reduced colonisation by ectomycorrhizal (ECM) fungi has previously been observed in declining native eucalypt forest (Ishaq et al. 2013, 2018; Sapsford 2017), and declining remnant forest in an agricultural region of south west Western Australia (Tommerup and Bougher 2000). The fruiting bodies of ectomycorrhizal fungi are a key dietary component of digging mammals in south west Western Australia (Christensen 1980; Lamont et al. 1985), and lower rates of foraging digging activity have been observed in areas where native eucalypt forests are in decline (Moore et al. 2014). If declining forests are less attractive to digging mammals, it may result in a cascade effect with reduced digging activity, lower rates of scat deposition and therefore potentially reduced colonisation by fungi, which may further exacerbate forest declines.
The woylie (Bettongia penicillata ogilbyi; Potoroidae) was once abundant across ~ 35% of the Australian mainland (Abbott 2008; Shortridge 1909), where it inhabited a broad range of habitats (Finlayson 1958; Start et al. 1998). Today, largely due to the impact of introduced predators, this species occupies less than 1% of its original range in the wild in south Western Australia, reinforced by additional populations within predator-proof sanctuaries (Wayne et al. 2015). Each woylie (~ 1.3 kg body mass) creates between 38 and 114 diggings per night searching for their main diet of hypogeous fungi, extrapolating to ~ 4.8 tonnes of soil/year per individual (Garkaklis et al. 2004). The decrease in woylie foraging has had a marked impact on soil turnover, soil dynamics, and nutrient cycling across these landscapes (Garkaklis et al. 2000, 2003).
Quenda (Isoodon fusciventer; Peramelidae) are common within south western Australia; however, their historical distribution of has contracted in the last 150 years (Abbott 2008). Quenda inhabit dense vegetation within native forest (Abbott 2008) but are also commonly seen in remnant vegetation within urban areas (Bryant et al. 2017; Hillman and Thompson 2016). The quenda is omnivorous, feeding on insects, plants, fruits, seeds and fungi (Quin 1985). Quenda are active diggers, creating characteristic cone shaped diggings which collect leaf litter and seeds (Valentine et al. 2013). A single quenda (1.1–1.7 kg body mass) can create ~ 45 digs per day, which equates to 3.9 tonnes of soil moved per year by each animal (Valentine et al. 2013). Their diggings markedly alter water infiltration, nutrient cycling, seedling recruitment (Valentine et al. 2017) and growth (Valentine et al. 2018).
Previous studies have shown that the absence or exclusion of digging mammals influences fungal communities (e.g. Clarke et al. 2015; Gehring et al. 2002), and it is therefore important to identify the impacts this may have on plant growth and health. Mycophagous mammals influence soil properties with their digging activity (Eldridge et al. 2015; Valentine et al. 2017), in addition to being vectors of fungal spores which in turn is likely to influence soil fungi assemblages (Tay et al. 2018). Seedlings grown in the spoil heap of quenda diggings exhibited improved growth compared to those grown in soil from within the digging or in undug soil (Valentine et al. 2018) and seedlings inoculated with quenda scats demonstrate a greater diversity of rhizosphere fungi compared with controls (Tay et al. 2018). The existence of woylies and quenda at high densities within large predator-proof wildlife sanctuaries provides the opportunity to examine their impact on communities of rhizosphere fungi and flow-on effects for seedling establishment.
‘Dug’ soil was collected from within predator-proof sanctuaries where woylies and quenda have been reintroduced and the turnover of soil is considerable. ‘Undug’ soil was collected from immediately outside these sanctuaries where lower densities of animals still dig, but the overall rate of soil turnover is considerably lower compared to within the sanctuary. We compared the rhizosphere fungi for seedlings grown in soil collected from inside and outside two predator-proof sanctuaries, and identified whether dug soils had improved seedling growth. We predicted that the communities of rhizosphere fungi in the presence of greater densities of woylies and quenda (within sanctuaries) would be distinctly different from those in the absence of such high densities (outside sanctuaries). For example, we predicted a greater abundance of hypogeous ectomycorrhizal species associated with seedlings grown on soil from within sanctuaries. We also predicted that seedlings grown in soil from within sanctuaries would have a larger biomass than seedlings grown in soil from outside due to the increased abundance and diversity of beneficial rhizosphere fungi.
Methods
Study sites
In October 2014, soil was collected from two predator-proof sanctuaries in south Western Australia where populations of woylies and quenda are maintained: Karakamia and Perup. Both sites are located in mesic forest, dominated by the forest canopy species Corymbia calophylla (marri), co-dominant with Eucalyptus marginata Donn ex Sm. (jarrah), and Eucalyptus wandoo Blakely (wandoo). These reserves protect important woylie populations, as well as populations of quenda. Both species make a significant contribution to soil turnover.
-
1.
Karakamia Sanctuary (Fig. 1a; − 31.823349°, 116.251425°) is a 275-ha predator-proof enclosure managed by Australian Wildlife Conservancy (AWC). Karakamia Sanctuary was fenced was fenced in 1994, 20 years prior to this study. The current population is estimated to be stable at ~ 249 woylies (95% credibility intervals 211–291), i.e. ~ 1 woylie/ha (M. Smith Regional Ecologist AWC pers. comm.). There are no woylies outside the sanctuary. Quenda are abundant inside the sanctuary (estimated population ~ 100–200; 0.3–0.7 quenda/ha, M. Smith Regional Ecologist AWC pers. comm.) and at (unknown) lower densities outside the sanctuary.
Fig. 1 -
2.
Perup Sanctuary (Fig. 1b; − 34.174328°, 116.569293°) is a 420-ha predator-proof enclosure maintained by the Western Australian Department of Biodiversity, Conservation and Attractions (DBCA 2013). The enclosure was established in November 2010 (4 years prior to this study) to protect an existing population of woylies. Upon completion of the fence in 2010, the enclosure was populated with 41 woylies from the surrounding area. Current population estimates suggest ~ 1 woylie/ha inside the sanctuary (M. Virgo Department of Biodiversity, Conservation and Attractions, pers. comm.). Woylies persist outside the sanctuary but at lower densities compared with inside the fence. Quenda are also present both inside the sanctuary (current estimate ~ 1.42 quenda/ha, M. Virgo Department of Biodiversity, Conservation and Attractions, unpublished data) and outside the sanctuary at lower densities. There are also 10 animal-proof exclosures within the main fence, each 10 × 10 m, constructed in 2010, which are monitored for vegetation changes.
Soil collection
A total of 30 soil cores were collected at each study site; 15 from within the sanctuary and 15 from the surrounding forest outside the sanctuary fence. In addition, 11 soil cores were collected at the Perup site from within six of the 10 vegetation monitoring exclosures. All cores were collected from within the rhizosphere of mature C. calophylla trees, located < 1 m from the base of randomly-selected trees.
Dry leaves and sticks were brushed from the surface of the soil, and then soil corers [100 × 270 mm (diameter × length) plastic PVC pipe with a sharpened cutting edge] were hammered into the ground to a depth of ~ 200 mm with a rubber mallet. Decomposed organic matter on the soil surface was collected with the soil core. The intact soil core was then transferred to new 160 × 160 mm (diameter x height) plastic pots. Asepsis was maintained throughout soil collection activities with the corer and digging tools wiped with methylated spirits between samples. Pots were kept covered during transport and efforts were made when handling pots to ensure no cross-contamination occurred.
Seedling biomass
Pots were seeded with C. calophylla seeds sourced from the Northern Jarrah Forest bioregion. Three seeds per pot were planted; seedlings were thinned at the cotyledon stage to one per pot. Seedlings were grown in an evaporatively-cooled glasshouse for 4 months from October 2014 until February 2015. Pots were watered automatically for only 30 s, three times a day, in an effort to reduce leaching from the free-draining pots. Pot locations were randomised weekly. In late January, seedlings exhibited symptoms of nutrient deficiency and were consequently treated with 50 mL of a general plant fertiliser (Thrive All Purpose liquid fertiliser; Yates, Padstow NSW) 0.25 g/L solution, twice weekly for 4 weeks before harvest.
Root harvesting and seedling measurements
Seedlings were harvested after 4 months and the roots manually extracted and gently washed to remove all soil. The fine roots (i.e. those most likely to contain mycorrhiza and other fungi of interest) were then stripped off by hand and stored at − 18 °C until analysis by High Throughput Sequencing (HTS). The harvested seedlings were dried at 51 °C for 72 h before being weighed.
DNA sequencing
For each sample, total DNA was extracted from a 0.05 g representative (mixed) subsample of fresh root material using the PowerPlant® Pro DNA Isolation Kit (MO BIO Laboratories 2014) following the manufacturer’s protocols. Extraction controls were undertaken to test the purity of the reagents used and detect any contamination introduced during the extraction process. The ITS2 region of the ribosome encoding genes was amplified using the fungal-specific primer fITS7 (GAACGCAGCRAAIIGCGATA; specific for higher fungi) (Ihrmark et al. 2012) and the general primer ITS4 (White et al. 1990) with adapters attached. Amplification was performed following the protocols outlined in Ihrmark et al. (2012) using HotStarTaq (Qiagen, Valencia, CA), 30 amplification cycles and an annealing temperature of 57 °C. The ITS4 primer contained a 10 base pair tag specific to each sample. Two replicate PCRs were performed per sample and these were combined following amplification. The amplified products were visualised on a 1% agarose gel and then mixed in similar concentrations before being cleaned using an Agencourt AMPure® XP PCR purification kit (Beckman Coulter Inc., MA, USA). The final library was then subjected to 454 sequencing on a Roche GS Junior at the Western Australian State Agricultural Biotechnology Centre, located at Murdoch University. Extraction controls and PCR controls were also tagged separately and sequenced to ensure no contamination was introduced during the amplification process; no sequencing reads were obtained from these controls.
Deconvolution (quality control and single-linkage clustering) was carried out in the Sequence Clustering and Analysis of Tagged Amplicons (SCATA) pipeline (scata.mykopat.slu.se). SCATA is a bioinformatics pipeline specially developed for processing fungal ITS data sets derived from 454-sequencing (Lindahl et al. 2013), the workflow of which has been used extensively detailed by Clemmensen et al. (2015). We used the settings described by Nguyen et al. (2017). Prior to clustering, all sequences were trimmed to 300 bp length and the primers removed. A clustering similarity of 98.5% was used, corresponding approximately to species level for ITS (Lindahl et al. 2013; Nguyen et al. 2017; Ottosson et al. 2015). Raw molecular data are stored at the Sequence Read Archive (SRA) curated by NCBI under the accession number SRP151221.
The most abundant genotype for each cluster was used to represent each OTU. Representative cluster sequences (operational taxonomic units or OTUs) were identified by searching against internally curated SCATA databases and by blasting against NCBI’s sequence database GenBank (Altschul et al. 1990) through Geneious. Internally-curated SCATA databases included a recent version of UNITE (Clemmensen et al. 2015; Kõljalg et al. 2013) and data collected for other studies of fungi in south Western Australia (Sapsford 2017; Tay et al. 2018). Putative species-level assignment was made based on best matches over the entire length of the query sequence and 98.5–100% sequence similarity, 94–98% sequence similarity for genus level, 90–93% family level and 80–89% sequence similarity for ordinal level, after Ottosson et al. (2015) and Nguyen et al. (2016). Similarity less than 80% was assigned to class level. Potentially similar OTUs were aligned in Geneious to confirm identification. The relative abundance of each OTU per sample was determined from the sequence reads as the number of reads for an OTU divided by the total number of reads for the sample (Nguyen et al. 2017).
Where identifications could be made, OTUs were designated into functional categories based on their putative life history following ecological guild assignment sensu FUNGuild (Nguyen et al. 2016). The life history of the majority of identified OTUs was determined based on literature searches. For a small number of OTUs, searches on NCBI revealed a close alignment with sequences from a known source (e.g. fruiting body, mycorrhizal root tip) and this information was used as an additional layer to guide putative life history classification. Guilds used in this study included ectomyccorhiza (ECM), arbuscular mycorrhiza (AM), ericoid mycorrhiza (ericoid), saprotroph, endophyte, or pathogen. Where guild membership was ambiguous (i.e. ECM or saprotroph), OTUs were assigned membership in both groups.
Data analysis
Seedling above-ground biomass
We compared the above-ground biomass by two-way factorial ANOVA with sanctuary and location (inside/outside enclosure) as independent factors. Additionally, we carried out a one-way ANOVA for the Perup data only with location (enclosure inside, enclosure outside and exclosure inside) as an independent factor as we had additional samples from exclosures within the sanctuary.
Fungal diversity and species richness
To compare the diversity of fungi present, we calculated the Shannon’s Diversity Index (PAST v3) and total number of fungal taxa and compared these data by two-way factorial ANOVA with site (Karakamia and Perup) and location (inside/outside sanctuary) as independent factors. We also carried out one-way ANOVA for the Perup data only as we had additional samples from exclosures within the sanctuary.
Fungal community composition
To compare the fungal community composition in the root samples, we performed non-metric Multidimensional Scaling (MDS) using Bray–Curtis similarity index (PAST v3) (Hammer and Harper 2013; Hammer et al. 2001) using the relative abundances of each fungal OTU (Nguyen et al. 2017; Ottosson et al. 2015). A two-way PERMANOVA was carried out, comparing sanctuary (Karakamia and Perup) and location (inside and outside the enclosure) as independent factors. For Perup, we also carried out a one-way PERMANOVA comparing fungal community composition by location (inside enclosure, outside enclosure, or within the animal exclosures). Two samples (one from each sanctuary) were marked outliers on the Multidimensional scaling plot (MDS) and were therefore excluded from analysis. These analyses were followed by similarity percentage (SIMPER) analyses to determine which OTUs contributed to the observed differences in fungal communities inside and outside the sanctuaries.
Results
Seedling above-ground biomass
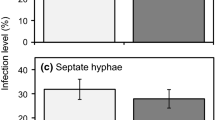
There was a significant effect of location of soil sample on C. calophylla seedling growth, with seedlings having significantly higher above-ground biomass (dry weight) when grown in soils taken from within the sanctuaries compared with outside (F1,54 = 16.91, p < 0.001; Fig. 2a). There was no significant difference in seedling biomass between sanctuaries (F1,54 = 0.19, p = 0.657) or for the sanctuary x location interaction term (F1,54 = 0.23, p = 0.628). There was a significant location (inside enclosure, outside enclosure, or within the animal exclosures) effect for Perup Sanctuary: seedlings grown in soil from inside the Sanctuary had significantly greater biomass compared with seedlings grown on soil collected from outside the enclosure or within the animal exclosure plots (F1,37 = 14.72, p < 0.001) (Fig. 2b).
Comparison of seedling shoot weight (g), diversity of fungal communities (represented by Shannon Diversity Index) and the total number of OTUs present in fungal root associations for Corymbia calophylla seedlings grown in soil taken from inside (I) and outside (O) the enclosures at Perup and Karakamia Sanctuaries (left column), south western Australia. The right column represents the samples from Perup Sanctuary only, where soil samples were collected from three locations: inside the predator-proof sanctuary (I; high density digging), outside the sanctuary (O; low density digging), and from the vegetation exclosure plots (E; no digging). Values are means ± 0.95% confidence intervals for n = 15 replicates for inside and outside sanctuaries, and n = 11 for Perup exclosures
Fungal diversity and species richness
There was no difference in rhizosphere fungal diversity between sanctuaries (F1,54 = 0.18, p = 0.668), but there was a significant location effect (F1,54 = 4.02, p = 0.045) with more fungal species identified for the samples grown on soil collected outside the fence (Fig. 2c). The sanctuary × location interaction term was not significant (F1,54 = 0.06, p = 0.804). For Perup, there was no significant location effect on the diversity of fungi present (F2,37 = 5.56, p = 0.062) (Fig. 2d).
There was no difference between sanctuaries (F1,54 = 2.81, p = 0.094) or location effect (F1,54 < 0.01, p = 0.955) on species richness (the number of fungal taxa present) (Fig. 2e). The sanctuary × location interaction term was also not significant (F1,54 = 0.10, p = 0.748). For Perup, there was no significant location effect on the number of fungal taxa present (F2,37 = 4.83, p = 0.089) (Figs. 2f).
Fungal community composition
There were marked differences in rhizosphere fungal community composition between the sanctuaries (two-way PERMANOVA; pseudo-F1,54 = 4.23, p < 0.001; Fig. 3) and with location (F1,54 = 2.42, p < 0.001). The sanctuary x location interaction term was not significant (F1,54 = 1.54, p = 0.078).
Multidimensional Scaled (MDS) plot showing the difference for fungal communities associated with Corymbia calophylla seedlings grown in soil from a inside (filled squares) and outside (open squares) Karakamia Sanctuary, south western Australia; and b inside (filled triangles), outside (open, inverse triangles) and exclosures within the sanctuary (open triangle) at Perup Sanctuary, south western Australia
At Karakamia, the rhizosphere fungal community composition for seedlings grown on soils collected from inside the sanctuary was significantly different from those grown on soil collected from outside (F1,54 = 1.97, p = 0.003; Fig. 4). At Karakamia, a total of 125 fungal OTUs were found exclusively on seedlings grown in soil collected from inside the enclosure and 137 for seedlings grown on soil from outside the enclosure; 36 OTUs were recorded for samples from both inside and outside. Specifically, there were similar numbers of saprophytes and ectomycorrhizal fungal OTUs found exclusively inside or outside the enclosure, but there were four times the number of arbuscular mycorrhizal fungal OTUs found exclusively outside the enclosure. There were also greater numbers of putatively hypogeous ectomycorrhizal fungi inside the enclosure. At Perup, there was no significant location effect on fungal community composition (F2,37 = 0.92, p = 0.550) (Figs. 1S, 2S).
Discussion
The impact of changes in populations of digging mammals on soil microbial communities is not well documented, despite the significant role microbes play in ecosystem health (Brundrett 2009; Clarke et al. 2015). In our study, Corymbia calophylla seedlings grew better (that is, had a greater above-ground biomass) in soil collected from within two predator-proof sanctuaries containing digging mammal populations compared with seedlings grown in soils from outside the sanctuaries. While there were no differences in the diversity or species richness for rhizosphere fungal communities isolated from these seedlings, the fungal community composition was significantly different, suggesting that changes in abundance of these mammal populations influences the overall distributions of fungal communities. Because colonisation by fungi is key for successful growth of many plant species around the world (Lodge 2000), it is likely that loss of these important mammals will have long-term impacts on tree health.
As a mycophagous species, woylies and quenda play a critical role in maintaining the diversity of rhizosphere fungi through their foraging activities and fungal spore dispersal. Previous scat analysis of Karakamia Sanctuary woylies indicated that fungi are the most abundant food type present (> 69% of undigested material), followed by plant material (9–17%) and invertebrates (< 5%) (Zosky et al. 2010). Woylies consume a diversity of fungi, with 32 spore classes identified from faecal samples (Zosky et al. 2010). At Perup, in a study carried out prior to the installation of the predator-proof enclosure, spores of the truffle Mesophellia trabalis Trappe, Castellano and Malajczuk were the most prevalent species of ectomycorrhizal fungi present in woylie scats (Lamont et al. 1985). Improved root tip colonisation of Mesophellia occurred for spores from woylie scats, suggesting that processing through the digestive tract of digging animals may assist the fungal lifecycle (Lamont et al. 1985). Similarly, quenda consume a diversity of fungal fruiting bodies and fungal spores from scats of quenda colonise eucalypt seedlings (Tay et al. 2018). In our study, fungi found both inside and outside the sanctuaries formed fruiting bodies that could be eaten and dispersed by woylies and quenda. There were many more putatively hypogeous fungi exclusively found inside the enclosure at Karakamia. The difference in fungal communities inside and outside the sanctuary and the improved seedling growth in soil collected from within the sanctuaries may be an indirect result of woylie and quenda activity. Increased plant growth associated with digging activities has also been noted by Travers et al. (2012). Our work suggests that digging mammals such as the woylie and quenda play an important part as dispersers of fungi and are important for plant growth in forest ecosystems.
It is likely that the length of time that the sanctuaries had been in existence influences the degree of difference in fungal communities on either side of the fence. The differences in fungal communities were most obvious for Karakamia Sanctuary, which had been in place for 20 years at the time of this study, and has markedly fewer digging mammals outside the sanctuary compared with inside. By contrast, Perup Sanctuary had been fenced only four years before this study and animals were also present outside the fence, albeit at lower densities. Therefore, although both sanctuaries have similar densities of digging animals presently, they have different histories. The ongoing volume of soil moved by these species is substantial. Given the soil turnover by woylies (4.8 tonnes per individual per year, Garkaklis et al. 2004) and quenda (3.9 tonnes per individual per year, Valentine et al. 2013), we estimated total soil turnover since establishment of the sanctuaries as 25.8 tonnes/ha in Perup (woylies total 4982 tonnes; quenda total 5838 tonnes) and 134.5 tonnes/ha in Karakamia (woylies total 22,550 tonnes; quenda total 14,430 tonnes) (from a starting population of 41 woylies at Perup, DBCA 2013; and 31 woylies at Karakamia, Pacioni et al. 2013 and assuming a population increase of 100% following the installation of fences until it reached current population levels). This intense digging activity most likely improves soil aeration, water infiltration and mixing of organic matter, which in turn will influence the soil microbial communities and therefore nutrient turnover. It would be informative to continue this analysis across time by comparing the impacts of digging mammals at these same sanctuaries in the future.
In this study, the changes observed in fungal communities in the presence of woylies and quenda are likely to substantially impact ecosystem health. We found different taxa of ectomycorrhizal fungi and saprotrophs in soil collected from within the sanctuaries compared with outside. These different taxa perform different ecological roles. Importantly, in our study, arbuscular mycorrhizal fungi were more prevalent outside the sanctuaries than inside. The type of mycorrhizal colonisation, whether it be arbuscular or ectomycorrhizal, may be an important bioindicator of ecosystem health (Brundrett and Tedersoo 2018). For example, in a study by Ishaq et al. (2013, 2018), arbuscular mycorrhizal fungi were more common on seedlings grown in soil taken from the rhizosphere of declining trees, while colonisation by ectomycorrhizal fungi was found to be greater in soil from beneath healthy trees. Thus, digging mammals may be critical to ecosystem health through their influence on fungal community structure and function.
The continued loss of digging mammals and the ecosystem processes they provide—changing soil properties through their digging actions, dispersing fungi, altering microclimates for seedling recruitment and growth—will have flow-on effects for forest health (Fleming et al. 2014). For example, the detection of fungal taxa exclusively inside the sanctuaries suggests that the loss of digging mammals could lead to co-extinction of fungi (Clarke et al. 2015). Co-extinction is predicted to be the most common form of species loss (Dunn et al. 2009; Koh et al. 2004). Indeed, the loss of mammals, and hence the loss the key functional microbes they disperse, could make it difficult for key overstorey and understory plant species to maintain health and resilience, increasing their vulnerability to plant pathogens such as Phytophthora. Furthermore, changes in functional microbe communities have implications for ecosystem processes such as nutrient cycling, decomposition rates, soil respiration, and the capacity of ecosystems to maintain soil organic carbon (Beare et al. 1992; McGuire and Treseder 2010; Van Der Heijden et al. 2008). Thus, although the loss of digging mammals alone may seem like an isolated issue, it has ecosystem-level implications. The loss of digging mammals therefore represents a critical loss in ecosystem functioning.
Conclusions
Our research demonstrates that the communities of rhizosphere fungi associated with a key forest canopy species are influenced by the presence of digging mammal species. Seedling growth of a key forest species is significantly greater in soil taken from sites within sanctuaries where these digging mammals are present. With the widespread loss of digging mammals across the Australian landscape, it is likely these ecosystems have changed drastically. Improving our knowledge of how digging mammals influence fungal communities and seedling development contributes to a better understanding of ecosystem functioning, revealing how these mammals can play a critical role in maintaining and restoring forests.
References
Abbott I (2008) Historical perspectives of the ecology of some conspicuous vertebrate species in south-west Western Australia. Conserv Sci West Aust 6:1–214
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Beare MH, Parmelee RW, Hendrix PF, Cheng W, Coleman DC, Crossley DA (1992) Microbial and faunal interactions and effects on litter nitrogen and decomposition in agroecosystems. Ecol Monogr 62:569–591
Brundrett MC (2008) Mycorrhizal associations: The Web Resource
Brundrett MC (2009) Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant Soil 320:37–77
Brundrett MC, Tedersoo L (2018) Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. https://doi.org/10.1111/nph.14976
Bryant GL, Kobryn HT, Hardy GES, Fleming PA (2017) Habitat islands in a sea of urbanisation. Urban For Urban Green 28:131–137
Burbidge AA, McKenzie NL (1989) Patterns in the modern decline of Western Australia’s vertebrate fauna: causes and conservation implications. Biol Conserv 50:143–198
Christensen PES (1980) The biology of Bettongia penicillata Gray, 1837, and Macropus eugenii (Desmarest, 1817) in relation to fire. Forests Department of Western Australia. Bulletin 91
Claridge AW, May TW (1994) Mycophagy among Australian mammals. Aust J Ecol 19:251–275
Clarke LJ, Weyrich LS, Cooper A (2015) Reintroduction of locally extinct vertebrates impacts arid soil fungal communities. Mol Ecol 24:3194–3205
Clemmensen KE, Finlay RD, Dahlberg A, Stenlid J, Wardle DA, Lindahl BD (2015) Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol 205:1525–1536
Courty P-E, Buée M, Diedhiou AG, Frey-Klett P, Le Tacon F, Rineau F, Turpault M-P, Uroz S, Garbaye J (2010) The role of ectomycorrhizal communities in forest ecosystem processes: new perspectives and emerging concepts. Soil Biol Biochem 42:679–698
DBCA (2013) Perup Sanctuary. (ed C. a. A. Western Australian Department of Biodiversity)
Dunn RR, Harris NC, Colwell RK, Koh LP, Sodhi NS (2009) The sixth mass coextinction: are most endangered species parasites and mutualists? Proc R Soc B 276:3037–3045
Eldridge DJ, James AI (2009) Soil-disturbance by native animals plays a critical role in maintaining healthy Australian landscapes. Ecol Manage Restor 10:S27–S34
Eldridge DJ, Woodhouse JN, Curlevski NJA, Hayward M, Brown MV, Neilan BA (2015) Soil-foraging animals alter the composition and co-occurrence of microbial communities in a desert shrubland. ISME J 9:2671–2681
Finlay RD (2008) Ecological aspects of mycorrhizal symbiosis: with special emphasis on the functional diversity of interactions involving the extraradical mycelium. J Exp Bot 59:1115–1126
Finlayson HH (1958) On Central Australian mammals (with notice of related species from adjacent tracts). Part III. The Potoroinae. Rec South Aust Mus 13:235–302
Fleming PA, Anderson H, Prendergast AS, Bretz MR, Valentine LE, Hardy GESJ (2014) Is the loss of Australian digging mammals contributing to a deterioration in ecosystem function? Mamm Rev 44:94–108
Fogel R, Trappe JM (1978) Fungus consumption (Mycophagy) by small animals. Northwest Sci 52:1–31
Garkaklis MJ, Bradley JS, Wooller RD (2000) Digging by vertebrates as an activity promoting the development of water-repellent patches in sub-surface soil. J Arid Environ 45:35–42
Garkaklis MJ, Bradley JS, Wooller RD (2003) The relationship between animal foraging and nutrient patchiness in south-west Australian woodland soils. Soil Res 41:665–673
Garkaklis MJ, Bradley JS, Wooller RD (2004) Digging and soil turnover by a mycophagous marsupial. J Arid Environ 56:569–578
Gehring CA, Wolf JE, Theimer TC (2002) Terrestrial vertebrates promote arbuscular mycorrhizal fungal diversity and inoculum potential in a rain forest soil. Ecol Lett 5:540–548
Hammer Ø, Harper DAT (2013) PAST: version 2.17c http://folk.uio.no/ohammer/past
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis. Palaeontol Electron 41:1–9
Hillman A, Thompson RCA (2016) Interactions between humans and urban-adapted marsupials on private properties in the greater Perth region. Aust Mammal 38:253–255
Ihrmark K, Bödeker ITM, Cruz-Martinez K, Friberg H, Kubartova A, Schenck J, Strid Y, Stenlid J, Brandström-Durling M, Clemmensen KE (2012) New primers to amplify the fungal ITS2 region–evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol Ecol 82:666–677
Ishaq L, Barber PA, Hardy GESJ, Calver M, Dell B (2013) Seedling mycorrhizal type and soil chemistry are related to canopy condition of Eucalyptus gomphocephala. Mycorrhiza 23:359–371
Ishaq L, Barber PA, Hardy GESJ, Dell B (2018) Diversity of fungi associated with roots of Eucalyptus gomphocephala seedlings grown in soil from healthy and declining sites. Australas Plant Pathol 47:155–162
Johnson CN (1995) Interactions between fire, mycophagous mammals, and dispersal of ectomycorrhizal fungi in Eucalyptus forests. Oecologia 104:467–475
Johnson CN (1996) Interactions between mammals and ectomycorrhizal fungi. Trends Ecol Evol 11:503–507
Koh LP, Dunn RR, Sodhi NS, Colwell RK, Proctor HC, Smith VS (2004) Species Coextinctions and the Biodiversity Crisis. Science 305:1632–1634
Kõljalg U, Nilsson RH, Abarenkov K, Tedersoo L, Taylor AFS, Bahram M, Bates ST, Bruns TD, Bengtsson-Palme J, Callaghan TM, Douglas B, Drenkhan T, Eberhardt U, Dueñas M, Grebenc T, Griffith GW, Hartmann M, Kirk PM, Kohout P, Larsson E, Lindahl BD, Lücking R, Martín MP, Matheny PB, Nguyen NH, Niskanen T, Oja J, Peay KG, Peintner U, Peterson M, Põldmaa K, Saag L, Saar I, Schüßler A, Scott JA, Senés C, Smith ME, Suija A, Taylor DL, Telleria MT, Weiss M, Larsson K-H (2013) Towards a unified paradigm for sequence-based identification of fungi. Mol Ecol 22:5271–5277
Lamont BB, Ralph CS, Christensen PES (1985) Mycophagous marsupials as dispersal agents for ectomycorrhizal fungi on Eucalyptus calophylla and Gastrolobium bilobum. New Phytol 101:651–656
Lindahl BD, Nilsson RH, Tedersoo L, Abarenkov K, Carlsen T, Kjøller R, Kõljalg U, Pennanen T, Rosendahl S, Stenlid J, Kauserud H (2013) Fungal community analysis by high-throughput sequencing of amplified markers—a user’s guide. New Phytol 199:288–299
Lodge DJ (2000) Ecto- or arbuscular mycorrhizas—which are best? New Phytol 146:353–354
Martin G (2003) The role of small ground-foraging mammals in topsoil health and biodiversity: implications to management and restoration. Ecol Manage Restor 4:114–119
McGuire KL, Treseder KK (2010) Microbial communities and their relevance for ecosystem models: decomposition as a case study. Soil Biol Biochem 42:529–535
McIlwee AP, Johnson C (1998) The contribution of fungus to the diets of three mycophagous marsupials in eucalyptus forests, revealed by stable isotope analysis. Funct Ecol 12:223–231
Moore TL, Craig MD, Valentine LE, Hardy GESJ, Fleming PA (2014) Signs of wildlife activity and Eucalyptus wandoo condition. Aust Mammal 36:146–153
Nguyen VP, Needham AD, Friend JA (2005) A quantitative dietary study of the ‘critically endangered’ Gilbert’s potoroo Potorous gilbertii. Aust Mammal 27:1–6
Nguyen NH, Song ZW, Bates ST, Branco S, Tedersoo L, Menke J, Schilling JS, Kennedy PG (2016) FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol 20:241–248
Nguyen D, Boberg J, Cleary M, Bruelheide H, Hönig L, Koricheva J, Stenlid J (2017) Foliar fungi of Betula pendula: impact of tree species mixtures and assessment methods. Sci Rep 7:41801
Nuske SJ, Vernes K, May TW, Claridge AW, Congdon BC, Krockenberger A, Abell SE (2017) Redundancy among mammalian fungal dispersers and the importance of declining specialists. Fungal Ecol 27:1–13
Ottosson E, Kubartová A, Edman M, Jönsson M, Lindhe A, Stenlid J, Dahlberg A (2015) Diverse ecological roles within fungal communities in decomposing logs of Picea abies. FEMS Microbiol Ecol 91:fiv012
Pacioni C, Wayne AF, Spencer PBS (2013) Genetic outcomes from the translocations of the critically endangered woylie. Curr Zool 59:294–310
Quin DG (1985) Observations on the diet of the southern brown bandicoot, Isoodon obesulus (Marsupialia: peramelidae), in southern Tasmania. Aust Mammal 11:15–25
Sapsford SJ (2017) Factors predisposing Corymbia calophylla to canker disease caused by Quambalaria coyrecup. PhD Thesis. Murdoch University, Perth
Short J, Smith A (1994) Mammal decline and recovery in Australia. J Mammal 75:288–297
Shortridge GC (1909) An account of the geographical distribution of the marsupials and monotremes of south-west Australia, having special reference to the specimens collected during the Balston Expedition of 1904–1907. Proc Zool Soc Lond 79:803–848
Start AN, Burbidge AA, Armstrong D (1998) A review of the conservation status of the woylie, Bettongia penicillata ogilbyi (Marsupialia: Potoroidae) using IUCN criteria. CALMScience 2:277–289
Tay N, Hopkins AJM, Ruthrof KX, Burgess T, Hardy GESJ, Fleming PA (2018) The tripartite relationship between a bioturbator, mycorrhizal fungi, and a key Mediterranean-type forest tree. Austral Ecol. https://doi.org/10.1111/aec.12598
Tommerup IC, Bougher NL (2000) The role of ectomycorrhizal fungi in nutrient cycling in temperate Australian woodlands. Surrey Beatty & Sons Pty., Ltd., Chipping Norton
Travers SK, Eldridge DJ, Koen TB, Soliveres S (2012) Animal foraging pit soil enhances the performance of a native grass under stressful conditions. Plant Soil 352:341–351
Valentine LE, Anderson H, Hardy GESJ, Fleming PA (2013) Foraging activity by the southern brown bandicoot (Isoodon obesulus) as a mechanism for soil turnover. Aust J Zool 60:419–423
Valentine LE, Bretz M, Ruthrof KX, Fisher R, Hardy GESJ, Fleming PA (2017) Scratching beneath the surface: bandicoot bioturbation contributes to ecosystem processes. Austral Ecol 42:265–276
Valentine LE, Ruthrof KX, Fisher R, Hardy GESJ, Hobbs RJ, Fleming PA (2018) Bioturbation by bandicoots facilitates seedling growth by altering soil properties. Funct Ecol. https://doi.org/10.1111/1365-2435.13179
Van Der Heijden MGA, Bardgett RD, Van Straalen NM (2008) The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett 11:296–310
Vernes K, Castellano M, Johnson CN (2001) Effects of season and fire on the diversity of hypogeous fungi consumed by a tropical mycophagous marsupial. J Anim Ecol 70:945–954
Wayne A, Maxwell MA, Ward CG, Vellios CV, Wilson I, Wayne JC, Williams MR (2015) Sudden and rapid decline of the abundant marsupial Bettongia penicillata in Australia. Onyx 49(1):175–185
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. Academic Press, San Diego
Woinarski JCZ, Burbidge AA, Harrison PL (2014) The action plan for Australian mammals 2012. CSIRO Publishing, Collingwood
Zosky K, Bryant K, Calver M, Wayne A (2010) Do preservation methods affect the identification of dietary components from faecal samples? A case study using a mycophagous marsupial. Aust Mammal 32:173–176
Acknowledgements
The authors would like to thank Bryony Palmer and Mike Smith from the Australian Wildlife Conservancy (AWC), and Adrian Wayne, Julia Wayne and Mark Virgo from the Department of Biodiversity, Conservation and Attractions for facilitating entry into the sanctuaries and for providing unpublished trapping data. We would also like to thank Pat Dundas, Judy Gardner (Scion, New Zealand) and Yvonne Lau for help collecting soil samples in the field. Thank you to the two anonymous reviewers who provided constructive comments to improve the manuscript. Support for this project was received through a Murdoch University Small Grants Scheme. SD, AH and KR were funded through the State Centre of Excellence for Climate Change, Woodland and Forest Health, which is a partnership between private industry, community groups, universities and the Government of Western Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by David Hawksworth.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dundas, S.J., Hopkins, A.J.M., Ruthrof, K.X. et al. Digging mammals contribute to rhizosphere fungal community composition and seedling growth. Biodivers Conserv 27, 3071–3086 (2018). https://doi.org/10.1007/s10531-018-1575-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-018-1575-1